Abstract
Rationale
Endothelial progenitor cell (EPC) survival and function in the injured myocardium is adversely influenced by hostile microenvironment like ischemia, hypoxia and inflammatory response, thereby compromising full benefits of EPC-mediated myocardial repair.
Objective
We hypothesized that interleukin-10 (IL-10) modulates EPC biology leading to enhanced survival and function following transplantation in the ischemic myocardium.
Methods and Results
Myocardial infarction (MI)-induced mobilization of bone marrow EPC (Sca-1+Flk1+ cells) into the circulation was significantly impaired in IL-10 KO-mice. Bone marrow transplantation (BMT) to replace IL-10 KO-marrow with WT-marrow attenuated these effects. Impaired mobilization was associated with lower SDF-1 expression levels in the myocardium of KO-mice. Interestingly, SDF-1 administration reversed mobilization defect in KO-mice. In vitro, hypoxia-mediated increases in CXCR4 expression and cell survival were lower in IL-10-deficient-EPCs. Furthermore, SDF-1-induced migration of WT-EPCs was inhibited by AMD3100, an inhibitor of CXCR4. To further study the effect of IL-10 on in vivo EPC survival and engraftment into vascular structures, GFP-labeled EPC were injected intramyocardially after induction of MI, and the mice were treated with either saline or recombinant IL-10. IL-10-treated group showed increased retention of transplanted EPCs in the myocardium and was associated with significantly reduced EPC apoptosis post-MI. Interestingly, increased EPC retention and their association with the vascular structures was observed in IL-10 treated mice. Increased EPC survival and angiogenesis in the myocardium of IL-10-treated mice corroborated with improved LV function, reduced infarct size and fibrosis in the myocardium. In vitro, IL-10-induced increase in VEGF expression in WT-EPC was abrogated by STAT3 inhibitor suggesting IL-10 signals via STAT3 activation.
Conclusions
Taken together, our studies demonstrate that MI-induced EPC mobilization was impaired in IL-10 KO-mice and that IL-10 increases EPC survival and function possibly via activation of STAT3/VEGF signaling cascades, leading to attenuation of MI-induced LV dysfunction and remodeling.
Keywords: Endothelial progenitor cells, survival, myocardial infarction, IL-10, bone marrow transplantation, inflammation, angiogenesis, left ventricular remodeling
Introduction
Several strategies to modify and enhance stem cell-mediated ischemic myocardial repair and regeneration have shown promising results 1-9. Increasing evidence from animal models of experimental ischemic injuries suggest that EPC participate in the process of neo-vascularization and tissue repair leading to enhanced recovery of ischemic myocardium 10-13. Furthermore, clinical trials involving bone marrow derived EPC transplantation for ischemic myocardium have confirmed this possibility 14-18. However, the adverse effects of ischemic tissue (hostile microenvironment) like inflammation and hypoxia on the survival and function of the mobilized/transplanted EPC during angio/vasculo-genesis and tissue repair is still a poses a challenge and research on the means to enhance stem cell survival and function is limited. Heart failure patients with high circulating levels of TNFα, a potent proinflammatory cytokine, were associated with significantly lower EPC counts as compared to patients treated with TNFα blocker 19. Most importantly, these EPCs exposed to prolonged inflammatory stimulus may be functionally impaired 20. Survival of EPCs following their intramyocardial transplantation is a strong indicator of favorable cardiovascular prognosis in cell-based therapy 21. These studies suggest that ischemic microenvironment including adverse inflammatory response has a deleterious effect on EPC survival and function. Therefore, modulation of local tissue microenvironment by anti-inflammatory factors either released by stem cells or through systemic delivery is important in conferring improved outcome after stem cell therapy. Previous studies from our laboratory and others 22-25 have shown that administration of IL-10, a potent anti-inflammatory cytokine, significantly improved post AMI LV functions, reduced cardiomyocyte apoptosis and fibrosis and enhanced neovascularization in the ischemic border-zone. Interestingly, whether attenuating the pro-inflammatory response on EPC biology can maximize the benefits of EPC-mediated myocardial repair in particular and cardiovascular events in general have not been explored yet. Therefore, we hypothesized that IL-10 modulates EPC biology and enhances its survival and function following transplantation in an ischemic myocardial tissue microenvironment leading to attenuation of MI-induced LV dysfunction and remodeling.
Methods and Materials
Comprehensive methods are available as an Online Supplement at http://circres.ahajournals.org.
Results
IL-10 KO mice display reduced MI-induced mobilization of BM-EPC
To determine whether IL-10 affects MI-induced mobilization of BM-EPCs into the circulation, we performed myocardial infarction (MI) and assessed EPC mobilization into the circulation by FACS analysis at 3 day post-MI. EPCs were identified as cells co-expressing Sca-1 and Flk-1. WT-mice showed increased number of Flk1+/Sca-1+ (double+) cells at 3d post-MI (Fig.1A&C, #p<0.05). Interestingly, IL-10 KO-mice showed diminished number of circulating EPCs after MI suggesting that IL-10 deficiency impairs mobilization of EPCs into the circulation in response to myocardial injury (Fig.1B&C, #p<0.05). Furthermore, we analyzed the number of circulating Sca-1+/CD31+ (double +cells) after 3 days post-MI. Interestingly, similar trend was observed (Online Figure I. #p<0.05) suggesting that IL-10 influences mobilization of other progenitor cells of endothelial lineage.
Figure 1.
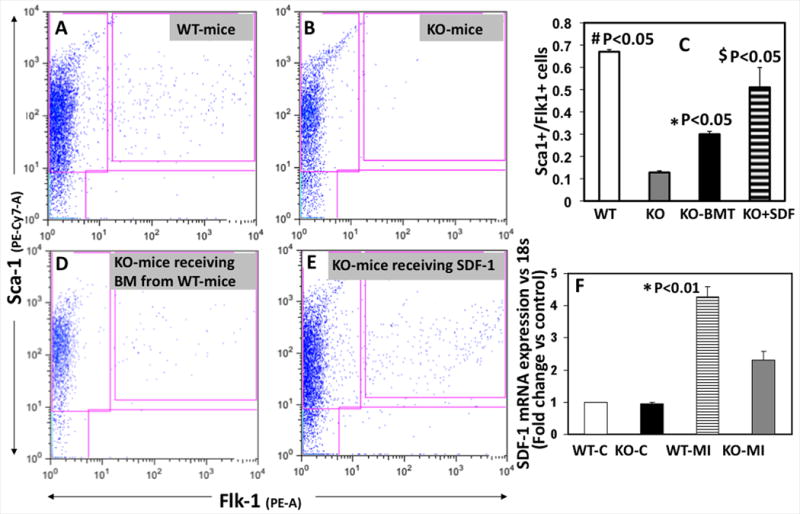
FACS analysis on peripheral blood mononuclear cells for MI-induced EPC mobilization [Sca1+/Flk1+] in WT (A), IL-10 KO-mice (B) and following bone marrow transplantation (D) or SDF-1 administration (E). C. Bar graph shows that Flk1+/Sca1+ cell mobilization was impaired in KO-mice as compared to WT-mice (#p<0.05). and BMT or SDF-1 administration attenuated mobilization in KO-mice (*$p<0.05 vs KO-mice). F. SDF-1 mRNA expression (RT-PCR) in border zone of myocardial tissue at 3 days after MI. mRNA expression normalized to 18S and depicted as fold change vs control (WT-C). SDF-1 expression was lower in KO mice as compared to WT mice (*P<0.05 WT-MI versus KO-MI).
