Abstract
Purpose
Post-myocardial infarction heart failure is a major health concern with limited therapy. Molecular revascularisation utilising granulocyte-macrophage colony stimulating factor(GMCSF) mediated endothelial progenitor cell(EPC) upregulation and stromal cell derived factor-1α(SDF) mediated myocardial EPC chemokinesis, may prevent myocardial loss and adverse remodeling. Vasculogenesis, viability, and haemodynamic improvements following therapy were investigated.
Procedures
Lewis rats(n=91) underwent LAD ligation and received either intramyocardial SDF and subcutaneous GMCSF or saline injections at the time of infarction. Molecular and haemodynamic assessments were performed at pre-determined time points following ligation.
Findings
SDF/GMCSF therapy upregulated EPC density as shown by flow cytometry (0.12±0.02 vs. 0.06±0.01% circulating lymphocytes,p=0.005), 48 hours following infarction. A marked increase in perfusion was evident 8 weeks after therapy, utilising confocal angiography (5.02 ± 1.7×10−2 vs. 2.03±0.7×10−2µm3blood/µm3 myocardial tissue, p=0.00004). Planimetric analysis demonstrated preservation of wall thickness (0.98 ± 0.09 vs. 0.67 ± 0.06 mm, p=0.003) and ventricular diameter (7.81±0.99 vs. 9.41±1.1mm, p=0.03). Improved haemodynamic function was evidenced by echocardiography and PV analysis (ejection fraction: 56.4±18.1 vs. 25.3±15.6%, p=0.001; preload adjusted maximal power: 6.6±2.6 vs. 2.7±1.4m Watts/µl2, p=0.01).
Conclusion
Neovasculogenic therapy with GMCSF mediated EPC upregulation and SDF mediated EPC chemokinesis maybe an effective therapy for infarct modulation and preservation of myocardial function following acute myocardial infarction.
Keywords: vasculogenesis, granulocyte-macrophage colony stimulating factor, stromal cell derived factor, endothelial progenitor cell, ischemia
INTRODUCTION
Acute ischaemia resulting from an acute MI greatly compromises myocardial function and begins a cycle of deleterious and irreversible myocardial remodeling. Present medical and surgical therapies provide limited efficacy in preventing progression of myocardial infarction to heart failure. Notably, complete revascularisation with either PCI or CABG is only possible in 63–80% of patients with ischaemic heart disease1. This warrants a search for novel molecular therapeutic modalities to treat acute post-infarct myocardial remodeling, regulate infarct modulation, and maximize salvage of viable myocardium immediately following MI, before the onset of irreversible myocardial damage.
Our group and others have demonstrated a reversal of ischaemia induced myocardial deterioration with enhanced perfusion.2–4 Endothelial progenitor cells (EPC) may provide a molecular means of enhancing myocardial microvascular perfusion in patients without traditional revascularisation options.5–10 Numerous studies have demonstrated EPC recruitment, migration, and vasculogenesis mediated by chemokines.7, 9, 11, 12 Stromal cell derived factor-1α (SDF) is a highly specific chemokine that interacts with the CXCR4 receptor on EPCs to induce migration and vasculogenesis.7, 13–17
Experimentally, SDF administration to ischemic hind limbs with the administration of an expanded population of EPCs resulted in recruitment of EPCs and therapeutic vasculogenesis.18 However, without the benefit of an expanded EPC pool, auto-amputation of the ischaemic limb resulted. Similarly, in a model of chronic, long-standing heart failure, as opposed to acute myocardial infarction, we have demonstrated therapeutic angiogenesis with granulocyte-macrophage colony stimulating factor (GMCSF) mediated expansion of the bone marrow-derived EPC population and SDF-mediated myocardial EPC chemokinesis.3, 19 Administration of isolated SDF or GMCSF did not provide a therapeutic benefit when compared to saline control alone. Isolated GMCSF therapy induced global bone marrow-derived EPC upregulation, but lacked a local chemotactic signal for EPC targeting to the ischaemic myocardium. While isolated SDF therapy provides the local chemotactic signal for EPCs, it lacks the ability to upregulate circulating EPC density.20 Therefore, for efficacious vasculogenesis a combination of EPC upregulation and SDF-mediated EPC chemokinesis is required.
Global upregulation of the bone marrow derived EPC population with GMCSF and local myocardial progenitor cell chemokinesis with SDF administration may provide a therapeutic means to enhance vasculogenesis and perfusion acutely, within the ischaemic myocardium. Though the benefits of vasculogenic therapy have been demonstrated in a model of established, chronic ischaemic cardiomyopathy, we believe that vasculogenic therapy acutely at the time of myocardial infarction will maximize myocardial salvage and preservation of myocardial function. We sought to study the regional molecular and global myocardial alterations that result from this neovasculogenic treatment immediately following acute myocardial infarction. If effective, this therapy can be straightforwardly and safely be translated to patients that present either immediately or within a few hours of acute myocardial infarction.
MATERIALS AND METHODS
Animal Care and Biosafety
Male adult, Lewis rats (250–300grams) were obtained from Charles River Laboratories (Boston, MA). Food and water were provided ad libitum. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). This study conforms to institutional ethical review and has been approved by the University of Pennsylvania Institutional Animal Care and Use Committee. All measurements were performed by investigators blinded to animal treatment.
Induction of Heart Failure
Male Lewis rats were anesthetised with intraperitoneal ketamine (50mg/kg) and xylazine (5mg/kg), endotracheally intubated with a 14-gauge angiocatheter and mechanically ventilated (Hallowell EMC) with 0.5% isoflurane maintenance anesthesia. A left 4th interspace thoracotomy was performed and the left anterior descending (LAD) coronary artery was ligated with a 7-0 prolene suture at the level of the left atrial appendage. This induced a consistent and reproducible anterolateral infarction of 35–40% of the left ventricle.21–25 Following LAD ligation the animals (n=91) were randomised to either saline control or therapy with SDF/GMCSF. The saline group received 200µl saline subcutaneously (sq) intraoperatively and on post-operative day 1 and a total of 250µl saline into 5 predetermined, evenly spaced, peri-infarct regions via direct intramyocardial injection with a 30gauge needle. The regions were chosen as the borderline tissue of visible myocardial ischaemia. The treatment group received 40µg/kg liquid sargamostim (GMCSF, Bayer Pharmaceuticals) subcutaneously, diluted in saline for a total volume of 200µl intraoperatively and on post-operative day 1. In addition, the treatment group received 3µg/kg recombinant human stromal cell derived factor-1α (R&D Systems, Minneapolis, MN), diluted in saline, via direct intramyocardial injection into the 5 predetermined peri-infarct regions. The thoracotomy was closed in 3 layers and the animals were allowed to recover. Subsets of animals were utilised for various physiologic and molecular assays over a range of time points.
