Abstract
OBJECTIVES
This study examined effects of iron deficiency anemia (IDA) on specific domains of infant cognitive function and the role of IDA-related socioemotional deficits in mediating and/or moderating these effects.
METHODS
Infants were recruited during routine 9-month visits to an inner-city clinic. IDA was defined as hemoglobin level <110 g/L with ≥2 abnormal iron deficiency indicators (mean corpuscular volume, red cell distribution width, zinc protoporphyrin, transferrin saturation, and ferritin). At 9 and 12 months, the Fagan Test of Infant Intelligence (FTII); A-not-B task; Emotionality, Activity, and Sociability Temperament Survey; and Behavior Rating Scale were administered. Analyses were adjusted for potential confounders, including age and sociodemographic variables.
RESULTS
Twenty-eight infants met criteria for IDA, 28 had nonanemic iron deficiency (NA ID) and 21 had iron sufficiency (IS). There was a linear effect for object permanence at 9 months: infants with IDA were least likely to exhibit object permanence, IS most likely, and NA ID intermediate. Infants with IDA and those with hemoglobin level ≤105 g/L showed poorer recognition memory on the FTII than infants without IDA. The Behavior Rating Scale orientation/engagement measure partially mediated these effects. Stronger effects of IDA on these outcomes were seen in infants who scored more poorly on the socioemotional measures.
CONCLUSIONS
These data indicate poorer object permanence and short-term memory encoding and/or retrieval in infants with IDA at 9 months. These cognitive effects were attributable, in part, to IDA-related deficits in socioemotional function. Children with poor socioemotional performance seem to be more vulnerable to the effects of IDA on cognitive function.
Keywords: iron deficiency anemia, infancy, recognition memory, object permanence, socioemotion, infant cognition
Iron-deficiency anemia (IDA) is a common cause of morbidity worldwide, affecting an estimated 1 to 2 billion people.1 The prevalence of IDA and iron deficiency (ID) has markedly declined in US infants since the 1980s, but rates among poor, minority, and/or immigrant infants remain high.2 Infants with IDA showed lower cognitive test scores than infants without IDA in all but 1 of 14 studies,3,4 and follow-up studies suggested that effects of chronic, severe ID in infancy on cognitive function persist later in life.5,6 Most studies also found that infants with IDA have lower motor test scores and poorer socioemotional behavior than their peers without IDA.3,4 To our knowledge, none has considered possible connections between IDA-related affective and cognitive deficits, with the exception of an early report by Lozoff et al7 of lower Bayley mental and motor scores in infants with IDA and abnormal affect.
Although IDA in infancy has consistently been associated with cognitive deficits on global developmental tests, few studies have examined specific domains of cognitive function in infancy. A preventive trial in Chile found that infants who did not receive iron supplementation exhibited slower processing speed on the Fagan Test of Infant Intelligence (FTII) than infants who received iron supplementation.8 In an inner-city US sample, we reported a developmental delay at 9 to 10 months in attention and memory in infants with IDA by using electroencephalogram event-related potentials (ERP); this delay was no longer evident at 12 to 13 months.9 We also found dose-response effects of ID (with or without anemia) on infant socioemotional behavior in this sample, specifically, increased shyness and decreased orientation/engagement, soothability, and positive affect and engagement.10 Infants who had ID without anemia showed effects that were intermediate between infants with IDA and iron sufficiency (IS).
The aims of this study were (1) to determine whether effects of IDA during infancy seen in the ERP assessments are specific to attention and memory or evident across a broader range of cognitive domains; (2) to test the hypothesis that effects of IDA on cognitive function are mediated by affective/emotional deficits; and (3) to examine whether the effects of IDA on cognitive function are exacerbated by affective/emotional differences between infants.
METHODS
Participants
Infants were recruited during routine 9-month visits to Children’s Hospital of Michigan, which serves an economically disadvantaged, inner-city population.10 Fewer than 10% of those contacted declined screening. Recruitment was restricted to black infants, who compose >90% of the clinic population. Of 881 infants screened between April 2002 and August 2005, 408 were not eligible for ≥1 reason: not black (73), low birth weight (130), multiple births (29), maternal perinatal complications (152), infant medical conditions (168), maternal age <18 years (43), heavy or unknown alcohol use (29), medicinal iron (22), or child in foster care (17). Of 473 who qualified, reasons for nonparticipation were lack of interest (61), insufficient blood (49), did not meet hematologic criteria or had insufficient iron measures to classify (35), or too old for neurobehavioral testing (86). Among the first 316 infants recruited, there were no differences between infants with blood work and those without regarding gender, birth weight, and gestational age or maternal age, education, and marital status.11 Infants without blood work had been hospitalized somewhat more often, and their mothers had more children (P < 0.05). All infants were given iron supplementation from the first visit onward regardless of iron status.