Reconstitution of WT bone marrow in IL-10 KO mice by bone marrow transplantation attenuates impaired EPC mobilization
In an independent experiment, we performed bone marrow transplantation (BMT) followed by myocardial infarction (MI) and assessed EPC mobilization into the circulation by FACS analysis at 3 day post-MI. Bone marrow transplantation (BMT) to replace IL-10 KO-marrow with WT-marrow followed by MI and FACS analysis showed that impaired EPC mobilization in IL-10 KO-mice was rescued by the reconstitution of wild type bone marrow (Fig.1C&D, *P<0.05).
SDF-1 administration reverses defect in MI-induced mobilization in IL-10 KO-mice
Up-regulation of SDF-1 in the infarcted hearts causes massive influx of CXCR4 positive stem cells from the bone marrow 26, 27. We determined the mRNA expression of SDF-1 in the border zone of myocardial infarct after MI by real time-PCR. As illustrated in Fig 1F, SDF-1 mRNA expression significantly increased in the myocardium after MI. Interestingly, MI-induced SDF-1 expression in the myocardium was lower in IL-10-deficient mice as compared to WT-mice at 3d after MI (Fig.1F, *p<0.05). To further demonstrate that MI-induced impaired mobilization in IL-10 KO-mice is mediated through SDF-1, we injected mouse recombinant SDF-1α (500ng/mouse/day for three days; R & D Systems, Minneapolis, MN) via i.p route after MI to examine if SDF-1 rescues IL-10 phenotype in IL-10 KO-mice. FACS analysis was performed for EPC mobilization at 3 days post-MI. SDF-1 injection increased MI-induced EPC mobilization as compared to KO-mice (Fig.1C&E, *p<0.05).
IL-10-deficient EPC shows reduced CXCR4 expression and are susceptible to inflammation or hypoxia induced apoptosis
Based on the above findings, we first postulated that IL-10 deficiency impairs MI-induced EPC mobilization through SDF1-CXCR4 signaling. To address this issue, we first analyzed CXCR4 expression levels by immunofluorescence staining of cultured EPCs derived from bone marrow of IL-10 WT (WT-EPC) and KO mice (IL-10-deficient; KO-EPC). Cells were treated with LPS (to simulate inflammation) or CoCl2 before the analysis of CXCR4 expression. As shown in Fig. 2, CXCR4 expression in response to LPS [dose and time that mimic excessive and prolonged inflammatory stimuli 28, 29], was higher in WT-EPC (IL-10+/+) as compared to IL-10-deficient EPC (KO-EPC) (Fig.2B). CoCl2-induced hypoxia has similar effects on EPCs (Online Figure II). CoCl2-induced hypoxic state was confirmed by increased HIF-1α levels as compared to untreated normoxic cells (Online Figure III).
Figure 2.
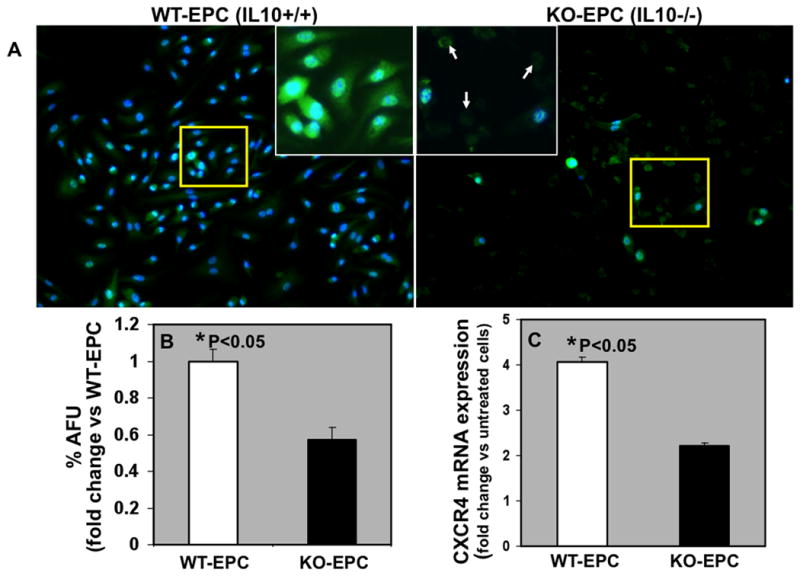
A. Immunofluroscence staining for CXCR4 protein expression (green) in EPCs from WT (WT-EPC) and IL-10 KO-mice (KO-EPC) and DAPI (blue) for nuclear staining. Inset is higher magnification of the yellow-boxed area. Also, IL-10 deficient EPCs showed increased cell death (arrows; rounding and no clear nuclear staining). B. Bar graph depicting semi-quantitative analysis of CXCR4 fluorescence signal expressed as % Arbitrary Fluorescence Units (%AFU). C. Real time-PCR data for CXCR4 mRNA expression in EPCs in response to LPS. mRNA expression normalized to 18S and depicted as fold change vs control untreated cells. CXCR4 expression (mRNA and protein) was lower in KO-EPCs as compared to WT-EPCs (*P<0.05 versus KO-EPC).
IL-10-deficiency impairs EPC migration toward SDF-1 through CXCR4 pathway
To first determine the migratory response of ex vivo expanded WT and IL-10 deficient EPCs toward a SDF-1 gradient, we performed a modified Boyden chamber migration assay (Fig. 3). As shown in Fig.3, migration of both WT and IL-10 deficient EPCs were enhanced towards SDF-1 gradient (P < 0.05, Fig. 3 a,b,d,e,h). However, IL-10-deficient (KO) EPCs showed significantly lower migration in response to SDF-1 as compared to WT-EPCs (P < 0.05, Fig. 3b,e,h). To further investigate if the differential migratory response of ex vivo expanded WT and IL-10 deficient EPCs toward a SDF-1 gradient are mediated through CXCR4, we incubated EPCs with 10μg/ml AMD3100 (CXCR4 antagonist). Incubation of EPC with the CXCR4 antagonist AMD3100 significantly blocked the increase in SDF-1 induced migration (P < 0.05, Fig. 3c,f,h). These data suggest that the impairment in MI-induced BM-EPC mobilization in IL-10-deficiency mice is mediated through SDF-1/CXCR4 signaling axis.
Figure 3.
