Summary
Follicular T helper cells (Tfh) provide critical help to B cells for germinal center (GC) formation. Mutations affecting SLAM-associated Protein (SAP) prevent GC formation due to defective T-B cell interactions, yet effects on Tfh cell differentiation remain unclear. We describe the in vitro differentiation of functionally-competent “Tfh-like” cells that expressed Interleukin-21, Tfh markers, and Bcl6, and rescued GC formation in SAP-deficient hosts better than other T helper (Th) cells. SAP-deficient Tfh-like cells appeared virtually indistinguishable from wildtype, yet failed to support GCs in vivo. Interestingly, both Tfh-like and in vivo-derived Tfh cells could produce effector cytokines in response to polarizing conditions. Moreover, Th1, Th2 and Th17 cells could be reprogrammed to obtain Tfh characteristics. ChIP-Seq analyses revealed positive epigenetic markings on Tbx21, Gata3 and Rorc in Tfh-like and ex vivo Tfh cells, and Bcl6 in non-Tfh cells, supporting the concept of plasticity between Tfh and other Th cell populations.
Introduction
Follicular T helper cells (Tfh) are key regulators of germinal center (GC) formation and T-dependent long-term humoral immunity (Crotty, 2011). First defined as CD4+ T cells located in human tonsillar GCs, Tfh cells in mice express CXCR5, ICOS, PD-1, and BTLA, molecules important for migration to B cell follicles and providing signals for initiation and maintenance of B cell GC responses (King et al., 2008). Tfh cells produce high amounts of the cytokine IL-21, a potent activator of GC B cell differentiation, immunoglobulin isotype switching, and plasma cell generation (Linterman et al., 2010; Spolski and Leonard, 2010; Zotos et al., 2010). Although it remains unclear whether Tfh cells are a distinct lineage, Tfh cells exhibit unique patterns of RNA and microRNA expression (Yu et al., 2009). Recent data have identified the transcription factor Bcl6 as a master regulator of Tfh cell generation (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009), further supporting that this is a distinct subpopulation of cells.
However, the concept of T helper (Th) cell lineages has been recently challenged by data supporting substantial plasticity between different effector cell populations (O’Shea and Paul, 2010; Wei et al., 2009; Zhou et al., 2009). Tfh cells within the GC can express cytokines characteristic of other Th cells, most notably IL-4 (characteristic of Th2 cells) (Fazilleau et al., 2009; King and Mohrs, 2009; Reinhardt et al., 2009; Smith et al., 2000; Yusuf et al., 2010; Zaretsky et al., 2009), but also IL-17 (Th17 cells) (Bauquet et al., 2009) and IFNγ (Th1 cells) (Johnston et al., 2009; Reinhardt et al., 2009; Smith et al., 2000). Furthermore, other data indicate that FoxP3+CD4+ regulatory T cells (Tregs) can differentiate into functional Tfh cells (Chung et al., 2011; Linterman et al., 2011; Tsuji et al., 2009). These data support the concept of reprogramming plasticity between polarized Th cell populations. Thus, whether Tfh cells are a separate cell population or a stage in effector T cell differentiation remains unclear. Because cytokines such as IL-4 and IFNγ induce B cell immunoglobulin class-switching, the relationship between Tfh and other CD4+ effector cells is of increasing importance for understanding regulation of mature antibody responses.
Recent studies have suggested that contact between T and B cells and/or sustained antigen stimulation are critical for Tfh cell generation (Deenick et al., 2010; Fahey et al., 2011; Fazilleau et al., 2009; Haynes et al., 2007; Johnston et al., 2009; Zaretsky et al., 2009). In this regard, mice deficient in the SLAM-associated protein (SAP) are of interest. These mice have impaired T cell help for GC generation, associated with selective defects in T-B cell adhesion (Cannons et al., 2010; Cannons et al., 2006; Crotty et al., 2003; Czar et al., 2001; Hron et al., 2004; Qi et al., 2008). However, the role of SAP in Tfh cell differentiation remains unclear. SAP-deficient CD4+ cells are activated normally by dendritic cells (DC) and initially express Tfh markers (Cannons et al., 2010; Kamperschroer et al., 2008; Qi et al., 2008). However, other data suggest that SAP-deficiency reduces or eliminates Tfh cells in GCs (Cannons et al., 2010; Linterman et al., 2009; Yusuf et al., 2010). Such data suggest that SAP affects a late stage required to generate functional Tfh cells, and that Tfh cell differentiation is a multistep process.
Given the critical role of Tfh cells in GC formation and long-term humoral immunity, understanding requirements for their differentiation and function is of great importance. For the differentiation of Th1, Th2, Th17 and Treg cells, in vitro culture studies have been invaluable for delineating requirements for cytokines, signaling proteins, and transcription factors, as well as evaluation of gene-expression and epigenetic modifications (Zhu et al., 2010). However, knowledge of Tfh cells is limited, in part, due to the lack of robust in vitro models. Recent studies have described in vitro generation of cells expressing IL-21 and other Tfh cell characteristics, but these cells were either primarily evaluated for gene expression or not evaluated for in vivo function (Nurieva et al., 2008; Suto et al., 2008).
We describe the in vitro differentiation of functionally competent IL-21-producing cells with Tfh-like properties. Importantly, transfer of low numbers of these cells induced GC formation in SAP-deficient hosts more effectively than other in vitro differentiated Th cells, suggesting they represent bona fide Tfh cell precursors. We have chosen the name “Tfh-like” cells for these in vitro differentiated IL-21 producing cells as they exhibit Tfh characteristics, but do not reside within B cell follicles. SAP-deficient Tfh-like cells were virtually indistinguishable from WT, yet nonetheless, failed to effectively contribute to Tfh cells and rescue GC formation in vivo. Evaluation of cytokine production as well as epigenetic chromatin modifications of genes encoding Th cell-specific transcription factors from either in vitro-generated Tfh-like cells or Tfh cells isolated directly ex vivo provided evidence for plasticity between Tfh-like and other Th cell populations, including Th1, Th2, and Th17 cells. Our results provide insight into the requirements for differentiation and plasticity of Tfh cells, which are critical for the generation of effective long-term humoral immunity.
Results
In vitro differentiated IL-21 producing cells have characteristics of Tfh cells
To evaluate differentiation of a Tfh-like cell population in vitro, we stimulated sorted naïve CD4+ T cells from OT-II mice in the presence of mitomycin-treated T-depleted splenocytes as antigen presenting cells (APCs) and neutralizing antibodies against IL-4, IL-12, IFNγ and TGF-β, along with IL-6 and IL-21, cytokines important for Tfh cell differentiation in vivo and IL-21 production in vitro (Eto et al., 2011; Nurieva et al., 2008; Suto et al., 2008; Vogelzang et al., 2008). Differentiation under these conditions resulted in a large population of IL-21 producing cells (Figures 1A, 1B and S1A), detected by intracellular staining with an IL-21R-Fc-chimera (IL-21R-Fc) (Suto et al., 2008). T cells deficient in STAT3, the major STAT activated by IL-6 and IL-21 (Wei et al., 2007) failed to produce IL-21, demonstrating a requirement for STAT3-activating cytokines, as well as the specificity of the IL-21R-Fc reagent (Figure S1B).
