Abstract
Multisensory neurons in cat SC exhibit significant postnatal maturation. The first multisensory neurons to appear have large receptive fields (RFs) and cannot integrate information across sensory modalities. During the first several months of postnatal life RFs contract, responses become more robust, and neurons develop the capacity for multisensory integration. Recent data suggest that these changes depend on both sensory experience and active inputs from association cortex. Here, we extend a computational model we developed (Cuppini et al 2010) using a limited set of biologically realistic assumptions to describe how this maturational process might take place. The model assumes that during early life, cortical-SC synapses are present but not active, and that responses are driven by non-cortical inputs with very large RFs. Sensory experience is modeled by a “training phase” in which the network is repeatedly exposed to modality-specific and cross-modal stimuli at different locations. Cortical-SC synaptic weights are modified during this period as a result of Hebbian rules of potentiation and depression. The result is that RFs are reduced in size and neurons become capable of responding in adult-like fashion to modality-specific and cross-modal stimuli.
Keywords: visual-acoustic neurons, anterior ectosylvian sulcus, enhancement, Hebb rule, learning mechanisms, inverse effectiveness principle, neural network modelling
Introduction
The multisensory neuron in the cat superior colliculus (SC) is a primary site of cross-modal convergence (Stein and Stanford 2008). The different tectopetal afferents to these neurons converge in a topographically organized manner so that the receptive fields of, for example, a visual-auditory neuron overlap one another in space (Stein and Meredith 1993). By integrating the visual and auditory inputs that are derived from a given event, such neurons can increase the magnitude of their responses, thereby increasing the physiological impact of the initiating event, and SC-mediated responses to it (Alvarado et al. 2009). Spatially disparate visual-auditory inputs are either not integrated or depress one another (e.g., Meredith and Stein 1986; Kadunce et al. 1997).
The alignment of a multisensory SC neuron’s receptive fields, and its ability to integrate its cross-modal inputs develop only gradually with postnatal experience (Wallace and Stein 1997). Presumably, these neurons are learning the statistical relationships of the different sensory cues that are derived from the same events. This leads their receptive fields to contract and align, and to the development of an ability to use its cross-modal inputs in concert. If provided only anomalous experience such as induced with consistently disparate visual-auditory cues, SC neurons learn these relationships as well. They develop visual-auditory receptive field misalignments and require spatially disparate cues to enhance physiological responses (Wallace and Stein 2007). In the absence of visual-auditory experience (e.g., dark rearing) multisensory neurons learn nothing about such events and retain much of their immaturity. Their receptive fields remain exceedingly large, show poor alignment, and are incapable of multisensory integration (Wallace et al. 2004).
The objective of the current study was to model a feasible neural mechanism underlying the experience-based processes that ultimately leads to normal multisensory integration. The model uses the visual-auditory neuron as an exemplar, incorporates what is known about its anatomical and physiological development, and is based on a network model that has been used to understand multisensory processes in adult SC neurons (Cuppini et al. 2010). It also incorporates a Hebbian learning mechanism that is used to describe processes driving the contraction of unisensory receptive fields and the alignment of different sensory maps (Zhang et al. 2000; Katz and Shatz 1996; Witten et al. 2008).
In cat’s SC, the maturation of the receptive field alignment and the multisensory integration appears to be guided by descending projections from association cortex (primarily from the anterior ectosylvian sulcus, AES, see Jiang et al. 2001; Jiang et al. 2006; Alvarado et al. 2009; Fuentes-Santamaria et al. 2009). This input exercises control over the host of other tectopetal afferents, and it is this cortico-SC projection that is believed to provide the SC with the experience-based information that guides its receptive field alignment, and the principles that ultimately govern how it integrates its multiple sensory inputs.
Methods
All model equations and parameter numerical values are provided in Supplementary material.