Of 242 potentially qualifying infants who were invited for neurobehavioral testing at the Child Development Research Laboratory, Wayne State University, 76 declined additional participation, 48 repeatedly missed appointments, 4 had high or missing lead values, 36 did not meet entrance criteria, and 1 had developmental delay. The neurobehavioral sample assessed at 9 to 10 months consisted of 113 healthy, term infants. Of these, 87 (77%) returned for 12-month neurodevelopmental testing. At both visits, infant weight, height, and head circumference were measured by trained staff and subsequently converted into z scores on the basis of Centers for Disease Control and Prevention norms.12 All infant assessments and maternal interviews were conducted by examiners who were blind with respect to infant iron status. The study design was approved by the Wayne State University and University of Michigan institutional review boards. Signed informed consent was obtained for the screening phase and neurobehavioral study.
Iron Status Assessment
Initial venous blood tests included a complete blood count, lead level, and zinc protoporphyrin–heme ratio, performed at the Detroit Medical Center, and serum iron, total iron-binding capacity, transferrin saturation, ferritin, transferrin receptor, and markers of inflammation performed at the laboratory of John Beard, Pennsylvania State University. Assay techniques and quality control have been reported previously.10 ID was defined as ≥2 abnormal iron measures, by using cutoffs from the Second National Health and Nutrition Examination Survey,13 the Third National Health and Nutrition Examination Survey,14 and Centers for Disease Control and Prevention15,16: mean corpuscular volume <74 fl; red cell distribution width >14%; zinc protoporphyrin–heme ratio (missing for 4 infants) >69 µmol/mol heme (corresponding to free erythrocyte protoporphyrin >80 µg/dL); transferrin saturation <12%; and ferritin <12 µg/L. IDA was defined as ID plus hemoglobin (Hb) level <110 g/L and nonanemic ID (NA ID) as ID with a higher Hb. IS was defined as Hb ≥115 g/L and no more than 1 abnormal iron measure. Of the 113 infants who underwent neurobehavioral testing, 77 met final iron status criteria: 28 IDA, 28 NA ID, and 21 IS. Given the relatively small sample size, this article focuses mainly on a comparison of the infants without anemia (NA ID + IS) with those with IDA. At the 9- to 10-month visit, infants were provided with oral iron (22 mg/day elemental iron).
Neurobehavioral Assessments
Visual Recognition Memory and Processing Speed.
Visual recognition memory was assessed at both visits by using the FTII.17 The infant, seated on the mother’s lap, is first shown 2 identical photographs and then a novel photograph paired with the familiar one. The normative response, preference for the novel stimulus, indicates ability to recall the familiar stimulus and discriminate it from the novel one. Infant fixation was recorded on a computer, and preference for novelty was computed by dividing duration of time looking at the novel stimulus by total time looking at the paired familiar and novel stimuli for each of the 10 problems. Mean length of look, a measure of processing speed,18,19 was computed for each problem by dividing the total duration looking time by the number of looks. A pattern of short looks is believed to reflect more rapid and efficient processing of information.20
Object Permanence and a Precursor of Executive Function
In the A-not-B task,21 the examiner hides a toy in 1 of 2 locations. Object permanence, the Piagetian task most strongly related to later IQ,22 is administered to determine whether the infant can retrieve the toy from the location where it is hidden.23 In subsequent trials, the examiner distracts the infant for 3 seconds after hiding the toy. When the infant succeeds in retrieving the toy 3 times, the examiner increases the length of the delay by 2 seconds, to a maximum of 11 seconds. Performance is assessed in terms of the longest delay at which the infant succeeds in retrieving the toy and perseverative errors, the percentage of trials during which the infant continues to search on the incorrect side. This paradigm is considered a precursor of executive function21 (ie, the ability to use new information to override a prepotent tendency to search where the toy was previously retrieved).24
Symbolic Play
Symbolic play with objects emerges in a hierarchical sequence of stages of increasing complexity.25 Complexity of play was assessed by using the procedure developed by Belsky et al26 and adapted by Jacobson et al.27 The infant is placed on the floor with a standard set of toys for 10 minutes of free play. Suggestion and modeling are then used to elicit progressively higher levels of play than those spontaneously exhibited by the infant. Elicited play level is scored as the highest of 14 levels successfully imitated by the infant. Level of play was scored by a single examiner, whose interobserver reliability calculated for a previous study on the basis of 33 assessments was 94.5% (range: 83%–100%).28
Affective Measures
As previously reported,10 mothers completed the 20-item Emotionality, Activity, and Sociability Temperament Survey29 at both visits. Internal consistency reliabilities for the Emotionality, Activity, and Sociability Temperament Survey ranged from r = −0.12 to 0.51 (median: 0.24). Two infant examiners completed a 30-item Behavior Rating Scale (BRS)30 at both ages. BRS composite affective factors include orientation/engagement and emotional regulation. Internal consistency reliabilities ranged from r = 0.20 to 0.80 (median: 0.50) for orientation/engagement and r = 0.00 to 0.65 (median: 0.33) for emotional regulation at 9 months. Mothers and examiners were unaware of the infant’s iron status at the time of assessment.