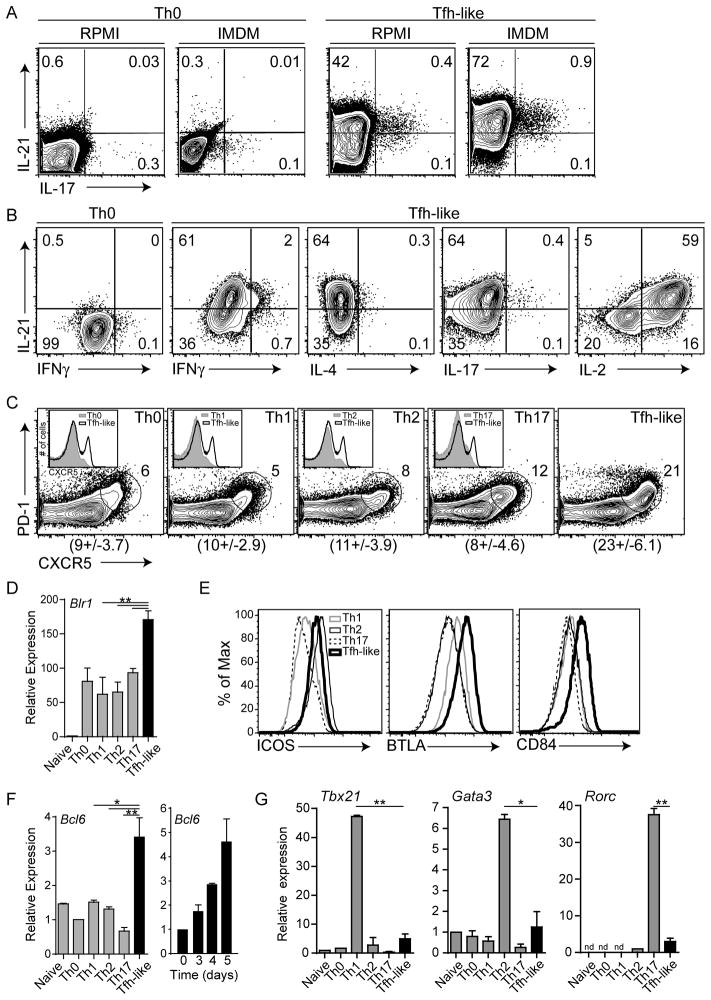
Figure 1. In vitro generated Tfh-like cells are distinct from other Th cells.
(A, B) Sorted naïve CD4+ T cells from WT mice were assessed for IL-21, IFNγ, IL-4, IL-17, and IL-2 cytokine production after 3.5 days in Tfh-like or Th0 culture conditions in RPMI (A) or IMDM media [(A) and (B)]. (C) In vitro differentiated Th1, Th2, Th17, and Tfh-like cells were gated on CD4+CD19−CD44+ cells and evaluated for surface CXCR5 and PD-1. B cells and T-B cell doublets were excluded by CD19 staining. Mean ± SD of %CXCR5+PD-1+ cells are shown below each plot. Inserts: CXCR5 expression on Tfh-like cells and respective Th cells (grey). Summaries of IL-21+ and CXCR5+PD-1+ cells are shown in Figures S1A and C. (D) Relative expression of Blr1, compared in WT naïve and in vitro differentiated Th0, Th1, Th2, Th17, and Tfh-like cells. Expression was calculated in reference to 18s rRNA, and normalized to naïve CD4+ T cells. (E) Surface expression of ICOS, BTLA, and CD84. Flow cytometry is representative of 5 or more independent experiments using 2–3 mice per genotype. (F–G) Relative expression of genes encoding Th cell-specific transcription factors Bcl6 (F) and Tbx21, Gata3 and Rorc (G) in Tfh-like and other Th cell populations. Expression was calculated in reference to 18s rRNA and normalized to Th0 cells (Bcl6), naïve CD4+ T cells (Tbx21 and Gata3) or Th2 cells (Rorc). Data in (D, F–G) are representative of at least 3 samples per group, with 2–3 mice per sample. ** = P < 0.005, * = P < 0.05, nd = not detectable.
In vitro differentiation of Th17 cells, another Th cell subset with high IL-21 expression, is enhanced by IMDM media, which is enriched with aryl hydrocarbons and amino-acid precursors (Veldhoen et al., 2009). Similarly, we found increased percentages of IL-21+ Tfh-like cells in IMDM media, compared to RPMI media (Figure 1A).
Further examination of in vitro Tfh-like cells revealed only low percentages of cells producing IFNγ, IL-4 or IL-17A (Figure 1B). Likewise, Tfh-like cells had abundant Il21 and low Ifng, Il4 and Il17a transcripts (Figure S1D). Interestingly, Tfh-like cells expressed more IL-2 than other Th cells (Figure 1B and data not shown), consistent with high IL-2 production in CXCR5hi human tonsillar T cells (Yu et al., 2009).
Tfh cells are distinguished by elevated expression of CXCR5, a chemokine receptor responsible for migration to B cell follicles (Schaerli et al., 2000), and PD-1, an inhibitory signaling molecule that regulates GC B cell survival (Good-Jacobson et al., 2010). These markers are induced upon T cell activation; however, highest expression is sustained on Tfh cells (Haynes et al., 2007). Although a distinct subpopulation of CXCR5+PD-1+ cells was seen in all in vitro differentiated cell populations, Tfh-like cells had the greatest percentage of CXCR5+PD-1+ cells (Figures 1C and S1C) with the highest CXCR5 intensity (Figure 1C inserts), and the greatest expression of Blr1, which encodes CXCR5 (Figure 1D). Tfh cells also express ICOS, BTLA, and the SLAM family member CD84 (Cannons et al., 2010; Chtanova et al., 2004; Nurieva et al., 2008). Whereas ICOS was induced on both Tfh-like and, as previously reported, Th2 cells (McAdam et al., 2000), BTLA and CD84 were distinctly elevated on Tfh-like cells (Figure 1E). Importantly, expression of CXCR5, PD-1, ICOS, BTLA and CD84 was comparable to that on CXCR5+PD-1+ Tfh cells directly isolated ex vivo (Figure S1E).
We further found that Tfh-like cells exhibited the greatest expression of Bcl6, which increased over 5 days in culture (Figure 1F). This expression was not secondary to B cell contamination, because similar results were seen with CD4+CD19− sorted cells. In contrast, expression of Tbx21, Gata3 and Rorc in Tfh-like cells was very low compared to Th1, Th2, and Th17 cells, respectively (Figure 1G). Thus, Tfh-like cells share phenotypic characteristics of Tfh cells, which differ from Th1, Th2, and Th17 subsets.