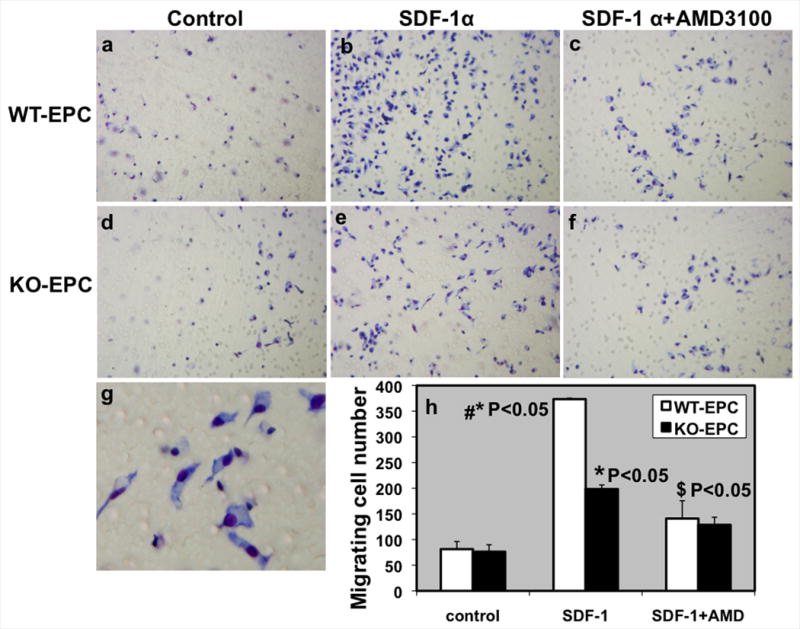
SDF-1 induced migration EPC from WT and IL-10-deficient mice. Migratory response of Ex vivo expanded EPCs toward 20ng/ml SDF-1 gradient was measured by modified Boyden chamber migration assay. a,d. Untreated control EPCs, SDF-1 stimulated migration (b,e) and EPCs incubated with AMD3100 (10μg/ml; CXCR4 inhibitor) (c,f). g. Higher magnification of cells attached to the membrane after migration towards SDF-1 gradient. h. Bar graph of migrated cell number after 18 hours of incubation. EPCs demonstrated a potent migratory activity toward SDF-1. SDF-1 induced migration was impaired in IL-10 deficient EPCs (KO-EPCs) as compared to WT-EPCs. *P<0.05, Control vs SDF-1; #P<0.05, SDF-1 induced, WT-EPC vs KO-EPC; $P<0.05, SDF-1 vs AMD3100.
Increased susceptibility of IL-10-deficient EPC to LPS/ CoCl2-induced apoptosis
We evaluated the effect of IL-10 phenotype on the survival of EPCs in response to inflammatory stimuli (LPS) or CoCl2-induced hypoxia, in vitro. EPCs derived from bone marrow of IL-10 WT (WT-EPC) and KO mice (KO-EPC) were incubated on 8-well glass slides coated with 0.1% fibronectin with 50ng/ml LPS or 100μM CoCl2 for 24h. Apoptosis was assessed using TUNEL staining. LPS-induced apoptosis was significantly higher in IL-10 deficient EPC as compared to WT-EPCs (Fig 4A&B; #p<0.01). Similar trends were observed in CoCl2 treated EPCs (Online Figure IV).
Figure 4.
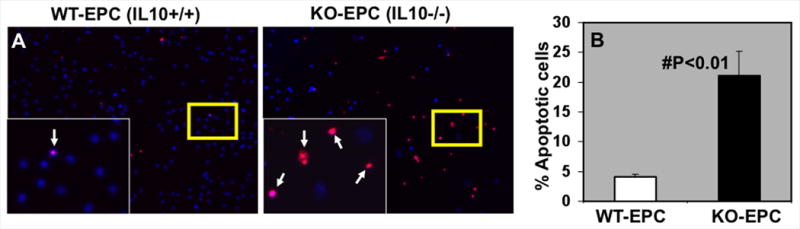
A. Inflammatory stimuli (LPS)-induced apoptosis (TUNEL+, red fluorescence) in EPC's isolated from WT and IL-10 KO-mice. DAPI (blue) was used for nuclear staining. Inset is higher magnification of the yellow-boxed area. B. LPS-induced EPC apoptosis was lower in WT-EPC as compared to EPC from KO-mice (#P<0.01).
Systemic IL-10 therapy enhances retention and survival of transplanted EPCs in the ischemic myocardium
We have previously reported that IL-10 significantly reduced post-MI inflammatory response in the myocardium 29. We examined the retention and survival of GFP+ EPCs (from Tie2GFP-Tg mice) following their transplantation in the ischemic myocardium (3d post-MI) by assessing the number of GFP+ cells (green) in the myocardium and their apoptosis (red) by TUNEL staining. Nuclei were stained blue with DAPI. As shown in Fig. 5A&B, mice receiving IL-10 had a higher number of GFP+ EPCs retained in the myocardium as compared to EPC+saline group (#P<0.01). Interestingly, in EPC+saline group, a large number of these GFP+ positive cells were undergoing apoptosis as compared to the mice that received IL-10 (Fig. 5A&C; #P<0.01). These data suggests that IL-10 protects transplanted EPC in the ischemic myocardium, thereby, increases the numerical availability (retention) of live EPCs leading to enhanced myocardial repair and LV function. We confirmed inflammatory response in the myocardium by assessing infiltration of macrophage and monocyte in the border zone of LV infarct by immunofluorescence staining of CD68-positive cells (CD68+ cells). The number of infiltrating CD68+ cells in the border zone of infarct increased at 3 days post-MI (P<0.01 vs. Sham, Online Figure V). IL-10 treatment significantly inhibited CD68+ cells infiltration at the injury site (P<0.01 vs. EPC+saline group, Online Figure V) with concurrent increase in EPC retention and survival (Fig. 5A&B). Furthermore, quantitative RT-PCR analysis revealed increased mRNA expression of various pro-inflammatory cytokines and chemokines (IL-1β, IL-6, TNF-α, MCP-1) in the myocardium at 3 days post-MI (P<0.01 vs. Sham, Online Figure VI), which was significantly reduced upon IL-10 treatment (P<0.05 vs. EPC+saline group, Online Figure VI).
Figure 5.
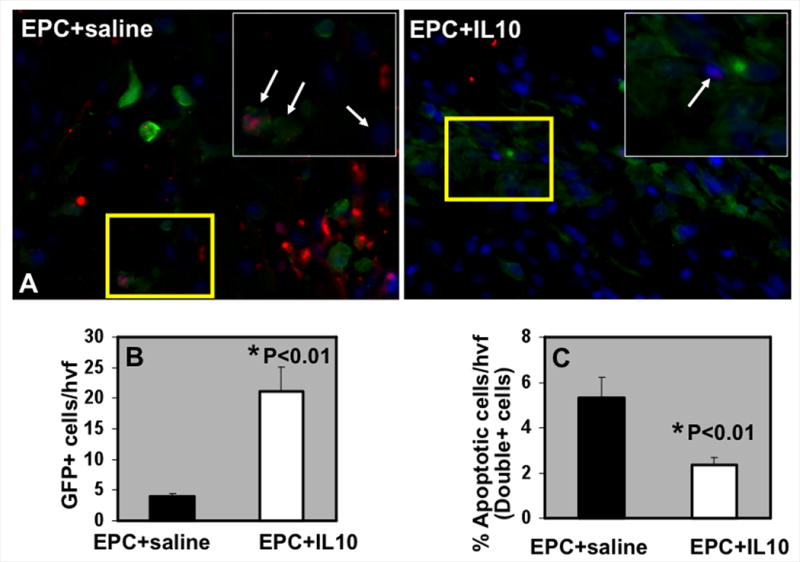
A. EPC retention and survival in the myocardium at 3 days post-MI in IL-10/saline treated mice. TUNEL staining for detecting apoptosis (Red) of EPC (GFP-positive, green fluorescence) and DAPI (blue) for nuclear staining. Inset is higher magnification of the yellow-boxed area. Arrows indicate GFP+TUNEL+ cells. B. Quantification of GFP+ (EPC) cells at 3 days post-MI. C. Quantitative analysis of GFP/TUNEL double-positive cells at 3 days post-MI. IL-10 increased GFP+ EPC retention and survival in the heart following transplantation, *P<0.01 vs EPC+saline group. hvf, high-power visual field.
