Abstract
It is unclear what level of neutralizing antibody is sufficient to protect cattle from experimental bovine papillomavirus type 4 (BPV4) challenge. Markedly lower, and often undetected, serum neutralizing antibody titers were associated with protection in cattle vaccinated with BPV4 L2 as compared to L1 VLP. We hypothesized that vaccination with concatemers of the N-terminal protective epitopes of L2 derived from multiple animal papillomavirus types would enhance the breadth and strength of immunity. Therefore we generated a multimeric L2 antigen derived from three bovine and three canine papillomavirus types with divergent phenotypes and purified it from bacteria. Mice vaccinated three times with this six type L2 vaccine formulated in alum or RIBI adjuvant generated robust serum neutralizing antibody titers against BPV1, BPV4 and canine oral papillomavirus (COPV). Furthermore, vaccination with this six type L2 vaccine formulated in alum, like BPV1 L1 VLP, protected the mice from experimental challenge with BPV1 pseudovirus.
Keywords: papillomavirus, L2, warts, sarcoid, vaccination
BACKGROUND
High risk human papillomaviruses (HPV) cause 5% of all cancer worldwide, and the low risk types are responsible for considerable morbidity (Parkin and Bray, 2006). While the HPVs do not productively infect non-human species and vice versa (Koller and Olson, 1972), a plethora of animal papillomaviruses induce papillomas and cancer that result in distress to the host and significant agricultural losses (Bernard et al., 2010; Munday and Kiupel, 2010). Ten different genotypes of bovine papillomavirus (BPV) have been described. BPV infection causes skin warts and urinary bladder cancer (BPV1 and BPV2), papillomatosis and cancers of the upper gastrointestinal tract (BPV4), papillomatosis of the penis (BPV1), teats and udder (BPV1, BPV5 and BPV6) (Nasir and Campo, 2008). The development of cancers can be enhanced by an interaction between BPV infection and consumption of carcinogens and immunosuppressants within bracken fern (Campo, 1987). Horses, donkeys and mules can develop sarcoids upon exposure to BPV1 and BPV2 that are locally invasive, refractory to treatment and often require culling of the animal (Nasir and Campo, 2008). Tumors can also be induced by challenge of genital mucosa or urinary bladder of cattle (Olson, Luedke, and Brobst, 1962). Canine oral papillomavirus (COPV) produces papillomas in the oropharynx of dogs that can spread to other regions and even progress to squamous cell carcinoma (SCC) (Bregman et al., 1987). A number of other canine papillomaviruses (CfPV2, CfPV3 and CfPV4) have also been associated with SCC, and feline (FdPV1 and FdPV2) and cottontail rabbit (CRPV) papillomaviruses can produce SCC in their respective hosts (Munday and Kiupel, 2010).
Licensed vaccines for the prevention of HPV infection are comprised of virus-like particles (VLPs) derived by recombinant expression of major capsid protein L1 (Roden and Wu, 2006). The impetus for their development came from the protection of rabbits, dogs and cattle from viral challenge by prior vaccination with L1 VLP generated from the genotype of papillomavirus used for challenge or passive transfer with immune serum (Breitburd et al., 1995; Christensen et al., 1996; Kirnbauer et al., 1996; Suzich et al., 1995). Passive transfer studies in the rabbit and dog models demonstrated that the neutralizing antibodies generated by L1 VLP vaccination are sufficient to mediate protection that is type restricted, although the minimal titers required for protection are not currently known (Breitburd et al., 1995; Suzich et al., 1995). Type-restriction in the protection of patients vaccinated with L1 VLP has triggered the development of a nonavalent L1 VLP formulation to broaden coverage to the majority of oncogenic HPV genotypes (Campo and Roden, 2010; Munoz et al., 2004). While formalin-treated papillomavirus vaccines have been used for veterinary applications, there are currently no commercial VLP-based vaccines for use in animals (Bell et al., 1994; Olson, Luedke, and Brobst, 1962; Olson, Robl, and Larson, 1968).
The diversity of papillomaviruses associated with disease in agriculturally significant and other domesticated species suggested that highly multivalent L1 VLP vaccines might be required even for individual species. Another possible prophylactic vaccine antigen is the minor capsid protein L2 (Campo and Roden, 2010). Studies in the cattle and rabbit challenge models suggest that vaccination with the amino terminus of L2 comprising residues 11–200 or 1–88 produced recombinantly in bacteria is protective (Campo, 1994; Chandrachud et al., 1995; Gambhira et al., 2007a). Furthermore vaccination of rabbits with residues 1–88 of L2 induces broadly cross-neutralizing antibodies and is sufficient for protection (Gambhira et al., 2007a; Pastrana et al., 2005). Vaccination of rabbits with L2 derived from HPV16 protects against challenge with either CRPV or ROPV, suggesting the possibility of broad protection using this antigen (Gambhira et al., 2007a). While neutralizing antibodies generated by L1 VLP recognize conformational epitopes, the neutralizing epitopes within L2 are predominantly linear (Christensen et al., 1991; Gambhira et al., 2007b; Knowles et al., 1997; Lin et al., 1992; Pilacinski et al., 1986). Thus, unlike L1 VLP, the breadth of protection can potentially be further enhanced by generating a multimer of the protective epitope derived from diverse papillomavirus types (Jagu et al., 2009). Herein we have explored the potential of this approach to derive a multimeric L2 vaccine for prevention of animal papillomavirus infections, and to examine the neutralizing antibody titers associated with protection.
RESULTS AND DISCUSSION
Passive transfer studies of L1 VLP or L2-specific neutralizing IgG protects naïve animals from experimental papillomavirus challenge suggesting that antibodies are at least sufficient to mediate immunity. However, the minimal serum titer of neutralizing antibodies necessary to effect protection against papillomavirus challenge is not currently defined, although recent studies suggest that it is very low (Day et al., 2010). To examine this question we exploited serum samples derived in earlier published challenge studies in which calves vaccinated twice with various BPV4 L2 polypeptides (Campo et al., 1997; Chandrachud et al., 1995) or VLPs (Kirnbauer et al., 1996) were subsequently challenged on the soft palate at ten sites with 1011 native BPV4 virions, and followed for the development of papillomas (Figure 1). These prior studies demonstrated that BPV4 VLPs and some, but not all L2 polypeptides protected the calves to varying degrees from experimental BPV4 challenge, thus providing an opportunity to correlate degree of protection with potentially relevant immune parameters, including BPV4 L1 and L2-specific and neutralizing antibody levels.