Control Variables
Maternal control variables that were examined for potential confounding effects included age at delivery, educational level, Hollingshead Scale for Socioeconomic Status,31 social support,32 verbal (Peabody Picture Vocabulary Test–Revised)33 and nonverbal (Raven Progressive Matrices)34 cognitive ability, Beck Depression Inventory,35 Spielberger State-Trait Anxiety Scale,36 quality of parenting and cognitive stimulation (Home Observation for Measurement of the Environment),37 and stressful life events.38 Infant control variables included age at visit, gender, growth indices, breastfeeding, and blood lead levels.
Data Analysis
The relation between object permanence and IDA was examined by using χ2. The continuous measures of cognitive function were examined in an analysis of covariance comparing the infants with and without IDA, after adjustment for potential confounders. A suggestive trend relating to recognition memory was examined further in an exploratory analysis comparing infants who had more marked IDA (Hb level ≤105 g/L plus ≥2 abnormal iron measures) with infants who did not have anemia on novelty preference by using independent sample t tests. Outcomes related to IDA were examined in multivariate analyses, in which all potential confounding variables that were even weakly related to the outcome measure (at P < .10) were controlled statistically.
Hierarchical multiple regression analysis was performed to determine whether any socioemotional measures that are affected by IDA mediate the observed effects of IDA on cognitive outcome. For each cognitive outcome related to IDA, IDA status and the potential confounders were entered in step 1; the socioemotional variable being examined was entered in step 2. When the standardized regression coefficient for IDA at step 1 was significantly reduced at step 2, we inferred that the socioemotional measure being examined partially mediated the effects of IDA on the cognitive outcome. Statistical significance for mediation was tested by evaluating the change in the regression coefficient for IDA from step 1 to step 2 by using the Difference in Coefficients Test.39,40 One-tail tests were used because the mediation hypothesis was unidirectional.
For examination of whether socioemotional factors exacerbated the effect of IDA on cognitive outcome, each socioemotional variable was dichotomized at the median.41 Analyses of the relation of IDA to the cognitive outcomes were then performed separately for each group. When the regression βs for IDA in relation to the cognitive outcome were at least 20% larger in 1 of the 2 groups, we inferred that the socioemotional factor on which the groups were dichotomized exacerbated the effect of IDA on the cognitive outcome in question.
RESULTS
Sample Characteristics
Infants with IDA were similar to infants without anemia in sociodemographic background but differed on the hematalogic indices and weight at both visits (Table 1). Infants with IDA weighed less and had lower weight-for-height z scores. Lead levels were low in all groups; none had levels >9 µg/dL, and >90% had levels <5 µg/dL. There were no differences in iron indices, IDA status, or sociodemographic background at 9 months between those who returned for testing at 12 months and those who did not (P > .15), except that mothers who were not seen at 12 months had completed fewer years of school (11.6 vs 12.4; P < .05), and infant length was slightly shorter for those seen only at 9 months (69.4 vs 71.0 cm; P = .05). After 3 months of treatment, iron status data at 12 months, available for 49 of the 77 infants, demonstrated persistent between-group differences in Hb (IDA 110 g/L vs non-IDA 119 g/L; P ≤ .01), although among infants with IDA, Hb had improved significantly (from 102 to 110 g/L; P ≤ .01).
TABLE 1.