General model structure
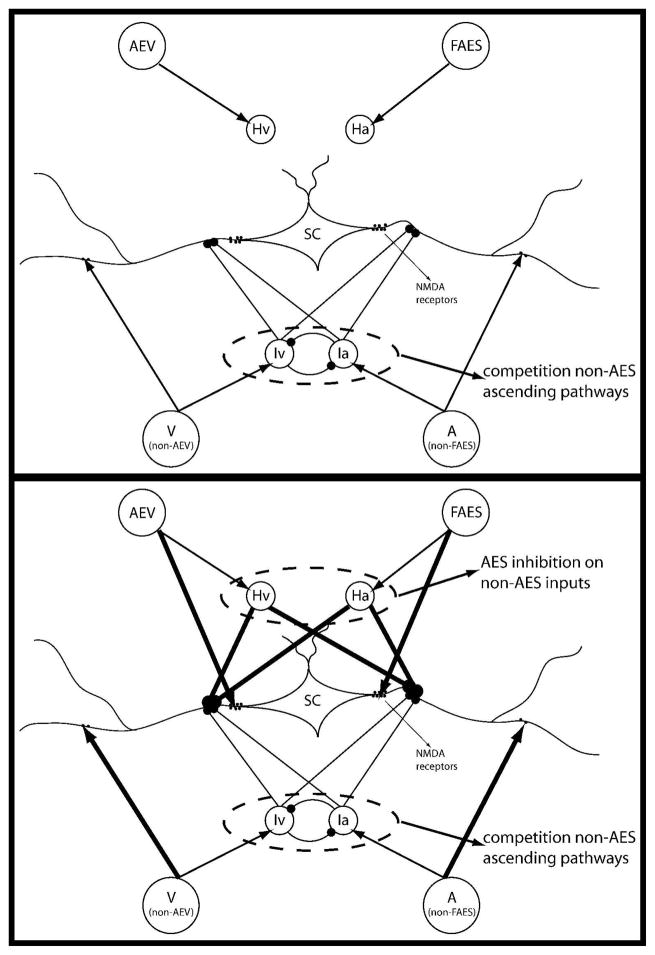
In this work we use a computational model developed previously (Cuppini et al, 2010). The former model was built to simulate an adult cat’s SC. Conversely, aim of the present study is to simulate and analyze the maturation of the SC circuitries, from an immature phase, lacking multisensory integrative capabilities, to a phase showing an adult-like behavior. In Figure 1 the structure of the network is presented in the immature stage, i.e. at approximately four weeks (upper panel) and in the maturity (bottom panel).
Fig. 1. The general structure of the network in immature (fig.1A) and in mature (fig.1B) phase.
The four projection areas make excitatory synapses with their target SC neurons and with their target interneurons (arrows). The interneurons provide two competitive mechanisms: 1) Ha and Hv provide the bases through which the inhibitory effect of AES is imposed on non-AES inputs; 2) Ia and Iv provide the substrate for a competition between two non-AES inputs in which the stronger one overwhelms the weaker.
The starting condition of our model approximately reflects the situation occurring at about four weeks. We made the realistic hypothesis that in the first four weeks after birth the appearance of a multisensory, but not integrative, nature is due to a physiological growth of targeting synapses from different non-AES unisensory input regions. What happens after these first weeks is due to a functional development, related to the experience, during which the non- effective synapses are strengthen on the basis of the input statistics. Accordingly, we assume that in the immature phase, at the beginning of the training period, the SC is targeted only by weak synapses from non-AES regions. Excitatory and inhibitory influences from AES subregions are immature and thus not effective, as commonly reported in the physiological literature (see, for instance, Stein et al. 2009) and supported from recent anatomical evidence (Fuentes-Santamaria et al. 2008). Hence they are initially set at zero.
Training Phase
In figure 1 we have evidenced which synapses are subjects of the training phase (black thick lines), and which are assumed to be fixed (black thin lines). This assumption agrees with several physiological evidences like: i) the inability of immature multisensory SC neurons to integrate stimuli form different sensory modalities, ii) the weak responses elicited in these neurons from both unisensory and multisensory stimulations, and iii) the widespread sensory RFs, compared with those in an adult-like conditions (see Stein 2005; Wallace et al. 2004; Wallace and Stein 1997). The first two evidences are obtained in the model as a consequence of the absence of AES influences on the SC neurons in the immature phase; the latter is reproduced assuming that the synapses from non-AES regions to the SC exhibit a wider spatial pattern than in the adult. We will discuss these findings later in the Result section, comparing simulated responses with in-vivo data in literature.
A normal development of the brain, in the real life, takes place through the interaction of the neural structures with thousands of modality-specific and cross-modal natural stimuli. In this case, we reproduced this process by presenting thousands (15.000) of modality-specific and cross-modal inputs to the network, to simulate the natural experience. We always used strong effective stimuli, to make shorter the developmental phase. Results will be presented with reference to the following statistics for the input stimuli: 80% cross-modal, 10% only visual, and 10% only auditory. The effect of other statistics will also be discussed. These stimuli were generated by a normal distribution of probability. Each stimulus lasted 50ms, during which, after an initial transient period, all the synaptic connections targeting the SC area (both from the AES cortex, from the non AES regions, and from inhibitory interneurons stimulated by the AES cortex, see Fig. 1) were crafted by using Hebbian algorithms of Long Term Potentiation (LTP) and Long Term Depression (LTD). In particular, in this work we chose a postsynaptic gaiting rule, which means that the training algorithm modifies only the synapses targeting an active neuron (in this case a SC neuron), and their strength is increased or decreased on the basis of the activity of the presynaptic neurons. In order to establish this correlation, the activity of the individual neurons (both presynaptic and postsynaptic) has been compared with a given threshold, to determine whether the neuron can be considered in the active state or in the inhibited state. The strengthening and depression processes are subjected to two more constraints: a normalization and a saturation rule. The first means that, during training, the overall sum of the synaptic strength entering an SC neuron cannot overcome a maximum value; this rule has been applied separately to the ascending and the descending projections to the SC. Furthermore, each single synapse cannot overcome a maximum value, nor decrease below zero. Both rules have a physiological reliability.