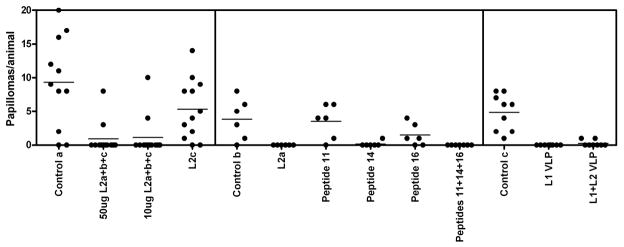
Figure 1.
Summary of three previously published BPV4 challenge studies in cattle receiving prophylactic vaccination with BPV4 L2 peptides or VLP. Animals in the control groups (Control a, Control b and Control c) received no vaccinations prior to challenge. The following polypeptide immunogens were used: L2a (BPV4 L2 11–200 fused with GST), L2b (BPV4 L2 201–326 fused with GST), L2c (BPV4 L2 327–454 fused with GST), and full length BPV4 L2 fused to GST. The following peptides were coupled to KLH and used as immunogens: peptide 11 comprising residues 101–120 of BPV4 L2 (TGVPIDPAVPDSSIVPLLES), peptide 14 comprising L2 residues 131–151(PGAEIEIIAEVHPPPVYEGPE), and peptide 16 comprising L2 residues 151–170 (EVTIGDIEEPPILEVVPETH), or a combination of the 3 peptides (Campo et al., 1997). BPV4 L1 only or L1+L2 VLPs were prepared using recombinant baculovirus (Kirnbauer et al., 1996). Left panel: Calves were vaccinated twice a month apart with 50μg or 10μg of L2a+b+c (a combination of L2a, L2b, L2c and full length BPV4 L2 in the ratio 5:5:5:1) or with 330μg L2c, all formulated in alhydrogel (Campo, 1997). Middle panel: Calves were vaccinated twice a month apart with 330μg of L2a, or 50μg of peptide 11, peptide 14, peptide 16, or all three together, in each case formulated in alhydrogel (Campo et al., 1997). Right panel: Calves were vaccinated twice a month apart with 150μg of BPV4 L1 VLPs or 200μg of L1+L2 VLPs, both formulated in alhydrogel (Kirnbauer et al., 1996). All animals were challenged on the soft palate two weeks after the final vaccination with 1011 BPV4 virions.
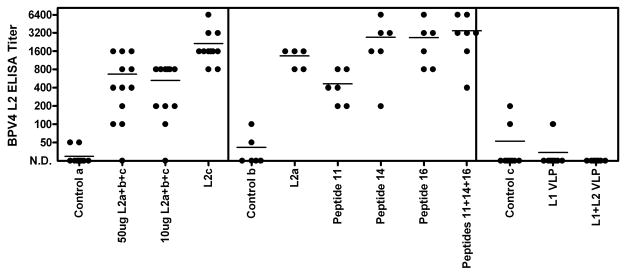
Initially, the ability of the vaccination with BPV4 L2-derived peptides or VLPs to induce specific serum antibody titers was examined by ELISA using full length BPV4 L2 protein purified from E. coli using a hexahistidine tag (Figure 2). Cattle vaccinated with L2a (BPV4 L2 11–200 fused with GST), L2b (BPV4 L2 201–326 fused with GST), L2c (BPV4 L2 327–454 fused with GST), or L2a+b+c (a combination of L2a, L2b, L2c and full length BPV4 L2 in the ratio 5:5:5:1) all formulated in alum adjuvant generated detectable titers of BPV4 L2-reactive serum IgG antibodies (Campo et al., 1997; Chandrachud et al., 1995). Conversely, these antibodies were lacking in the control animals and those vaccinated with either L1 only VLPs or VLPs containing L1 and L2. The failure of immunization with L1+L2 VLPs to induce significant L2-specific antibody responses has been observed previously (Roden et al., 2000), and might suggest that the majority of L2 is buried below the capsid surface (Day et al., 2008), and, as a minor and widely spaced constituent of the capsid, L2 is immunologically subdominant to L1(Chackerian, Lowy, and Schiller, 2001).
Figure 2.
Assessment of BPV4 L2-specific serum immunoglobulin responses to vaccination by ELISA. Sera from cattle vaccinated with BPV4 L2-derived peptides or VLPs from the prior studies summarized in Figure 1 were tested for specific serum antibody titers by ELISA using full length BPV4 L2 protein. The serum samples tested in the left panel, middle panel and right panel are described respectively in (Campo et al., 1997; Chandrachud et al., 1995; Kirnbauer et al., 1996).
Unfortunately native BPV4 virions do not produce a readily detectable phenotype upon infection of cells in tissue culture that can be utilized in the measurement of viral titers and neutralizing antibodies. A model in which bovine fetal palate chips are infected in vitro with native BPV4 in the presence of serum and then engrafted subcutaneously into the flank of nude mice has demonstrated that vaccination with BPV4 L2 induces neutralizing antibodies (Gaukroger et al., 1996). However, to simplify the measurement of neutralizing antibody titers we developed BPV4 pseudovirions that encapsidate a reporter plasmid expressing secreted alkaline phosphatase (Buck et al., 2004; Pastrana et al., 2004). The BPV4 L1 and L2 genes were synthesized as codon-modified constructs and each inserted into the pcDNA3.1(-) vector to enhance their expression in mammalian cells as previously described for BPV1 (Buck et al., 2004). The BPV4 L1 and L2 expression constructs and the pSEAP reporter plasmid were co-transfected in to 293TT cells and after 72h the cells were harvested and lysed with detergent (Supplementary Figure 1). The cell lysates were separated by density centrifugation and the fractions typically containing particles analyzed by SDS-PAGE and Coomassie blue staining and Western blot for the presence of either BPV4 L1 or L2 (Supplementary Figure 1) and titrated for their ability to transfer the reporter plasmid to fresh 293TT cells. The fraction containing the greatest titer for delivery of the secreted alkaline phosphatase (SEAP) reporter exhibited the highest level of L2 and a high level of L1, and thus it was utilized for the development of an in vitro neutralization assay for BPV4.