Sample Characteristics by Iron Group
| Characteristic | No Anemia | IDA | |||
|---|---|---|---|---|---|
| N | Mean or % (SD) |
N | Mean or % (SD) |
t or χ2 | |
| Infant characteristics | |||||
| Hb, g/L | 49 | 121 (0.6) | 28 | 102 (0.5) | −14.53a |
| Female gender, % | 49 | 38.8 | 28 | 50.0 | 0.92 |
| Age at 9-mo visit, mo | 49 | 9.8 (0.3) | 28 | 9.6 (0.4) | −1.40 |
| Age at 12-mo visit, mo | 38 | 12.7 (0.6) | 24 | 12.6 (0.5) | −0.69 |
| Breast-fed, % | 49 | 53.0 | 28 | 32.0 | −3.15b |
| Lead, µg/dL | 49 | 2.6 (2.1) | 28 | 2.4 (1.5) | −0.57 |
| 9 mo | |||||
| Weight for age z score | 49 | 0.1 (1.2) | 28 | −0.5 (1.9) | −2.40c |
| Height for age z score | 49 | −0.4 (1.2) | 28 | −0.5 (0.9) | −0.40 |
| Weight for height z score | 49 | 0.9 (1.3) | 28 | 0.2 (1.1) | −2.65d |
| Head circumference, cm | 49 | 45.2 (1.6) | 28 | 44.6 (1.6) | −1.03 |
| 12 mo | |||||
| Weight for age z score | 38 | 0.6 (1.5) | 24 | 0.2 (1.1) | −2.30c |
| Height for age z score | 38 | −0.1 (1.0) | 24 | −0.4 (0.8) | −1.21 |
| Weight for height z score | 38 | 0.8 (1.2) | 24 | 0.0 (2.2) | −1.80c |
| Head circumference, cm | 38 | 46.4 (1.5) | 24 | 46.1 (1.3) | −0.70 |
| Maternal characteristics | |||||
| Age, y | 47 | 24.6 (5.8) | 28 | 23.9 (5.6) | −0.52 |
| Education, y | 48 | 12.3 (1.3) | 20 | 12.3 (1.6) | 0.21 |
| Verbal competence (PPVT-R33) | 45 | 72.5 (14.4) | 27 | 70.7 (12.8) | −0.54 |
| Raven Progressive Matrices34 | 37 | 35.2 (10.3) | 24 | 35.1 (9.5) | −0.40 |
| Hollingshead Scale for Socioeconomic Status31 | 47 | 28.3 (7.7) | 26 | 28.1 (9.4) | −0.11 |
| Social supporte | 38 | 3.3 (0.5) | 22 | 3.5 (0.3) | 1.69 |
| HOME score37 | 38 | 31.1 (6.1) | 23 | 32.4 (5.2) | 0.86 |
| Beck Depression Inventory35 | 46 | 6.2 (4.9) | 27 | 6.1 (5.0) | −0.11 |
| Spielberger State-Trait Anxiety Scale36 | 37 | 35.8 (9.4) | 24 | 32.8 (9.1) | −1.22 |
| Life Experience Survey–Maternal38 | 38 | 21.3 (18.0) | 22 | 24.1 (18.1) | 0.87 |
PPVT-R indicates Peabody Picture Vocabulary Test-Revised; HOME, Home Observation for Measurement of the Environment–Revised.
P ≤ .001.
P ≤ .10.
P ≤ .05.
P ≤ .01.
Crnic’s adaptation of a scale by Henderson.32
Neurocognitive Outcomes
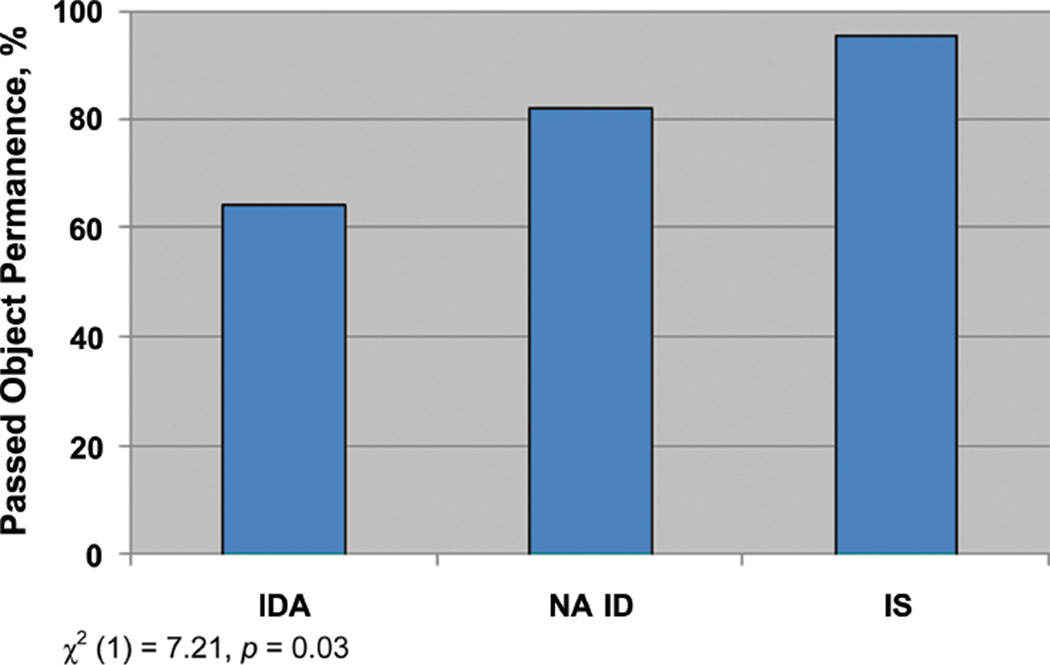
At 9 to 10 months of age, the infants with IDA were significantly less likely to exhibit object permanence (odds ratio: 0.25 [95% confidence interval: 0.08–0.80]; Table 2). This finding was examined further in a comparison of the IDA, NA ID, and IS groups (Fig 1), which showed a clear dose-response effect of iron status on object permanence. The threshold for this effect was between the IDA and all infants without anemia; only 64.3% of the former passed object permanence, compared with 87.8% of the latter (, P = .02). There was also a suggestive trend for IDA to be associated with less novelty preference on the Fagan recognition memory test (Table 3). When infants with more marked IDA (Hb ≤105 g/L) were compared with infants who were did not have anemia (Hb ≥110 g/L), those with IDA showed less novelty preference on the FTII (mean: 56.7 vs 60.3, respectively; t80 = 2.10, P < .05). In posthoc analyses, the threshold for this effect was between the infants with IDA and NA ID (mean: 56.7 vs 61.3, respectively; Bonferroni P < .05). There was no difference in novelty preference between the group with NA ID and the group with IS (mean: 61.3 and 60.2, respectively; Bonferroni P > .20). There was no difference in processing speed on the Fagan test or on executive function or perseverative errors measures on the A-not-B task. No group differences were evident on the assessment of complexity of symbolic play or any cognitive measure at 12 to 13 months.
