Somatic cells can be reprogrammed to induced pluripotent stem (iPS) cells by retroviral transduction of four transcription factors, Oct4, Sox2, Klf4 and c-Myc (Takahashi et al., 2006; Takahashi et al., 2007; reviewed in Jaenisch and Young, 2008). While the reprogrammed pluripotent cells are thought to have great potential for regenerative medicine (Hanna et al., 2007; Wernig et al., 2008b), genomic integrations of the retroviruses, especially c-Myc, increase the risk of tumorigenesis (Okita et al., 2007). Recently, iPS cells have been generated without c-Myc retrovirus (Myc[-]), but in the absence of exogenous c-Myc the efficiency and kinetics of reprogramming are significantly reduced (Nakagawa et al., 2008; Wernig et al., 2008a). We report here that soluble Wnt3a promotes the generation of iPS cells in the absence of c-Myc retrovirus. These data demonstrate that signal transduction pathways and transcription factors can act coordinately to reprogram differentiated cells to a pluripotent state.
Naturally occurring signaling molecules that modulate the expression of endogenous ES cell transcription factors are promising candidates for soluble factors that enhance reprogramming. The Wnt signaling pathway contributes to the maintenance of pluripotency in mouse and human ES cells (Sato et al., 2004; Ogawa et al., 2006; Singla et al., 2006; Cai et al., 2007), as well as the self-renewal of undifferentiated adult stem cells in multiple tissues (Reya and Clevers, 2005). In addition, initial studies of iPS cell generation suggested that constitutively active β-catenin, another downstream component of the Wnt pathway, might promote reprogramming of fibroblasts to pluripotency (Takahashi and Yamanaka, 2006). Studies of Tcf3, one of the key transcriptional regulators downstream of the Wnt pathway in embryonic stem cells, have revealed that this factor co-occupies almost all promoter regions occupied by ES cell specific transcription factors, including Oct4 and Nanog, and can regulate the expression of key ES cell transcription factors (Cole et al, 2008; Tam et al., 2008; Yi et al., 2008).
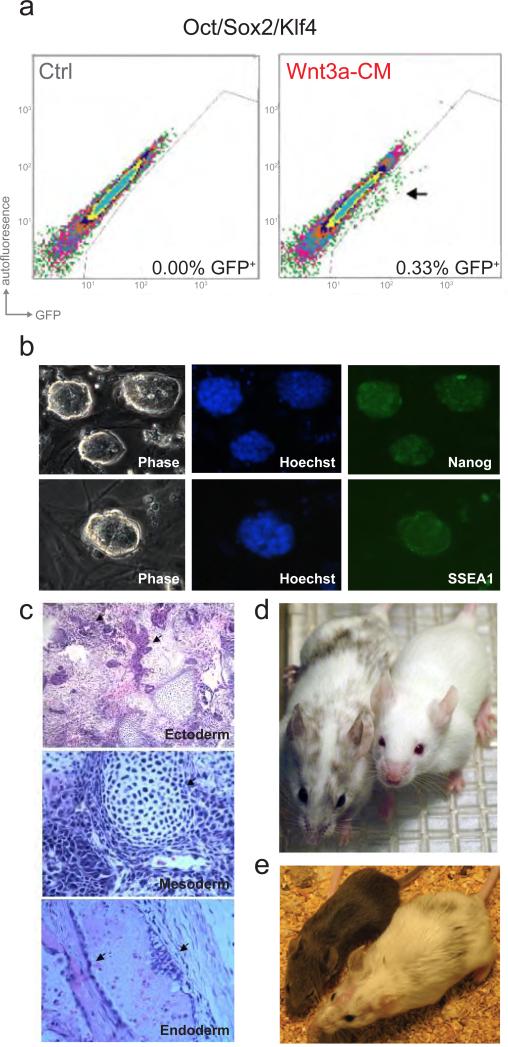
Since the Wnt pathway is intimately connected to the core circuitry of pluripotency, we hypothesized that the stimulation of the pathway using soluble factors could modulate the efficiency of inducing pluripotency in somatic cells. Here we examined the influence of Wnt stimulation on the reprogramming of murine fibroblasts in the absence of c-Myc retrovirus. For this, cells with GFP driven by the endogenous Oct4 promoter (Meissner et al., 2007) were infected with doxycycline (DOX)-inducible lentiviruses encoding Oct4, Sox2 and Klf4. Oct4/Sox2/Klf4 infected cells were either cultured in standard ES cell medium or in Wnt3a-conditioned medium (Wnt3a-CM) and were analyzed for GFP expression by flow cytometry at days 10, 15 and 20 after DOX induction. No GFP positive cells were present