IL-10 therapy augments EPC-mediated neo-vascularization in the ischemic myocardium
To determine the effect of IL-10 on EPC-mediated neovascularization including engraftment of the transplanted EPC into the vascular structures, we assessed capillary density and the number of GFP+ EPC (green) incorporated into the capillaries (red; identified by CD31+ immunofluorescence staining)29 in the myocardium at 28 days post-MI. Figure 6 illustrates that the mice receiving IL-10 showed increased number of GFP+ EPC in the border zone of infarct (Fig 5A) associated with enhanced capillary density (Fig 5B&C; *P<0.05). EPCs were either seen in the vicinity of the existing vascular structures (green and red color) or incorporated into the vascular structures (yellow), suggesting possibilities of both incorporation into vascular structures and paracrine mechanisms aiding in neovascularization. Interestingly, the number of GFP+ EPCs co-localized to CD31+ vascular structures (yellow) were higher (Fig 5B&D; #P<0.01) in IL-10 treated mice. In contrast, both the number and co-localization of GFP+ cells with CD31+ vascular structures was significantly lower in the myocardium of mice receiving EPC+Saline.
Figure 6.
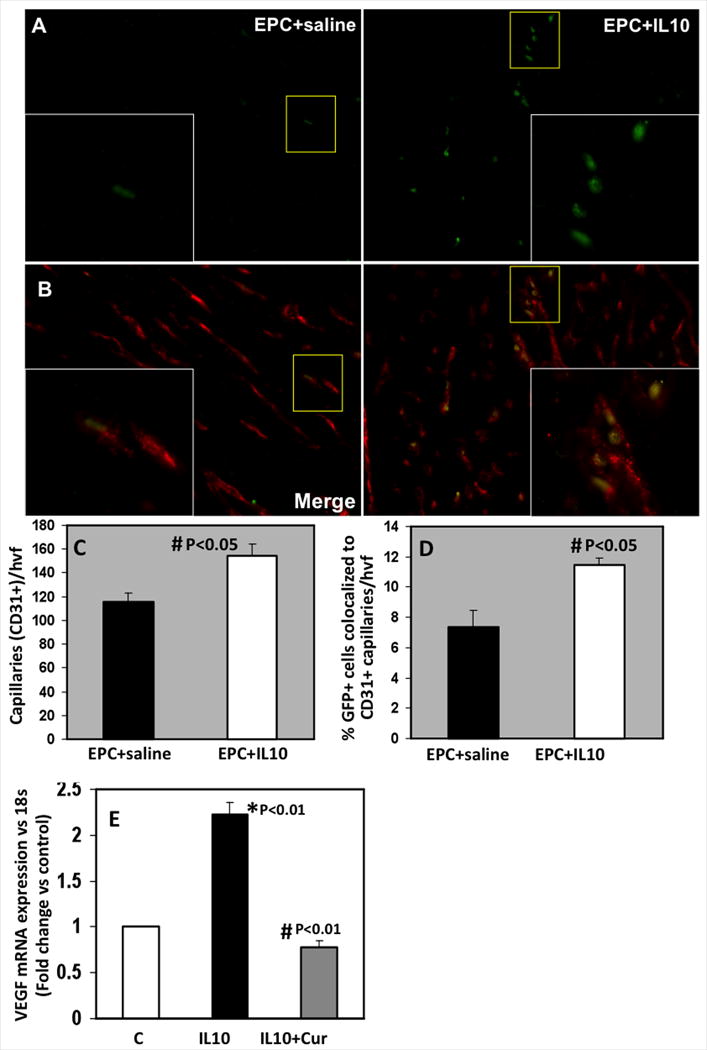
A,B. EPC-mediated neovascularization in border zone of LV infarct at 28 days post-MI. Engraftment of EPC (GFP+, green fluorescence) into vascular structures (CD31 staining for capillaries, red fluorescence) is seen as yellow structures. However, some cells are not incorporated (green). Inset is higher magnification of the yellow-boxed area. Bar graph shows quantitative analysis of CD31+ capillaries per high-power visual field (hvf) (C) and number of GFP+ cells associated with CD31+ vasculature (D). Capillary density and EPC engraftment into vascular structures was higher in IL-10 treated mice (#P<0.05). E. Effect of STAT3 on VEGF-A mRNA expression in EPC cells. Curcurbitacin I (Cur, STAT3 inhibitor) treated cells inhibited IL-10 induced VEGF-A. mRNA expression normalized to 18S and depicted as fold change vs control (c) untreated cells. *P<0.01 vs control cells; #P<0.01 vs IL-10 treated cells.
IL-10-induced up-regulation of VEGF expression in EPC's is mediated through STAT-3
VEGF and STAT3 play an important role in neovascularization during ischemic injury 30-32. Our previous studies 29 have shown that IL-10 activate STAT3 and increases VEGF expression in the myocardium after MI. We speculated that EPC-mediated increased neovascularization in IL-10-treated mice might be through STAT3/VEGF signaling mechanism. To determine this possibility, we pretreated EPCs with curcurbitacin I (stat3 inhibitor, 1 μM) for 1 hr and 10ng/ml IL-10 vs. PBS for an additional 6 hours. VEGF mRNA expression was quantified by real time-PCR analysis (Fig 6E). IL-10 treatment significantly increased VEGF expression (P<0.01 vs. control cells, Fig 6E). However, inhibition of STAT-3 with curcurbitacin I significantly decreased IL-10-induced VEGF gene expression (P<0.01 vs. IL-10-treated cells, Fig 6E), therefore suggesting that IL-10-induced VEGF in EPCs is mediated through STAT-3 transcription factor.
IL-10 enhances EPC-induced LV functional recovery after MI
LV function was assessed by echocardiography as described earlier 29 at 7, 14 and 28 days, post-MI (Figure 7). The results are available as an Online Supplement at http://circres.ahajournals.org.
Figure 7.