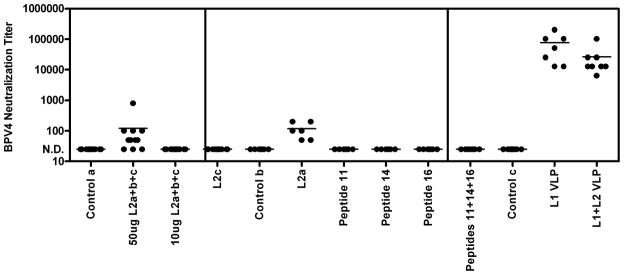
The in vitro neutralization assay for BPV4 was thus utilized to assay the titers of the sera of the calves vaccinated with the various BPV4 L2 constructs or VLPs (Campo et al., 1997; Chandrachud et al., 1995; Kirnbauer et al., 1996). Figure 3 shows that the sera of calves vaccinated with BPV4 L1 VLPs or L1+L2 VLPs all exhibited high neutralizing antibody titers that were absent from the sera of control animals (Kirnbauer et al., 1996). Interestingly, sera of calves vaccinated with BPV4 L2a, alone or mixed with other L2-derived peptides, contained much lower levels of BPV4 neutralizing antibodies than the sera of VLP-vaccinated calves (Campo et al., 1997; Chandrachud et al., 1995). BPV4 neutralizing antibodies were not detected in the sera of calves vaccinated with BPV4 L2c, or KLH-coupled small synthetic L2 peptides (peptide 11 comprising BPV4 L2 residues 101–120, peptide 14 comprising L2 residues 131–151, or peptide 16 comprising L2 residues 151–170 or a mixture of all three), although we cannot rule out the possibility of neutralizing antibody titers of <25 due to non-specific background at high serum concentrations.
Figure 3.
Assessment of titers for BPV4 pseudovirion neutralization of sera of vaccinated cattle. Sera from cattle vaccinated with BPV4 L2-derived peptides or VLPs were tested for in vitro neutralization titers using BPV4 pseudovirions carrying the secreted alkaline phosphatase reporter. The serum samples tested in the left panel, middle panel and right panel are as in Figure 1 (described respectively in (Campo et al., 1997; Chandrachud et al., 1995; Kirnbauer et al., 1996).
These in vitro neutralization titers for BPV4 were compared to the previously published outcome of challenge of the same calves with BPV4 two weeks after their second immunization (Campo et al., 1997; Chandrachud et al., 1995; Kirnbauer et al., 1996). Vaccination with BPV4 L1 VLP protected all 8 animals against papillomas after BPV4 challenge, whereas 2 of 7 calves vaccinated with L1+L2 VLPs developed a small number of papillomas, and 9 of 10 control animals developed papillomas, mostly in larger numbers (Figure 1). However, all of the VLP immunized animals developed robust serum titers of BPV4 neutralizing antibodies that were L1-specific as determined by BPV4 L1 VLP ELISA (not shown).
Vaccination of calves with 50μg of L2a+b+c covering the entire protein protected 10 of 12 calves from experimental challenge with BPV4 (Figure 1). Conversely, 9/11 control animals developed multiple papillomas. Low titers of BPV4 neutralizing antibodies were detected in the sera of all but three calves vaccinated with 50μg of L2a+b+c in alum, and none of the sera from the control animals exhibited detectable neutralizing antibody titers. Surprisingly, none of the animals vaccinated with 10μg of L2a+b+c contained detectable neutralizing antibody titers, yet 10/12 vaccinated animals remained free of papillomas after experimental BPV4 challenge (Campo et al., 1997; Chandrachud et al., 1995).
In the prior studies an effort was made to map the protective epitopes (Campo et al., 1997; Chandrachud et al., 1995). The L2b peptide was not found to be immunogenic in the calves vaccinated with L2a+b+c, and thus protection studies were performed with the L2a and L2c antigens. Vaccination with L2c polypeptide was not protective as papillomas developed in 10 of 12 vaccinated calves, and no neutralizing antibodies were detected in their sera, despite robust L2-specific antibody titers measured by L2 ELISA. Conversely, none of 6 calves vaccinated with L2a developed papillomas after challenge, and all 6 contained detectable titers of BPV4 neutralizing antibodies, albeit much lower than those observed for VLP-vaccinated animals (Figure 1).
Mapping of the epitopes recognized by animals vaccinated with L2a using overlapping peptides spanning residues 1–200 of BPV4 L2 indicated recognition of peptide 11 comprising residues 101–120 (TGVPIDPAVPDSSIVPLLES), peptide 14 comprising L2 residues 131–151(PGAEIEIIAEVHPPPVYEGPE), and peptide 16 comprising L2 residues 151–170 (EVTIGDIEEPPILEVVPETH) by ELISA (Knowles et al., 1997). Calves were vaccinated with each peptide individually, a control peptide, or a mixture of all three peptides coupled to KLH, and all animals receiving an L2-derived peptide developed reactive serum antibodies as determined by L2 ELISA. Calves vaccinated with control peptide, peptide 11 alone, or peptide 16 alone developed papillomas after experimental challenge (5/6, 5/6, and 4/6 respectively, Figure 1), whereas calves vaccinated with peptide 14 alone or the mixture of peptides 11, 14 and 16 were protected as only 1/6 and 0/6 animals developed papillomas after experimental challenge with BPV4. This earlier study indicated that peptide 14 is a protective epitope (Campo et al., 1997), although no in vitro neutralizing antibodies were detected in the sera of these animals (Figure 3). Since passive transfer of an L2-specific neutralizing monoclonal antibody (Gambhira et al., 2007b) or small volumes of immune serum (Day et al., 2010) confers protection against experimental challenge in a mouse model, this suggests that either very low levels of neutralizing antibodies are sufficient for protection and that our in vitro neutralization assay lacks the sensitivity to detect these titers, or that T cell-based responses to L2 might also contribute to protection. Indeed, it is noteworthy that three of the 6 animals vaccinated with peptide 14 developed small translucent dome-shaped lesions termed plaques (typically seen before the appearance of typical frondy papillomas actively producing virus), yet they did not develop papillomas (Campo et al., 1997). This suggests the possibility of immune-mediated clearance of these plaques, but it is hard to exclude that partial neutralization of the inocula resulted in low level abortive infections. Jarrett et al vaccinated cattle with BPV2 L2 90–467 and this was not protective, but it did enhance regression of papillomas (Jarrett et al., 1991). This is consistent with studies in BPV1 mapping the location of the neutralizing epitopes principally to the first 88 residues of BPV1 L2 (Pastrana et al., 2005), and raises the possibility of a T cell-mediated clearance through recognition of residues 90–467. Conversely, vaccination with L2 1–88 or 11–200 of CRPV protects rabbits from cutaneous challenge with CRPV virions but not CRPV genomic DNA, and the resulting papillomas grow at the same rate in L2-vaccinated and control NZW rabbits (Gambhira et al., 2007a). The latter discrepancy might reflect either the location of the T cell epitopes of CRPV L2 after residue 200, or an incomplete late expression program for CRPV in domestic NZW rabbits as compared with cottontail rabbits, the natural host for this virus.