TABLE 2.
Object Permanence by Iron Status
| Object Permanence |
Infants Assessed at 9 mo, n (%) | ||
|---|---|---|---|
| No Anemia |
IDA | Total | |
| Pass | 43 (87.8) | 18 (64.3) | 61 (79.2) |
| Fail | 6 (12.2) | 10 (35.7) | 16 (20.8) |
, P = .02.
FIGURE 1.
Proportion of children who passed object permanence task by iron status group.
TABLE 3.
Neurobehavioral Outcomes by Iron Status, Adjusted for Potential Confounders
| Outcome | No Anemia | IDA | F | ||
|---|---|---|---|---|---|
| N | Mean | N | Mean | ||
| 9 mo | |||||
| FTII | |||||
| Mean novelty preference, % | 49 | 60.9 | 28 | 58.1 | −1.80a |
| Processing speed, mean look time, msb | 49 | 1.6 | 28 | 1.6 | −0.08 |
| A-not-B | |||||
| Perseverative errors, %c | 49 | 52.3 | 27 | 48.0 | −1.08 |
| Longest delay, sd | 43 | 1.4 | 18 | 1.7 | 0.41 |
| 12 mo | |||||
| FTII | |||||
| Mean novelty preference, % | 37 | 60.6 | 23 | 59.7 | −0.59 |
| Processing speed, mean look time, mse | 37 | 1.5 | 23 | 1.6 | 1.04 |
| A-not-B | |||||
| Perseverative errors, %f | 38 | 45.6 | 24 | 42.9 | −0.54 |
| Longest delay, sg | 36 | 2.7 | 22 | 3.5 | 1.07 |
| Highest level of elicited play | 34 | 9.2 | 22 | 8.6 | −0.79 |
P = .08.
Adjusted for Life Events Scale–Mother, level of social services support, anxiety (state), and anxiety (trait).
Adjusted for maternal age.
Adjusted for infant age at visit and maternal IQ.
Adjusted for breastfeeding, maternal IQ, maternal education, and level of social services support.
Adjusted for maternal IQ.
Adjusted for Life Events Scale–Mother, level of social services support, and anxiety (state).
Mediation and Effect Modification by Socioemotional Behavior
As previously reported, infants in this sample with IDA had lower BRS orientation/engagement scores than infants without anemia and were rated as shyer by their mothers.10 Addition of orientation/engagement to the hierarchical regression of IDA on object permanence decreased the standardized regression coefficient for IDA by 19.6% (t = 1.89, P < .05, 1-tailed), calculated as recommended by MacKinnon et al40 (Table 4). Similarly, the inclusion of orientation/engagement when examining the relation of more marked IDA to Fagan novelty preference resulted in a decrease in the coefficient for IDA by 24.0% (t = 1.70, P < .05, 1-tailed; Table 4). In contrast, inclusion of shyness ratings in the regression analyses did not alter the relation of IDA to novelty preference (t = 0.48, nonsignificant) and only slightly reduced the relation of IDA to object permanence (t = 1.00, nonsignificant). Thus, orientation/engagement seems to partially mediate the effects of IDA on both object permanence and novelty preference. The effects of IDA on object permanence and novelty preference (Table 5) were seen mainly in infants who scored below the median on measures of emotionality, sociability, shyness, and orientation and those who scored above the median on activity.