Echocardiographic Assessment of Hemodynamic Function
Eight weeks following LAD ligation, when the Lewis rat is known to be in ischaemic heart failure, haemodynamic function was assessed and the hearts were explanted for molecular biologic analysis. Closed chest myocardial function was assessed utilizing echocardiography (Phillips Sonos 5500 revD system with an S12 probe at 12MHz and a 3cm depth of penetration). ECG electrodes were placed on both front limbs and the right hind limb. Wall motion score index (WMSI) was analysed utilising the American Society of Echocardiography regional wall motion grading scale (1=normal, 2=hypokinetic, 3=akinetic, 4=dyskinetic, 5=aneurysmal), whereby six regions were scored at the basal and mid-papillary level utilising parasternal short axis views. The apex was scored with parasternal long-axis visualisation. The WMSI was determined by the average of the 13 segments. Ventricular measurements were performed according to the American Society for Echocardiography leading-edge method. M-mode was used to define left ventricular lateral wall diameters.26, 27
Invasive Haemodynamic Analysis
Following echocardiographic analysis, a midline sternotomy was performed and a 2.5mm ascending aortic flow probe (Transonic Systems) was placed for Doppler analysis of cardiac output. A 2Fr pressure-volume catheter (Millar Instruments) was inserted into the left ventricle via the apex for analysis of left ventricular function. Multiple haemodynamic parameters were measured (heart rate, end-systolic volume, pressure, end-systolic pressure, end-diastolic pressure, ejection fraction, cardiac output, stroke work, dp/dt, power, contractility), recorded, and quantified with Chart v4.1.2 software (AD Instruments) and the ARIA 1 Pressure-Volume Analysis software (Millar Instruments). In addition to steady-state haemodynamic parameters, contractility was determined from pressure-volume relationships obtained by reducing pre-load via occlusion of the inferior vena cava. Volume measurements were calibrated by two-point linear interpolation with fixed volume cuvettes of heparinized rat blood and parallel conductance was excluded with the hypertonic saline injection technique. Following haemodynamic analysis, the hearts underwent perfusion of tomato lectin. Subsequently, the hearts were explanted for molecular biologic analysis. The hearts were distended at a fixed pressure and snap frozen in liquid nitrogen.
Quantitation of Myocardial Perfusion
Eight weeks following LAD ligation, 200µg of fluorescein labeled Lycopersicon esculentum (tomato) lectin (Vector Laboratories) was injected into the supra-diaphragmatic inferior vena cava and allowed to circulate for 10 minutes. Tomato lectin binds to the surface N-acetylglucosamine oligomers of endothelial cells lining perfused vessels.28 Direct contact of lectin with endothelial cells is requisite for lectin labeling, therefore only perfused vessels will be labeled.
Following lectin perfusion, the hearts were explanted and snap frozen in liquid nitrogen. One-hundred and twenty sequential images were obtained through 100 µm thick myocardial sections at the level of the papillary muscle utilising scanning laser confocal microscopy (z-series, 25× air magnification, Zeiss LSM-510 Meta Confocal Microscope). Three-dimensional reconstructions of the image stacks were created utilizing Volocity Software v.3.61 (Improvision). Pixels delineating labeled vasculature and total tissue sections were quantified, allowing determination of total perfusion per mass of myocardial tissue. Measurements were made in 2 peri-infarct and remote myocardial regions for each sample. Peri-infarct myocardium was defined as one microscopic field lateral to the edge of the scar (no myocardium present).25 Myocardium was examined on both sides of the myocardial scar by blinded investigators to ensure comparable assessment between therapy and control groups.
Quantification of Myocardial Neovasculogenesis
Myocardial vascular density was determined by labeling the ischaemic myocardial regions for CD31, 8 weeks following LAD ligation. Sections were treated with mouse anti-rat CD31 antibody (BD Biosciences) and goat anti-mouse FITC secondary antibody (Abcam). Immunocytochemical vascular density analysis was performed in a group-blinded manner in four fields per specimen and averaged for each heart (20×, Leica DM5000B microscope, Leica Application Suite v2.2.0). In order to confirm previous findings that a combination of GMCSF mediated EPC upregulation and SDF chemokinesis is required for therapeutic vasculogenesis, SDF only and GMCSF only groups were also investigated.
Myocardial Viability Analysis
Myocardial viability was assessed using a combination of apoptosis and myofilament density analysis on animals 8 weeks following LAD ligation. Apoptotic fraction was determined utilising a TUNEL assay (CardioTACS In Situ Apoptosis Detection System; Trevigen). After fixation in 3.7% formaldehyde, 10µm frozen sections were permeabilised with cytonin for 45 minutes. Apoptotic, nuclei were labeled with TdT and viable nuclei were counterstained. Total and apoptotic nuclei were counted in 5 separate peri-infarct and remote myocardial regions. Apoptotic fraction was determined as apoptotic nuclei/total nuclei per 40× high power field ×100. Positive control with fragmentation of DNA with nuclease was performed to validate the assay.
Myocardial sections were counterstained with haematoxylin and eosin to delineate myofilament structure. Myofilament density (total cardiomyocytes/hpf) was determined in 5 separate peri-infarct borderzone regions in a 40× high power microscopic field, as a measure of myocardial density. Myofilament size was determined in 10 adjacent myofilaments in each field. In order to ensure similarity in baseline structure, myofilament size and density analysis was performed in 5 separate remote, non-infarct myocardial regions.
Assessment of Ventricular Geometry
To determine preservation of ventricular geometry, 10µm myocardial sections, at the level of the papillary muscles, underwent Masson’s Trichrome staining. Peri-infarct borderzone wall thickness, left ventricular diameter, left ventricular chamber circumference and scar fraction, was evaluated on animals 8 weeks following LAD ligation, using digital planimetric software (Scion Image v.4.0.3.2). Peri-infarct borderzone wall thickness was measured on each side of the zone of fibrosis and averaged. Left ventricular diameter was measured in the greatest transverse diameter. Scar fraction was defined as scar length/left ventricular circumference ×100.
Assessment of Circulating Endothelial Progenitor Cell Density
To quantify endothelial progenitor cell upregulation with SDF/GMCSF neovasculogenic therapy, flow cytometry of peripheral blood was utilised. Animals underwent mid-LAD ligation and were randomised to treatment with either subcutaneous GMCSF and intramyocardial SDF or saline control injections. Forty-eight hours following LAD ligation the animals were sacrificed and circulating blood was obtained from the ascending aorta. Rat circulating progenitor cells were obtained from the mononuclear cell (MNC) fraction of blood. MNC were separated from peripheral blood collected into sodium-heparin Vacutainer™ tubes (BD Biosciences) via centrifugation (350xg; 25 min; 4 °C) through Histopaque 1077 (Sigma Aldrich). Red blood cells were lysed with 1X ammonium chloride solution (BD Biosciences). Cells were then counted and treated with 5%(v/v) mouse serum in modified Hank’s Balanced Salt Solution supplemented with 2% (v/v) Fetal Bovine Serum (FACS buffer) so as to block non specific binding.