The previous Hebbian rules have different consequences on synapse maturation, and on the dimensions of the SC Receptive Fields, depending on whether modality-specific or cross-modal stimuli are used.
Unisensory stimulation
When we present a unisensory stimulus to the network, for example a visual stimulus V, two input areas are contemporary activated: one in the AES cortex, (the AEV subregion), and the other in a non-AES region (the non-AEV area). In the immature period, some SC neurons are activated by inputs coming from the (visual) ascending pathway. The training algorithm postulates a modification of the synapses targeting to those SC neurons whose activity overcome a fixed threshold. As a consequence, connections between the active unisensory input neurons (in the example those belonging to the AEV and non-AEV regions), and the active SC neurons are strengthened (LTP postsynaptic gating), while, thanks to the LTD rule, projections from silent neurons of the input regions are weakened (they can be from all the four areas: i.e. all input neurons whose RFs do not contain the input). With unisensory stimulation it is possible to strengthen only one sensory pathway at time, while the other is contemporary depressed. This mechanism does not lead to the appearance of multisensory integrative capability in case of alternate unisensory training since potentiation in one modality always occurs together with depression in the other modality, at the same spatial position.
Cross-modal stimulation
When, instead, we present a multisensory input to the network, all four input areas are activated contemporary along with the SC neurons. In this case the LTP is applied to both sensory modality pathways at the same time, causing a reinforcement of synapses from both AEV and FAES. Parallel to this effect, as described above, those neurons that are not stimulated by the external input, and are silent, weak their synapses to neurons active in the SC area. These two mechanisms produce two effects: 1) during the development, in presence of multisensory experiences, the synapses between AES subregions and SC are simultaneously strengthened and the SC can acquire integrative capabilities like those present in the adulthood; 2) the SC loses some connections from non-AES regions, and this has the effect of reducing the RFs of the mature SC even in absence of AES subregions.
The simulated stimuli (V and A) during the training period were either coincident in time and position or disparate in time. Here for simplicity we used stimuli with high levels of intensity. The statistics of training inputs used in the presentation of results is the following: 10% visual stimuli only, 10% auditory stimuli only, 80% visual and auditory spatial coincident stimuli. Alternative statistics, which differ for what concerns the percentage of multisensory vs. unisensory inputs, and the presence of a moderate percentage of cross-modal inputs in spatial disalignment, have also been tested, as commented in section results.
In conclusion, the model makes use of some assumptions which require a posteriori validation, and of some elements taken from known anatomical-physiological features.
Anatomical/physiological knowledge include: i) the presence of separate descending and ascending paths; ii) the presence of unisensory RFs topographically organized; iii) the idea that AES connections in the immature phase are still latent but highly plastic; iv) the fact that the non-AES pathways in the immature phase have moderate strength and quite high RFs.
Aspects still hypothetical and which require validation are: i) the presence of separate descending and ascending inhibitory mechanisms, with a shunting inhibition effect on ascending synapses; ii) the learning rules adopted (i.e., Hebbian potentiation with a post-synaptic gating mechanism and a forgetting factor for depotentiation); iii) the balance between the different learning rates for the various synapses.
Results
The responses of a single simulated neuron in the model (position 26) to modality-specific and cross-modal stimuli at different developmental stages is presented to illustrate how they change as a consequence of experience. Randomness in the system can slightly alter the developmental outcome, but the presented results are the most typical.
Immature behaviour
A first set of simulations was performed to evaluate the neuron’s behaviour before sensory experience. Two different testing paradigms were used, both involving modality-specific and cross-modal stimuli. In the first paradigm we tested the network with simulated stimuli having the same fixed location but different intensities to evaluate the neuron’s “dynamic ranges” (Fig. 2), in the second we tested the network with stimuli having the same (strong) intensity but different locations to evaluate the target neuron’s receptive fields (Fig. 3). In each case the results of model simulations are discussed with reference to those obtained from physiological recordings of individual SC neurons.
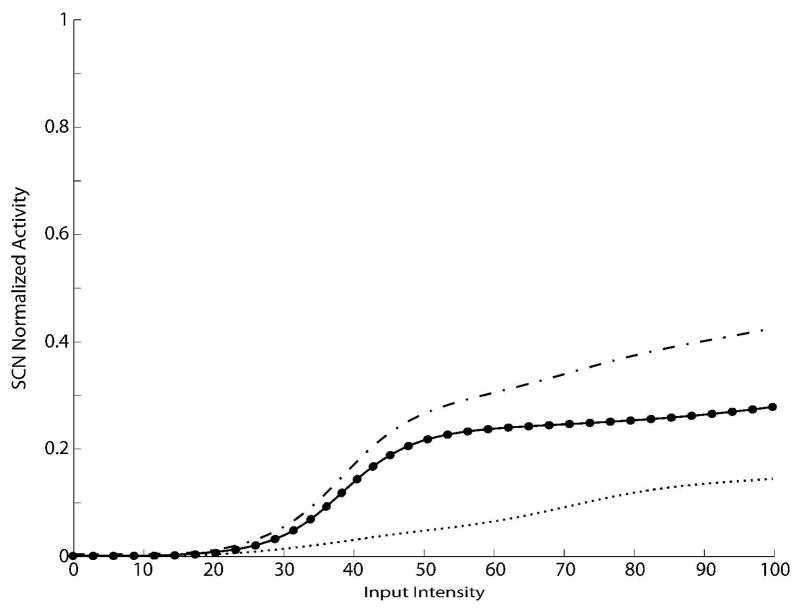
Fig. 2. SC Dynamic Ranges in the newborn.