While several studies raise the possibility that neutralizing epitopes are present further toward the C-terminus of L2 (Campo et al., 1997; Christensen et al., 1991; Kawana et al., 1999; Lin et al., 1992), vaccination with concatemers of L2 11–88 derived from diverse HPV genotypes induces robust titers of antibodies neutralizing numerous medically significant HPV genotypes within the mucosatropic α species of papillomaviruses (Jagu et al., 2009). Since the papillomavirus genotypes impacting agricultural and domesticated animals, notably cattle and dogs, belong to distinct species of papillomaviruses (Bernard et al.), we generated a multimer of L2 residues spanning the putative furin cleavage site to residue 88 derived from three bovine and three canine papillomavirus genotypes; BPV1, BPV4, BPV5, COPV, CaPV2 and CaPV3 (Richards et al., 2006). The construct was generated by direct synthesis with codon utilization optimized for bacterial expression and inserted into the expression vector pET28a in frame with a hexahistidine tag. This construct, herein termed ‘animalx6’, was produced in Rosetta cells upon IPTG induction and affinity purified under denaturing conditions using a Ni-NTA column and exchanged into PBS by dialysis.
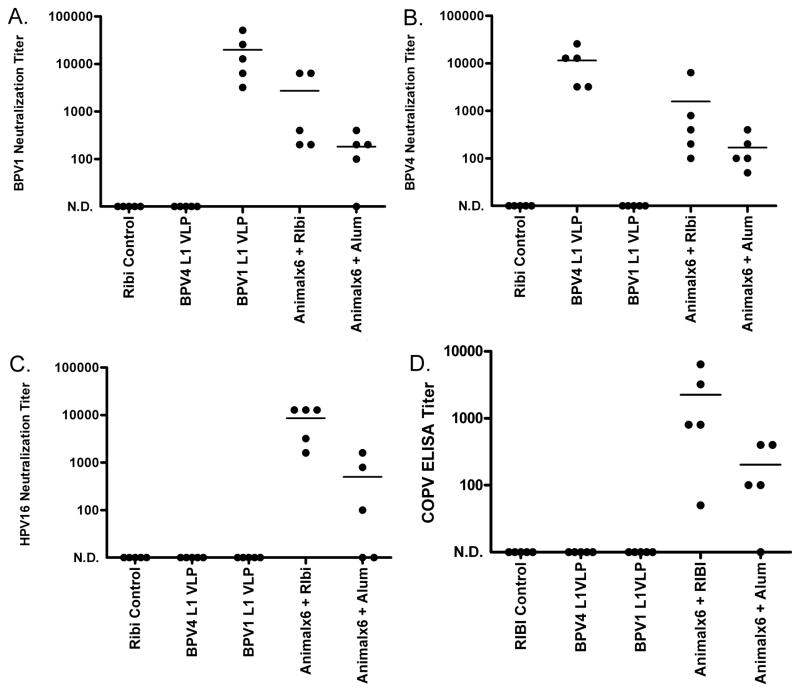
The immunogenicity of the animalx6 construct was tested in Balb/c mice (Figure 4). Two weeks after three immunizations of 25μg of protein formulated in alum or RIBI adjuvant system, sera was harvested from 5 mice and tested for in vitro neutralizing antibody titer. Additional mice were vaccinated in parallel with either Sigma adjuvant system alone or 25μg of BPV4 L1 VLP in alum, or BPV1 L1 VLP in alum on the same schedule. The sera of mice vaccinated with BPV1 L1 VLP exhibited high titers of neutralizing antibody against BPV1, but not BPV4 or HPV16 (Figure 4). Likewise, the sera of mice vaccinated with BPV4 L1 VLP had consistently high neutralizing antibody titers for BPV4, but none were detected for neutralization of BPV1 or HPV16, as expected for the type-restricted protection induced by L1 VLP vaccination. Interestingly, sera from mice vaccinated with the animalx6 protein neutralized BPV1, BPV4 and HPV16, with generally higher titers in the sera of mice vaccinated using RIBI as compared to the alum formulation of the animalx6 antigen (Figure 4). The antisera from mice vaccinated with the animalx6 antigen in either adjuvant also contained robust IgG titers that recognized COPV pseudovirions by ELISA. Pooled antisera from mice vaccinated with the animalx6 antigen in either alum or RIBI adjuvant were able to neutralize wart-derived COPV virions in vitro at 1:200, demonstrating that neutralization is not an artifact of the pseudovirion system. Therefore, the animalx6 vaccine induces a neutralizing antibody response against a broad range of papillomavirus types.
Figure 4.