Test samples were incubated for 30 minutes on ice with biotin-conjugated mouse anti-rat VEGFR2 antibody (Novus Biologicals), phycoerythrin-conjugated (PE) mouse anti-rat cKit (Abcam), and Alexa 647-conjugated mouse anti-rat CD45 antibodies (Serotec). After washing with cold FACS buffer, cells were incubated on ice for 15 minutes with fluorescein (FITC)-conjugated streptavidin (BD Biosciences). A portion of the MNC fraction was used for “Fluorescence Minus One” (FMO) and compensation staining [FITC-conjugated mouse anti-rat CD4 antibody (Serotec), PE-conjugated mouse anti-rat CD4 antibody (BD Boiosciences), or Alexa 647-conjugated mouse anti-rat CD4 antibody (Serotec)]. FMO’s were used to identify negative populations. Compensation controls were created using cells labeled with. Cell viability was assessed utilising 7AAD (BD Biosciences) labeling. EPCs were defined as CD45dimVEGFR2+cKit+ mononuclear cells. All cell samples were analysed using a Becton Dickinson FACSCalibur flow cytometer. Data analysis was performed using Flow Jo 8.8.3 (Tree Star Inc.). 29–33
Statistical Analysis
Quantitative data are expressed as means ± standard error of the mean (SEM). Statistical significance was evaluated using the unpaired Student’s t test for comparison between two means. For comparison between more than two means statistical analyses were performed using one way analysis of variance and Tukey-Kramer HSD to test for differences among means. A p-value of less than 0.05 was considered statistically significant.
RESULTS
SDF/GMCSF Therapy Upregulated EPC Density
Lymphocyte density analysis of circulating blood 48 hours post-LAD ligation demonstrates a statistically significant increase in total circulating lymphocyte count with SDF/GMCSF therapy (7.9±1.5 vs. 3.7±0.6 million lymphocytes/cc, n=7, p=0.006). This increase in circulating lymphocyte population, of which EPCs are a subset, is likely due to GMCSF administration. The increase in lymphocyte density validates our conceptual treatment paradigm in which the EPC population is increased by GMCSF, providing more cells for SDF-mediated myocardial EPC targeting.
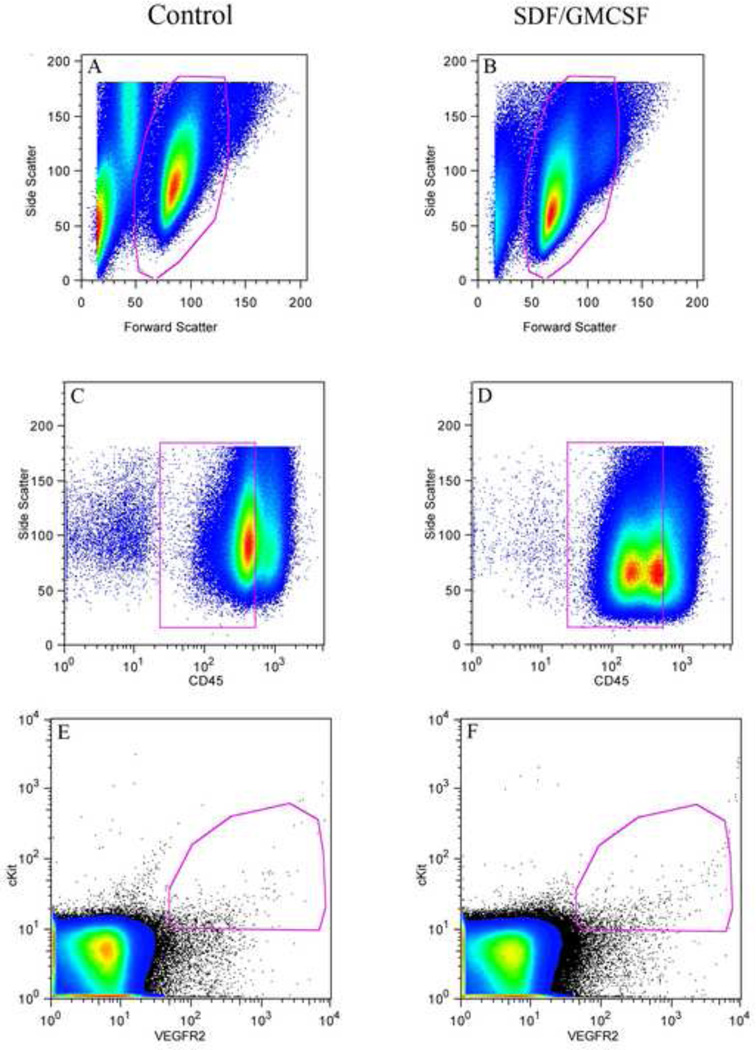
Flow cytometric analysis demonstrates a statistically significant increase in CD45dimVEGFR2+cKit+ determined circulating endothelial precursor cell concentration with SDF/GMCSF therapy when compared to saline control (0.12±0.02 vs. 0.06±0.01% circulating lymphocytes, n=6, p=0.005), Figure 1. Therefore, it appears that SDF/GMCSF therapy upregulates circulating endothelial precursor cells by nearly 100 percent.
Figure 1.
Representative flow cytometric plots of circulating blood 48 hours following acute LAD ligation. (A + B) Pseudo-color plots delineating the desired viable (7AAD−) lymphocyte population selected utilising side and forward scatter. (C + D) Lineage negative, CD45dim lymphocytes. (E) EPC density in a representative saline control animal, as determined by co-labeling for VEGFR2 and cKit (CD45dim). (F) EPC density in a representative SDF/GMCSF control animal, as determined by co-labeling for VEGFR2 and cKit (CD45dim).
Bone Marrow Endothelial Precursor Cell Upregulation is Required for Therapeutic SDF Mediated Chemokinesis and Vasculogenesis
Immunocytochemical analysis did not demonstrate a significant increase in vasculature with either isolated GMCSF or isolated SDF therapy when compared to saline control. But, combination therapy with GMCSF and SDF resulted in a marked increase in vasculature (28.8±1.6 vessels/hpf, n=5) within the treated myocardium; resulting in a statistically significant increase in myocardial vasculature when compared to saline control (18.1±0.9 vessels/hpf, n=5), GMCSF only (19.1±1.2 vessels/hpf, n=5), and SDF only (21.1±1.3 vessels/hpf, n=5) groups. This data confirms previous findings by our group and others that bone marrow derived EPC upregulation with GMCSF in addition to targeted myocardial chemokinesis with SDF are required for therapeutic angiogenesis. Sole therapy with isolated GMCSF or SDF does not provide an adequate stimulus for therapeutic vasculogenesis.