with or without Wnt3a-CM treatment on day 10 or day 15. By day 20 a small population of GFP expressing cells was detected only in the cells cultured in Wnt3a-CM (Figure 1a). The Wnt3a-CM exposed cultures formed GFP expressing colonies with morphology typical for ES or iPS cells (Figure 1b). However, unlike four factor transduced cells, which usually form a highly heterogeneous population of cells when propagated without selection, the Oct4/Sox2/Klf4/Wnt3a-CM colonies appeared homogenously ES-like, similar to previously reported Myc[-] iPS clones (Nakagawa et al., 2008).
Figure 1. Wnt3a promotes reprogramming of somatic cells to pluripotency.
a. Scatter plots comparing GFP intensity to autofluoresence, using flow cytometry, in Oct4-GFP cells on day 20 post-induction of Oct4/Sox2/Klf4, reveal a GFP expressing population of cells (indicated with an arrow) only with Wnt3a-CM treatment (right), not in the control (Ctrl) Oct4/Sox2/Klf4-infected cells cultured in standard ES cell medium (left). b. Phase-contrast micrographs (Phase, left) of GFP expressing Myc[-] cells derived with Wnt3a-CM treatment and without any genetic selection. Immunostaining reveals induction of pluripotency markers, Nanog (upper) and SSEA-1(lower) in Wnt3a-CM treated Myc[-] cells. c. Wnt3a-CM treated Myc[-] lines formed teratomas when injected into SCID mice subcutaneously. Teratomas from Oct4/Sox2/Klf4/Wnt3a-CM iPS lines showed evidence of differentiated cells of three germ layers similar to teratomas formed from V6.5 mES injections. Arrows indicated neural tissue in (upper), cartilage in (middle), and endodermal cells in (lower), d. Oct4/Sox2/Klf4/Wnt3aCM iPS lines derived without selection gave rise to chimeric mice (as shown on the left) with agouti coat color and pigmented eyes (in contrast to wild type Balb/c mouse, right) providing evidence of contribution to somatic cells. e. Agouti coat color of offspring (left) of chimeric mouse (right) confirmed that the Oct4/Sox2/Klf4/Wnt3a-CM iPS line generated here is germline-competent.
Several assays were performed to characterize the developmental potential of Myc[-] iPS cells derived with Wnt3a-CM treatment. Immunocytochemistry confirmed the expression of markers of pluripotency, including the nuclear factor Nanog and the surface glycoprotein SSEA1 (Figure 1b). Functional assays confirmed that, like ES cells, these iPS cells were pluripotent. When injected into SCID mice subcutaneously, the Myc[-] iPS cells gave rise to teratomas with histological evidence of cells differentiating into all three germ layers (Figure 1c). More importantly, Myc[-] iPS cells derived with Wnt3a-CM treatment contributed to the formation of somatic tissues as well as the germline in chimeric mice (Figures 1d and 1e and Supplemental Table S1). These results indicate that Wnt3a-CM treated Myc[-] clones are pluripotent cells that are morphologically and functionally indistinguishable from ES cells.
