Abstract
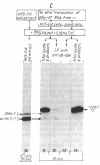
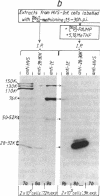
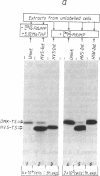
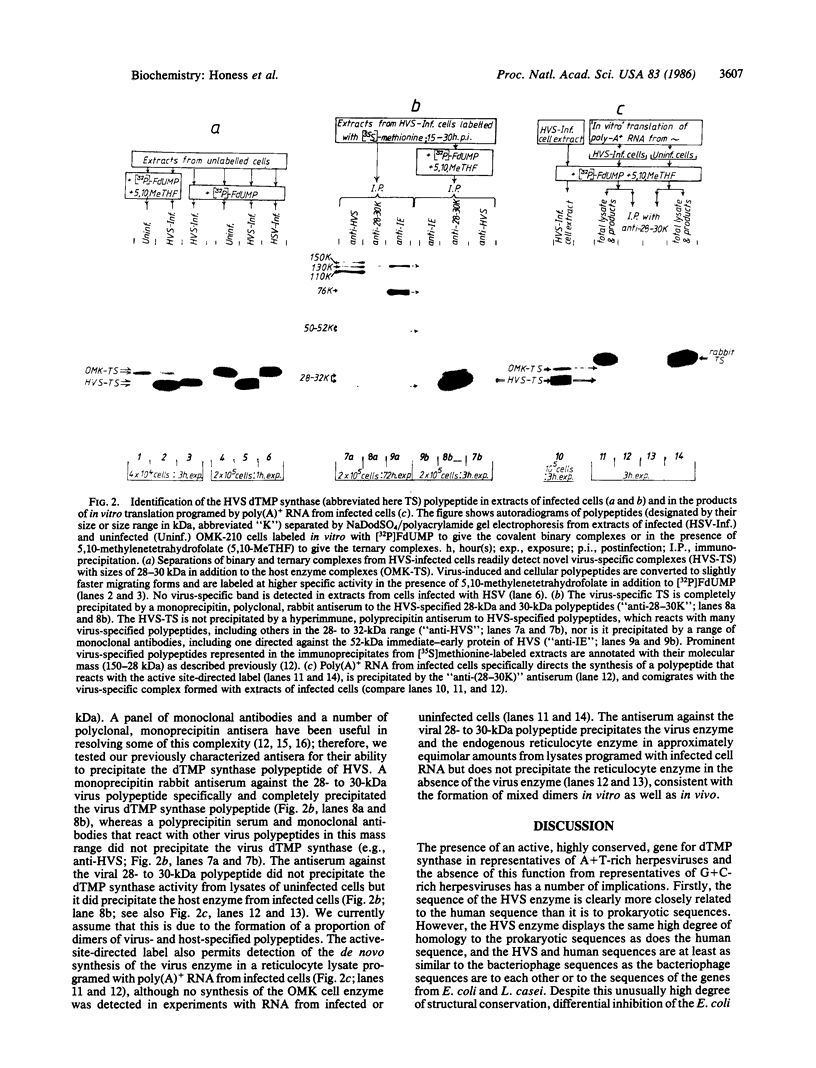
Herpesvirus saimiri (HVS) is the prototype member of a distinctive subset of lymphotropic herpesviruses (the gamma 2 subgroup) with A+T-rich coding sequences. In this paper, we show that cells productively infected with HVS contain high concentrations of a virus-specified thymidylate synthase (5,10-methylenetetrahydrofolate:dUMP C-methyltransferase, EC 2.1.1.45); we identify the active polypeptide and present the sequence of the virus gene. The predicted amino acid sequence of the 294-residue subunit of the virus enzyme is 70% homologous with the sequence of the human enzyme and about 50% homologous with prokaryotic thymidylate synthases, illustrating the remarkable structural constraints imposed by the thymidylate synthase function. However, the presence of the enzyme is not a conserved property of herpesviruses. We find no evidence for a virus-encoded thymidylate synthase activity (or a homology to a thymidylate synthase sequence) in G+C-rich representatives of alpha 1 (e.g., herpes simplex viruses, 66-68% G+C), beta (i.e., human cytomegalovirus, 58-59% G+C), and gamma 1 (i.e., Epstein-Barr virus, 60% G+C) herpesvirus subgroups. The production of excess thymidylate by a virus thymidylate synthase in cells infected with an A+T-rich herpesvirus would provide one plausible source of biased mutations by the virus-encoded replicative enzymes, which we have previously suggested as the likely general cause of differences in the mean nucleotide compositions of herpesvirus genomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayusawa D., Iwata K., Seno T., Koyama H. Conditional thymidine auxotrophic mutants of mouse FM3A cells due to thermosensitive thymidylate synthase and their prototrophic revertants. J Biol Chem. 1981 Dec 10;256(23):12005–12012. [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Belfort M., Maley G., Pedersen-Lane J., Maley F. Primary structure of the Escherichia coli thyA gene and its thymidylate synthase product. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4914–4918. doi: 10.1073/pnas.80.16.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemer W., Knust E., Angermüller S., Fleckenstein B. Immediate-early transcription of Herpesvirus saimiri. J Virol. 1984 Aug;51(2):452–457. doi: 10.1128/jvi.51.2.452-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou H. C., Weller S. K., Coen D. M. Mutations in the herpes simplex virus major DNA-binding protein gene leading to altered sensitivity to DNA polymerase inhibitors. Virology. 1985 Sep;145(2):213–226. doi: 10.1016/0042-6822(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., Maley F., Belfort M. Intervening sequence in the thymidylate synthase gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1984 May;81(10):3049–3053. doi: 10.1073/pnas.81.10.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J. DNA sequence of the US component of the varicella-zoster virus genome. EMBO J. 1983;2(12):2203–2209. doi: 10.1002/j.1460-2075.1983.tb01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W. Herpes simplex and 'the herpes complex': diverse observations and a unifying hypothesis. The eighth Fleming lecture. J Gen Virol. 1984 Dec;65(Pt 12):2077–2107. doi: 10.1099/0022-1317-65-12-2077. [DOI] [PubMed] [Google Scholar]