In vitro differentiated SAP-deficient Tfh-like cells resemble WT Tfh-like cells
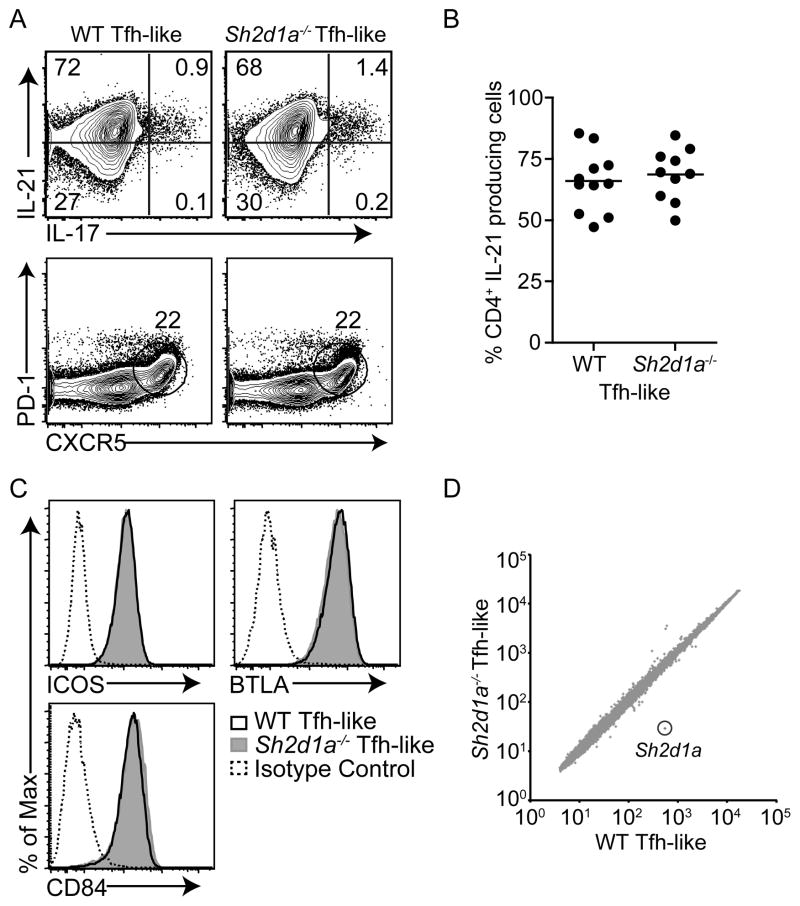
T cells deficient in SAP are unable to provide contact-dependent signals necessary for GC formation, despite expression of Tfh cell markers required for follicular localization and B cell help (Qi et al., 2008). However, SAP-deficient (Sh2d1a−/−) mice have also been reported to have lower numbers of Tfh cells and reduced IL-21 production from splenocytes (Cannons et al., 2010; Linterman et al., 2009). Under Tfh-like culture conditions, percentages of IL-21+ and CXCR5+PD-1+ populations of Sh2d1a−/− cells were similar to WT (Figures 2A and 2B), as was expression of ICOS, BTLA, and CD84 (Figure 2C). Moreover, global gene expression in Sh2d1a−/− Tfh-like cells closely resembled that of WT Tfh-like cells (WT vs. Sh2d1a−/−, R2 = 0.93051) (Figure 2D). Indeed, the gene most differentially expressed (19x) was Sh2d1a; only two other genes (Itsn and Rab4a) were identified with greater than a two-fold reduction in expression, in comparison to WT Tfh-like cells. Thus, in vitro differentiated Sh2d1a−/− Tfh-like cells were virtually indistinguishable from WT.
Figure 2. In vitro differentiated Sh2d1a−/− Tfh-like cells resemble WT Tfh-like cells.
(A–C) WT and Sh2d1a−/− sorted naïve CD4+ T cells cultured under Tfh-like conditions and evaluated for IL-21 and IL-17 production (A, top and B), surface CXCR5+PD-1+ (A, bottom), and ICOS, BTLA, and CD84 (C). (D) Microarray pairwise comparison of gene expression in WT and Sh2d1a−/− Tfh-like cells (from independent biological triplicates, using 2–3 mice per experiment).
In vitro differentiated WT, but not Sh2d1a−/− Tfh-like cells, induce GC formation
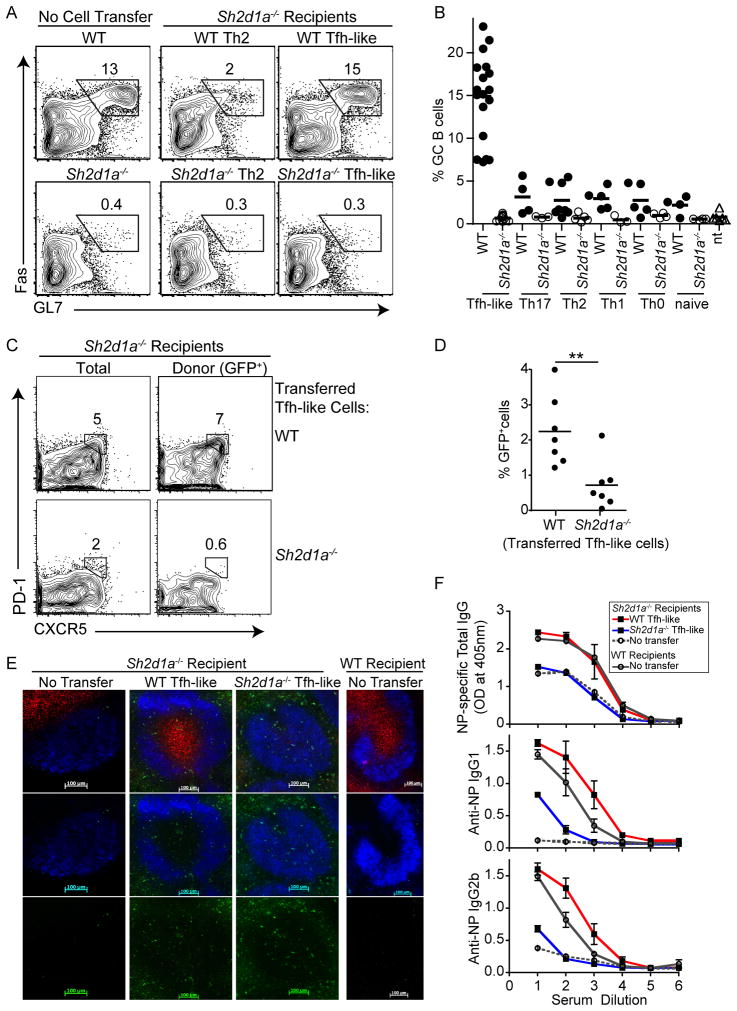
To evaluate the ability of in vitro differentiated Tfh-like cells to initiate and maintain GC responses in vivo, we adoptively transferred low numbers (4×104) of GFP+ OT-II in vitro differentiated cells into Sh2d1a−/− recipients, which lack GCs. Following immunization with NP-OVA, a specific T-dependent antigen, a subset of B cells downregulate IgD and express GC markers, including Fas and GL7. In the absence of transferred cells, very low percentages of B220+IgDloGL7hiFashi B cells were detected in the draining lymph nodes of Sh2d1a−/− hosts six days post-immunization (Figure 3A, left panels). However, transfer of WT Tfh-like cells resulted in robust generation of GCs in Sh2d1a−/− recipients, equivalent to that seen in immunized WT mice (Figures 3A upper right panel and 3B). Notably, WT Tfh-like cells improved GC formation significantly better than WT naïve, Th0, Th1, Th2 or Th17 cells (P<0.005, Figures 3A, 3B and S2A). Consistent with the role of CXCR5 in homing to B cell zones, sorted CXCR5+ Tfh-like cells were more efficient than CXCR5− cells, yet both could provide help for GC formation (data not shown). Similarly, WT transferred Tfh-like cells contributed to the CD4+CD44hiCXCR5+PD-1+ Tfh population in Sh2d1a−/− hosts (Figure 3C). Thus, in vitro differentiated Tfh-like cells contribute to functional Tfh cells to support GC formation in vivo and do so more effectively than other Th cell populations, including Th0, Th1, Th2, and Th17 cells.