Activity of SC neurons in response to different inputs in the newborn. The x-axis reports the magnitude of simulated external inputs in arbitrary units, while the y-axis the normalized activity evoked in the stimulated SC neurons. The activity was assessed by stimulating the model with auditory (small dotted line), visual (big dotted line) and cross-modal (solid line) inputs, presented in the center of their relative RFs, at various intensities. Before the training phase, the SC shows no multisensory integration and the cross-modal responses are no bigger than the best unisensory response. Note that the predicted sum (dash-dotted line) far outpaces the actual multisensory response.
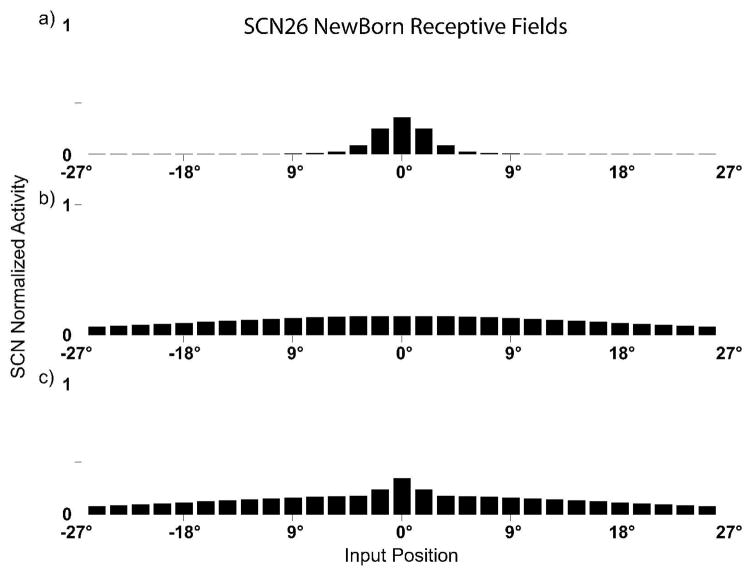
Fig. 3. SC RFs and responses in the newborn.
Responses in a simulated immature SC neuron to different spatial configurations of modality-specific (only visual, panel a, only auditory, panel b) and cross-modal stimuli (panel c). Note the huge receptive fields (especially the auditory and the cross-modal) of an actual immature SC neuron. In panel c the neuron is incapable of integrating their two cross-modal inputs, responds to stimuli presented in a very huge portion of the space, and have responses equivalent to those of the stronger of the two, independently to their relative spatial configurations.
At the immature stage the unisensory responses are weak, with saturation at about 0.1–0.2 (10–20% of the maximum activity), a finding that parallels the physiology (Wallace and Stein 1997). Also, and more important, multisensory enhancement to spatially concordant cross-modal stimuli is not present at any location in the dynamic range; in other words, the multisensory response is not significantly greater than the response to the more effective of the two unisensory components. These results are due to the network’s initial architecture: when a modality-specific input is simulated in the model in the immature phase, only the non-AES inputs are effective in generating responses from the SC neuron. Because of the WTA mechanism in the ascending afferent pathways, cross-modal inputs compete and the SC neuron is driven only by the more effective sensory modality, and thus there is not an enhanced multisensory response. In the example in Fig. 2 the simulated neuron was more sensitive to the visual modality. In this test we used two modality-specific inputs of the same relative intensity, hence the responses to cross-modal inputs were the same obtained with visual stimulations.
When input intensity is fixed but stimulus location varies, the modality-specific and cross-modal receptive fields (or spatial profile of responsiveness) are revealed (Fig. 3). As illustrated, the spatial tuning of the simulated SC neuron is very poor at this immature stage, which is consistent with experimental observations (Wallace and Stein 1997). The tuning is especially broad in the auditory modality, as observed empirically (Wallace and Stein 1997). Also, as observed empirically, the neuron can respond to multiple spatial locations but does not integrate concordant stimuli presented at any of them (Wallace and Stein 1997).
Developmental Phase
The model in its naïve state was then repeatedly stimulated with modality-specific and cross-modal stimuli (see Training) in order to simulate the experience of young animals with real stimuli in a normal environment. The weights from the projections descending from AES were adjusted according to Hebbian dynamics and waxed and waned as expected. An example can be found in Figure S1 of the supplementary material. Initially only the non-FAES inputs show any selectivity, while the FAES inputs are weak and not influential. The large RFs of a single SC neuron is attributable to the diffuse nature of the non-FAES projection. After maturation, the projection from non-FAES has considerably sharpened in its tuning; however, it is not nearly as sharp and strong as the now-mature projection from FAES. These simulations are believed to reflect biological processes involving synaptic strengthening, weakening, and pruning.