Acute Neovasculogenic Therapy Enhanced Myocardial Perfusion
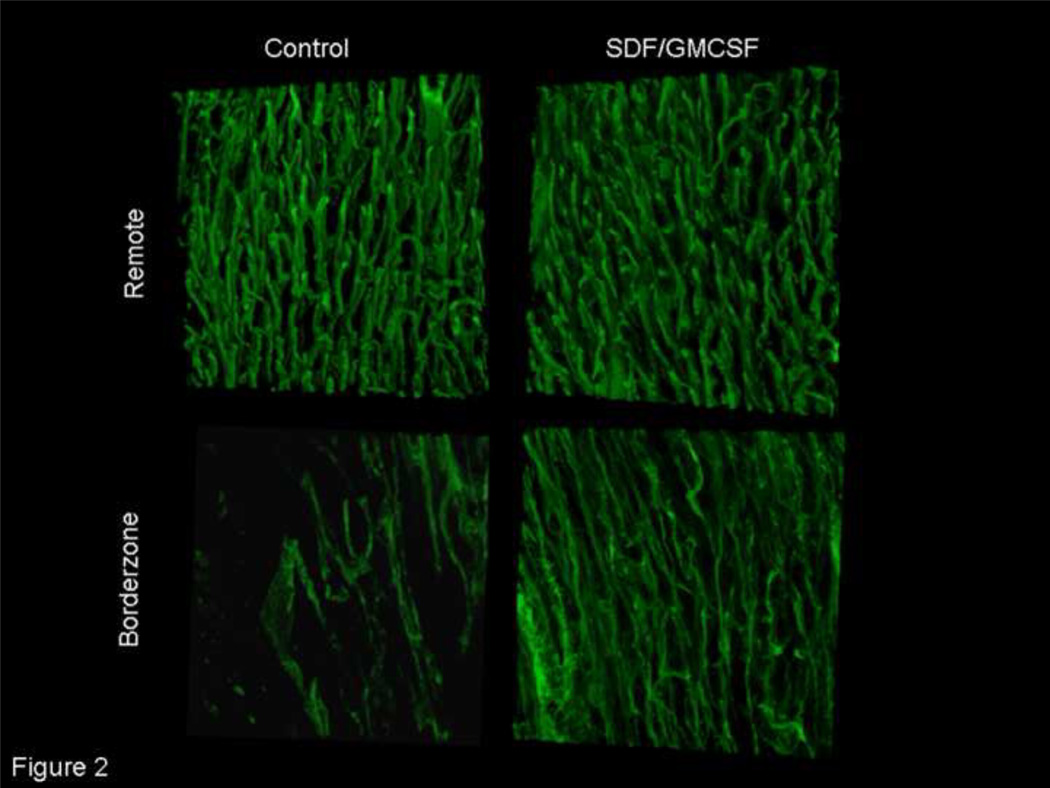
Three-dimensional, confocal microscopy allowed imaging of myocardial sections perfused with tomato lectin. Analysis of remote myocardial vasculature did not reveal a difference in myocardial perfusion (11.6±6.0 vs. 12.9±2.1 µm3 blood/µm3 myocardial tissue, n=7, p=NS), thereby serving as an internal control to validate the assay and demonstrate similar baseline morphology between the two experimental groups. Qualitative analysis revealed a visually apparent increase in borderzone myocardial perfusion with SDF/GMCSF therapy, Figure 2. The neovasculature demonstrated a branching pattern characteristic of functional blood vessels. Three-dimensional quantitative analysis of myocardial peri-infarct perfusion demonstrated a nearly 2.5 fold increase in myocardial perfusion with SDF/GMCSF treatment (5.02±1.7×10−2 vs. 2.03±0.7×10−2 µm3blood/µm3myocardial tissue, n=7, p=0.00004).
Figure 2.
Three dimensional microvascular lectin angiogram demonstrating enhanced myocardial perfusion following neovasculogenic therapy. Representative microvascular angiograms from remote, non-ischaemic and peri-infarct borderzone myocardium of saline control and SDF/GMCSF treated groups. (25× magnification)
Acute Neovasculogenic Therapy Decreased Apoptosis and Enhanced Myocardial Viability
TUNEL labeling demonstrated a statistically significant decrease in peri-infarct borderzone apoptotic fraction with neovasculogenic SDF/GMCSF therapy as compared to saline control (2.78±0.43 vs. 4.40±0.42 %, n=6, p=0.001). There was no difference in remote myocardial apoptotic fraction between the 2 groups, validating the assay and denoting comparable baseline myocardial viability (1.07±0.40 vs. 0.84±0.10%, n=6, p=NS).
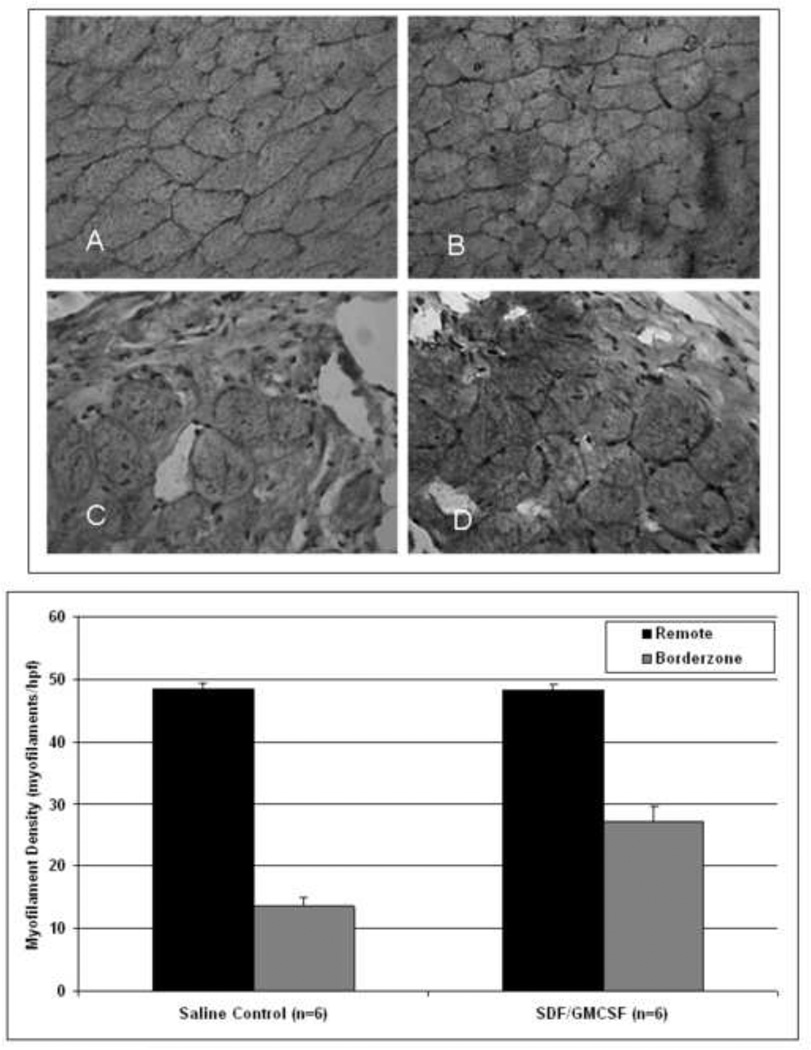
Borderzone myofilament density analysis revealed a statistically significant increase in cardiomyocyte density with SDF/GMCSF therapy when compared to saline control (27.0±2.6 vs. 13.7±1.3 myofilaments/hpf, n=6, p=0.00003). Both the decrease in apoptotic fraction and increase in myofilament density correspond to the enhanced myocardial perfusion that was demonstrated in the treatment group. Analysis of remote, intact myocardial density demonstrated similar myofilament structure and density, thereby validating the assay as an internal control (48.3±0.9 vs. 48.4±1.1 myofilaments/hpf, n=6, p=NS), Figure 3.
Figure 3.
1) Representative haematoxylin and eosin stained images of myofilament density within the remote (A=Saline Control, B=SDF/GMCSF) and borderzone myocardium (C=Saline Control, D=SDF/GMCSF). 2) Myofilament density within the remote and borderzone myocardium. (40× magnification).