Figure 3. WT Tfh-like cells promote GC response in Sh2d1a−/− mice.
GC B cells (B220+IgDloGL7hiFashi) in draining lymph nodes six days post-immunization with NP-OVA in Alum. (A) Left: WT or Sh2d1a−/− mice without cell transfers; middle and right: transfer of WT or Sh2d1a−/− in vitro generated Th2 or Tfh-like cells into Sh2d1a−/− hosts one day prior to immunization. (B) Summary of % GC B cells in Sh2d1a−/− mice after cell transfer and immunization. Immunized WT mice had an average of 14.35 +/− 0.65 SEM % GC B cells. Transferred cells: WT, filled circles; Sh2d1a−/, open circles; no cells transferred (nt), open triangles. P<0.005 for WT Tfh-like cell transfers compared to all other cell transfers. (C) Tfh cells (CXCR5+PD-1+) in Sh2d1a−/− mice after transfer of Tfh-like cells and immunization. Left: total Tfh cells (gated on CD4+CD44hi); right: transferred donor cells (gated on CD4+CD44hiGFP+). (D) Summary of % GFP+ cells, 6 days post-immunization in draining LNs of Sh2d1a−/− mice that received WT or Sh2d1a−/− Tfh-like cells. Data points represent one mouse, from three or more independent experiments. (E) Immunofluorescence of draining lymph node sections. Top Panel: GFP+ OT-II cells (GFP+, green); GC (PNA, red); follicle (IgD, blue), 6 days after immunization. Middle panel: IgD and GFP+ cells only. Lower panel: GFP staining alone for better visualization. (F) NP-specific antibody titers (total IgG, IgG1, IgG2b) measured by ELISA. Data in (A), (C) and (F) are representative of three or more independent experiments.
In contrast, Sh2d1a−/− Tfh-like cells failed to rescue GC responses (Figures 3A lower panels and 3B), despite having an almost identical phenotype to WT cells in vitro. Histological examination confirmed GC formation in Sh2d1a−/− recipients that received WT, but not Sh2d1a−/− Tfh-like cells. Transferred GFP+ WT Tfh-like cells were found within the GC structure, as well as in the B cell follicles and in the T cell zone along the follicle borders (Figure 3E). In contrast, although GFP+Sh2d1a−/− Tfh-like cells were found in B cell follicles, they failed to induce GCs. Evaluation by flow cytometry four and eight days after immunization revealed that Sh2d1a−/− Tfh-like cells mainly contributed to the CXCR5+PD-1+ population at early times post-immunization (Figure S2B and data not shown). Moreover, by day six after immunization, a greater percentage of WT Tfh-like GFP+CXCR5+PD-1+ cells were found in the draining lymph nodes than transferred Sh2d1a−/− cells (Figures 3C and D). Thus, Sh2d1a−/− Tfh-like cells are either not retained or expanded within the lymph node environment. Interestingly, other WT Th cell populations could contribute to Tfh cells when transferred in vivo, although with delayed efficiency, suggesting these cells acquired Tfh-like properties to promote GC formation (Figures S2B and data not shown).
Sh2d1a−/− mice also have reduced antigen-specific immunoglobulins after T-dependent immunization. Transfer of WT Tfh-like cells into Sh2d1a−/− recipients prior to immunization greatly enhanced NP-specific antibody titers, in some cases even above those in WT immunized mice (Figure 3F). Transfer of WT Th1 and Th2 cells also improved NP-specific total IgG, IgG1 and IgG2b antibody titers, albeit less well than Tfh-like cells and with different efficiency, depending on the isotype (Figure S2C, (Cannons et al., 2006)). In contrast, Sh2d1a−/− Tfh-like cells failed to rescue NP-specific IgG titers. Thus, although Sh2d1a−/− and WT Tfh-like cells are phenotypically indistinguishable, only WT Tfh-like cells functioned as Tfh cells in vivo to rescue GC responses in Sh2d1a−/− mice, and did so better than other CD4+ effector Th cells.
Tfh-like cells can acquire properties characteristic of other effector Th cells
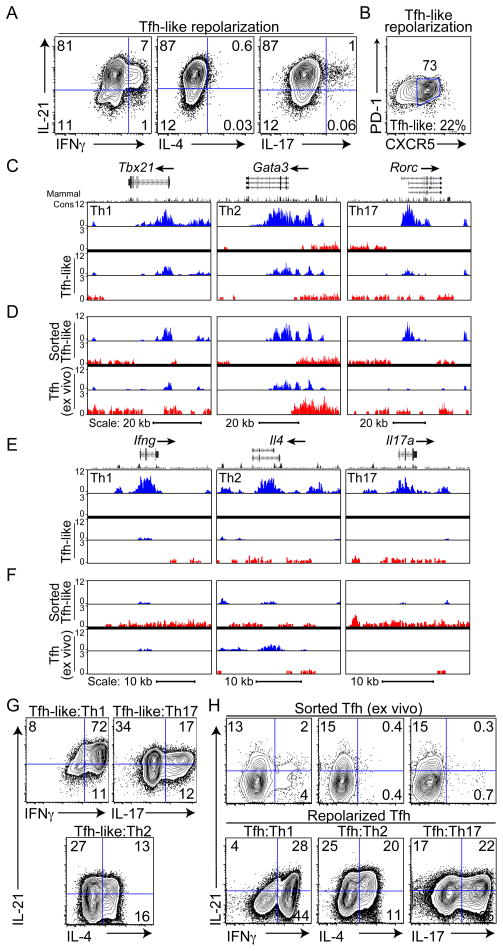
Several recent reports have shown overlapping cytokine patterns between Tfh cells and other Th cell lineages in response to specific parasitic infections, suggesting plasticity in cytokine production by Tfh cells. It has also recently been appreciated that plasticity between Th cell populations is associated with the presence of active epigenetic modifications of the genes encoding Th lineage-restricted transcription factors, even in lineages where these factors are not expressed (Wei et al., 2009). Thus, although the presence of positive chromatin modifications, such as lysine 4 trimethylation of histone H3 (H3K4me3) and negative modifications, such as H3K27me3, largely conform to the patterns of cytokine expression of distinct Th cells, the genes encoding the regulatory transcription factors exhibit positive (H3K4me3) chromatin modifications in multiple effector Th cell populations, suggesting they are poised for expression (Wei et al., 2009). To evaluate epigenetic modifications of the genes encoding the master regulatory transcription factors in Tfh-like cells, we first subjected CD4+ T cells to two rounds of polarization in Tfh-like conditions. Although sequential polarizations did not increase the percentage of IL-21+ cells (Figure 4A and data not shown), the majority of cells became CXCR5+PD-1+ (Figure 4B).