TABLE 4.
Potential Mediating Effects of Socioemotional Measures in the Relation of IDA to Object Permanence and Novelty Preference
| Parameter | IDA to Object Permanence |
IDA to Novelty Preference |
|||
|---|---|---|---|---|---|
| r | β1 | β2 | r | β3 | |
| EAS | |||||
| IDA | 0.29a | .26b | .24a | 0.24a | .24c |
| Shyness | −0.13 | −.12 | −.06 | 0.04 | .09 |
| BRS | |||||
| IDA | 0.31a | .28a | .24a | 0.25a | .19c |
| Orientation/engagement | 0.27b | .24a | .20c | 0.30a | .24c |
Pvalues are 1-tailed. β1 indicates coefficient in regression model including infant age at 9-month visit; β2, coefficient from the last step of the regression model including infant age at 9-month visit; β3, coefficient from the last step of the regression model including IDA and the given socioemotional variable; EAS, Emotionality, Activity, and Sociability Temperament Survey.
P ≤ .05.
P ≤ .01.
P ≤ .10.
TABLE 5.
Comparing the Effects of IDA on Object Permanence and Novelty Preference Between Upper and Lower 50% Groups for Each Socioemotional Measure
| Socioemotional Measurea | Object Permanence | Novelty Preference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower 50% | Upper 50% | Lower 50% | Upper 50% | |||||||
| N | r | β | N | r | β | N | R | N | r | |
| EAS | ||||||||||
| Emotionality | 41 | 0.48b | .47b | 34 | 0.10 | .03 | 37 | 0.42b | 31 | 0.18 |
| Activity | 37 | 0.26 | .26 | 38 | 0.35c | .35c | 35 | 0.20 | 33 | 0.39c |
| Sociability | 37 | 0.39c | .36c | 38 | 0.10 | .05 | 34 | 0.39c | 34 | 0.00 |
| Shyness | 40 | 0.60d | .58d | 35 | −0.02 | −.05 | 36 | 0.38c | 32 | 0.28 |
| BRS | ||||||||||
| Orientation/engagement | 39 | 0.43b | .37c | 37 | 0.09 | .09 | 35 | 0.38c | 35 | 0.15 |
| Emotional regulation | 37 | 0.45b | .40b | 39 | 0.08 | .07 | 32 | 0.25 | 36 | 0.38c |
EAS indicates Emotionality, Activity, and Sociability Temperament Survey.
β1 controls for infant age at 9-month visit.
P ≤ .01.
P ≤ .05.
P ≤ .001.
DISCUSSION
We previously reported a developmental delay in infants with IDA from this sample in attention, stimulus encoding, and memory updating by using ERP assessments.9 One aim of this study was to determine whether these effects were evident across a range of cognitive tasks or seem to be specific to memory processing. Five aspects of cognitive processing were assessed on relatively narrow-band infant tests: recognition memory, processing speed, object permanence, A-not B, and symbolic play. Among these, IDA affected 2 measures that relate most directly to memory encoding and retrieval: object permanence and recognition memory. The effect on recognition memory was evident only in the subgroup of infants who had IDA and had Hb ≤105 g/L. These findings are consistent with the attention and memory effects seen in the ERP assessments. Moreover, maternal characteristics were unrelated to object permanence or novelty preference, indicating that these findings did not reflect sociodemographic differences.
Given that the IDA effects on object permanence and novelty preference were no longer evident at 12 to 13 months, the results reported here indicate either a developmental delay or improvement as a result of initiation of iron supplementation at 9 to 10 months. The relatively small numbers of infants in each group make it difficult to choose between these interpretations. We were unable to supervise iron administration, and response to iron could not be determined for infants who did not come for a repeat blood test. The degree of IDA in this population was relatively mild and duration probably quite short, because infants generally received iron-fortified formula. More pervasive deficits might well be evident with more severe and/or prolonged IDA.
IDA is associated with fatigue and decreased attention, play, and motivation42; similar decreases in activity and more submissive behavior and interaction have been noted in animal studies.43,44 Our mediation analyses suggest that the effects of IDA on both object permanence and recognition memory are attributable, in part, to the poorer orientation/engagement that is associated with IDA. Thus, these cognitive deficits seem to be attributable, in part, to the reduced ability of the infant with IDA to engage actively with the environment. Moreover, the effects of IDA on cognitive function were seen most clearly in the infants who scored more poorly on measures of socioemotional function and orientation/engagement, suggesting that these socioemotional and attentional deficits can increase the infant’s vulnerability to the cognitive effects of IDA.
