Abstract
The apical plasma membrane domain of retinal pigment epithelial (RPE) cells in the eye faces the outer segment portions of rods and cones and the inter-photoreceptor matrix in the subretinal space. Two important receptor-mediated interactions between the apical surface of the retinal pigment epithelium (RPE) and adjacent photoreceptors are adhesion ensuring outer segment alignment and diurnal phagocytosis of shed outer segment fragments contributing to outer segment renewal. Both depend on the apical distribution of the integrin family adhesion receptor αvβ5 as lack of αvβ5 in mice causes weakened retinal adhesion and asynchronous phagocytosis. With age, lack of αvβ5 leads to accumulation of harmful lipofuscin in the RPE and to vision loss. Here, we discuss three different possible mechanisms that could generate the exclusive apical distribution of αvβ5 integrin receptors in the RPE. (1) αvβ5 could be apical in the RPE because RPE attachment to neural retina generally or αvβ5 ligands specifically in the subretinal space stabilize apical but not basolateral αvβ5 surface receptors. (2) αvβ5 could be apical in the RPE because it resides in a complex with other components of the phagocytic machinery that assembles at the apical, phagocytic surface of the RPE. (3) αvβ5 could be apical due to mechanisms intrinsic to this receptor protein and specifically to its β5 integrin subunit.
15.1 Introduction
Post-mitotic retinal pigment epithelial cells (RPE) in the eye form a stationary monolayer epithelium whose lateral junctions seal off the neural retina from the underlying vascularized choroidal tissue forming the outer blood-retinal barrier. The plasma membrane of each RPE cell is strictly divided by a tight junction permeability barrier. The RPE's basolateral domain faces Bruch's membrane, a multi-layer basement membrane rich in adhesive glycoproteins such as laminin and collagen IV connecting to the vascularized choroid. The RPE's apical plasma membrane faces the avascular subretinal space where it adheres to components of the interphotoreceptor matrix and possibly the outer segment plasma membrane of photoreceptor rods and cones. These apical interactions are unusual compared to most other epithelial tissues that line fluid-filled lumina. Distinct protein distributions at its basolateral and apical surfaces are an obvious prerequisite for functions of the RPE associated with its control of molecular flux into and out of the neural retina, such as vectorial transport of ions, water, and metabolites. Moreover, other functions of RPE cells also take place exclusively at one of their surface domains. Diurnal phagocytosis of shed photoreceptor outer segment fragments and mechanically stable adhesion likely mostly to interphotoreceptor extracellular matrix components are two receptor protein-dependent functions that take place at the apical surface of the RPE in the eye (Finnemann and Chang 2008). To qualify for an essential role in the molecular machineries involved in these RPE functions membrane candidate proteins must therefore localize at least in part to the apical surface of the RPE in situ.
15.2 Functions of Apical αvβ5 Integrin Receptors in Retinal Phagocytosis and Adhesion
Diurnal synchronized phagocytosis of photoreceptor outer segment tips shed daily by photoreceptor cells is an essential task of the RPE deficiencies of which cause retinal degeneration in animal models and cause some forms of human retinitis pigmentosa. Prompted by the initial observation that onset of expression at the apical surface of the integrin family adhesion receptor αvβ5 correlates exactly with the begin of daily shedding and phagocytosis in maturing rat RPE (Finnemann and Bonilha 1997) we studied RPE phagocytosis in knockout mice lacking the β5 inte-grin subunit and thus αvβ5 integrin receptors (Nandrot and Kim 2004). As young adults, β5 integrin knockout mice had normal retinal morphology and function but we counted similar numbers of outer segment derived phagosomes in their RPE cells at all times of day. This was in sharp contrast to strain- and age-matched wild-type mice whose RPE contained phagosomes only within 3 h following light onset, similar to earlier observations by others. Furthermore, RPE cells of β5 knockout mice at old age contained excess numbers of autofluorescence inclusions resembling lipofuscin granules that increasingly accumulate in human RPE with age. At the same age, photoreceptor function measured by electroretinography was con siderably impaired in β5 knockout mice suggesting that accumulation of debris accumulating with age in β5 knockout RPE is harmful for the retina. Finally, RPE cells isolated from young β5 knockout mice in culture demonstrated normal morphology but dramatically reduced binding activity towards isolated photoreceptor outer segment fragments. Notably, lack of αvβ5 in situ or in RPE in culture abolished the stimulation of tyrosine kinases focal adhesion kinase (FAK) and Mer tyrosine kinase (MerTK) both of which are essential for POS engulfment. Taken together, these results identified a critical function for αvβ5 receptors in synchronizing diurnal RPE phagocytosis likely by stimulating rhythmic downstream tyrosine kinase signaling.