It is worth noting that the model predicts a rapid transition of SC neurons from non-integrative to integrative. In fact, descending synapses require a long training period before reaching a strength sufficient to significantly affect the SC response. As soon as this strength value has been reached, the descending paths rapidly drive the SC behavior, causing a sudden increase in the SC response and very rapid maturation.
Additional simulations were performed with different scheduling ratios. If the percentage of cross-modal stimuli is reduced, the model needs more training steps but settles at a comparable final adult configuration. If the percentage of cross-modal stimuli is excessively reduced (approximately below 50%) the SC neurons remain unisensory. The modality-specific stimulation that was stronger during training establishes the response. If a moderate percentage of cross-modal spatially-misaligned stimuli is introduced, the training is further slowed down.
Mature behaviour
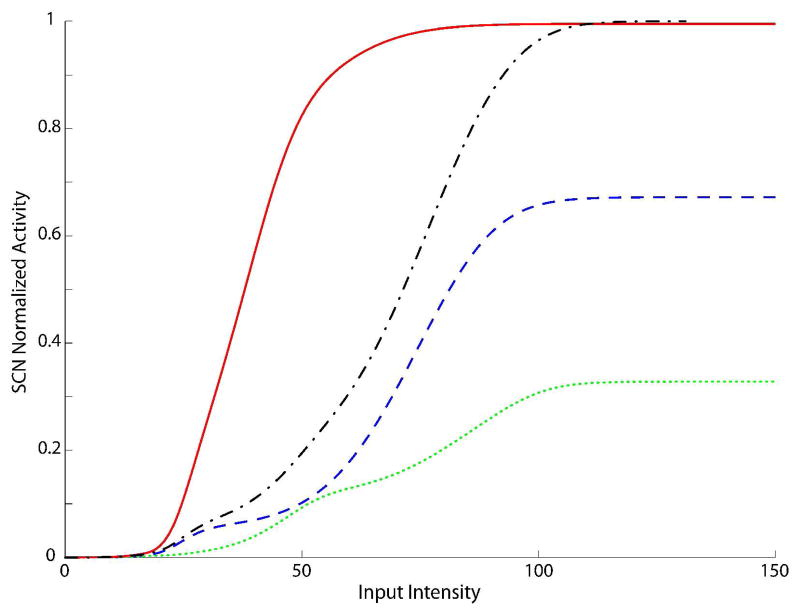
At the end of this training process (15000 inputs) we used the same testing paradigm used to evaluate the SC neuron behaviour in the immature phase. In testing the dynamic range, we find much more robust modality-specific responses, and critically, enhanced multisensory responses to the presentation of concordant cross-modal cues at intensity levels between threshold and saturation (Fig. 4). The model robustly accounts for the main results on adult SC neurons and produces responses very similar to those reported in the literature (Perrault Jr et al. 2005; Stanford et al. 2005; Stein and Meredith 1993): a) multisensory enhancement; b) multisensory responses evidence a greater dynamic range than unisensory responses; c) the response to cross-modal stimuli shifts from superadditive to additive and finally to subadditive at higher levels of stimulus effectiveness, in agreement with the principle of inverse effectiveness.
Fig. 4. SC Dynamic Ranges after the development.
Activities elicited in the model by stimulating the network with auditory (dotted line), visual (dashed line) and cross-modal (solid line) inputs, at various intensities. The x-axis reports the magnitude of simulated external inputs in arbitrary units, while the y-axis the normalized activity evoked in the stimulated SC neurons. The stimuli were presented in the center of the RF of the observed SC neuron. Note that the model in this phase shows a response to a cross-modal stimulation greater than the predicted sum (dash-dotted line) of the two modality-specific responses, that means multisensory neurons have acquired the integrative capabilities during the training.
Similarly, after training the individual SC neuron now exhibits RFs (Fig. 5) that closely match those reported in the empirical literature (Stein and Meredith 1993; Meredith and Stein 1986; Meredith and Stein 1996). In contrast to those in the neonate, the mature SC neuron has more tightly constrained RFs for both visual and auditory sensory modalities and they are more closely in register with one another. Moreover, when stimulated with a weak cross-modal coincident stimulus inside the overlapped region of the RFs, the responses of the neuron can be greater than the arithmetical sum of the two unisensory responses obtained by its two unisensory components by themselves (as shown also in Fig. 5). It is interesting to note that multisensory enhancement produced by concordant cross-modal stimuli can be observed throughout the entire extent of the overlapping portions of the RFs; that is, even at the borders.