SDF/GMCSF Therapy Preserved Ventricular Geometry
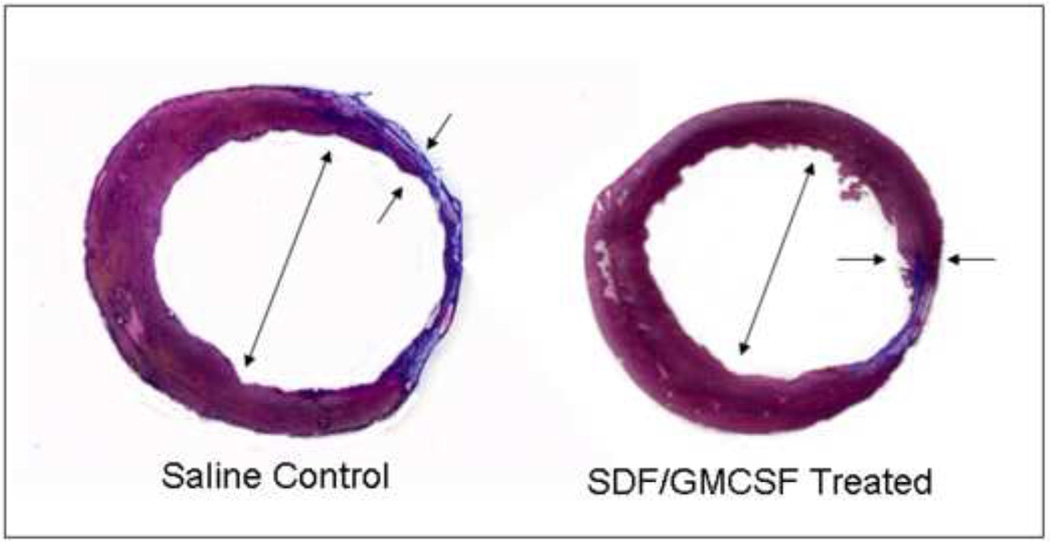
There is a statistically significant increase in peri-infarct ventricular wall thickness in the SDF/GMCSF treatment group when compared to saline control, Table 1. Quantitative analysis denotes a greater preservation of ventricular geometry with therapy as noted by diminished ventricular diameter, limited ventricular circumference, and smaller scar fraction, Figure 4. It should be noted that a scar fraction of 40.2%, 8 weeks post-LAD ligation, in the control group corroborates previous findings by other investigators.22
Table 1.
Digital Left Ventricular Planimetric Measurements 8 weeks Post-Left Anterior Descending Coronary Artery Ligation.
| Saline Control (n=7) |
SDF/GMCSF (n=7) |
p= | |
|---|---|---|---|
| Peri-Infarct Wall thickness (mm) | 0.67±0.06 | 0.98±0.09 | 0.003 |
| Ventricular Diameter (mm) | 9.41±1.1 | 7.81±0.99 | 0.03 |
| Ventricular Circumference (mm) | 28.10±3.47 | 24.56±1.74 | 0.05 |
| Scar Fraction (%) | 40.2±9.1 | 19.7±9.7 | 0.01 |
Figure 4.
Representative cross-sectional Masson’s Trichrome stained sections of (A) saline control and (B) SDF/GMCSF treated myocardial specimens. Small arrows indicate regions of myocardial peri-infarct borderzone measurements. Large arrows indicate ventricular diameter. Collagen of scar is stained blue and viable myocardium is stained red.
Neovasculogenic Therapy Augments Global Myocardial Haemodynamic Function
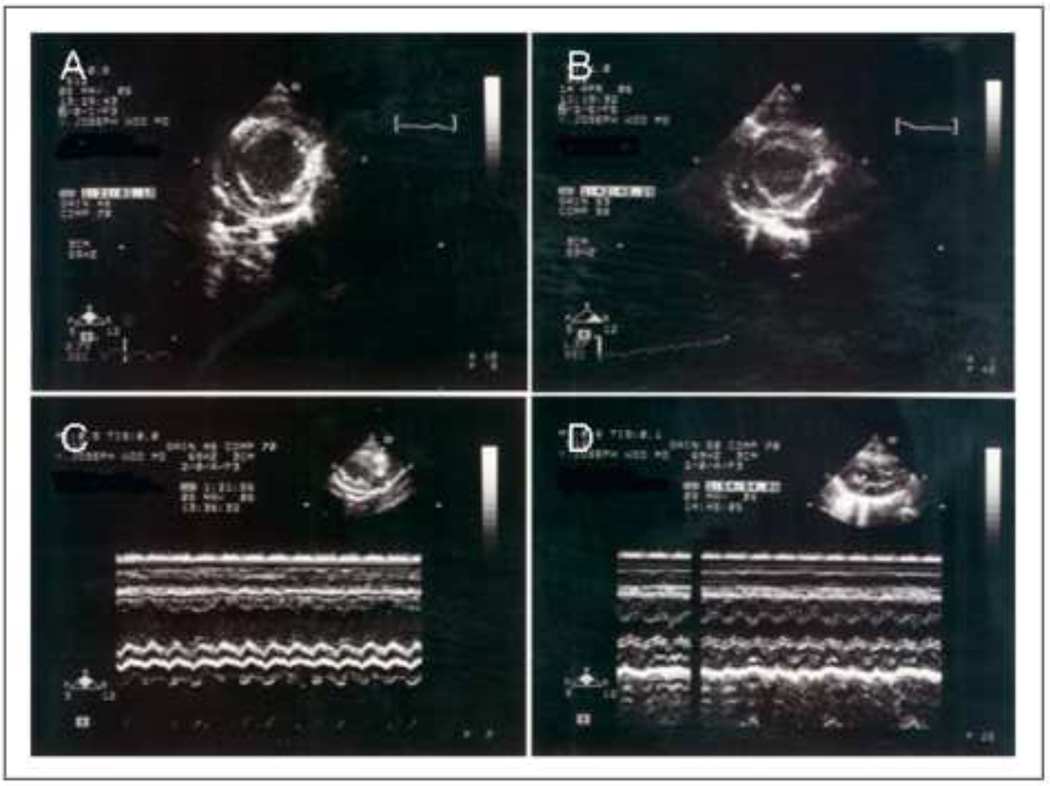
Echocardiographic analysis performed 8 weeks following LAD ligation demonstrated a statistically significant improvement in haemodynamic function with SDF/GMCSF therapy as demonstrated by left ventricular end-systolic volume, ejection fraction, and fractional shortening, Table 2. Parasternal short axis images at the level of the papillary muscle demonstrated a significantly higher ejection fraction and confirmed preservation of ventricular geometry, Figure 5. M-mode images demonstrated a statistically significant preservation of left ventricular lateral wall thickness with SDF/GMCSF treatment. Therapy with SDF/GMCSF resulted in preservation of myocardial contractility as denoted by improvements in wall motion score index (1.2±0.1 vs. 1.8±0.3, n=7, p=0.001). There was no difference in right ventricular volumes.
Table 2.
Echocardiographic Parameters 8 weeks following LAD Ligation.
| Saline Control (n=7) |
SDF/GMCSF (n=7) |
p= | |
|---|---|---|---|
| Heart Rate (bpm) | 225±25 | 212±20 | NS |
| Right Ventricular End-Diastolic Diameter (mm) | 1.61±0.18 | 1.67±0.39 | NS |
| Left Ventricular End-Diastolic Diameter (mm) | 7.23±1.02 | 5.34±0.61 | 0.005 |
| Left Ventricular End-Systolic Diameter (mm) | 6.61±1.48 | 3.88±1.24 | 0.016 |
| Fractional Shortening (%) | 12.1±6.9 | 35.6±12.2 | 0.02 |
| Ejection Fraction (%) | 25.3±15.6 | 56.4±18.1 | 0.001 |
| Left Ventricular End-Systolic Volume (µl) | 350±190 | 110±80 | 0.01 |
| Left Venticular Lateral Wall Thickness, End-Systole (mm) | 2.26±0.42 | 3.98±0.28 | 0.00001 |
| Left Ventricular Lateral Wall Thickness, End-Diastole (mm) | 2.05±0.38 | 2.84±0.36 | 0.01 |
Figure 5.