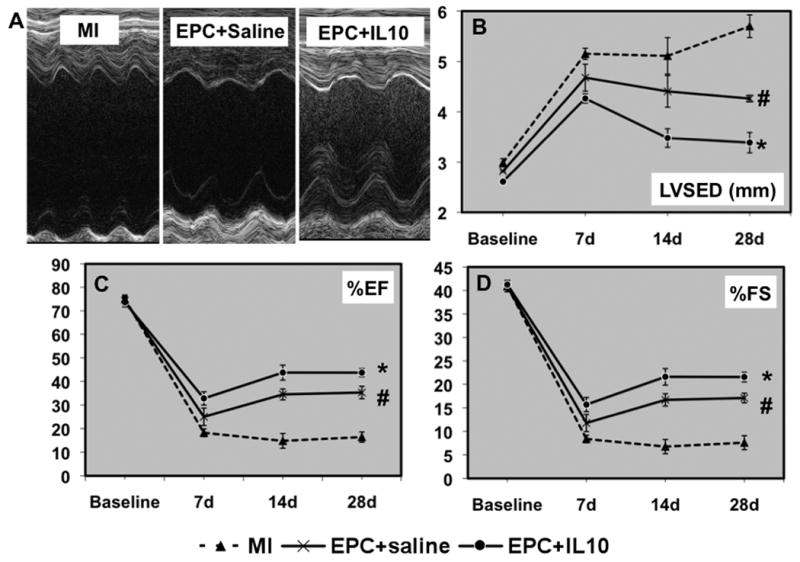
A. M-mode echocardiographic tracings at baseline and 7, 14 and 28 days of MI in EPC+saline and EPC+IL-10 groups. Analysis of LV diameter in systole (B) and %EF (C) and %FS (D) calculations. IL-10 administration significantly improved LV function with decreased LVESD and increased %EF and %FS, as compared to EPC+saline group. LVESD, LV end-systolic diameter; %EF, percent ejection fraction; %FS, percent fractional shortening; #P<0.05 vs MI; *P<0.05 vs EPC+saline; #P<0.05 vs MI alone group.
Replacement of KO bone marrow by WT bone marrow attenuates MI-induced LV dysfunctions in IL-10 KO mice
MI was performed after 6 weeks of BMT in IL-10 KO-mice (receiving WT-marrow; KO-BMT) and WT-mice (receiving IL-10-deficient marrow; WT-BMT). LV function was assessed by echocardiography at 7, 14 and 28 days using m-mode tracings (Online Figure VII,A). The results are available as an Online Supplement at http://circres.ahajournals.org.
Discussion
Coronary artery disease is a leading cause of morbidity and mortality. There are several repair mechanisms that are now thought to involve circulating EPCs mobilized from the bone marrow and home to sites of ischemic injury. A growing body of evidence in experimental models of cardiac injury suggests that stem cell treatment or transplantation of stem cells remodels and regenerates injured tissue, improves function, and protects tissue from further insult. Indeed, these encouraging results have led to phase I and II clinical trials involving endothelial progenitor cell therapy for a variety of diseases 14, 15, 33. However, stem cells transplanted in the ischemic myocardium are susceptible to adverse tissue microenvironment like prolonged inflammation, reduced oxygen supply and free radical damage, thereby compromising their full therapeutic benefits. Previous studies from our laboratory 28, 29 have shown that IL-10, an anti-inflammatory cytokine, attenuates inflammatory response in the myocardium and improves LV function and remodeling. However, if IL-10 mediated inhibition of inflammation in the injured myocardium can also modulate the survival and function of the transplanted EPCs is not known. Therefore, we tested if IL-10 (primarily an anti-inflammatory cytokine) may modulate EPC biology leading to enhanced survival and function following transplantation in an ischemic myocardium and therefore attenuate MI-induced LV dysfunction and remodeling. We report that this indeed is the case. Several lines of evidence support our conclusions: a) in mice genetically deficient in IL-10 (KO), myocardial ischemia induced mobilization of BM-EPC is impaired; b) transplantation of wilt type bone marrow in IL-10 KO mice rescues the BM-EPC mobilization, conversely replacement of WT marrow with Il-10 KO marrow in WT mice induces impaired EPC mobilization; c) co-administration of IL-10 enhances the retention and survival of the intramyocardially transplanted EPCs; d) enhanced EPC survival in the ischemic myocardium is associated with augmentation of EPC-mediated neovascularization and further improvements in the LV function when compared to EPC transplantation alone; and e) mechanistically Il-10 effects on EPCs appear to be mediated through SDF-1/CXCR4 and STAT-3/VEGF signaling mechanisms.
Endothelial cell proliferation, migration and sprouting of preexisting blood vessels were considered to be the principal source of new vessel formation. However, evidence suggests that mobilization of endothelial progenitor cells (EPCs) from the bone marrow and their subsequent participation in new vessel formation are essential for repair of injured myocardium 34. In the present study, flow-cytometry analyses indicated that circulating Sca-1/Flk1 double-positive cell (EPCs) following MI was diminished in IL-10 KO-mice as compared to WT-mice, suggesting that IL-10 plays a crucial role in mobilization of EPCs. Bone marrow transplantation (BMT) to replace IL-10 KO-marrow with WT-marrow attenuated these effects, therefore further validating the effect of IL-10 on EPC mobilization. Impaired mobilization in IL-10 KO-mice was associated with reduced Stromal cell-derived factor 1α (SDF-1α) levels in the border zone of myocardial infarct, suggesting that MI-induced EPC mobilization defect in KO-mice might be mediated through SDF-1/CXCR4. The reversal of mobilization defect in KO-mice by SDF-1 administration further confirmed this possibility. SDF-1α is a chemotactic factor for EPCs in humans; SDF-1α induces EPC migration in vitro 35. SDF-1/CXCR4 signaling axis regulates BM endothelial progenitor cell mobilization, survival and angiogenesis, as well as several intracellular signaling pathways 26, 27, 36. Thus, SDF-1α and CXCR4 are key regulators of EPC mobilization and recruitment. Previous report has suggested that up-regulation of SDF-1α in the infarcted myocardium causes massive influx of CXCR4 positive stem cells from the bone marrow 37. One major finding of the present study is that IL-10-deficient EPCs showed reduced functional expression of CXCR4 and associated with reduced SDF-1 induced EPC migration in vitro. It has been demonstrated that CXCR4 plays a key role in modulating EPC migration and homing 38. Previous investigation suggested that EPC with reduced functional CXCR4 expression or impaired CXCR4 signaling show decreased migratory capacity 39. As expected, CXCR4 blocker, AMD3100, abolished the effects of IL-10/SDF-1 on EPC migration. These observations provide clear evidence that the effect of IL-10 on BM-EPC mobilization is mediated through SDF-1/CXCR4 signaling axis.
In the present study, we used Cobalt chloride (CoCl2) to study hypoxia-induced CXCR4 expression and cell death in WT and IL-10-deficient EPCs. The hypoxic state of EPCs was confirmed by an increase in hypoxia inducible transcription factor 1α (HIF1α). CoCl2 has been widely used as a hypoxia mimic in both in vivo and in vitro studies and is known to activate hypoxic signaling by stabilizing HIF1α 40. However, with varying time and dose of exposure, CoCl2 can activate a number of other signaling pathways 41, 42. Increased apoptosis in CoCl2 treated cells is in agreement with the previous findings that prolonged hypoxia can also induce genes involved in cell death by enhancing the expression of two BH3 domain containing cell death genes, BNip3 and NIX 41. An interesting finding in the present work is that IL-10-deficient EPCs showed increased cell death in response to CoCl2-induced hypoxia. The signaling mechanism involved in this process is not clear. However, it can be speculated that Heme oxygenase (HO-1) might be playing a significant cytoprotective role in WT-EPCs 43. HO-1 is highly inducible in response to various stimuli, including oxidative stress, heavy metals, UV radiation, and inflammation.