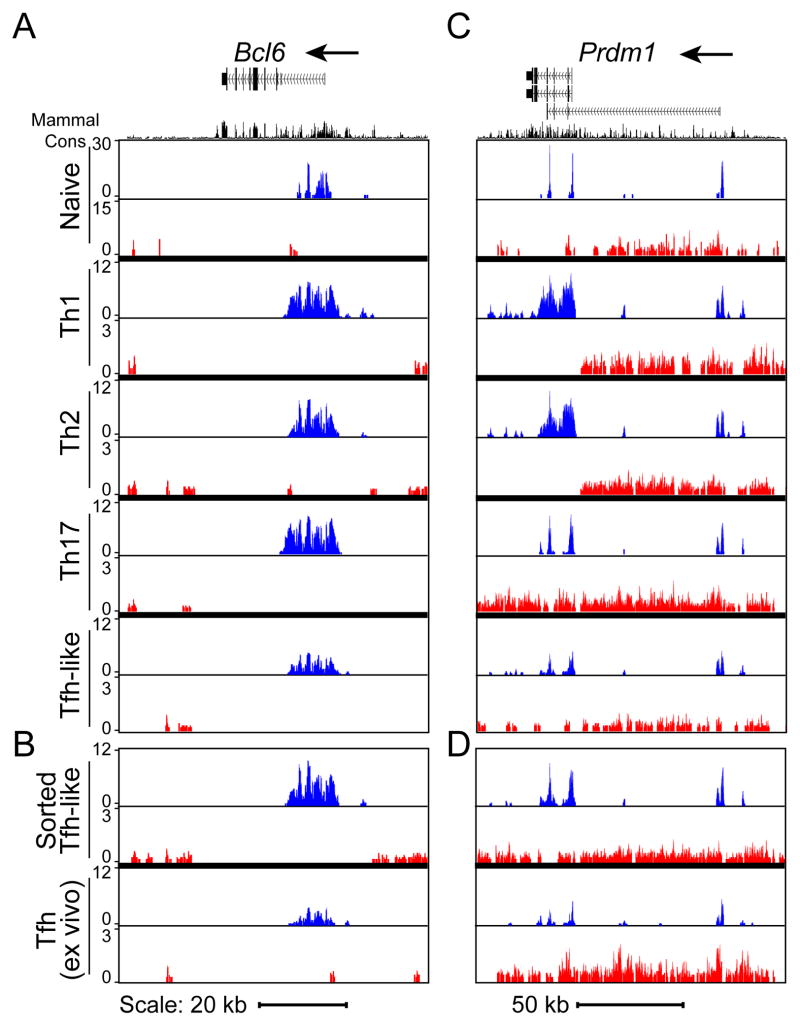
Figure 4. Epigenetic state and cytokine plasticity of Tfh-like cells.
Evaluation of cytokine production (A) and CXCR5+PD-1+ expression (B) after 2 rounds of Tfh-like polarization. Percentage of CXCR5+PD-1+ cells after one round of Tfh-like polarization is listed below (B). Data are representative of 3 or more independent experiments. (C–F) Genome browser view of ChIP-Seq profile maps. (C, D) Distributions of H3K4me3 (blue) and H3K27me3 (red) on genes encoding Th cell-determining transcription factors, Tbx21 (chr11:96,942,182–96,993,793), Gata3 (chr2:9,764,070–9,826,790), and Rorc (chr3:94,143,165–94,220,573) shown for (C) double-polarized in vitro generated Th cells and Tfh-like cells, and (D) sorted CD4+CD19−CD44hiCXCR5+PD-1+ in vitro differentiated Tfh-like cells and Tfh cells isolated ex vivo from SRBC-immunized mice. Biological triplicates of Th cell cultures with comparison to data from (Wei et al., 2009) are shown in Figure S3A. (E,F) Distributions of H3K4me3 (blue) and H3K27me3 (red) on Th cell-specific cytokine genes Ifng (chr10:117,864,373–117,896,679), Il4 (chr11:53,418,049–53,445,596), and Il17a (chr1:20,710,659-20,734,904) shown for (E) double-polarized in vitro generated Th cells and Tfh-like cells and (F) sorted CD4+CD19−CD44hiCXCR5+PD-1+ in vitro Tfh-like cells and ex vivo Tfh cells. Gene structure is from the UCSC Genome Browser. Where no signals were detected, corresponding columns are blank. Vertical scales are noted. (G) Cytokine production examined after repolarization of Tfh-like cells under Th1, Th17, and Th2 conditions. (H) Cytokine production of sorted Tfh cells after isolation ex vivo and rested one day in the presence of IL-2 (top) and after repolarization under Th1, Th2, and Th17 conditions (bottom). Data in (G) and (H) are representative of three or more independent experiments.
We then performed high-density sequence analyses of H3K4me3 and H3K27me3 chromatin immunoprecipitations (ChIP-Seq) on double-polarized Tfh-like cells. Although the vast majority of Tfh-like cells produced only IL-21, H3K4me3 histone modifications were observed on the genes encoding transcription factors considered master regulators of other effector CD4+ T cell populations, including Tbx21 (Th1), Gata3 (Th2), and Rorc (Th17) (Figures 4C, see S3A for comparison of independent biological triplicate samples). These findings are consistent with the ability of Tfh cells to express other effector cytokines depending on the in vivo challenge (King and Mohrs, 2009; Poholek et al., 2010; Reinhardt et al., 2009; Yusuf et al., 2010; Zaretsky et al., 2009).
One concern with such studies is that these findings result from heterogeneous cell populations. To start to address these issues, we sorted CD4+CD19−CD44hiCXCR5+PD-1+ cells from in vitro Tfh-like cultures, as well as from mice six days post-immunization with SRBC. At this time point, GCs are well formed in WT mice. In both CXCR5+PD-1+ sorted Tfh-like and Tfh cells isolated directly ex vivo, positive marks were detected on the effector Th cell-determining transcription factor loci Tbx21, Gata3 and Rorc (with the exception of weaker H3K4me3 marks on Rorc in ex vivo Tfh cells, Figure 4D), suggesting these genes were poised for activation.
Consistent with their low expression by in vitro Tfh-like cells, the genes encoding effector cytokines characteristic of other CD4+ effector T cells (Ifng, Il4 and Il17a) showed either low or no positive marks in the total Tfh-like population in comparison to Th1, Th2, and Th17 cells, respectively (Figure 4E). Even with further purification by cell sorting, positive marks were detected on Ifng, Il4 and Il17 in the CXCR5+PD-1+ population from in vitro-differentiated Tfh-like cells and on Ifng and Il4 in Tfh cells isolated directly ex vivo (Figure 4F), suggesting both populations had the potential for expression of these effector cytokines. Positive marks were detected in Tfh-like and Tfh cells on the Il21 and Il2 loci, consistent with their high expression (Figures S3B).
To determine whether these chromatin modifications correlated with potential for cytokine production, we repolarized pre-differentiated Tfh-like cells under Th1, Th2, or Th17 conditions. Cytokine analysis revealed that these repolarized Tfh-like cells acquired the ability to produce IFNγ, IL-4 or IL-17, respectively (Figure 4G), while still producing IL-21, suggesting that the acquisition of effector cytokine production could occur from the IL-21 producing population. Increased expression of the genes encoding Tbet, GATA3 and RORγt were also observed under appropriate conditions of repolarization (Figure S3C). To confirm these findings in Tfh cells isolated ex vivo, we sorted CD4+CD19−CD44hiCXCR5+PD-1+ Tfh cells from mice immunized with SRBCs. Cells were cultured and cytokine production examined either prior to or after restimulation under Th1, Th2, and Th17 polarizing conditions. The primary cytokine detected in ex vivo Tfh cells was IL-21; however, these cells could produce Th1, Th2 and Th17 cytokines upon repolarization (Figure 4H). Although we cannot rule out the effects of heterogeneity even in the sorted cell populations, these results suggest that both in vitro and early stage ex vivo-derived Tfh-like cells retain the ability to express cytokines of other effector T cells.