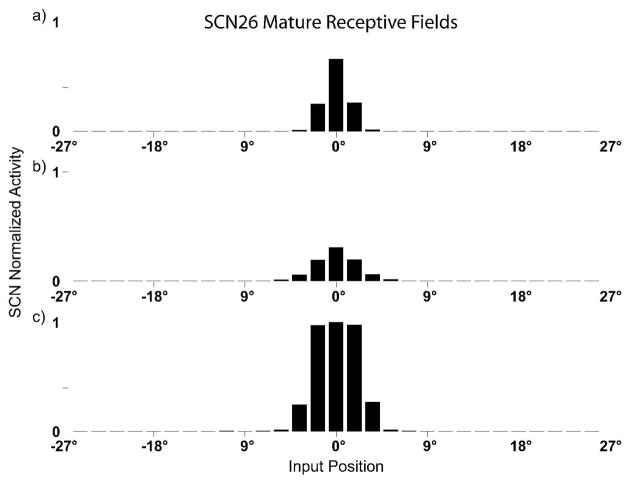
Fig. 5. SC RFs and responses after the development.
Responses in a simulated mature SC neuron to different spatial configurations of modality-specific (only visual, panel a, only auditory, panel b) and cross-modal stimuli (panel c). Note the contraction of receptive fields compared to those shown in Fig.3. In this neuron the RFs are clearly in spatial register and overlapped each other. In panel c the neuron shows its integrative capabilities: the multisensory enhancement is produced by concordant cross-modal stimuli and it can be observed throughout the entire extent of the overlapping portions of the RFs.
Finally, it is interesting to note that the mature AES inhibitory projections to the SC eliminate the non-AES influences through the interneurons Ha and Hv (see Figure 1). But if the AES is deactivated, these interneurons are not excited (since they receive excitation from the AES only), hence they do not send their inhibitory influence to the SC. Consequently, the effect of the ascending path to the SC becomes evident again, in agreement with experimental results (Wallace and Stein 1994; Jiang et al. 2001; Alvarado et al. 2009) and with simulation results of our previous paper (Cuppini et al. 2010).
Discussion
Among the many features that characterize SC multisensory maturation are two that are of principal importance in the current context: aligning the multiple receptive fields of individual multisensory neurons, and developing an ability to integrate their cross-modal inputs. These features develop in parallel: once a neuron’s receptive fields had contracted to approximately 150% of their adult size, the probability that it will integrate its different sensory inputs has increased dramatically (Wallace and Stein 1997). The present study suggests that a single model (see Cuppini et al. 2010) can provide a basis for understanding the mechanisms involved in both processes.
The model begins at the immature stage at which multisensory neurons have appeared, but still have very large receptive fields and lack the capacity for multisensory integration (Stein et al. 1973, Wallace and Stein 1997). The model shows how the SC can realistically incorporate the statistics of unisensory and multisensory experience to produce a mature state using simple learning rules similar to those that have previously been suggested in empirical studies of receptive field contraction and alignment that leads to the spatial register of different sensory maps (Zhang et al. 2000; Katz and Shatz 1996; Witten et al. 2008)
Model Assumptions and Essential Behavior
The model makes several basic assumptions based on empirical findings in the literature. Among these is that the initial visual and auditory afferents derived from non-AES pathways (these include, but are not limited to, subcortical projections) have developed extensive and weak synaptic inputs with little spatial tuning at birth (Wallace and Stein 1997). The spatial tuning is slightly tighter in the visual pathway, an assumption consistent with observations that it provides a guide for the development of spatial tuning in the auditory pathway (e.g., Luksch H. et al. 2000; King et al. 1988; Knudsen and Brainard 1991; Wallace et al. 2004). Cortical projections from AES, which later provide significant contributions to SC activity, are intact but functionally ineffective.
Simple Hebbian mechanisms then refine these initial biases as the model is repeatedly exposed to modality-specific and correlated cross-modal signals that drive activity in SC neurons. Hence, a main assumption is that activity in the individual SC neuron is an important component of learning. Simulated cross-modal stimuli produce activity in unisensory AES and non-AES neurons responsive to the simulated regions of space in which inputs are presented, which then selectively activate SC neurons that have a slight a priori bias to the same location. Typically the non-AES visual inputs drive this selection. The Hebbian learning rule then selectively strengthens connections linking the active presynaptic and postsynaptic neurons while weakening projections from inactive modality-specific inputs, as suggested by Mize et al. (1992). The consequence of this pruning, in combination with the competitive inhibitory influences, is that the RFs of individual neurons shrink in size and (as a consequence of the correlated experience) enhance their spatial register. As this occurs, the inputs from AES become refined and very powerful. Upon achieving maturity, the RFs of SC neurons primarily reflect their converging inputs from AES. In this mature state, multisensory integration in the model is critically dependent on the integrity of these cortico-collicular projections, a finding which matches the results of many recent physiological and anatomical studies (Wallace and Stein 1994; Jiang et al. 2001; Alvarado et al. 2009).