Immune response of mice vaccinated with a multimeric L2 vaccine derived from six animal papillomaviruses. Sera from mice vaccinated three times at 2 week intervals with BPV4 or BPV1 L1 VLPs formulated in alum or the multimeric L2 vaccine (Animalx6) formulated in either alum or RIBI adjuvant were tested for in vitro neutralization titers using BPV1 pseudovirions (panel A), BPV4 pseudovirions (panel B) or HPV16 pseudovirions (panel C), each carrying a secreted alkaline phosphatase reporter. Sera were also analyzed using COPV pseudovirion ELISA (panel D).
The BPV/cattle and COPV/dog challenge models require considerable infrastructure and resources, and therefore we sought an alternative approach to test the protective efficacy of the animalx6 vaccine. Roberts et al have developed a mouse vaginal challenge model using HPV pseudovirions delivering a reporter construct (Roberts et al., 2007). Since BPV1 and BPV2 infect mouse cells (Dvoretzky et al., 1980), we examined whether pseudovirions derived from BPV1, BPV4 or COPV and carrying the luciferase reporter could detectably infect the mouse vaginal tract. Only BPV1 pseudovirions generated a detectable infectious signal, which may reflect differences in efficiency of pseudovirion assembly or tropism since BPV1 has been shown to infect the vagina, as well as skin and urinary bladder of cattle (Olson, Luedke, and Brobst, 1962). These findings suggested that vaginal challenge of mice with BPV1 pseudovirions might provide a feasible alternative to the challenge of calves with BPV1 virus.
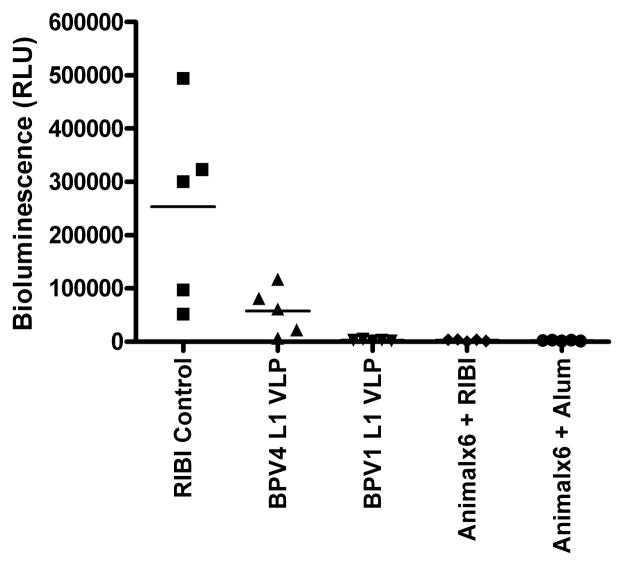
The groups of mice vaccinated with animalx6 protein formulated in either alum or RIBI, RIBI alone or L1 VLPs derived from BPV1 or BPV4 and formulated in alum were thus challenged intra-vaginally with BPV1 pseudovirions carrying the luciferase reporter. Three days later, the extent of infection was assayed by instillation of luciferin and imaging of the resultant bioluminescence. In mice receiving RIBI alone, a high bioluminescent signal was observed, and this was absent from mice that had been vaccinated with BPV1 VLPs (Figure 5). A prior study in cattle by Jarrett et al indicated that vaccination with virions of one BPV type generally failed to protect against challenge by other BPV genotypes, although there may be significant cross-protection between BPV1 and BPV2 (Jarrett et al., 1990; Shafti-Keramat et al., 2009). While BPV1 L1 VLP vaccination completely protected the mice (p<0.01), vaccination with BPV4 L1 VLPs weakly protected mice from challenge with BPV1 pseudovirus (p<0.05). Conversely, no significant infection was observed in either group of mice vaccinated with the animalx6 protein in alum or RIBI (p<0.01), as observed in the group vaccinated with BPV1 L1 VLPs.
Figure 5.
Protection of mice from genital challenge with BPV1 by L1 VLP or L2 multimer vaccines. Mice vaccinated three times at 2 week intervals with BPV4 or BPV1 L1 VLPs formulated in alum or the multimeric L2 vaccine (Animalx6) formulated in either alum or RIBI adjuvant were challenged with BPV1 pseudovirions carrying a luciferase reporter construct. Infection was assessed by measuring bioluminescence at 3 days post challenge.
These findings support earlier studies suggesting that immunization with concatemers of conserved polypeptides derived from the N-terminus of L2 (between the putative furin cleavage site and residue 88, see Supplementary Figure 2) has promise as a prophylactic vaccine for the prevention of disease in animals caused by diverse papillomavirus types (Chandrachud et al., 1995; Jagu et al., 2009). Vaccination with L2 induces neutralizing antibodies of significantly lower titers than L1 VLP, but they are nonetheless protective in several animal models, and more broadly acting than L1 VLP-induced responses (Campo and Roden, 2010). While L1 VLP vaccination elicits lasting immunity to natural infection, it remains unclear how long L2 vaccination maintains protection. However, protection of cattle from experimental BPV4 challenge one year after vaccination with L2 has been observed (McGarvie et al., 1994). Although an additional contribution by T cell-mediated responses remains a possibility (Jarrett et al., 1991), passive transfer studies have indicated that immunity generated by L2 or L1 VLP vaccination can be mediated by neutralizing antibodies alone (Day et al.; Gambhira et al., 2007b; Schiller, Day, and Kines). That no potent therapeutic effect has been demonstrated for an L1 VLP vaccine in humans (Hildesheim et al., 2007; Koutsky et al., 2002) suggests that a minimal titer of neutralizing antibodies may be required to provide sterilizing immunity. Although it remains possible that a capsid antigen-specific cytotoxic T cell response may also contribute to protection, vaccination with HPV6 L2 (fused with E7) in a potent adjuvant failed to enhance the regression rate of genital warts (Vandepapeliere et al., 2005), and vaccination with HPV16 L2 (fused with E6 and E7) failed to trigger dramatic regression of HPV16+ high grade VIN/VaIN (Davidson et al., 2003; Fiander et al., 2006; Smyth et al., 2004), although both induced a vaccine-specific T cell response. Likewise, vaccination with L1 VLP also induces a T cell response in patients (Pinto et al., 2003; Pinto et al., 2006) without apparently impacting existing disease (Hildesheim et al., 2007; Koutsky et al., 2002). Nevertheless, a more detailed subset analysis may reveal a therapeutic effect and/or that capsid antigen-specific T cell-mediated responses do contribute to protection, as has been suggested from some animal and clinical studies (Govan, Rybicki, and Williamson, 2008; Jarrett et al., 1991; Koutsky et al., 2002; Zhang et al., 2000).