Bcl6 exhibits H3K4me3 modifications in multiple Th cell populations
To further evaluate the potential plasticity among effector Th cells, we compared epigenetic modifications of Bcl6 in naïve CD4+ cells to Tfh-like and other Th cell populations that had undergone two rounds of polarization. As expected, predominantly positive marks (H3K4me3) were detected in Tfh-like and ex vivo Tfh cells at the Bcl6 locus (Figures 5A, B). Surprisingly, Th1, Th2 and Th17 cells all displayed H3K4me3 markings at the Bcl6 locus (Figures 5A and S4A for independent biological triplicate samples), suggestive of the ability of these cells to acquire characteristics of Tfh cells.
Figure 5. Histone H3K4me3 and H3K27me3 modifications of Bcl6 and Prdm1.
H3K4me3 (blue) and H3K27me3 (red) modifications on (A, B) Bcl6 (chr16:23,941,577-24,012,259) and (C, D) Prdm1 (chr10:44,133,792-44,279,402) loci in (A, C) naïve CD4+ T cells, and double-polarized Th1, Th2, Th17, Tfh-like, and (B, D) CD4+CD19−CD44hiCXCR5+PD-1+ sorted Tfh-like cells and Tfh cells isolated ex vivo from SRBC-immunized mice. Biological triplicates for Th cell cultures are shown in Figure S4.
Expression of Bcl6 is antagonized by Blimp1, encoded by Prdm1, which is highly expressed in non-Tfh CD4+ T helper cell lineages (Crotty, 2011; Johnston et al., 2009). Both H3K4me3 and H3K27me3 modifications of Prdm1 were detected in all T helper cell populations, including the total (Figures 5C and S4B) and sorted CXCR5+PD-1+ Tfh-like cells, and, importantly, the ex vivo Tfh cells from immunized mice (Figure 5D). The presence of positive marks on Bcl6 and Prdm1 in multiple Th cell populations supports the potential for reprogramming between Tfh cells and other effector CD4+ T cells.
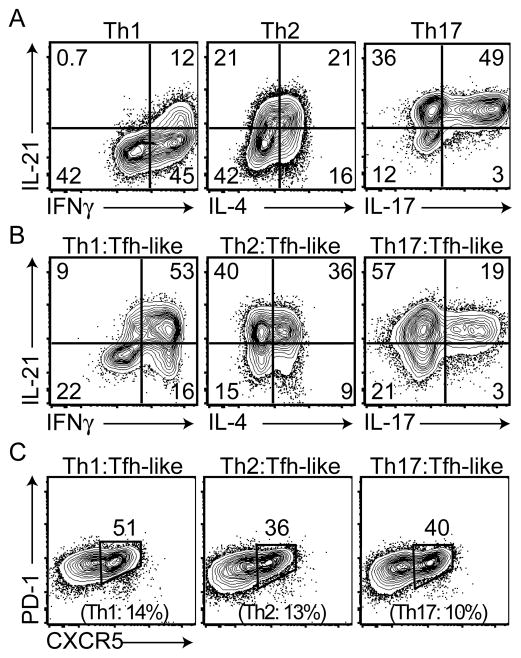
To further evaluate the relationship between Tfh-like cells and other effector cell populations, we examined cytokine expression by Th1, Th2 and Th17 cells (Figure 6A). As previously demonstrated, all three populations expressed Il21 transcripts (Figure S1D) (Spolski and Leonard, 2010). Surprisingly, high percentages of these cells produced IL-21, perhaps due to culture conditions in IMDM. Although the majority of our Th1 cells only produced IFNγ (Figure 6A), our Th2 cultures contained cells that only produced IL-4, along with populations that produced both IL-4 and IL-21, or only IL-21 (Figure 6A). Under Th17 conditions, the majority of the cytokine-producing cells produced both IL-17 and IL-21, along with populations that expressed only IL-17 or IL-21 (Figure 6A right panel). After expansion in IL-2, cells were repolarized under Tfh-like conditions and evaluated for cytokine production. Repolarized Th1 and Th2 cells had a greater capability to produce IL-21, in conjunction with maintaining production of their primary effector cytokines IFNγ or IL-4 (Figure 6B). In repolarized Th17 cells, an increase of the sole IL-21 producing population was observed. Notably, CXCR5+PD-1+ populations were enhanced in all the Tfh-like repolarized cell subsets (Figure 6C) and increased Bcl6 mRNA was observed in repolarized Th2 and Th17 cells (Figure S5). These results suggest that Th1, Th2, and Th17 cells retain the potential to express Tfh cell characteristics upon restimulation in the appropriate cytokine environment.
Figure 6. Repolarization of Th1, Th2, and Th17 cells in Tfh-like conditions.
(A) Cytokine production from polarized Th1, Th2, and Th17 cells. (B) Cells were rested, repolarized under Tfh-like conditions, and evaluated for cytokine production (B) and surface markers CXCR5 and PD-1 (C). The percentages of CXCR5+PD-1+ cells of the original polarized Th1, Th2, and Th17 cell populations are listed in parentheses. Data are representative of three or more independent experiments.
Discussion
Using defined in vitro conditions, we have examined the differentiation of naïve CD4+ T cells into cells with characteristics of Tfh cells, including the ability to provide efficient help for GC formation in vivo. These in vitro generated Tfh-like cells are notable for their high expression of BTLA, ICOS, CD84, IL-21 and IL-2, low expression of other effector cytokines, and the presence of a subpopulation with increased CXCR5 and PD-1, all of which are comparable to Tfh cells isolated ex vivo. Furthermore, in comparison to Th0, Th1, Th2, and Th17 populations, these cells exhibit greater expression of Bcl6.
With some exceptions, Tfh cells are found to reside within the GC as well as along the boundaries of the T-B cell zones, and may require prolonged B cell or other secondary APC interactions to acquire full effector cell function (Baumjohann et al., 2011; Cannons et al., 2010; Crotty, 2011; Deenick et al., 2010; Goenka et al., 2011; Qi et al., 2008). Although our Tfh-like cells are generated in vitro, the ability of these cells to potently induce GCs when transferred in vivo strongly argues that they contribute to functional Tfh cells. For this reason, we refer to them as “Tfh-like”, but alternatively they may be considered a “pre-Tfh” cell due to their resemblance to Tfh cells early in humoral responses, or a “Th21” cell, due to their cytokine expression being primarily limited to IL-21. Whether these in vitro differentiated cells represent bona fide Tfh cells, versus a Tfh cell precursor, may require further evaluation. However, these cells have permitted examination of questions about early Tfh differentiation and epigenetic modifications, which, importantly, we were able to confirm with primary Tfh cells isolated directly ex vivo from mice. Strikingly, both ex vivo and in vitro cells exhibit evidence for multi-potential cytokine production, accompanied by epigenetic modifications consistent with plasticity of expression of regulatory transcription factors.