Previous reports have suggested that prolonged and sustained pro-inflammatory response in the myocardium could result in adverse outcomes leading to LV dysfunction and remodeling 23, 44, 45. Our recent studies have shown that IL-10 attenuates MI-induced inflammatory response and associated LV dysfunction and remodeling 28, 29. A longstanding body of evidence on stem cell therapy describes the challenges in overcoming the susceptibility of transplanted stem cells in a hostile inflammatory and ischemic tissue microenvironment 46-48. Previous reports have suggested that prolonged inflammation has been implicated with reduced EPC mobilization, cell death and functional impairment 19, 20, 49-51. Here, we investigated whether IL-10 enhances EPC survival and incorporation of these EPCs into sites of neovascularization after MI, and whether this enhancement leads to greater preservation of myocardial function and integrity. IL-10 was originally characterized for its ability to inhibit the synthesis of proinflammatory cytokines, but evidence is growing that it directly regulates the growth and survival of noninflammatory cells, as well [reviewed in Ref 52]. The present study reveals that mice receiving IL-10 had a higher number of GFP+ EPCs retained in the myocardium as compared to EPC+saline group. Interestingly, in EPC+saline group, a large number of these GFP+ positive cells were undergoing apoptosis as compared to the mice that received IL-10. In addition, in vitro experiments show that IL-10 deficient EPCs show diminished protection against inflammatory stimuli (LPS) or CoCl2-mediated hypoxia-induced apoptosis, which is consistent with recent findings that the death of both primary oligodendrocyte progenitor cells and astrocytes was inhibited by IL-10 53. These studies imply that IL-10 protects transplanted EPC in the ischemic myocardium, thereby, increases the numerical availability (retention) of live EPCs leading to significantly enhanced myocardial repair and LV function. Moreover, evidence exists that not only the cell number but also functional properties of EPC determine the therapeutic success in autologous stem cell transplantation 15. Recent findings from therapeutic interventions in humans suggest that the microvasculature may be a viable target for cardiovascular therapy 33. Most compelling, however, are clinical trial data suggesting that protection or restoration of the myocardial microvasculature may provide benefits and that strategies designed to restore microvascular integrity can improve long-term outcomes 54. In the present study, mice that received IL-10 showed enhanced neovascularization and co-localization of EPCs to CD31+ vascular structures. These EPCs were either seen in the vicinity of the existing vessels or incorporated into the vascular structures, suggesting possibilities of both engraftment into vascular structures and paracrine mechanisms aiding in neovascularization. In vitro, inhibition of STAT3 with curcurbitacin I in EPC's resulted in reduced IL-10-mediated VEGF expression. Previous studies have suggested that IL-10 signaling is mediated through STAT3 55, which regulates a number of key cellular processes including pro-survival and pro-angiogenic VEGF signaling pathways 31. However, the requirement of endogenous STAT3 for mediating IL-10-induced EPC survival and EPC-mediated neovascularizaton signaling has not been explored so far. Our previous reports have shown that IL-10 increases post-MI neovascularization associated with STAT3 activation and VEGF expression in the myocardium 29. Taken together, our study suggests that IL-10 mediated increased angiogenesis in LV after MI might be a synergy of STAT3 activation in the myocardium/EPC and VEGF expression in the EPC. However, it should be noted that STAT3/VEGF might not be the only signaling mechanisms. A higher HIF1α mRNA expression level in WT-EPCs as compared to IL-10 deficient EPCs (although not significant) suggests this possibility. IL-10 has been shown to modulate other downstream signaling target like NFkB and HO-1 43, 56.These findings along with the EPC survival data suggests that IL-10 protects transplanted EPC in the ischemic myocardium and enhances EPC-mediated neo-vascularization, post-MI.
In animals, the preservation or restoration of micro-vessels correlates with better left ventricular (LV) function after MI, and therapies designed to enhance the microvascular circulation have been shown to preserve cardiac function and integrity 57. In the present study, LV function assessment revealed that mice receiving EPC along with IL-10 showed much improved LV function than those receiving EPC alone. Improved LV function was associated with reduction in infarct size in IL-10 treated group. From the above observations, it could be postulated that both increased neo-angiogenesis and transdifferentiation of EPCs into cardiomyocyte might have contributed to enhanced EPC-mediated improvement of LV function in IL-10-treated mice. Furthermore, the reduction in infarction size could be due to formation of new myocytes or due to increased survival of myocytes in the infarcted area due to reduced apoptosis 58. Recent studies suggest that progenitor cells can transdifferentiate into other lineages 59. EPCs can contribute to not only vasculogenesis but also myogenesis in the ischemic myocardium in vivo, either through transdifferentiation 60 or through activation of resident cardiac stem cells 61. However, IL-10 mediated transdifferentiation potential of endothelial progenitor cells (EPCs) in the present study was not explored. Recent studies have also indicated that immunomodulation with anti-inflammatory paracrine factors including IL-10 released by stem cells are important in conferring improved outcome after stem cell therapy 22, 46, 47. Interestingly, bone marrow mononuclear cells (BM-MNCs) transplanted in the infarcted mouse hearts secreted significant amounts of IL-10 and the cardiac protection was associated with decreased T lymphocyte accumulation, reactive hypertrophy, and myocardial collagen deposition 22. Together, these findings provide enticing evidence that IL-10 significantly enhances the survival and angiogenic potential of EPCs, therefore leading to preservation of LV function and integrity in the setting of MI.
Supplementary Material
Figure 8.
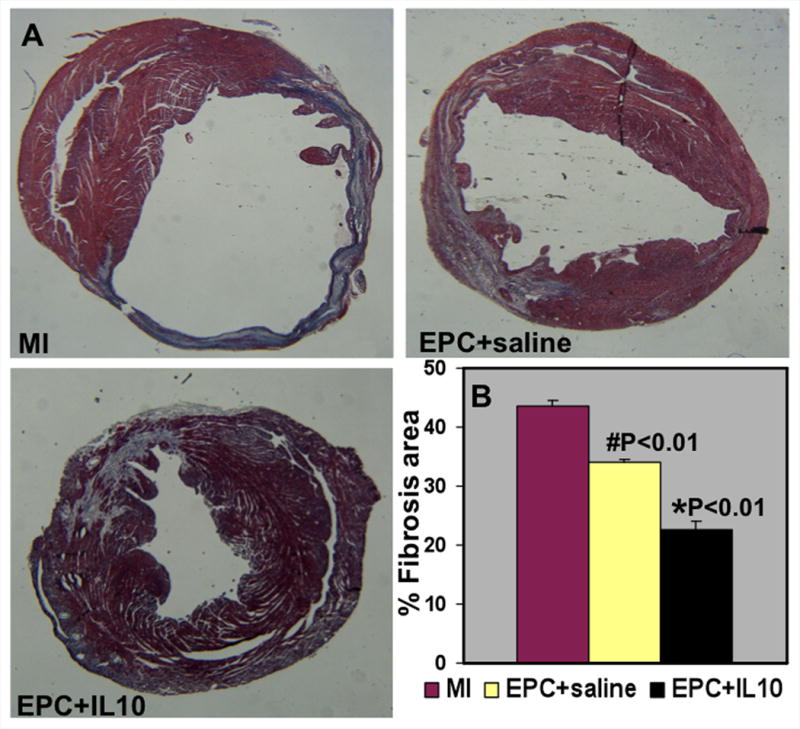
A. Trichrome stained heart sections (28 days post-MI). B. Quantitative analysis of fibrosis area at 28 days post-MI. Mice that received EPC+IL-10 showed lower fibrosis area when compared to EPC+saline group. #P<0.01 vs MI; *P<0.01 vs EPC+saline.