No neutralizing antibody was detected in a number of cattle vaccinated with L2 polypeptides and immune to experimental BPV4 challenge. If protection in these animals is not mediated by T cell-based immunity, then it is likely that the current in vitro neutralizing assay lacks the sensitivity to detect low titer yet protective antibody levels, at least for those specific for L2. This may reflect lower L2 as compared to L1-specific antibody avidity and/or differing mechanisms and timing of neutralization in vivo as compared to infection of the 293TT cells used in the in vitro neutralization assays (Kines et al., 2009). These observations suggest that such low titer protective responses might be better detected by passive transfer into mice prior to challenge (Day et al., 2010). T cell responses may also contribute to protection and additional studies are warranted to define mechanisms and relevant protective epitopes.
METHODS
Production of the Animalx6 protein
L2 residues spanning the putative furin cleavage site to residue 88 derived from three bovine and three canine papillomavirus genotypes; BPV1, BPV4, BPV5, COPV, CaPV2 and CaPV3 were codon-modified and the sequence (see supplementary information and Supplementary Figure 2) was synthesized by Biobasic Inc, and then subcloned as a BamHI-XhoI fragment into the pET28a vector (Novagen) for expression in E. coli as a hexahistidine-tagged recombinant polypeptide expressed in E. coli BL21 (Rosetta cells, Novagen) (Pastrana et al., 2005). The hexahistidine-tagged recombinant L2 polypeptide animalx6 was affinity purified by binding to a nickel-nitrilotriacetic acid (Ni-NTA) column (Qiagen) in 8M urea (using the QiaExpressionist standard purification protocol for denaturing conditions) and then dialyzed in cassettes (Pierce) against Dulbecco’s phosphate buffered saline (PBS). Purity was monitored by SDS-PAGE and protein concentration determined by bicinchoninic acid test (Pierce) using a bovine serum albumen standard (Jagu et al., 2009).
Neutralization assays
The BPV1 and HPV16 pseudovirion in vitro neutralization assays were performed as described earlier (Jagu et al., 2009; Pastrana et al., 2004) and the secreted alkaline phosphatase content in the clarified supernatant was determined using the p- Nitrophenyl phosphate tablets (Sigma, St. Louis, MO) dissolved in diethanolamine and absorbance measured at 405nm. Constructs and detailed protocols for the preparation of the pseudovirions can be found at http://home.ccr.cancer.gov/lco/. Titers were defined as the reciprocal of the highest dilution that caused a 50% reduction in A405. For the generation of BPV4 pseudovirions, the codon-modified BPV4 L1 and L2 sequences (see supplementary information) were synthesized by Blue Heron Biotech and each sublconed into pcDNA3.1(-) using XbaI. The BPV4 L2 sequence was also subcloned into pET28a for expression in bacteria, and production of mouse antiserum to full length 6His-tagged BPV4 L2 protein by vaccination of Balb/c mice three times with 25μg of antigen in RIBI adjuvant. BPV4 pseudovirions were prepared as described previously by co-transfection of the BPV4 L1 and L2 expression plasmids with the pSEAP reporter construct into 293TT cells. Expression of the BPV4 L1 and L2 proteins was verified by Western blot using mouse monoclonal antibody 1H8 (Chemicon) to L1 and mouse antiserum to 6His-BPV4 L2 respectively. BPV1 and BPV4 L1 VLPs were generated by transfection of the 293TT cells with only the L1 expression vector. The COPV L1 and L2 codon modified constructs are described by (Palmer et al, submitted).
COPV Neutralization Assay
Canine CPEK cells were trypsinized and seeded at 5 × 105 per well in 6-well plates. The next day, cultures were infected with 200 wart-derived COPV (CPV1) particles per cell in 1 ml of medium in the presence of PBS control or indicated diluted antisera. Cultures received 2-ml additional medium 24 h post-infection. Total RNAs were isolated using TRIzol (Invitrogen) according to the manufacturer’s instructions. All reactions containing RNAs from virus-infected cells were performed in triplicate. cDNA were made using SuperScript® III One-Step RT-PCR System (Invitrogen). SYBR Green real-time qRT-PCR was performed on the Bio-Rad iCycler MyiQ (BioRad) for quantification of viral E1^E4 mRNA. The primers for CPV1 E1^E4: sense primer, 5′-GCTAGAAAAGTGCCGCCG GA-3′, anti-sense primer, 5- TGGGGAACCGCGACGACCTC-3′). The canine HPRT mRNA (sense primer, 5′-CTCACTATGATCCACCACG-3′, anti-sense primer, 5- TAGCCTCCATAACCTCCAC-3′) was used as internal RNA control for the relative quantification. Primer concentration in the reaction mixture was 5 pmol/ml. Cycling conditions were 95 degrees for 10 min followed by 42 cycles of 94 degrees for 15 s and 60 degrees for 1 min. All samples were run in triplicate. Relative quantities of viral target cDNA were determined using iQ™5 Optical System Software (BioRad).
Enzyme-linked immunosorbent assays (ELISA)
Immobilon plates (Nunc) were coated with 100ng/well of 6His-BPV4 L2 (prepared in E. coli) or BPV4 L1 VLPs (produced in 293TT) or COPV L1/L2 pseudovirions (Palmer et al, submitted) in PBS overnight at 4°C. Wells were then blocked with SeaBlock (Pierce) for 1h at room temperature, and incubated with 2-fold dilutions of bovine sera for 1h at room temperature, or blocked with BSA for 1h at room temperature, and incubated with 2-fold dilutions of mouse antisera for 1h at room temperature. Following a wash step with PBS-0.01 % (v/v) Tween 20, either peroxidase-labeled rabbit anti-bovine Ig (KPL) diluted 1:5,000 in SeaBlock was added for 1h, or peroxidase-labeled anti-mouse Ig (GE) diluted 1:5,000 in 1% BSA-PBS was added for 1h. The plates were then washed and developed with 2, 2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid solution (Roche) for 10min and the absorbance at 405nm read with Benchmark Plus (Biorad) microtiter plate reader.