We have used this system to probe the requirements for differentiation of Tfh-like populations. Similar to studies on IL-17a (Veldhoen et al., 2009), we found that IMDM also enhanced generation of IL-21 expressing cells, suggesting that IMDM components enhance production of multiple cytokines. We further found a requirement for STAT3 for in vitro production of IL-21 in these culture conditions. Although the requirements for IL-6 and IL-21 for Tfh cells and GC formation in vivo are controversial (Eddahri et al., 2009; Eto et al., 2011; Nurieva et al., 2008; Poholek et al., 2010; Vogelzang et al., 2008), blockade of IL-6 and IL-21 prevented IL-21 production in Tfh-like culture conditions (data not shown). Whether other cytokines or pathways may substitute in vivo or in vitro will require further examination. It is notable that patients expressing a dominant-negative mutation of STAT3 still develop GC responses, suggesting that there may be multiple pathways of Tfh differentiation, an idea that would be consistent with the concept of Th cell plasticity.
Because prolonged interactions with B cells or other APCs have been implicated in development of Tfh cells in vivo, we utilized mitomycin C-treated T-depleted splenocytes as APCs to preserve the antigen presenting function of B cells (Webb et al., 1985). Although in our hands isolated splenic CD11c+ DCs and T-depleted splenocytes induced similar amounts of IL-21 and CXCR5, T cells activated with T-depleted splenocytes induced twice the percentage of GC B cells (data not shown). Thus, although DCs can confer Tfh phenotypes upon CD4+ T cells, the presence of B cells and/or prolonged antigen stimulation may be important for Tfh differentiation and function.
Supporting this idea, SAP-deficiency did not intrinsically alter the initiation of a Tfh differentiation program, although it prevented Tfh-like cells from functioning in vivo, consistent with defects in T-B cell interactions (Cannons et al., 2010; Qi et al., 2008). These results were not due to reduced efficiency; increasing the number of transferred cells did not improve GC formation (data not shown). However, whether these observations result from an inability of Sh2d1a−/− T cells to undergo a late differentiation process is not clear. Indeed, although IL-21 (Spolski and Leonard, 2010), ICOS (Tafuri et al., 2001), CXCR5 (Ansel et al., 1999), and PD-1 (Agata et al., 1996) are induced by initial T cell activation, Tfh cells may specifically maintain or increase their expression, perhaps due to prolonged or secondary antigen encounter (Kerfoot et al., 2011; Kitano et al., 2011). Our findings are consistent with the concept that Tfh cell differentiation is a multistep process that requires secondary interactions after initial activation by DCs (Baumjohann et al., 2011; Choi et al., 2011; Deenick et al., 2010; Goenka et al., 2011).
Recent studies suggest that Tfh cells exhibit features overlapping with other effector Th cell populations (Spolski and Leonard, 2010). Consistent with this idea, we found that in vitro Tfh-like cells can be induced to produce other effector cytokines. Likewise, other effector CD4+ T cell populations, including Th1, Th2, and Th17, can be induced to express Tfh markers. Our results provide evidence for reprogramming capacity at least between early Tfh-like cells and other effector Th cell populations. This reciprocal plasticity in cytokine production does not appear to result solely from the presence of non-differentiated cells in the culture, since many of the Tfh-like cells produced both IL-21 and other effector cytokines upon exposure to secondary polarization. Notably, our findings were confirmed with sorted CXCR5+PD-1+ Tfh cells isolated directly ex vivo, which also could be induced to produce IFN-γ, IL-4, and IL-17 after exposure to polarizing cytokines.
This potential for plasticity is reinforced by evaluation of the chromatin landscape of the genes encoding the master regulators of Th cell differentiation. Although variability was observed in certain markings, Tfh-like cells consistently displayed active marks on the Tbx21, Gata3 and Rorc loci, even though the vast majority of these cells expressed IL-21 in the absence of other cytokines. Importantly, positive chromatin modifications on Tbx21, Gata3 and Rorc were also observed in sorted in vitro CXCR5+PD-1+ Tfh-like cells, as well as Tfh cells sorted directly ex vivo.
Conversely, in most non-Tfh-like cells, including naïve cells, Bcl6 and Prdm1 exhibited active chromatin marks. The universal presence of H3K4me3 marks on Bcl6 may reflect the ability of multiple Th cell populations (including naïve cells) to initially express markers of Tfh cells upon activation. Even though recent data demonstrate an early bifurcation of Tfh and non-Tfh cells (Choi et al., 2011), our data suggest these populations retain the potential to express both Bcl6 and Blimp1, and therefore maintain the ability to adopt Tfh or other Th properties in the proper context. Indeed, Bcl6 is co-expressed with and regulates other Th cell-specific transcription factors, suggesting it is the balance of transcription factors that determines cell phenotype (O’Shea and Paul, 2010; Oestreich et al., 2011; Zhou et al., 2009; Zhu et al., 2010). The presence of active chromatin modifications on genes encoding Th effector lineage-specific transcription factors suggest that Tfh-like cells have a global reciprocal reprogramming capacity with other effector Th cell populations. Although we cannot rule out these findings could reflect heterogeneity in our cultured cells or even in Tfh cells sorted from immunized mice at the early time points used in this study (six days post-immunization), our results suggest Tfh cells constitute a state of differentiation for CD4+ effector cells that can be associated with multiple cytokine-producing capacities.
Our findings therefore argue that CD4+ T cells can gain distinct cytokine expression profiles of given effector Th cell populations upon activation by DCs, yet still maintain the potential to adopt Tfh cell characteristics. This flexibility would permit CD4+ T cells to migrate to the B cell follicle for induction of GCs, even after expression of other cytokine producing fates, allowing them to orchestrate appropriate antibody class switching and production of specific immunoglobulin subclasses suited for a given infection. Conversely, cells that adopt Tfh characteristics, such in the context of prolonged or repeated stimulation, may still possess the potential to produce other effector cytokines, thereby allowing appropriate contouring of humoral responses. Our work, therefore, suggests flexibility in effector T cell responses that permit CD4+ T cells to effectively coordinate integrated immune responses to pathogens. Whether such plasticity can be exploited to better induce specific antibody isotypes may be important for development of optimal long-term humoral immunity in response to vaccines and other immune challenges.
Experimental Procedures
Mice
Sh2d1a−/− mice (Czar et al., 2001) were backcrossed to C57BL6/J for 10 generations. CD4-Cre Stat3fl/fl mice were described (Wei et al., 2007). C57BL/6, Ub-GFP expressing, and OVA323–339-specific TCR-transgenic OT-II mice were from Jackson Laboratory. Mice were maintained and used under specific-pathogen-free conditions in accordance of NIH institutional guidelines for animal welfare.