Novelty and Significance.
What is known?
Recruitment of endothelial progenitor cells (EPC) to the site of injury is fundamental for neovascularization of ischemic tissue.
Chronic pro-inflammatory response in the myocardium after infarction is associated with cardiomyocyte loss and fibrosis leading to LV dysfunction and adverse remodeling.
IL-10, an anti-inflammatory cytokine, attenuates inflammatory response in the myocardium and improves LV function and adverse remodeling.
The microenvironment of the ischemic tissue adversely effects EPC survival and function
What new information does this article contribute?
IL-10 enhances survival and function of EPC following transplantation in an ischemic myocardial tissue.
We demonstrate that this action is principally due to the activation of an angiogenic mechanism involving STAT3-dependent induction of the vascular endothelial growth factor (VEGF).
Deletion of IL-10 in mice reduces EPC mobilization from the bone marrow after myocardial infarction.
Summary of the Novelty and Significance
Adverse microenvironment of ischemic tissue has a detrimental effect on the survival of mobilized/transplanted EPC, which could compromise their full benefits in cell-based therapy. Prolonged pro-inflammatory response in the myocardium after MI results in cardiomyocyte loss and fibrosis leading to LV dysfunction and adverse remodeling. Therefore, modulation of local tissue pro-inflammatory response by anti-inflammatory factors either released by stem cells or through systemic delivery is important in improving outcome after stem cell therapy. This study demonstrates that MI-induced mobilization of BM-EPC is impaired in IL-10-deficient mice; IL-10 co-administration enhances the retention and survival of the intramyocardially transplanted EPCs, and it augments EPC-mediated neovascularization. Mechanistically, the effects of IL-10 on EPCs are mediated through SDF-1/CXCR4 and STAT-3/VEGF signaling mechanisms. These findings suggest a potential therapeutic role for IL-10 in enhancing the regenerative effects of EPC cell-based therapies by augmenting cell retention and survival on one hand, while modulating EPC biology and EPC-mediated neovascularization on the other., Signaling downstream of IL-10 could be targeted to promote stem cell mobilization, homing and survival for improving the therapeutic efficacy of EPCs
Acknowledgments
Sources of Funding: Work described in this manuscript was in part supported by American Heart Association National-The Davee Foundation SDG grant 0930219N (P.K.) and National Institute of Health grants HL091983 and HL105597 (R.K.).
Non-standard Abbreviations and Acronyms
- MI
myocardial infarction
- EPC
Endothelial progenitor cell
- BMT
bone marrow transplantation
- GFP
green fluorescent protein
- KO-EPC
EPCs from IL-10 knock-out (deficient) mice
- WT-EPC
EPCs from wild-type mice
- Sca-1
stem cell antigen 1
- Flk1
fetal-liver kinase 1
- FACS
fluorescence-activated cell sorting
- LPS
lipopolysaccharide
- LV
left ventricle
- hvf
high-power visual field
Footnotes
Disclosures: None
THIS PAPER HAD A CONSULTING EDITOR: Mark A. Sussman:
In August 2011, the average time from submission to first decision for all original research papers submitted to Circulation Research was 16 days.
References
- 1.Cottage CT, Bailey B, Fischer KM, Avitable D, Collins B, Tuck S, Quijada P, Gude N, Alvarez R, Muraski J, Sussman MA. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. 2010;106(5):891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Amario D, Cabral-Da-Silva MC, Zheng H, Fiorini C, Goichberg P, Steadman E, Ferreira-Martins J, Sanada F, Piccoli M, Cappetta D, D'Alessandro DA, Michler RE, Hosoda T, Anastasia L, Rota M, Leri A, Anversa P, Kajstura J. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res. 2011;108(12):1467–1481. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.D'Amario D, Fiorini C, Campbell PM, Goichberg P, Sanada F, Zheng H, Hosoda T, Rota M, Connell JM, Gallegos RP, Welt FG, Givertz MM, Mitchell RN, Leri A, Kajstura J, Pfeffer MA, Anversa P. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108(7):857–861. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer KM, Din S, Gude N, Konstandin MH, Wu W, Quijada P, Sussman MA. Cardiac progenitor cell commitment is inhibited by nuclear Akt expression. Circ Res. 2011;108(8):960–970. doi: 10.1161/CIRCRESAHA.110.237156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogorek B, Ferreira-Martins J, Arranto C, D'Amario D, del Monte F, Urbanek K, D'Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, Leri A. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123(12):1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Kanashiro-Takeuchi RM, Schulman IH, Hare JM. Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madonna R, Rokosh G, De Caterina R, Bolli R. Hepatocyte growth factor/Met gene transfer in cardiac stem cells--potential for cardiac repair. Basic Res Cardiol. 2010;105(4):443–452. doi: 10.1007/s00395-010-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111(9):1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 9.Spyridopoulos I, Sullivan AB, Kearney M, Isner JM, Losordo DW. Estrogen-receptor-mediated inhibition of human endothelial cell apoptosis. Estradiol as a survival factor. Circulation. 1997;95(9118519):1505–1514. doi: 10.1161/01.cir.95.6.1505. [DOI] [PubMed] [Google Scholar]
- 10.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96(1):127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 11.Masuda H, Kalka C, Takahashi T, Yoshida M, Wada M, Kobori M, Itoh R, Iwaguro H, Eguchi M, Iwami Y, Tanaka R, Nakagawa Y, Sugimoto A, Ninomiya S, Hayashi S, Kato S, Asahara T. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101(6):598–606. doi: 10.1161/CIRCRESAHA.106.144006. [DOI] [PubMed] [Google Scholar]
- 12.Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Bohm M, Nickenig G. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107(12810616):3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 13.Zuba-Surma EK, Guo Y, Taher H, Sanganalmath SK, Hunt G, Vincent RJ, Kucia M, Abdel-Latif A, Tang XL, Ratajczak MZ, Dawn B, Bolli R. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J Cell Mol Med. 2011;15(6):1319–1328. doi: 10.1111/j.1582-4934.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 15.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108(14557356):2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 16.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115(17562958):3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 17.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106(12370212):1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 18.Trachtenberg B, Velazquez DL, Williams AR, McNiece I, Fishman J, Nguyen K, Rouy D, Altman P, Schwarz R, Mendizabal A, Oskouei B, Byrnes J, Soto V, Tracy M, Zambrano JP, Heldman AW, Hare JM. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J. 2011;161(3):487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D, Weigel G, Schwarzinger I, Wolozcszuk W, Steiner G, Smolen JS. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111(2):204–211. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 20.Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol. 2006;26(2):257–266. doi: 10.1161/01.ATV.0000198239.41189.5d. [DOI] [PubMed] [Google Scholar]