Mouse vaccination and challenge
Studies were performed with the approval of the Johns Hopkins University Animal Care and Use Committee. Female Balb/c 6–8 weeks old mice were purchased from the National Cancer Institute (Frederick, MD, USA) and kept in the animal facility of the Johns Hopkins University (Baltimore, MD, USA) at least one week prior to use. The mice were vaccinated in groups of 5 animals three times at two week intervals s.c. with 25 μg of L2 animalx6 protein in 50 μg of aluminum hydroxide (alum) alone, or with RIBI adjuvant (2% squalene, 50μg MPLA, 50μg trehalose dicorynomycolate, Sigma S6322) or with 5 μg of BPV1 L1 VLP or BPV4 L1 VLP adsorbed on to alum. Serum samples were obtained by tail vein bleeds two weeks and four months after the final immunization. Two weeks after the final immunization, mice received 3mg of Depo-provera (Pfizer) diluted in 100 μl of sterile PBS in a subcutaneous injection. Four days later the pseudovirus challenge inoculum was administered as a 20 μl dose composed of purified BPV4 pseudovirus carrying the luciferase reporter gene mixed in 2% (v/v) carboxymethyl cellulose (CMC) (Sigma C5013). The virus was delivered in two doses. Half was deposited into mouse vagina by using a M50 positive displacement pipette (Gilson). A cytobrush cell collector was inserted in the vagina and turned clockwise and counter clockwise for 10 times and the remaining 10 μl introduced. Three days later, the mice were anesthetized and luciferin (40 μl at concentration of 7 mg/ml) was deposited intravaginally, and their images were acquired for 10 min using Xenogen IVIS200.
Statistical analysis
Comparison of Log10 transformed titers by ANOVA with Bonferroni correction for multiple comparisons and graphical presentation of titers as box/whisker plots were performed using GraphPad Prism 4 software. Significance was set a p<0.05. Titers less than 25 were arbitrarily set at 10 for graphical purposes.
Supplementary Material
Research Highlights.
Veterinary vaccines are needed against diverse animal papillomaviruses
Concatenated L2 N-termini from six different animal papillomavirus types
Vaccination with L2 concatemer induced broadly neutralizing antibodies
Vaccination with L2 concatemer protected the mice from challenge with BPV1 pseudovirus
Low serum neutralizing antibody titers associated with protection of L2-vaccinated cattle
Acknowledgments
This study was funded by PHS grants CA133749, CA118790 and P50 CA098252 and RC2 CA148499 to RBSR and WKH, and by the American Cancer Society RSG 116279, and by Cancer Research UK to MSC. MSC was supported by Cancer Research UK. We thank Martin Müller (DKFZ, Germany), Christopher Buck and John Schiller (National Cancer Institute, Bethesda, MD) for sharing codon-optimized L1 and L2 genes.
Footnotes
Competing Interests: SJ and RBSR are co-inventors on L2 patents licensed to Shantha Biotechnics, Ltd., PaxVax, Inc., GlaxoSmithKline and Acambis, Inc. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies. MSC is an inventor of patents for the use of papillomavirus L2 protein and L2 peptides. The patents are managed by Cancer Research Technology, UK.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell JA, Sundberg JP, Ghim SJ, Newsome J, Jenson AB, Schlegel R. A formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology. 1994;62(4):194–8. doi: 10.1159/000163910. [DOI] [PubMed] [Google Scholar]
- Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401(1):70–9. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman CL, Hirth RS, Sundberg JP, Christensen EF. Cutaneous neoplasms in dogs associated with canine oral papillomavirus vaccine. Vet Pathol. 1987;24(6):477–87. doi: 10.1177/030098588702400602. [DOI] [PubMed] [Google Scholar]
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69(6):3959–63. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78(2):751–7. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo MS. Papillomas and cancer in cattle. Cancer Surv. 1987;6(1):39–54. [PubMed] [Google Scholar]
- Campo MS. Vaccination against papillomavirus in cattle. Curr Top Microbiol Immunol. 1994;186:255–66. doi: 10.1007/978-3-642-78487-3_13. [DOI] [PubMed] [Google Scholar]
- Campo MS. Vaccination against papillomavirus in cattle. Clin Dermatol. 1997;15(2):275–83. doi: 10.1016/s0738-081x(96)00165-4. [DOI] [PubMed] [Google Scholar]
- Campo MS, O’Neil BW, Grindlay GJ, Curtis F, Knowles G, Chandrachud L. A peptide encoding a B-cell epitope from the N-terminus of the capsid protein L2 of bovine papillomavirus-4 prevents disease. Virology. 1997;234(2):261–6. doi: 10.1006/viro.1997.8649. [DOI] [PubMed] [Google Scholar]
- Campo MS, Roden RB. Papillomavirus prophylactic vaccines: established successes, new approaches. J Virol. 2010;84(3):1214–20. doi: 10.1128/JVI.01927-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J Clin Invest. 2001;108(3):415–23. doi: 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrachud LM, Grindlay GJ, McGarvie GM, O’Neil BW, Wagner ER, Jarrett WF, Campo MS. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology. 1995;211(1):204–8. doi: 10.1006/viro.1995.1392. [DOI] [PubMed] [Google Scholar]
- Christensen ND, Kreider JW, Kan NC, DiAngelo SL. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181(2):572–9. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol. 1996;70(2):960–5. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, Dobson J, Roberts JS, Hickling J, Kitchener HC, Stern PL. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63(18):6032–41. [PubMed] [Google Scholar]
- Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. J Virol. 2008;82(9):4638–46. doi: 10.1128/JVI.00143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, Schiller JT. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8(3):260–70. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I, Shober R, Chattopadhyay SK, Lowy DR. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980;103(2):369–75. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, Baldwin PJ, Sterling JC, Kitchener HC. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006;16(3):1075–81. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, Roden RB. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N-terminus of HPV16 minor capsid antigen L2. J Virol. 2007a;81(21):13927–13931. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of Human Papillomavirus L2. J Virol. 2007b;81(24):13927–31. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaukroger JM, Chandrachud LM, O’Neil BW, Grindlay GJ, Knowles G, Campo MS. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J Gen Virol. 1996;77(Pt 7):1577–83. doi: 10.1099/0022-1317-77-7-1577. [DOI] [PubMed] [Google Scholar]