Representative end-diastolic, parasternal, short axis echocardiographic views at the level of the papillary muscle of the left ventricle, 8 weeks following LAD ligation, demonstrating significant dilatation and lateral wall thinning of the control hearts when compared to the SDF/GMCSF group (A= Saline Control, B= SDF/GMCSF). M-mode echocardiographic images delineating left ventricular dilatation and lateral wall thinning (C= Saline Control, D=SDF/GMCSF).
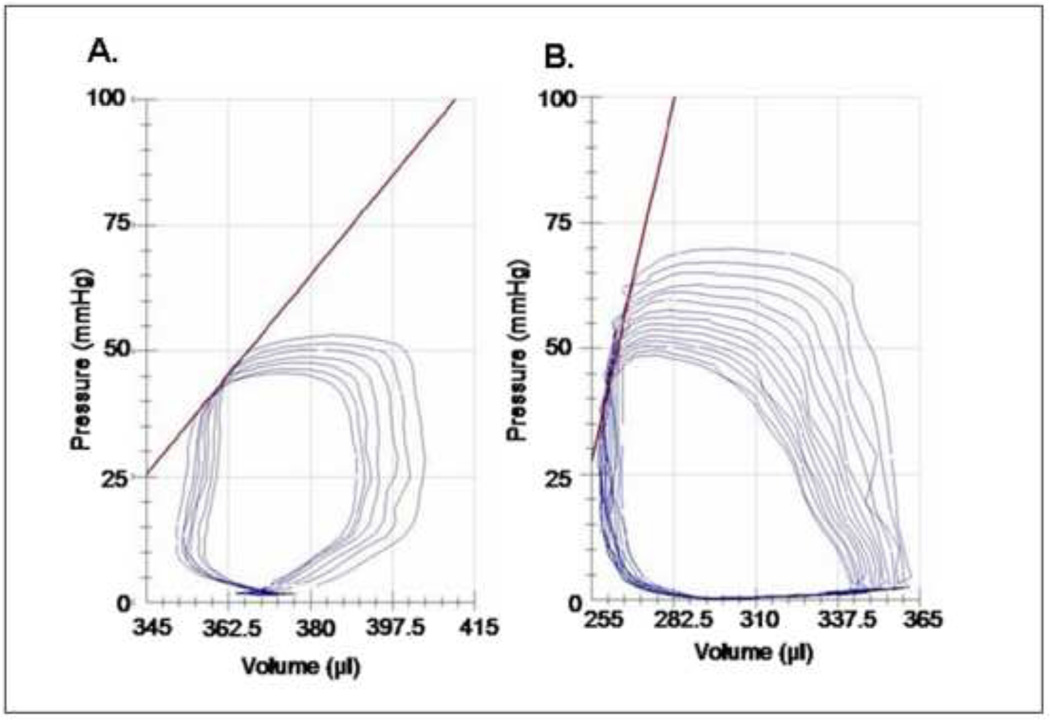
In vivo, invasive haemodynamic analysis utilising an intra-ventricular pressure-volume catheter and ascending aortic flow probe demonstrated a significant increase in global haemodynamic function with SDF/GMCSF administration. There was a statistically significant increase in maximum pressure, dP/dt, ejection fraction, cardiac output, maximal power, preload adjusted maximal power, and contractility, Figure 6. Though not statistically significant, there was a large difference in end-systolic volume that approached significance. In addition to improvements in systolic haemodynamic parameters, there was a significant improvement in min dP/dt, denoting improved diastolic relaxation in the SDF/GMCSF group. Pressure-volume loops from each group are demonstrated, Figure 6. Variability was noted in measured volumes between the pressure-volume loops and echocardiography. Since echocardiographic data was collected on closed chest-animals, we believe this to be a more physiologically accurate assessment.
Figure 6.
Pressure-volume loops from (A) saline control and (B) SDF/GMCSF treated hearts 8 weeks following LAD ligation. Pressure-volume loops were obtained with an intra-venticular left ventricular pressure-volume catheter during occlusion of the inferior vena cava. Slope of contractility is represented in red.
DISCUSSION
Therapeutic post-myocardial infarction upregulation and targeting of endothelial precursor cells, as outlined in this study, demonstrates efficacy in preserving ventricular structure and function, and preventing the onset of ischemic heart failure. Therapy resulted in enhanced progenitor cell density, vasculogenesis, and perfusion. Augmenting myocardial perfusion increased myocardial viability and preservation; ultimately translating into increased function.
Myocardial ischaemia induces altered cardiomyocyte metabolism, apoptosis, and mitochondrial damage resulting in inefficient cardiac function.34, 35 A reduction in perfusion diminishes myocardial function proportional to the degree of ischaemia.36 Ultimately, a deleterious cycle of cell slippage, progressive ventricular dilatation, and depressed myocardial function ensues, resulting in ischaemic heart failure. Restoration of myocardial perfusion attenuates cellular ischaemia, restores cellular energetics, and prevents adverse remodeling, thereby preventing the progression to heart failure.
In this study, we have noticed benefits associated with improvements in myocardial perfusion. Enhanced perfusion correlated with enhanced cardiomyocyte integrity, preservation of efficient myocardial geometry, and enhanced myocardial function. Benefits associated with enhanced perfusion have also been demonstrated in human clinical trials utilising exogenous administration of cultured EPCs.2, 37–41 A major limitation of these delivery systems is the requisite cell isolation, exogenous cell replication, limited cell engraftment, and delivery of viable cells with potential infectious complications. The inherent advantage of the proposed neovasculogenic strategy is the utilisation of a pre-existing endogenous reparative process. GMCSF is routinely utilised to enhance lymphocyte populations in the bone marrow transplant population, and as such has proven clinically both beneficial and safe. SDF is a recombinant protein that does not rely on adenoviral replication or the delivery of infectious matter. As demonstrated in this manuscript, sole therapy with SDF or GMCSF did not demonstrate a significant difference in CD31 determined vasculogenesis, thereby implying the need for combination therapy as utilised in this therapeutic model.
Abbott and colleagues have demonstrated a statistically significant upregulation of myocardial SDF levels as early as 48 hours following myocardial infarction in a murine model.20 Upregulation of, the endogenous chemokine, SDF plays a pivotal role in mediating myocardial neovasculogenesis to attenuate ischaemia. Yet, the significant loss of contractile mass, ventricular dilatation and loss of myocardial function following MI implies that the unenhanced, native reparative process is not sufficient to reverse myocardial injury.