- 21.Swijnenburg RJ, Schrepfer S, Cao F, Pearl JI, Xie X, Connolly AJ, Robbins RC, Wu JC. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells Dev. 2008;17(6):1023–1029. doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burchfield JS, Iwasaki M, Koyanagi M, Urbich C, Rosenthal N, Zeiher AM, Dimmeler S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ Res. 2008;103(2):203–211. doi: 10.1161/CIRCRESAHA.108.178475. [DOI] [PubMed] [Google Scholar]
- 23.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13(16):1877–1893. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 24.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165(5):2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 25.Stumpf C, Petzi S, Seybold K, Wasmeier G, Arnold M, Raaz D, Yilmaz A, Daniel WG, Garlichs CD. Atorvastatin enhances interleukin-10 levels and improves cardiac function in rats after acute myocardial infarction. Clin Sci (Lond) 2009;116(1):45–52. doi: 10.1042/CS20080042. [DOI] [PubMed] [Google Scholar]
- 26.Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, Zhang L, Huang Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36(4):644–650. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Li C, Chen Y, Hao Y, Zhou W, Chen C, Yu Z. Hypoxia enhances CXCR4 expression favoring microglia migration via HIF-1alpha activation. Biochem Biophys Res Commun. 2008;371(2):283–288. doi: 10.1016/j.bbrc.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 28.Krishnamurthy P, Lambers E, Verma S, Thorne T, Qin G, Losordo DW, Kishore R. Myocardial knockdown of mRNA-stabilizing protein HuR attenuates post-MI inflammatory response and left ventricular dysfunction in IL-10-null mice. Faseb J. 2010 doi: 10.1096/fj.09-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res. 2009;104(2):e9–18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95(2):187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 31.Hilfiker-Kleiner D, Limbourg A, Drexler H. STAT3-mediated activation of myocardial capillary growth. Trends Cardiovasc Med. 2005;15(4):152–157. doi: 10.1016/j.tcm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, Xie K. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22(3):319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 33.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. Intramyocardial, Autologous CD34+ Cell Therapy for Refractory Angina. Circ Res. 2011;109(4):428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103(23):2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 35.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185(1):111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273(36):23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 37.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 38.Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, Ito A, Misener S, Tanaka T, Klyachko E, Kobayashi K, Tongers J, Roncalli J, Tsurumi Y, Hagiwara N, Losordo DW. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107(24):11008–11013. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honold J, Lehmann R, Heeschen C, Walter DH, Assmus B, Sasaki K, Martin H, Haendeler J, Zeiher AM, Dimmeler S. Effects of granulocyte colony simulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arterioscler Thromb Vasc Biol. 2006;26(10):2238–2243. doi: 10.1161/01.ATV.0000240248.55172.dd. [DOI] [PubMed] [Google Scholar]
- 40.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82(12):3610–3615. [PubMed] [Google Scholar]
- 41.Vengellur A, LaPres JJ. The role of hypoxia inducible factor 1alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci. 2004;82(2):638–646. doi: 10.1093/toxsci/kfh278. [DOI] [PubMed] [Google Scholar]
- 42.Xi L, Taher M, Yin C, Salloum F, Kukreja RC. Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of HIF-1alpha and AP-1 and iNOS signaling. Am J Physiol Heart Circ Physiol. 2004;287(6):H2369–2375. doi: 10.1152/ajpheart.00422.2004. [DOI] [PubMed] [Google Scholar]
- 43.Ricchetti GA, Williams LM, Foxwell BM. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol. 2004;76(3):719–726. doi: 10.1189/jlb.0104046. [DOI] [PubMed] [Google Scholar]
- 44.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115(11):1398–1407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 45.Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110(20):3221–3228. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 46.Bishopric NH. Mesenchymal stem cell-derived IL-10 and recovery from infarction: a third pitch for the chord. Circ Res. 2008;103(2):125–127. doi: 10.1161/CIRCRESAHA.108.180596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Surgically relevant aspects of stem cell paracrine effects. Surgery. 2008;143(5):577–581. doi: 10.1016/j.surg.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabelink TJ, de Boer HC, de Koning EJ, van Zonneveld AJ. Endothelial progenitor cells: more than an inflammatory response? Arterioscler Thromb Vasc Biol. 2004;24(5):834–838. doi: 10.1161/01.ATV.0000124891.57581.9f. [DOI] [PubMed] [Google Scholar]
- 49.Andreou I, Tousoulis D, Tentolouris C, Antoniades C, Stefanadis C. Potential role of endothelial progenitor cells in the pathophysiology of heart failure: clinical implications and perspectives. Atherosclerosis. 2006;189(2):247–254. doi: 10.1016/j.atherosclerosis.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 50.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin C, Kotlarz D, Mueller M, Fuchs M, Hornig B, Haller H, Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110(14):1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 51.Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rossig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111(9):1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- 52.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 53.Molina-Holgado E, Vela JM, Arevalo-Martin A, Guaza C. LPS/IFN-gamma cytotoxicity in oligodendroglial cells: role of nitric oxide and protection by the anti-inflammatory cytokine IL-10. Eur J Neurosci. 2001;13(3):493–502. doi: 10.1046/j.0953-816x.2000.01412.x. [DOI] [PubMed] [Google Scholar]
- 54.Moreno R, Hernandez-Antolin R, Alfonso F, Macaya C. Diabetes mellitus and acute myocardial infarction: more data supporting a poorer microvasculature reperfusion. Am Heart J. 2003;146(2):E6. doi: 10.1016/S0002-8703(03)00166-2. [DOI] [PubMed] [Google Scholar]
- 55.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172(1):567–576. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 56.Dhingra S, Sharma AK, Arora RC, Slezak J, Singal PK. IL-10 attenuates TNF-alpha-induced NF kappaB pathway activation and cardiomyocyte apoptosis. Cardiovasc Res. 2009;82(1):59–66. doi: 10.1093/cvr/cvp040. [DOI] [PubMed] [Google Scholar]
- 57.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107(3):461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 58.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 59.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107(7):1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 60.Murasawa S, Kawamoto A, Horii M, Nakamori S, Asahara T. Niche-dependent translineage commitment of endothelial progenitor cells, not cell fusion in general, into myocardial lineage cells. Arterioscler Thromb Vasc Biol. 2005;25(7):1388–1394. doi: 10.1161/01.ATV.0000168409.69960.e9. [DOI] [PubMed] [Google Scholar]
- 61.Brunner S, Huber BC, Fischer R, Groebner M, Hacker M, David R, Zaruba MM, Vallaster M, Rischpler C, Wilke A, Gerbitz A, Franz WM. G-CSF treatment after myocardial infarction: impact on bone marrow-derived vs cardiac progenitor cells. Exp Hematol. 2008;36(6):695–702. doi: 10.1016/j.exphem.2008.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