Model Predictions
Development
The model makes certain predictions about the properties of SC neurons during development that differ from those of other models. Unlike models that emphasize the topographic alignment of the RFs within populations of neurons or across topographic maps, the present model focuses on the individual neuron. This leaves these individual neurons unconstrained by the topographic processes on-going in neighbouring neurons, and can lead to different predictions. The model accurately predicts that rearing animals with spatially-disparate cross-modal stimuli can lead to spatially misaligned RFs and anomalous patterns of integration as shown empirically (Wallace and Stein 2007). However, it also predicts that these changes would only occur for neurons whose RFs encompass the exposure site(s): the receptive fields of other neurons would exhibit normally aligned RFs. Animals reared without correlated cross-modal experience would not develop the capacity for multisensory integration, a finding consistent with physiological studies (Wallace et al. 2004). The model also predicts that the speed of RF contraction increases during development (its developmental trajectory is approximately sigmoid) because as the descending pathways from AES become active on any given SC neuron, that target neuron is activated more strongly, and higher activity levels result in faster circuit development and faster RF contraction. Deactivating, or removing AES early in life would slow RF contraction and alignment and yield neurons incapable of multisensory integration, a prediction consistent with the empirical findings of Jiang, Jiang and Stein (2006). In fact, the same effect can be seen in the model if only the visual AES inputs are kept inactive during development, a finding that would be predicted based on the synergy among converging cortico-collicular afferents from AES demonstrated by Alvarado et al. (2009). Finally, the model assumes that the inhibitory influences from AES (i.e., via GABAergic SC interneurons, see Fuentes-Santamaria et al. 2009) are also trained via a similar Hebbian mechanism. The latter aspect is essential because it produces the competitive mechanism between AES inputs and the non-AES projections
Adult
The model also makes certain predictions for the behavior of SC neurons in the adult state. The first is that, insofar as the learning mechanisms controlling changes in AES are still in place in the adult, the principles of multisensory integration in the SC should be plastic. Restricting visual-auditory neurons to experience with only auditory inputs (e.g., by housing the animals in a dark room) should, over time, degrade the influence of other sensory modalities. Similarly, repeatedly exposing animals to, for example, spatially disparate configurations of cross-modal stimuli should, over time, shift the RFs of SC neurons, thereby degrading their alignment and leading to more efficacious integration of spatially disparate stimuli consistent with the animal’s experience. This has already been noted in developing animals (see Wallace and Stein 2007), and would be expected to occur in the adult with similar cross-modal experience, albeit changes would be smaller and less dramatic due to the synaptic pruning that had already taken place during development.
The model also accurately replicates the multisensory integrative capability of SC neurons; for example the principle of inverse effectiveness and superadditivity (stronger cross-modal signals generate less proportional enhancement, see Stein et al. 2010 for a review), and its dependence on the functional integrity of inputs from AES (Wallace and Stein 1994; Jiang et al. 2001; Alvarado et al. 2009; see also Stein 2005). In the absence of AES, or before it is functionally mature, the responses of multisensory SC neurons to visual-auditory cross-modal stimuli reflect the results of the competition between their respective inputs mediated by non-AES pathways. The neurons are driven by the most effective of the two. However, once AES projections are functionally active, the converging visual and auditory cortico-collicular influences can be processed simultaneously by their target SC neurons, producing appreciable multisensory enhancement.
Model Limitations and Future Directions
There are a number of important limitations and simplifications of the model that may be the subject of future studies. One noteworthy simplification is that, unlikely inhibitory projections from interneurons stimulated by AES, direct lateral inhibitory connections between SC multisensory neurons are not modelled. These will have to be included and trained in order for the model to account for multisensory depression.
Another limitation is that the model does not include the possible contributions of a cortical region neighbouring AES, specifically the rostral portion of the lateral suprasylvian sulcus (rLS), also shown to have the capacity to modulate multisensory integration in the adult (Jiang et al. 2001). However, this limitation is not critical as its neonatal removal can often be compensated for by an expanded role of AES (Jiang et al. 2006). The model is also limited by the use of a narrow set of the input statistics of the training paradigm and the learning rate used in the algorithm. A greater number of alternative schedules is necessary in future works, (including a certain percentage of auditory and visual inputs in disparate positions, and performing a sensitivity analysis on the learning rates) to test the training mechanisms in the model and generate new testable predictions. Unfortunately, there is no data currently available that would allow us to set these parameters in a realistic way. However, these issues are being explored empirically, and these data are likely to be available in the future.
Supplementary Material
Acknowledgments
Supported by NIH grants NS036916 and EY016716.