Like diurnal phagocytosis, robust adhesion of the apical aspect of the RPE to the retina generally and the interphotoreceptor matrix and outer segments specifically is enormously important for retinal health. Its disruption in retinal detachment rapidly leads to a variety of well described stress responses in the neural retina (Fisher and Lewis 2005). If prolonged, retinal detachment will result in photoreceptor apoptotic cell death and hence vision loss (Cook and Lewis 1995). The receptor proteins on the RPE's apical surface that are responsible for retinal adhesion are only poorly characterized. Since αvβ5 integrin promotes adhesion to extracellular matrices in other tissues, we tested if apical αvβ5 receptors may contribute to retinal adhesion. β5 integrin knockout mice do not exhibit retinal detachment. However, using semi-quantitative detachment assays we demonstrated that resistance to shear forces is considerably reduced in these mice indicating weakened retinal adhesion (Nandrot et al. 2006). Since β5 knockout retinal adhesion differed to similar extent from wild-type retinal adhesion at all ages examined, we concluded that this impairment was not a consequence of asynchronous phagocytosis and lipofuscin accumulation. Instead, we concluded that αvβ5 integrin receptors fulfill two distinct functions at the apical surface of the RPE, retinal adhesion and phagocytosis.
15.3 Apical Polarity of αvβ5 Integrin Receptors is Independent of the Neural Retina
Integrin receptors that reside on cellular surfaces are likely engaged in receptor-ligand interactions. Unoccupied integrins may indicate lack of proper tissue context and have been demonstrate to be sufficient to induce apoptotic cell death (Frisch and Screaton 2001). Thus, it is generally thought that, at steady-state, integrins only localize to surfaces where appropriate ligands are available. Related to this, once ligand-bound, integrin receptors are more likely to persist at the cell surface for longer periods of time than unoccupied integrin receptors. Apical polarity of αvβ5 receptors in the RPE may thus be a consequence of unique availability of stabilizing ligands at the apical surface. This would imply that ligands for αvβ5 may be scarce or even absent at the basolateral surface of the RPE. However, this is an unlikely scenario.
First, RPE cells in the eye express numerous integrin receptors. All integrin receptors found to be expressed by the RPE in the retina besides αvβ5 show a highly polarized basolateral distribution. This includes the integrin receptor, αvβ3, which is most related to αvβ5 sharing the αv subunit and with overlapping if not identical ligand binding preferences (Finnemann and Bonilha 1997). Our knowledge of integrin ligands available to RPE cells in the eye at either surface aspect are not fully characterized but the joint ligand for αvβ3 and αvβ5, vitronectin, localizes to Bruch's membrane. We have previously identified the extracellular RGD-domain glycoprotein MFG-E8 as sole ligand that activates αvβ5 downstream signaling toward MerTK in the retina that is essential for diurnal phagocytosis (Nandrot and Anand 2007). Mice lacking MFG-E8 lack the diurnal rhythm of RPE phagocytosis exactly like mice lacking αvβ5. This correlation is supported by RPE culture studies showing that recombinant MFG-E8 enhances wild-type phagocytosis and restores phagocytosis by MFG-E8 knockout RPE cells to wild-type levels but has no effect on uptake by β5 knockout RPE cells. These data demonstrate that MFGE8 is the only essential ligand for the phagocytic function of αvβ5 in the retina. Notably, mice lacking MFG-E8 have only minimally reduced retinal adhesion in contrast to β5 knockout mice. This implies that the retinal adhesive function of αvβ5 uses ligands other than MFG-E8 in the subretinal space. At this time, these ligands remain unidentified. However, αvβ3 can bind MFG-E8 like αvβ5 but exclusively localizes to the basolateral and not the apical surface of the RPE in the retina. Taken together, these results suggest that ligands for αvβ5 exist at both apical and basolateral surfaces of the RPE rendering selective retention at the apical surface unlikely.