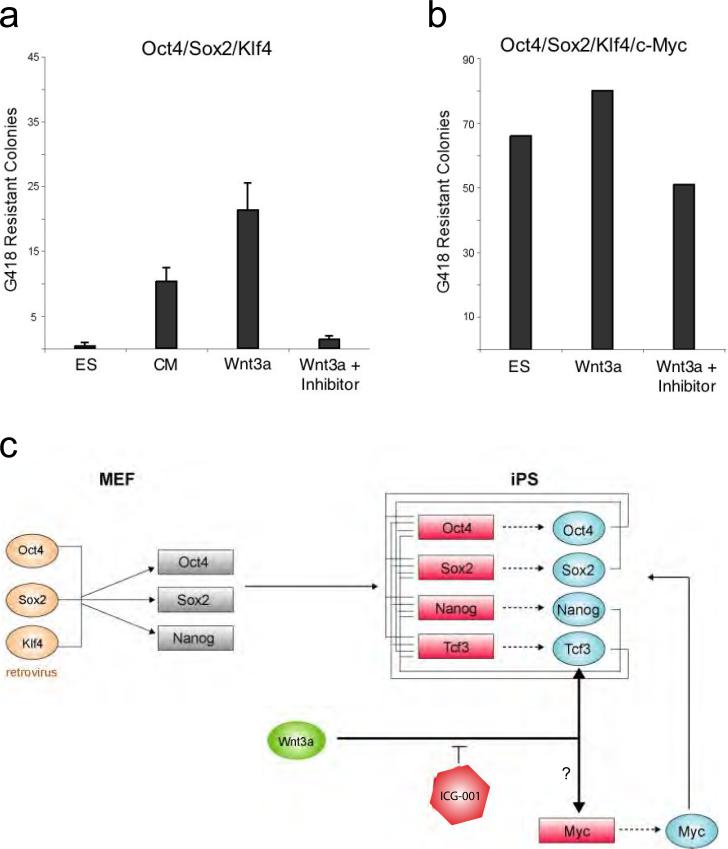
To quantify the effects of Wnt3a-CM, triplicate experiments were performed on Oct4/Sox2/Klf4-inducible MEFs carrying a G418 resistance cassette downstream of the Oct4 promoter (Figure 2a). G418 was added to the cultures at 15 days after infection to select for cells that had reactivated the Oct4 locus. When scored on day 28 after infection, only a few Myc[-] G418 resistant colonies (between 0-3 colonies forming on each ten centimetre plate) were detected in standard ES cell culture conditions. In contrast, ~20 fold more drug resistant colonies formed when G418 selection was initiated on Wnt3a-CM-treated cells, consistent with the conclusion that activation of the Wnt pathway enhances reprogramming. It should be noted that conditioned medium from control fibroblasts (L cells, ATCC) lacking Wnt3a over-expression also caused a moderate increase in the number of G418-resistant colonies relative to standard ES medium, suggesting that normal fibroblasts may secrete factors, perhaps including Wnt3a, that promote reprogramming. So far, we have been unable to recapitulate the effects of Wnt3a-CM on reprogramming with small molecule inhibitors of glycogen synthase kinase-3 (Gsk3) that modulate the Wnt pathway and promote the self-renewal of ES cells (Sato et al., 2004; Ying et al., 2008) (Supplementary Figure S1). While there are many plausible explanations for this negative result, one possibility is that the half-life of these chemicals is too short to provide sustained stimulation of the Wnt-pathway required to enhance reprogramming.