Footnotes
Note: Paper originally presented at the IMRF 2010
References
- Alvarado JC, Stanford TR, Rowland BA, Vaughan JW, Stein BE. Multisensory integration in the superior colliculus requires synergy among corticocollicular inputs. J Neurosci. 2009;29:6580–6592. doi: 10.1523/JNEUROSCI.0525-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppini C, Ursino M, Magosso E, Rowland BA, Stein BE. An emergent model of multisensory integration in superior colliculus neurons. Front Integr Neurosci. 2010;22:4–6. doi: 10.3389/fnint.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Santamaria V, Alvarado JC, McHaffie JG, Stein BE. Axon morphologies and convergence patterns of projections from different sensory-specific cortices of the anterior ectosylvian sulcus onto multisensory neurons in the cat superior colliculus. Cereb Cortex. 2009;19:2902–2915. doi: 10.1093/cercor/bhp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Santamaria V, McHaffie JG, Stein BE. Maturation of multisensory integration in the superior colliculus: Expression of nitric oxide synthase and neurofilament SMI-32. Brain Res. 2008;1242:13–23. doi: 10.1016/j.brainres.2008.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. Neonatal cortical ablation disrupts multisensory development in superior colliculus. J Neurophysiol. 2006;95:1380–1396. doi: 10.1152/jn.00880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Wallace MT, Jiang H, Vaughan JW, Stein BE. Two cortical areas mediate multisensory integration in superior colliculus neurons. J Neurophysiol. 2001;85:506–522. doi: 10.1152/jn.2001.85.2.506. [DOI] [PubMed] [Google Scholar]
- Kadunce DC, Vaughan JW, Wallace MT, Benedek G, Stein BE. Mechanisms of within- and cross-modality suppression in the superior colliculus. J Neurophysiol. 1997;78:2834–2847. doi: 10.1152/jn.1997.78.6.2834. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- King AJ, Hutchings ME, Moore DR, Blakemore C. Developmental plasticity in the visual and auditory representations in the mammalian superior colliculus. Nature. 1988;332:73–76. doi: 10.1038/332073a0. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Brainard MS. Visual instruction of the neural map of auditory space in the developing optic tectum. Science. 1991;253:85–87. doi: 10.1126/science.2063209. [DOI] [PubMed] [Google Scholar]
- Koch C. Biophysics of computation: Information Processing in Single Neurons. New York: Oxford University Press; 1998. [Google Scholar]
- Luksch H, Gauger B, Wagner H. A candidate pathway for a visual instructional signal to the barn owl’s auditory system. J Neurosci. 2000;20:RC70, 1–4. doi: 10.1523/JNEUROSCI.20-08-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res. 1986;365:350–354. doi: 10.1016/0006-8993(86)91648-3. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Spatial determinants of multisensory integration in cat superior colliculus. J Neurophysiol. 1996;75:1843–1857. doi: 10.1152/jn.1996.75.5.1843. [DOI] [PubMed] [Google Scholar]
- Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res. 1992;90:219–48. doi: 10.1016/S0079-6123(08)63616-X. [DOI] [PubMed] [Google Scholar]
- Perrault TJ, Jr, Vaughan JW, Stein BE, Wallace MT. Superior colliculus neurons use distinct operational modes in the integration of multisensory stimuli. J Neurophysiol. 2005;93:2575–2586. doi: 10.1152/jn.00926.2004. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Quessy S, Stein BE. Evaluating the operations underlying multisensory integration in the cat superior colliculus. J Neurosci. 2005;25:6499–6508. doi: 10.1523/JNEUROSCI.5095-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE. The development of a dialogue between cortex and midbrain to integrate multisensory information. Exp Brain Res. 2005;166:305–315. doi: 10.1007/s00221-005-2372-0. [DOI] [PubMed] [Google Scholar]
- Stein BE, Burr D, Constantinidis C, Laurienti PJ, Alex Meredith M, Perrault TJ, Jr, Ramachandran R, Röder B, Rowland BA, Sathian K, Schroeder CE, Shams L, Stanford TR, Wallace MT, Yu L, Lewkowicz DJ. Semantic confusion regarding the development of multisensory integration: a practical solution. Eur J Neurosci. 2010;31:1713–20. doi: 10.1111/j.1460-9568.2010.07206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Labos E, Kruger L. Sequence of changes in properties of neurons of superior colliculus of the kitten during maturation. J Neurophysiol. 1973;36:667–679. doi: 10.1152/jn.1973.36.4.667. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, Rowland BA. The neural basis of multisensory integration in the midbrain: its organization and maturation. Hear Res. 2009;258:4–15. doi: 10.1016/j.heares.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Perrault TJ, Jr, Hairston WD, Stein BE. Visual experience is necessary for the development of multisensory integration. J Neurosci. 2004;24:9580–9584. doi: 10.1523/JNEUROSCI.2535-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Cross-modal synthesis in the midbrain depends on input from cortex. J Neurophysiol. 1994;71:429–432. doi: 10.1152/jn.1994.71.1.429. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Development of multisensory neurons and multisensory integration in cat superior colliculus. J Neurosci. 1997;17:2429–2444. doi: 10.1523/JNEUROSCI.17-07-02429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Early experience determines how the senses will interact. J Neurophysiol. 2007;97:921–926. doi: 10.1152/jn.00497.2006. [DOI] [PubMed] [Google Scholar]
- Witten IB, Knudsen EI, Sompolinsky H. A Hebbian learning rule mediates asymmetric plasticity in aligning sensory representations. J Neurophysiol. 2008;100:1067–1079. doi: 10.1152/jn.00013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Poo M. Visual input induces long-term potentiation of developing retinotectal synapses. Nat Neurosci. 2000;3:708–715. doi: 10.1038/76665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.