Cell Isolation and T helper cell differentiation
Mouse CD4+ T cells were purified from spleens and lymph nodes by negative selection (CD4+ T cell isolation kit, Miltenyi Biotec). Naïve (CD4+CD62L+CD44loCD25−) or Tfh (CD4+CD19−CD44hiCXCR5+PD-1+) cells from mice immunized with SRBC (Colorado Serum Company) (Cannons et al., 2006) were sorted on a FACSAria (>95% purity). Differentiation of Th cells was modified from previous protocols (Gomez-Rodriguez et al., 2009; Nurieva et al., 2008; Suto et al., 2008): T-depleted APCs were prepared by incubating splenocytes from C57BL/6 mice with anti-Thy-1 (BD Bioscience) at 4°C for 20 min, followed by rabbit complement (Cedarlane Laboratories) at 37°C for 45 min, and treatment with mitomycin-C (50 ug/ml, Sigma, 37°C for 45 min), followed by four washes. 2×105 naïve sorted OT-II cells were co-cultured with 1×106 APCs in 1ml/well of a 48-well plate, in the presence of 0.1 (Th0, Th2) or 1.0 (Th1, Th17, Tfh-like) μg/ml of OVA323–339 (AnaSpec) for 3–4 days in IMDM media (Invitrogen) under different skewing conditions: Th0: αIFNγ (XMG1.2), αIL-12 (C17.8), αIL-4 (11B11, 10 μg/ml); Th1: αIL-4 plus IL-12 (20ng/ml); Th2: αIFN-γ, αIL-12, plus IL-4 (40ng/ml); Th17: αIFNg, αIL-4, αIL-12, plus TGF-β (5 ng/ml, R&D), IL-6 (20 ng/ml); and Tfh-like: αIFNγ, αIL-4, αIL-12, αTGF-β (1D11, 20 μg/ml, antibodies from BioXCell), IL-6 (100 ng/ml), IL-21 (50 ng/ml, cytokines from Peprotech). After initial culture, Th cells were either analyzed or further expanded in IL-2 (50 U/ml) for 3 days, followed by a second round of differentiation for an additional 3 days.
Intracellular cytokine analysis and Flow Cytometry
Differentiated cells were restimulated with PMA (20ng/ml) plus ionomycin (1μg/ml, Sigma) at 37°C for 4–5h in the presence of GolgiStop (BD) for the last 2.5h. Cells were fixed and permeabilized with Cytofix/Cytoperm Solution (BD Bioscience). All subsequent washes and antibody dilutions were made with permeabilization buffer (eBioscience). Cells were incubated with IL-21 R/Fc chimera (R&D Systems) for 1h, washed and stained with PE-labeled affinity-purified F(ab′)2 goat anti-human Fcγ antibody (Jackson ImmunoResearch Laboratories) for 30 min (modified from (Suto et al., 2008)), washed twice and stained with antibodies to IL-4 (11B11), IFNγ (XMG1.2), IL-17A (17B7), and CD4 (RM4-5) (eBioscience) for 1h. CXCR5 staining used biotinylated anti-CXCR5 (2G8, BD Bioscience) for 1h on ice, followed by APC or PE-Cy7-labeled streptavidin (BD Bioscience). Secondary controls without primary antibodies confirmed staining specificity. Additional information provided in supplemental material.
Adoptive-Transfer, Immunizations, and ELISAs
4×104 in vitro differentiated GFP+OT-II T cells were retro-orbitally transferred into Sh2d1a−/− recipients one day prior to subcutaneous immunization in each hind limb with 50 μg of NP13-OVA (Biosearch Technologies) emulsified in Alum (Pierce). Draining lymph nodes were analysed on specified days post-immunization. ELISAs were performed as described (Cannons et al., 2006).
ChIP-Seq
ChIP-Seq experiments were performed essentially as described previously (Wei et al., 2009). In brief, T cells were treated with Micrococcal nuclease (MNase) to generate mononucleosomes. Chromatin from naïve and in vitro differentiated Th cells (1×107 cells) was immunoprecipitated with antibodies against histone H3K4me3 (Abcam ab8580, Milipore 04-745) or H3K27me3 (Upstate 07-449, Milipore CS200603). For sorted Tfh cells, cells were immediately treated with MNase and native fragmented chromatin was stored at −80C. Chromatin preparation from sorted samples (CXCR5+PD-1+ sorted Tfh-like, a total of 3×106 cells (H3K4me3) and 7×106 cells (H3K27me3); CXCR5hiPD-1hi ex vivo Tfh, 3×106 cells (H3K4me3), and 5×106 cells (H3K27me3)) were pooled together and simultaneously processed as described above. Immunoprecipitated DNA fragments were blunt-ended, ligated to the Illumina adaptors, and sequenced with the Illumina GAII sequencer.
Data Analyses (ChIP-Seq)
Illumina provided pipeline analysis was performed to obtain sequence reads of 25 bp and uniquely matching reads were mapped to the mouse genome (mm9) using the SICER peak calling program as described (Wei et al., 2009). Only uniquely matching reads were retained. Data from (Wei et al., 2009) were also reanalyzed using the mm9 version of the mouse genome for comparison and the output data was converted to browser-extensible data (BED) files in a window size of 200 bp as described previously (Wei et al., 2009), with or without (for H3K27me3) peak calling. Results were uploaded for viewing and comparative analyses in the UCSC genome browser.
Statistical Analyses
Statistical differences between analyzed groups were calculated with the paired Student’s t test in Prism (Graph Pad) and Excel (Microsoft). Values of P < 0.05 are considered significant.
Supplementary Material
Acknowledgments
We thank J. Gomez-Rodriguez, J. Zhu, and K. Zhao for helpful advice and J. Reilley and A. Venegas for technical assistance. This work was funded in part by the intramural research programs of the NHGRI and NIAMS, NIH. K.L. was supported by a PRAT Fellowship from the NIGMS, NIH.
Footnotes
Accession Numbers
Sequencing and gene expression data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo) under accession numbers x and y.
Supplemental Information includes five figures and additional experimental procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct Waves of BCL6 Expression during T Follicular Helper Cell Development. J Immunol. 2011;187:2089–2092. doi: 10.4049/jimmunol.1101393. [DOI] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011 doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinct transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci USA. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, Andris F. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113:2426–2433. doi: 10.1182/blood-2008-04-154682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N, Mark L, MacHeyzer-Williams LJ, MacHeyzer-Williams MG. Follilcular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenka R, Barnett LG, Silver JS, O’Neill PJ, Hunter CA, Cancro MP, Laufer TM. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol. 2011;187:1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, Schwartzberg PL. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31:587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Hron JD, Caplan L, Gerth AJ, Schwartzberg PL, Peng SL. SH2D1A regulates T-dependent humoral autoimmunity. J Exp Med. 2004;200:261–266. doi: 10.1084/jem.20040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamperschroer C, Roberts DM, Zhang Y, Weng N-p, Swain SL. SAP enables T cells to help B cells by a mechanism distinct from Th cell programming or CD40 ligand regulation. J Immunol. 2008;181:3994–4003. doi: 10.4049/jimmunol.181.6.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Ann Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang Y-h, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2 or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Huang AC, Weinmann AS. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J Exp Med. 2011;208:1001–1013. doi: 10.1084/jem.20102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. In Vivo Regulation of Bcl6 and T Follicular Helper Cell Development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlies germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Pottage L, Thomas ER, Leishman AJ, Doig TN, Xu D, Liew FY, Garside P. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J Immunol. 2000;165:3136–3144. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. IL-21 and T follicular helper cells. Int Immunol. 2010;22:7–12. doi: 10.1093/intimm/dxp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto A, Kashiwakuma D, Kagami S-i, Hirose K, Watanabe N, Yokotte K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, Nakajima H. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1376. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeman A, Boucher LM, Bouchard D, Chan VSF, Duncan G, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Webb SR, Li JH, Wilson DB, Sprent J. Capacity of small B cell-enriched populations to stimulate mixed lymphocyte reactions: marked differences between irradiated vs. mitomycin C-treated stimulators. Eur J Immunol. 1985;15:92–96. doi: 10.1002/eji.1830150118. [DOI] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal Center T Follicular Helper Cell IL-4 Production Is Dependent on Signaling Lymphocytic Activation Molecule Receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.