Second, if specific apical ligands available to αvβ5 generate the strict apical polarity observed for this receptor, disruption of the native apical interactions of RPE cells would likely promote αvβ5 redistribution. Earlier studies aiming to identify molecular mechanisms involved in generating specific protein polarity in the RPE have shown that some transmembrane proteins that distribute apically in the RPE in the retina are non-polar or basolateral in RPE cell in culture. This has been particularly well studied for two type I transmembrane proteins, the Ig-CAM family cell adhesion receptor N-CAM and the matrix metalloproteinase protein EMMPRIN (Marmorstein and Gan 1998; Gundersen and Powell 1993). Both are commonly expressed by epithelial tissues and cell lines. Both are basolateral in kidney epithelium as well as in the best-characterized culture model for cell polarity, the kidney epithelium derived Madin Darby Kidney (MDCK) cell line. Both mostly distribute to the apical surface of RPE cells in the retina but rapidly relocalize in RPE in culture. N-CAM assumes a strictly lateral localization likely contributing to adhesive contacts between neighboring RPE cells in culture. EMMPRIN is non-polar in culture. While the precise interacting molecules remain to be identified, these data suggest that the steady-state apical distribution of both N-CAM and EMMPRIN in RPE in situ is a consequence of molecular interactions of the RPE's apical surface that take place in the subretinal space.
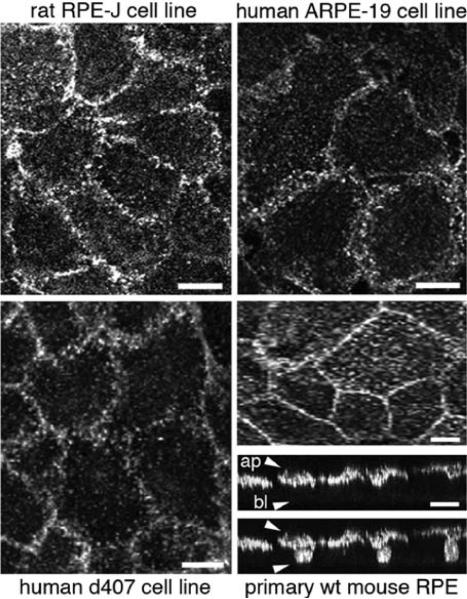
Yet, αvβ5 receptors maintain their apical steady-state polarity even in RPE cells in tissue culture. Indeed, immunofluorescence microscopy of αvβ5 surface receptors in primary, unpassaged mouse RPE, the immortalized rat RPE cell line RPE-J, the human spontaneously immortalized cell line ARPE-19 and the human RPE derived d407 cell line demonstrates that like RPE in the eye facing the interphotoreceptor matrix with its ligand MFG-E8 all these RPE model cells possess apical αvβ5 despite great species and phenotypical discrepancies among them otherwise (Fig. 15.1). These findings demonstrate that the apical polarity of αvβ5 is maintained by RPE cells autonomously independent of their apposition to and interactions with the neural retina and the MFG-E8-rich interphotoreceptor matrix.
Fig.15.1.
Apical polarity of αvβ5 integrin receptors in RPE cells in culture. Rat RPE-J, human ARPE-19 and human d407 RPE cell lines, as indicated, were grown to confluence on glass coverslips and labeled live on ice with αvβ5 surface dimer-specific antibody P1F6. 3D projections representing the upper 2 μm of apical aspects of cells are shown. Wild-type 129 strain mouse RPE was isolated in patches from 10-day-old mouse eyes, cultured for 4 days before fixation and labeling with antibody recognizing the β5 integrin cytoplasmic domain. Images were acquired by laser scanning confocal microscopy. Representative whole cell maximal projections of the same field are shown in x–y plane and in x–z plane. x–z projection is shown with (upper panel) and without (lower panel) nuclei counterstaining. Approximate locations of apical (ap) and basolateral (bl) surfaces of cells are indicated by arrowheads in the upper panel. Scale bar is 10 μm for cell lines and 20 μm for primary RPE
15.4 Apical Polarity of αvβ5 Receptors is Independent of the Essential Engulfment Receptor MerTK
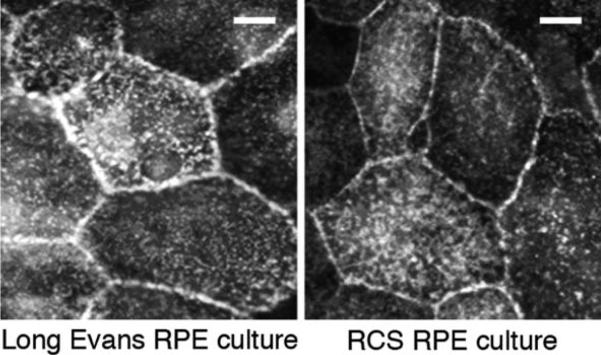
Phagocytic mechanisms involve the coordinated activities of numerous cell surface receptors, associated cytosolic proteins and the actin cytoskeleton. As outlined earlier, RPE phagocytosis in the eye and in culture involves αvβ5 integrin recognition of its ligand MFG-E8, which likely acts to bridge shed POS and αvβ5. In the mouse retina, this interaction is required for subsequent maximal stimulation of MerTK via FAK causing the burst of engulfment activity that characterizes the response to POS of wild-type RPE. Additional receptor proteins such as the receptor for modified lipids, CD36, likely contribute to RPE phagocytosis as well although their precise roles remain unresolved thus