- Govan VA, Rybicki EP, Williamson AL. Therapeutic immunisation of rabbits with cottontail rabbit papillomavirus (CRPV) virus-like particles (VLP) induces regression of established papillomas. Virol J. 2008;5:45. doi: 10.1186/1743-422X-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. Jama. 2007;298(7):743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RB. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst. 2009;101(11):782–92. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett WF, O’Neil BW, Gaukroger JM, Smith KT, Laird HM, Campo MS. Studies on vaccination against papillomaviruses: the immunity after infection and vaccination with bovine papillomaviruses of different types. Vet Rec. 1990;126(19):473–5. [PubMed] [Google Scholar]
- Jarrett WF, Smith KT, O’Neil BW, Gaukroger JM, Chandrachud LM, Grindlay GJ, McGarvie GM, Campo MS. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology. 1991;184(1):33–42. doi: 10.1016/0042-6822(91)90819-w. [DOI] [PubMed] [Google Scholar]
- Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J Virol. 1999;73(7):6188–90. doi: 10.1128/jvi.73.7.6188-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106(48):20458–63. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Chandrachud LM, O’Neil BW, Wagner ER, Grindlay GJ, Armstrong A, McGarvie GM, Schiller JT, Lowy DR, Campo MS. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219(1):37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- Knowles G, Grindlay GJ, Campo MS, Chandrachud LM, O’Neil BW. Linear B-cell epitopes in the N-terminus of L2 of bovine papillomavirus type 4. Res Vet Sci. 1997;62(3):289–91. doi: 10.1016/s0034-5288(97)90207-1. [DOI] [PubMed] [Google Scholar]
- Koller LD, Olson C. Attempted transmission of warts from man, cattle, and horses and of deer fibroma, to selected hosts. J Invest Dermatol. 1972;58(6):366–8. doi: 10.1111/1523-1747.ep12540579. [DOI] [PubMed] [Google Scholar]
- Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347(21):1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- Lin YL, Borenstein LA, Selvakumar R, Ahmed R, Wettstein FO. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992;187(2):612–9. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- McGarvie GM, Chandrachud L, Gaukroger JM, Grindlay GJ, O’Neil BW, Baird JW, Wagner ER, Jarrett WFH, Campo MS. Vaccination of cattle with L2 protein prevents BPV-4 infection. In: Stanley MA, editor. Immunology of Human Papillomaviruses. Plenum Press; New York: 1994. pp. 283–290. [Google Scholar]
- Munday JS, Kiupel M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet Pathol. 2010;47(2):254–64. doi: 10.1177/0300985809358604. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, Shah KV, Meijer CJ. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111(2):278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- Nasir L, Campo MS. Bovine papillomaviruses: their role in the aetiology of cutaneous tumours of bovids and equids. Vet Dermatol. 2008;19(5):243–54. doi: 10.1111/j.1365-3164.2008.00683.x. [DOI] [PubMed] [Google Scholar]
- Olson C, Luedke AJ, Brobst DF. Induced immunity of skin, vagina, and urinary bladder to bovine papillomatosis. Cancer Res. 1962;22:463–8. [PubMed] [Google Scholar]
- Olson C, Robl MG, Larson LL. Cutaneous and penile bovine fibropapillomatosis and its control. J Am Vet Med Assoc. 1968;153(9):1189–94. [PubMed] [Google Scholar]
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Kruger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321(2):205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, Christensen ND, Lowy DR, Schiller JT, Roden RB. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337(2):365–72. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Pilacinski WP, Glassman DL, Glassman KF, Reed DE, Lum MA, Marshall RF, Muscoplat CC, Faras AJ. Immunization against bovine papillomavirus infection. Ciba Found Symp. 1986;120:136–56. doi: 10.1002/9780470513309.ch10. [DOI] [PubMed] [Google Scholar]
- Pinto LA, Edwards J, Castle PE, Harro CD, Lowy DR, Schiller JT, Wallace D, Kopp W, Adelsberger JW, Baseler MW, Berzofsky JA, Hildesheim A. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188(2):327–38. doi: 10.1086/376505. [DOI] [PubMed] [Google Scholar]
- Pinto LA, Viscidi R, Harro CD, Kemp TJ, Garcia-Pineres AJ, Trivett M, Demuth F, Lowy DR, Schiller JT, Berzofsky JA, Hildesheim A. Cellular immune responses to HPV-18, -31, and -53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology. 2006;353(2):451–62. doi: 10.1016/j.virol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Richards RM, Lowy DR, Schiller JT, Day PM. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc Natl Acad Sci U S A. 2006;103(5):1522–7. doi: 10.1073/pnas.0508815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13(7):857–61. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006;6(10):753–63. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden RB, Yutzy WHt, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270(2):254–7. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 118(1 Suppl):S12–7. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafti-Keramat S, Schellenbacher C, Handisurya A, Christensen N, Reininger B, Brandt S, Kirnbauer R. Bovine papillomavirus type 1 (BPV1) and BPV2 are closely related serotypes. Virology. 2009;393(1):1–6. doi: 10.1016/j.virol.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, Pawlita M, Man S, Hickling JK, Fiander AN, Tristram A, Kitchener HC, Offringa R, Stern PL, Van Der Burg SH. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004;10(9):2954–61. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92(25):11553–7. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepapeliere P, Barrasso R, Meijer CJ, Walboomers JM, Wettendorff M, Stanberry LR, Lacey CJ. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005;192(12):2099–107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]
- Zhang LF, Zhou J, Chen S, Cai LL, Bao QY, Zheng FY, Lu JQ, Padmanabha J, Hengst K, Malcolm K, Frazer IH. HPV6b virus like particles are potent immunogens without adjuvant in man. Vaccine. 2000;18(11–12):1051–8. doi: 10.1016/s0264-410x(99)00351-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.