Figure 2. Wnt/ß-catenin stimulation enahances iPS colony formation in absence of c-Myc retrovirus.
a. Counts are shown for G418-resistant colonies in Oct4/Sox2/Klf4 over-expressing MEFs cultured in ES cell media, control L cell conditioned media (ATCC), Wnt3a over-expressing conditioned media (ATCC), and Wnt3a over-expressing conditioned media with ICG-001 (4μM). Selection was initiated on day 15 post-induction, and colonies were assessed on day 28. Wnt3a-CM treatment was maintained for 6-9 days after selection was initiated. Mean number of counts from triplicate experiments is displayed with error bars indicating S.D. b. Counts are shown for G418-resistant colonies (in a 32cm2 area) in Oct4/Sox2/Klf4/c-Myc over-expressing MEFs cultured in ES cell media, Wnt3a over-expressing conditioned media (ATCC), and Wnt3a over-expressing conditioned media with ICG-001 (4μM). Selection was initiated on day 10 post-induction, Wnt3a-CM was maintained for the first 6-9 days of selection, and colonies were assessed on day 20. c. Wnt stimulation promotes the formaton of iPS cells in the absence of c-Myc transduction. This could be due to: i) direct regulation by the Wnt pathway of key endogenous pluripotency factors, such as Oct4, Sox2 and Nanog as suggested by genomic studies in ES cells (Cole et al., 2008), ii) Wnt pathway-induced activation of endogenous Myc (He et al., 1998; Cole et al., 2008), or other cell proliferation genes, accelerating the sequential process of forming iPS colonies.
To independently assess the effect of Wnt3a on reprogramming, we cultured cells in the presence of ICG-001 (Teo et al., 2005; McMillan and Kahn, 2005), an inhibitor of the Wnt/ß-catenin pathway. Figure 2a (right columns) shows that 4μM ICG-001 strongly inhibited the effect of Wnt3a-CM on Myc[-] iPS formation. The effects of Wnt3a-CM and ICG-001 were also examined in MEFs over-expressing all four reprogramming factors, including c-Myc (Figure 2b). High numbers of G418 resistant colonies were observed in both standard ES cell media (66 colonies/32cm2) and Wnt3a-CM in four factor reprogrammed cells, with only a subtle increase in the number of colonies with Wnt3a-CM (80 colonies/32cm2). In contrast to the dramatic effect of ICG-001 on Myc[-] cells, at the same dose, the compound had only a subtle effect on the number of G418 colonies in c-Myc transduced cells, and a relatively high number of resistant colonies were observed under these conditions (51 colonies/32cm2) (Figure 2b). When the experiment was repeated with higher doses of ICG-001, iPS colony numbers were further reduced, but even at 25μM multiple Oct4/Sox2/Klf4/c-Myc iPS colonies were observed (mean= 8 colonies/32cm2) (Supplemental Figure S2). These data suggest that Wnt signaling and c-Myc could have overlapping, but not completely redundant roles in the induction of pluripotency.
Recent reports have demonstrated that Myc is dispensable for inducing pluripotency in MEFs (Nakagawa et al., 2008; Wernig et al., 2008a), establishing Oct4, Sox2 and Klf4 as a core set of reprogramming factors, although a slightly different combination of factors also functions in human cells (Yu et al., 2007). Epigenetic reprogramming of MEFs with defined factors is a gradual process, where transcription factors progressively re-establish the core circuitry of pluripotency over the course of weeks (Brambrink et al, 2008; Stadtfeld et al., 2008). Myc accelerates this process (Nakagawa et al., 2008; Wernig et al., 2008a), conceivably because it enhances cell proliferation allowing the changes to unfold more rapidly. Additionally, Myc is suspected to have widespread effects on chromatin state (Knoepfler, 2008; Kim et al., 2008) and could facilitate the productive binding of the core reprogramming factors to their appropriate genomic targets (Jaenisch and Young, 2008).
The Wnt signaling pathway has been shown to connect directly to the core transcriptional regulatory circuitry of ES cells, suggesting a mechanism by which this pathway could directly promote the induction of pluripotency in the absence of c-Myc transduction (Figure 2c). In ES cells, Tcf3 occupies and regulates the promoters of Oct4, Sox2 and Nanog (Cole et al., 2008; Tam et al., 2008; Yi et al., 2008). In MEFs, these endogenous pluripotency transcription factors are silenced. During reprogramming, as exogenous Oct4, Sox2 and Klf4 contribute to the reactivation of the endogenous pluripotency factors (Jaenisch and Young, 2008), Wnt signaling could directly potentiate the effect of these transcription factors, as it does in ES cells (Cole et al., 2008).