far. Given the close functional interaction of αvβ5 with MerTK we hence hypothesized that the apical polarity of αvβ5 may be a result of its integration into the complex phagocytic machinery of the RPE, which exists solely at the apical surface of the RPE in the eye. This would predict that αvβ5 apical polarity persists in RPE in culture as commonly studied primary and permanent RPE cell culture models (some of them mentioned above) retain specific phagocytic activity toward POS. We therefore studied whether αvβ5 was apical in mutant RPE cells that lack phagocytic function. Royal College of Surgeons (RCS) rats carry a mutation that eliminates MerTK protein expression. As a result, RCS RPE cells are unable to engulf POS in vivo and in vitro. Despite their phagocytic incompetence, RCS RPE cells in primary culture possess apical αvβ5 integrin receptors at equal levels as RPE cells isolated from wild-type rats (Fig. 15.2). This suggests that neither MerTK specifically nor a functional engulfment mechanism generally are required for the apical polarity of αvβ5 integrin receptors in RPE cells.
Fig.15.2.
Apical polarity of αvβ5 integrin receptors in MerTK-mutant RCS RPE cells. Wild-type Long Evans and mutant RCS rat RPE cells were isolated in patches from 10-day-old rat eyes and cultured for 4 days before live labeling on ice with αvβ5 surface dimer-specific antibody P1F6. Representative epifluorescence images are shown. Scale bar is 20 μm
15.5 Motifs of the β5 Integrin Subunit Cytoplasmic Domain that May Promote Apical Polarity of αvβ5 Integrin Receptors
As discussed thus far, available data do not support a critical role for either neural retina apposition and specific ligands of the subretinal space, or for the essential phagocytic receptor MerTK and a functional phagocytic machinery in causing the unique apical polarity of αvβ5 integrin receptors in the RPE. We therefore hypothesize that αvβ5 receptors traffic to or are specifically retained at the apical surface of the RPE as a result of specific motifs inherent to αvβ5 receptors. Because the αv subunit is not specific to αvβ5 but also forms basolateral αvβ3 receptors in the RPE, we will focus on possible contributions to receptor polarity of the β5 integrin protein subunit and particularly its cytoplasmic domain.
The β5 cytoplasmic domain consists of 60 amino acids (Legate and Fassler 2009). The β5 cytoplasmic tail is responsible for interaction with FAK in transfected cells (Eliceiri and Puente 2002) and FAK resides in the apical αvβ5 integrin complex in RPE cells where it is critical for stimulating MerTK and POS engulfment (Finnemann 2003). It contains an NPxY motif that is important for recruitment and binding of cytoplasmic proteins forming adhesive complexes in other proteins such as the actin binding protein talin (Horwitz and Duggan 1986; Calderwood 2004). Talin interacts with αvβ5 integrin receptors in transfected cells and this depends on the β5 subunit (Singh and D'Mello 2007). While this domain is thus likely important for αvβ5 function in RPE as well, it is also present in β1 and β3 integrin cytoplasmic domains and hence, does not explain the unique apical polarity of αvβ5. However, overall only 28 amino acids of the terminal 42 are identical between β5 and β3 tails. Single and di-leucine motifs contribute to trafficking mechanisms in other proteins (Deora and Gravotta 2004; Hunziker and Fumey 1994). Notably, in the β5 integrin the × position of the NPxY motif is leucine while in the β3 integrin the × position is isoleucine. Finally, The β5 integrin tail contains an inserted stretch of eight amino acids close to the carboxiterminus that has no homology to either β1 or β3 integrin and that effectively extends the β5 tails (Legate and Fassler 2009). Taken together, both β5 and β3 integrin subunits form phagocytic receptors with the αv subunit with very similar functions but in RPE cells only αvβ5 but not αvβ3 heterodimers localize to the apical plasma membrane. We therefore hypothesize that the unique residues and motifs of the β5 integrin cytoplasmic domain are responsible for the unique polarity of αvβ5 receptors.