In the primary care setting, screening for IDA often occurs at 9 to 12 months; however, our findings suggest that cognitive (and socioemotional) deficits may be apparent even earlier. Furthermore, the linear effects of iron status on object permanence raise concern about infants who have NA ID and may thus be missed by standard Hb screening. This pattern was even more pronounced for socioemotional behavior, leading us to speculate that the socioemotional domain might be particularly sensitive to ID, at least in infants similar in age to those in our sample and/or with relatively mild ID.
CONCLUSIONS
Our findings provide evidence of specific deficits in cognitive processing (attention and memory) with IDA during an important period of infant development before the usual age for IDA screening in the primary care setting. Deficits in these processes, which have demonstrated predictive validity for later cognitive function, have implications for intellectual function in childhood. Furthermore, these early cognitive deficits seem to be mediated, in part, by the effects of IDA on the infant’s ability to engage affectively with the environment, and socioemotional deficits seem to increase the infant’s vulnerability to the cognitive effects of early IDA.
WHAT’S KNOWN ON THIS SUBJECT.
IDA in infancy is associated with cognitive deficits, which may persist later in life. Socioemotional deficits are also consistently observed in infants with IDA, but it is not known whether these deficits affect cognitive function.
WHAT THIS STUDY ADDS.
With these findings the authors demonstrate specific IDA-related deficits in attention and recognition memory, which were found to be partially mediated by IDA effects on socioemotional measures. Infants with socioemotional deficits seem to be more vulnerable to cognitive effects of IDA.
ACKNOWLEDGMENTS
This study was supported by a program project grant from the National Institutes of Health (P01 HD39386, Brain and Behavior in Early Iron Deficiency, Dr Lozoff, principal investigator) and a grant from the Joseph Young, Sr, Fund from the State of Michigan to Dr Jacobson.
We thank the study families for participation; our colleague the late John Beard (Pennsylvania State University) for performing additional iron status measures and advising on their interpretation; our collaborators Charles A. Nelson and Rosa Angulo-Barroso for contributions to study design; and Tal Shafir, Renee Sun, Jigna Zatakia, Margo Laskowski, Brenda Tuttle, Douglas Fuller, Agustin Calatroni, Yuezhou Jing, Niko Kaciroti, and Kelly Zidar for work on data collection and analysis.
ABBREVIATIONS
- IDA
iron-deficiency anemia
- ID
iron deficiency
- FTII
Fagan Test of Infant Intelligence
- ERP
electroencephalogram event-related potentials
- IS
iron sufficiency
- Hb
hemoglobin
- NA ID
nonanemic ID
- BRS
Behavior Rating Scale
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Iron Deficiency Anemia: Prevention, Assessment and Control—Report of a Joint WHO/UNICEF/UNU Consultation. Geneva, Switzerland: World Health Organization; 1998. World Health Organization/United Nations Children’s Fund/United Nations University. [Google Scholar]
- 2.Brotanek JM, Halterman J, Auinger P, Flores G, Weitzman M. Iron deficiency, prolonged bottle-feeding, and racial/ethnic disparities in young children. Arch Pediatr Adolesc Med. 2005;159(11):1038–1042. doi: 10.1001/archpedi.159.11.1038. [DOI] [PubMed] [Google Scholar]
- 3.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131(2S–2):649S–668S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 4.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(5 pt 2):S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004. pp. 2141–2165. [Google Scholar]
- 7.Lozoff B, Wolf AW, Urrutia JJ, Viteri FE. Abnormal behavior and low developmental test scores in iron-deficient anemic infants. J Dev Behav Pediatr. 1985;6(2):69–75. [PubMed] [Google Scholar]
- 8.Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants [published correction appears in Pediatrics. 2004;113(6):1853] Pediatrics. 2003;112(4):846, 854. [PubMed] [Google Scholar]
- 9.Burden MJ, Westerlund AJ, Armony-Sivan R, et al. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120(2) doi: 10.1542/peds.2006-2525. Available at: www.pediatrics.org/cgi/content/full/120/2/e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152(5):696–702. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozoff B, Angelilli M, Zatakia J, Jacobson SW, Calatroni A, Beard J. Iron status of inner-city African-American infants. Am J Hematol. 2007;82(2):112–121. doi: 10.1002/ajh.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden C, Grummer-Strawn LM, et al. CDC Growth Charts: United States. Hyattsville, MD: US Department of Health and Human Services; 2000. NCHS Advance Data Report No. 314. [Google Scholar]