Several studies have demonstrated a positive correlation between the presence of coronary artery disease/cardiovascular events and reduced circulating populations of endothelial progenitor cells.42–44 Moreover, EPCs from patients with coronary artery disease appear to have impaired reactivity to SDF as well as decreased CXCR4-mediated down stream signaling.45 Additionally, there is a significant reduction in colony forming and vasculogenic capacity of haematopoetic progenitors in the setting of chronic illness or chronic ischaemic heart disease.15, 46, 47 These findings provide evidence for reduced endogenous neovasculogenic capabilities in patients with preexistent cardiovascular disease. As previously discussed, upregulation of the circulating EPC population may be required to provide a significant population of EPCs available for SDF chemokinesis and vasculogenesis. Intramyocardial SDF administration likely enhances chemokine concentration to supratherapeutic levels. This strategy may enhance myocardial EPC targeting and upregulate CXCR4-mediated vasculogenic activity, thereby providing therapeutic vasculogenesis and myocardial preservation.
Early neovasculogenic intervention prior to the loss of significant myocardial mass, as utilised in this study, demonstrates a dramatic preservation of myocardial geometry and function as compared to intervention following the onset of congestive heart failure. Acute neovasculogenic intervention appears to minimise infarct formation and progression. Therefore, we advocate early neovasculogenic intervention prior to the onset of heart failure.
The therapeutic strategy utilized in this study can be translated to patients suffering from an acute myocardial infarction. At the time of presentation either percutaneous or minimally invasive, thoracoscopic techniques can be utilised for intramyocardial administration of SDF. This therapy can be utilised either in combination with PCI or CABG, or as sole-therapy. Further studies to determine efficacy and safety are necessary before this therapy can be translated to clinical utilisation. Though clinically utilized GMCSF dosages can be utilised in humans, further pharmacokinetic studies in humans will have to be performed in order to determine optimal SDF dosing.
In conclusion, it appears that neovasculogenic therapy with GMCSF-mediated EPC upregulation and SDF-mediated EPC chemokinesis may be an effective therapy for infarct modulation and preservation of myocardial function following acute myocardial infarction.
CONCLUSION
This novel neovasculogenic therapy with SDF/GMCSF is an effective therapy for progenitor cell mediated myocardial vasculogenesis with enhanced myocardial viability and subsequent preservation of myocardial function, in a pre-clinical model of acute myocardial infarction.
Table 3.
Invasive Haemodynamic Parameters 8 weeks following LAD Ligation
| Saline Control (n=9) |
SDF/GMCSF (n=8) |
p= | |
|---|---|---|---|
| Heart Rate (bpm) | 197±19 | 195±33 | 0.91 |
| End-Systolic Volume (µl) | 219±89 | 118±57 | 0.07 |
| Maximum Pressure (mmHg) | 64±13 | 84±6 | 0.001 |
| Minimum Pressure (mmHg) | 6.3±4.1 | 0.3±0.1 | 0.003 |
| End-Systolic Pressure (mmHg) | 62±13 | 76±7 | 0.01 |
| End-Diastolic Pressure (mmHg) | 9.1±4.1 | 3.2±0.8 | 0.005 |
| Ejection Fraction (%) | 25.3±9.5 | 53.7±10.0 | 0.0008 |
| Cardiac Output (µl/min) [Determined by flow probe] | 22.5±3.0 | 32.1±3.3 | 0.001 |
| Stroke Work (mmHg*µl) | 2219±748 | 6821±1280 | 0.00003 |
| dP/dt Max (mmHg/sec) | 2156±677 | 3939±463 | 0.00002 |
| dP/dt Min (mmHg/sec) | −1829±679 | −4042±1123 | 0.0009 |
| Pressure at dVdt Max (mmHg) | 11.1±4.5 | 1.7±1.5 | 0.0009 |
| Pressure at dP/dt Max (mmHg) | 39.8±10.5 | 52.0±6.7 | 0.02 |
| Maximal Power (mWatts) | 13.4±5.1 | 24.0±6.7 | 0.01 |
| Preload Adjusted Maximal Power (mWatts/µl2) | 2.7±1.4 | 6.6±2.6 | 0.01 |
| Slope of Contractility (mmHg/µl) | 0.63±0.19 | 1.59±0.73 | 0.02 |
ACKNOWLEDGEMENTS
This work was supported in part by grants from the National Institutes of Health (NIH), National Heart Lung and Blood Institute RO1 HL089315 (Y. Joseph Woo, M.D.), NIH National Heart Lung and Blood Institute/Thoracic Surgery Foundation for Research and Education HL072812 (Y. Joseph Woo, M.D.), American Heart Association 0465519U (Y. Joseph Woo, M.D.), and National Institutes of Health, National Heart Lung Blood Institute – Ruth L. Kirschstein National Research Service Award, Individual Fellowship 1 F32 HL79769-01 (Pavan Atluri, M.D.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Boodhwani M, Sodha NR, Laham RJ, Sellke FW. The future of therapeutic myocardial angiogenesis. Shock. 2006 Oct;26(4):332–341. doi: 10.1097/01.shk.0000225318.08681.a7. [DOI] [PubMed] [Google Scholar]
- 2.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002 Dec 10;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 3.Woo YJ, Grand TJ, Berry MF, Atluri P, Moise MA, Hsu VM, Cohen J, Fisher O, Burdick J, Taylor M, Zentko S, Liao G, Smith M, Kolakowski S, Jayasankar V, Gardner TJ, Sweeney HL. Stromal cell-derived factor and granulocyte-monocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. The Journal of thoracic and cardiovascular surgery. 2005 Aug;130(2):321–329. doi: 10.1016/j.jtcvs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Numaguchi Y, Sone T, Okumura K, Ishii M, Morita Y, Kubota R, Yokouchi K, Imai H, Harada M, Osanai H, Kondo T, Murohara T. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006 Jul 4;114(1 Suppl):I114–I119. doi: 10.1161/CIRCULATIONAHA.105.000588. [DOI] [PubMed] [Google Scholar]
- 5.Simons M. Angiogenesis: where do we stand now? Circulation. 2005 Mar 29;111(12):1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 6.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature medicine. 2001 Apr;7(4):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 7.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature medicine. 2004 Aug;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 8.Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O'Malley P, Rocic P, Focardi M, Chilian WM. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005 Oct 4;112(14):2108–2113. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 9.Atluri P, Woo YJ. Pro-angiogenic cytokines as cardiovascular therapeutics: assessing the potential. BioDrugs. 2008;22(4):209–222. doi: 10.2165/00063030-200822040-00001. [DOI] [PubMed] [Google Scholar]
- 10.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. The New England journal of medicine. 2006 Sep 21;355(12):1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 11.Huusko J, Merentie M, Dijkstra MH, Ryhanen MM, Karvinen H, Rissanen TT, Vanwildemeersch M, Hedman M, Lipponen J, Heinonen SE, Eriksson U, Shibuya M, Yla-Herttuala S. The effects of VEGF-R1 and VEGF-R2 ligands on angiogenic responses and left ventricular function in mice. Cardiovascular research. 2009 Dec 28; doi: 10.1093/cvr/cvp382. [DOI] [PubMed] [Google Scholar]
- 12.Zhu XY, Zhang XZ, Xu L, Zhong XY, Ding Q, Chen YX. Transplantation of adipose-derived stem cells overexpressing hHGF into cardiac tissue. Biochemical and biophysical research communications. 2009 Feb 20;379(4):1084–1090. doi: 10.1016/j.bbrc.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. Journal of molecular and cellular cardiology. 2007 Apr;42(4):792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirshahi F, Pourtau J, Li H, Muraine M, Trochon V, Legrand E, Vannier J, Soria J, Vasse M, Soria C. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thrombosis research. 2000 Sep 15;99(6):587–594. doi: 10.1016/s0049-3848(00)00292-9. [DOI] [PubMed] [Google Scholar]