- Kenny E., Atkinson T., Hartley B. S. Nucleotide sequence of the thymidylate synthetase gene (thyP3) from the Bacillus subtilis phage phi 3T. Gene. 1985;34(2-3):335–342. doi: 10.1016/0378-1119(85)90142-8. [DOI] [PubMed] [Google Scholar]
- Knust E., Dietrich W., Fleckenstein B., Bodemer W. Virus-specific transcription in a Herpesvirus saimiri-transformed lymphoid tumor cell line. J Virol. 1983 Nov;48(2):377–383. doi: 10.1128/jvi.48.2.377-383.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Schirm S., Dietrich W., Bodemer W., Kolb E., Fleckenstein B. Cloning of Herpesvirus saimiri DNA fragments representing the entire L-region of the genome. Gene. 1983 Nov;25(2-3):281–289. doi: 10.1016/0378-1119(83)90232-9. [DOI] [PubMed] [Google Scholar]
- Maley G. F., Bellisario R. L., Guarino D. U., Maley F. The primary structure of Lactobacillus casei thymidylate synthetase. III. The use of 2-(2-nitrophenylsulfenyl)-3-methyl-3-bromoindolenine and limited tryptic peptides to establish the complete amino acid sequence of the enzyme. J Biol Chem. 1979 Feb 25;254(4):1301–1304. [PubMed] [Google Scholar]
- Maley G. F., Maley F., Baugh C. M. Differential inhibition of host and viral thymidylate synthetases by folylpolyglutamates. J Biol Chem. 1979 Aug 25;254(16):7485–7487. [PubMed] [Google Scholar]
- Maley G. F., Maley F., Baugh C. M. Studies on identifying the folylpolyglutamate binding sites of Lactobacillus casei thymidylate synthetase. Arch Biochem Biophys. 1982 Jul;216(2):551–558. doi: 10.1016/0003-9861(82)90244-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Ahmed F., Dunlap R. B. Isolation of the covalent binary complex of 5-fluorodeoxyuridylate and thymidylate synthetase by trichloroacetic acid precipitation. Biochem Biophys Res Commun. 1984 Oct 15;124(1):37–43. doi: 10.1016/0006-291x(84)90912-4. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R. E., Honess R. W., O'Hare P. Proteins specified by herpesvirus saimiri: identification and properties of virus-specific polypeptides in productively infected cells. J Gen Virol. 1983 Jan;64(Pt 1):19–35. doi: 10.1099/0022-1317-64-1-19. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Newman C., Honess R. W. A single major immediate-early virus gene product is synthesized in cells productively infected with herpesvirus saimiri. J Gen Virol. 1984 Jul;65(Pt 7):1215–1219. doi: 10.1099/0022-1317-65-7-1215. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Newman C., Honess R. W. Isolation and characterization of monoclonal antibodies to structural and nonstructural herpesvirus saimiri proteins. J Virol. 1984 Dec;52(3):872–883. doi: 10.1128/jvi.52.3.872-883.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S., Perriard E. R. A filter assay for thymidylate synthetase using 5-fluoro-2'-deoxyuridylate as an active site titrant. Biochemistry. 1974 Jan 29;13(3):467–470. doi: 10.1021/bi00700a011. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi K., Kaneda S., Ayusawa D., Shimizu K., Gotoh O., Seno T. Nucleotide sequence of a functional cDNA for human thymidylate synthase. Nucleic Acids Res. 1985 Mar 25;13(6):2035–2043. doi: 10.1093/nar/13.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. J., Banks L. M., Purifoy D. J., Powell K. L. Interactions between herpes simplex virus DNA-binding proteins. J Gen Virol. 1984 Nov;65(Pt 11):2033–2041. doi: 10.1099/0022-1317-65-11-2033. [DOI] [PubMed] [Google Scholar]
- veer Reddy G. P., Pardee A. B. Inhibitor evidence for allosteric interaction in the replitase multienzyme complex. Nature. 1983 Jul 7;304(5921):86–88. doi: 10.1038/304086a0. [DOI] [PubMed] [Google Scholar]