- 13.Assessment of the Iron Nutrition Status of the US Population Based on Data Collected in the Second National Health and Nutrition Survey, 1976–1980. Bethesda, MD: Federation of American Societies for Experimental Biology; 1984. Life Sciences Research Office. [Google Scholar]
- 14.Looker AC, Dallman P, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277(12):973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 15.Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(3):1–29. [PubMed]
- 16.Centers for Disease Control. Healthy People 2000 National Health Promotion and Disease Prevention Objectives, Final Review. Hyattsville, MD: Department of Health and Human Services; 2001. [Google Scholar]
- 17.Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. In: Lipsitt LP, editor. Advances in Infancy Research. Vol 2. NJ: Ablex; 1983. pp. 31–78. Norwood. [Google Scholar]
- 18.Colombo J, Mitchell DW. Individual differences in early visual attention: fixation time and information processing. In: Colombo J, Fagen J, editors. Individual Differences in Infancy: Reliability, Stability, Prediction. Hillsdale, NJ: Lawrence Erlbaum; 1990. pp. 193–227. [Google Scholar]
- 19.Colombo J, Mitchell DW, Coldren JT, Freeseman LJ. Individual differences in infant visual attention: are short lookers faster processors or feature processors? Child Dev. 1991;62(6):1247–1257. [PubMed] [Google Scholar]
- 20.Jacobson SW, Jacobson JL, O’Neill JM, Padgett RJ, Frankowski JJ, Bihun JT. Visual expectation and dimensions of infant information processing. Child Dev. 1992;63(3):711–724. [PubMed] [Google Scholar]
- 21.Diamond A. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Dev. 1985;56(4):868–883. [PubMed] [Google Scholar]
- 22.Wachs TD. Relation of infants’ performance on Piaget scales between twelve and twenty-four months and their Stanford-Binet performance at thirty-one months. Child Dev. 1975;46:929–935. [Google Scholar]
- 23.Uzgiris C, Hunt JM. Assessment in Infancy: Ordinal Scales of Psychological Development. Urbana, IL: University of Illinois Press; 1975. [Google Scholar]
- 24.Diedrich FJ, Thelen E, Smith LB, Corbetta D. Motor memory is a factor in infant perseverative errors. Dev Sci. 2000;3(4):479–494. [Google Scholar]
- 25.McCune-Nicholic L. Toward symbolic functioning: structure of early pretend games and potential parallels with language. Child Dev. 1981;52(3):785–797. [Google Scholar]
- 26.Belsky J, Garduque L, Hrncir E. Assessing performance, competence, and executive capacity in infant play: relations to home environment and security of attachment. Dev Psychol. 1984;20(3):406–417. [Google Scholar]
- 27.Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Kaplan-Estrin MG. Teratogenic effects of alcohol on infant development. Alcohol Clin Exp Res. 1993;17(1):174–183. doi: 10.1111/j.1530-0277.1993.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Dev. 1993;64(6):1706–1721. [PubMed] [Google Scholar]
- 29.Buss AH, Plomin R. Temperament: Early Developing Personality Traits. Hillsdale, NJ: Lawrence Erlbaum; 1984. [Google Scholar]
- 30.Bayley N. Bayley Scales of Infant Development. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 31.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [unpublished manuscript] [Google Scholar]
- 32.Crnic KA, Greenberg MT, Ragozin AS, Robinson NM, Basham RB. Effects of stress and social support on mothers and premature and full-term infants. Child Dev. 1983;54(1):209–217. [PubMed] [Google Scholar]
- 33.Dunn LM, Dunn LM. PPVT Manual for Forms L and M. Circle Pines, MN: American Guidance Service; 1981. [Google Scholar]
- 34.Raven JC, Court JH, Raven J. Raven Matrices Progressive Manual. Madrid, Spain: Publicaciones De Psicologia Aplicada; 1996. [Google Scholar]
- 35.Beck AT, Steer RA, Brown GK. Beck Depression Inventory II. San Antonio, TX: Psychological Corporationss; 1996. [Google Scholar]
- 36.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 37.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas Press; 1984. [Google Scholar]
- 38.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46(5):932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 39.Clogg CC, Petkova E, Shihadeh ES. Statistical methods for analyzing collapsibility in regression models. J Educ Stat. 1992;17(1):51–74. [Google Scholar]
- 40.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychol Bull. 1993;114(2):376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- 42.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child Dev. 1998;69(1):24–36. [PubMed] [Google Scholar]
- 43.Coe CL, Lubach GR, Schneider ML. Neuromotor and socio-emotional behavior in the young monkey are presaged by prenatal conditions. In: Lewis M, Ramsey D, editors. Stress and Soothing. Hillsdale, NJ: Lawrence Erlbaum; 1999. pp. 19–38. [Google Scholar]
- 44.Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51(1):47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]