Additionally, Wnt could serve to activate endogenous c-Myc directly, thereby substituting for exogenous c-Myc (Figure 2c). Indeed, c-Myc is a well-established target of the Wnt pathway in colorectal cancer cells (He et al., 1998). In ES cells, Tcf3 occupies the c-Myc promoter, and Wnt3a positively contributes to expression of the gene (Cole et al., 2008). However, quantitative PCR studies did not reveal Wnt3a-CM-dependent activation of c-Myc during the first 48 hours after induction of Oct4/Sox2/Klf4 in MEFs (Supplementary Figure S3). Although we cannot exclude that Wnt3a enhances c-Myc expression in the rare cells that will eventually become iPS cells, the expression data suggest that the role of Wnt in reprogramming could be independent of c-Myc induction, consistent with recent findings in ES cells (Ying et al., 2008). Wnt signaling and c-Myc could play partially redundant functional roles in iPS cell generation. For example, Wnt-induced effects on cell proliferation could help to accelerate the sequence of events that lead to the generation of Myc[-] iPS colonies.
A major goal of current research is to identify transient cues that can reprogram somatic cells, eliminating the need for retroviruses. The studies described here establish that Wnt stimulation can be used to enhance the efficiency of reprogramming in combination with nuclear factors, Oct4, Sox2 and Klf4. By enhancing the efficiency of reprogramming in the absence of c-Myc retrovirus, soluble Wnt, or small molecules that modulate the Wnt signaling pathway, will likely prove useful in combination with other transient cues, yet to be identified, that can replace the remaining retroviruses.
Supplementary Material
Acknowledgements
We thank members of the Jaenisch, Young and Kahn laboratories, especially Jamie Newman, Megan Cole and Tony Lee for discussions and critical review of the manuscript. We are grateful to Jamie Newman, Megan Cole, and Sarah Johnstone for sharing data prior to publication and for Wnt3a conditioned media, and to Seshamma Reddy, Jessica Dausman, Ruth Flannery and Betty Zhou for technical assistance. We also thank Tom DiCesare for graphic assistance. This work was supported by NIH grants 5-RO1-HDO45022, 5-R37-CA084198, and 5-RO1- CA087869 to RJ and by NIH grant HG002668 and a grant from the Whitehead Institute to RY. RJ is an advisor to Stemgent which has obtained a license from MIT to distribute some of the reagents used in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Ye Z, Zhou BY, Mali P, Zhou C, Cheng L. Promoting human embryonic stem cell renewal or differentiation by modulating Wnt signal and culture conditions. Cell Res. 2007;17:62–72. doi: 10.1038/sj.cr.7310138. [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes and Development. 2008 doi: 10.1101/gad.1642408. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Young RA. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS. Why Myc? An unexpected ingredient in the stem cell cocktail. Cell Stem Cell. 2008;2:18–21. doi: 10.1016/j.stem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- McMillan M, Kahn M. Investigating Wnt signaling: a chemogenomic safari. Drug Discov Today. 2005;10:1467–1474. doi: 10.1016/S1359-6446(05)03613-5. [DOI] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B. Tcf3 Regulates Embryonic Stem Cell Pluripotency and Self-Renewal by the Transcriptional Control of Multiple Lineage Pathways. Stem Cells. 2008 doi: 10.1634/stemcells.2007-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo JL, Ma H, Nguyen C, Lam C, Kahn M. Specific inhibition ofn CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc Natl Acad Sci U S A. 2005;102:12171–12176. doi: 10.1073/pnas.0504600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008a;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008b;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Pereira L, Merrill BJ. Tcf3 Functions as a Steady State Limiter of Transcriptional Programs of Mouse Embryonic Stem Cell Self Renewal. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.