- 15.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004 Apr 6;109(13):1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 16.Schuh A, Liehn EA, Sasse A, Hristov M, Sobota R, Kelm M, Merx MW, Weber C. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic research in cardiology. 2008 Jan;103(1):69–77. doi: 10.1007/s00395-007-0685-9. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Yu J, Li Y, Li D, Yan D, Qu Z, Ruan Q. CXCR4 positive bone mesenchymal stem cells migrate to human endothelial cell stimulated by ox-LDL via SDF-1alpha/CXCR4 signaling axis. Experimental and molecular pathology. 2009 Dec 16; doi: 10.1016/j.yexmp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003 Mar 11;107(9):1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 19.Atluri P, Liao GP, Panlilio CM, Hsu VM, Leskowitz MJ, Morine KJ, Cohen JE, Berry MF, Suarez EE, Murphy DA, Lee WM, Gardner TJ, Sweeney HL, Woo YJ. Neovasculogenic therapy to augment perfusion and preserve viability in ischemic cardiomyopathy. The Annals of thoracic surgery. 2006 May;81(5):1728–1736. doi: 10.1016/j.athoracsur.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004 Nov 23;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 21.Jayasankar V, Pirolli TJ, Bish LT, Berry MF, Burdick J, Grand T, Woo YJ. Targeted overexpression of growth hormone by adenoviral gene transfer preserves myocardial function and ventricular geometry in ischemic cardiomyopathy. Journal of molecular and cellular cardiology. 2004 Apr;36(4):531–538. doi: 10.1016/j.yjmcc.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Liu YH, Yang XP, Nass O, Sabbah HN, Peterson E, Carretero OA. Chronic heart failure induced by coronary artery ligation in Lewis inbred rats. The American journal of physiology. 1997 Feb;272(2 Pt 2):H722–H727. doi: 10.1152/ajpheart.1997.272.2.H722. [DOI] [PubMed] [Google Scholar]
- 23.Jayasankar V, Woo YJ, Bish LT, Pirolli TJ, Chatterjee S, Berry MF, Burdick J, Gardner TJ, Sweeney HL. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation. 2003 Sep 9;108 Suppl 1:II230–II236. doi: 10.1161/01.cir.0000087444.53354.66. [DOI] [PubMed] [Google Scholar]
- 24.Woo YJ, Panlilio CM, Cheng RK, Liao GP, Atluri P, Hsu VM, Cohen JE, Chaudhry HW. Therapeutic delivery of cyclin A2 induces myocardial regeneration and enhances cardiac function in ischemic heart failure. Circulation. 2006 Jul 4;114(1 Suppl):I206–I213. doi: 10.1161/CIRCULATIONAHA.105.000455. [DOI] [PubMed] [Google Scholar]
- 25.Jayasankar V, Bish LT, Pirolli TJ, Berry MF, Burdick J, Woo YJ. Local myocardial overexpression of growth hormone attenuates postinfarction remodeling and preserves cardiac function. The Annals of thoracic surgery. 2004 Jun;77(6):2122–2129. doi: 10.1016/j.athoracsur.2003.12.043. discussion 2129. [DOI] [PubMed] [Google Scholar]
- 26.Morgan EE, Faulx MD, McElfresh TA, Kung TA, Zawaneh MS, Stanley WC, Chandler MP, Hoit BD. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am J Physiol Heart Circ Physiol. 2004 Nov;287(5):H2049–H2053. doi: 10.1152/ajpheart.00393.2004. [DOI] [PubMed] [Google Scholar]
- 27.Brown L, Fenning A, Chan V, Loch D, Wilson K, Anderson B, Burstow D. Echocardiographic assessment of cardiac structure and function in rats. Heart lung &circulation. 2002;11(3):167–173. doi: 10.1046/j.1444-2892.2002.00148.x. [DOI] [PubMed] [Google Scholar]
- 28.Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WM. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. The American journal of pathology. 2003 Jan;162(1):183–193. doi: 10.1016/S0002-9440(10)63809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arteriosclerosis thrombosis and vascular biology. 2003 Jul 1;23(7):1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 30.Rothe G, Barlage S, Schmitz G, Kloushe M. Flow cytometric assessment of haematopoietic stem and progenitor cells. J Lab Med. 2003;27:175–181. [Google Scholar]
- 31.Marsboom G, Pokreisz P, Gheysens O, Vermeersch P, Gillijns H, Pellens M, Liu X, Collen D, Janssens S. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem cells (Dayton Ohio) 2008 Apr;26(4):1017–1026. doi: 10.1634/stemcells.2007-0562. [DOI] [PubMed] [Google Scholar]
- 32.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. The Journal of clinical investigation. 2006 Jul;116(7):1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagley RG, Rouleau C, St Martin T, Boutin P, Weber W, Ruzek M, Honma N, Nacht M, Shankara S, Kataoka S, Ishida I, Roberts BL, Teicher BA. Human endothelial precursor cells express tumor endothelial marker 1/endosialin/CD248. Molecular cancer therapeutics. 2008 Aug;7(8):2536–2546. doi: 10.1158/1535-7163.MCT-08-0050. [DOI] [PubMed] [Google Scholar]
- 34.Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation. 2000 Nov 14;102(20 Suppl 4):IV14–IV23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin IJ, Schneider MD. Learning from failure: congestive heart failure in the postgenomic age. The Journal of clinical investigation. 2005 Mar;115(3):495–499. doi: 10.1172/JCI200524477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canty JM, Jr, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circulation research. 2004 Apr 30;94(8):1142–1149. doi: 10.1161/01.RES.0000125628.57672.CF. [DOI] [PubMed] [Google Scholar]
- 37.Erbs S, Linke A, Adams V, Lenk K, Thiele H, Diederich KW, Emmrich F, Kluge R, Kendziorra K, Sabri O, Schuler G, Hambrecht R. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: first randomized and placebo-controlled study. Circulation research. 2005 Oct 14;97(8):756–762. doi: 10.1161/01.RES.0000185811.71306.8b. [DOI] [PubMed] [Google Scholar]
- 38.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003 May 13;107(18):2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 39.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003 Nov 4;108(18):2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 40.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006 Nov 14;114(20):2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 41.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. The New England journal of medicine. 2006 Sep 21;355(12):1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 42.Powell TM, Paul JD, Hill JM, Thompson M, Benjamin M, Rodrigo M, McCoy JP, Read EJ, Khuu HM, Leitman SF, Finkel T, Cannon RO., 3rd Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arteriosclerosis thrombosis and vascular biology. 2005 Feb;25(2):296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005 Jun 7;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 44.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. The New England journal of medicine. 2003 Feb 13;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 45.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circulation research. 2005 Nov 25;97(11):1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 46.Zhuo Y, Li SH, Chen MS, Wu J, McDonald Kinkaid HY, Fazel S, Weisel RD, Li RK. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: Combined consequences for cell therapy in older recipients. The Journal of thoracic and cardiovascular surgery. 2009 Nov 18; doi: 10.1016/j.jtcvs.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 47.Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, Sellke FW. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009 Feb;296(2):H428–H434. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]