15.6 Perspective
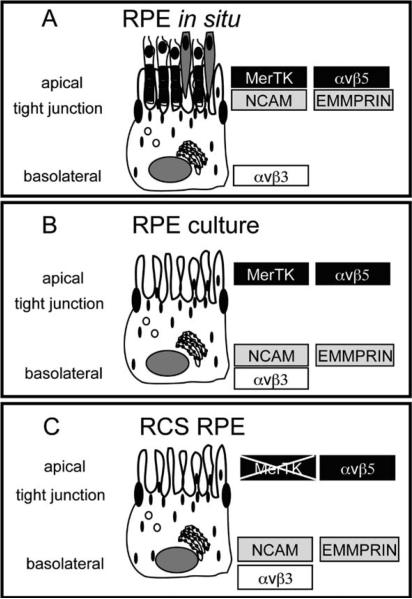
αvβ5 integrin receptors fulfill two distinct and equally important functions at the apical surface of the RPE by contributing to retinal adhesion and by synchronizing diurnal POS phagocytosis. All evidence suggests that the unique apical polarity of αvβ5 receptors in the RPE is not merely a consequence of ligand-induced stabilization or of anchorage to the MerTK dependent engulfment machinery. Rather, we propose that RPE cells use a trafficking pathway to specifically sort αvβ5 to the apical surface. This pathway is likely to recognize motifs of the β5 cytoplasmic domain. Comparing trafficking and complex formation among αvβ5 receptors with cytoplasmic deletions and point mutations will be our future approach to identify trafficking-relevant residues and motifs of the β5 integrin cytoplasmic tail.
Fig.15.3.
Summary of steady-state polarity of selected transmembrane proteins in different RPE models as discussed in the text. a. Polarity in the RPE in the eye. b. Polarity in RPE cells in culture. c. Polarity in MerTK-deficient RCS RPE cells in culture
Acknowledgement
This work was supported by NIH grant R01-EY13295 to SCF.
ABBREVIATIONS
- POS
shed photoreceptor outer segment fragments
- RPE
retinal pigment epithelium
References
- Calderwood DA. Talin controls integrin activation. Biochem Soc Trans. 2004;32(Pt3):434–437. doi: 10.1042/BST0320434. [DOI] [PubMed] [Google Scholar]
- Cook B, Lewis GP, et al. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36(6):990–996. [PubMed] [Google Scholar]
- Deora AA, Gravotta D, et al. The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium. Mol Biol Cell. 2004;15(9):4148–4165. doi: 10.1091/mbc.E04-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP, Puente XS, et al. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157(1):149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22(16):4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, et al. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94(24):12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Chang Y. Photoreceptor-RPE interactions physiology and molecular mechanisms. In: Tombran-Tink J, Barnstable CJ, editors. From ophthalmology research: visual transduction and non-visual light perception. Humana Press; Totowa, NJ: 2008. [Google Scholar]
- Fisher SK, Lewis GP, et al. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005;24(3):395–431. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Gundersen D, Powell SK, et al. Apical polarization of N-CAM in retinal pigment epithelium is dependent on contact with the neural retina. J Cell Biol. 1993;121(2):335–343. doi: 10.1083/jcb.121.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A, Duggan K, et al. Interaction of plasma membrane fibronectin receptor with talin – a transmembrane linkage. Nature. 1986;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13(13):2963–2969. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122(Pt 2):187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Gan YC, et al. Apical polarity of N-CAM and EMMPRIN in retinal pigment epithelium resulting from suppression of basolateral signal recognition. J Cell Biol. 1998;142(3):697–710. doi: 10.1083/jcb.142.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, et al. Novel role for alphavbeta5-integrin in retinal adhesion and its diurnal peak. Am J Physiol Cell Physiol. 2006;290(4):C1256–C1262. doi: 10.1152/ajpcell.00480.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, et al. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci USA. 2007;104(29):12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, et al. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med. 2004;200(12):1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, D'Mello V, et al. A NPxY-independent beta5 integrin activation signal regulates phagocytosis of apoptotic cells. Biochem Biophys Res Commun. 2007;364(3):540–548. doi: 10.1016/j.bbrc.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]