Abstract
The cognitive control of attention involves maintaining task rules in working memory (or “online”), monitoring reward and error rates, filtering distractors, and suppressing prepotent and competitive responses. Weak attentional control increases distractibility and causes attentional lapses, impulsivity and attentional fatigue. Levels of tonic cholinergic activity (changes over tens of seconds or minutes) modulate cortical circuitry as a function of the demands on cognitive control. Increased cholinergic modulation enhances the representation of cues, by augmenting cue-evoked activity in thalamic glutamatergic afferents, thereby increasing the rate of detection. Such cholinergic modulation is mediated primarily via α4β2* nicotinic acetylcholine receptors. Animal experiments and clinical trials in adult patients with ADHD indicate that attentional symptoms and disorders may benefit from drugs that stimulate this receptor. Tonic cholinergic modulation of cue-evoked glutamatergic transients in prefrontal regions is an essential component of the brain’s executive circuitry. This circuitry model guides the development of treatments of deficits in attentional control.
Keywords: Attention, Top-down Control, Cognitive Impulsivity, Acetylcholine, Glutamate, Nicotine, Dopamine
Control of attention in health and disease
Picture yourself working as an air traffic controller. Your task is to follow several aircraft represented as blinks on your screen, ensuring separation, altitude, speed, direction, and so forth. At the same time you are constantly checking for new planes entering your sector, and perhaps you are also receiving information about the changing status in adjacent sectors and watch an incoming storm front. These tasks require an enormous capacity for sustaining and shifting attention, over relatively long periods of time, and the management of attentional resources across competing sub-tasks.
Which are the mechanisms that suppress the urge to disengage from this task at regular intervals, to dream about your upcoming vacation, or simply to take a break from watching an array of monitors and instead look out of the window? Which are the mechanisms that allow you to filter intruding thoughts about pleasurable activities or the troubles in your family? In short, what controls sustaining attention to the task and minimizes distractibility?
Attentional control mechanisms (Figure 1) are severely impaired in patients with neuropsychiatric and neurodegenerative disorders, including schizophrenia and ADHD. Beginning with Kreapelin’s (1912) rather modern, dynamic conceptualization of the long-term consequences of the increased distractibility of schizophrenic patients for their conception of the world, and followed by the description and classification of patients’ self-reports by McGhie and Chapman (1961) (“…I jump from one thing to another…it’s difficult to concentrate on any one sound…”), contemporary research has continued to classify the limited attentional resources and poor attentional control as hallmarks of, and perhaps essential contributors to, the cognitive impairments of these patients (e.g., Elvevag, Weinberger, Suter, & Goldberg, 2000; Lesh, Niendam, Minzenberg, & Carter, 2011; Luck, Ford, Sarter, & Lustig, 2011; Luck & Gold, 2008; Nuechterlein, Luck, Lustig, & Sarter, 2009; Silver & Feldman, 2005). Moreover, theories have continued to postulate that the emergence of positive symptoms is causally related to attentional control deficits. Such deficits allow irrelevant items to become attentionally bound and therefore signifiant components of the perception of a scene (e.g., Collerton, Perry, & McKeith, 2005). Therefore, such items gain control of behavioral and cognitive processes and further impair the efficacy if attentional control. The more these interacting processes escalate “…the less coherent and uniform is the conception of the external world” (Kraepelin, 1912; p. 22).
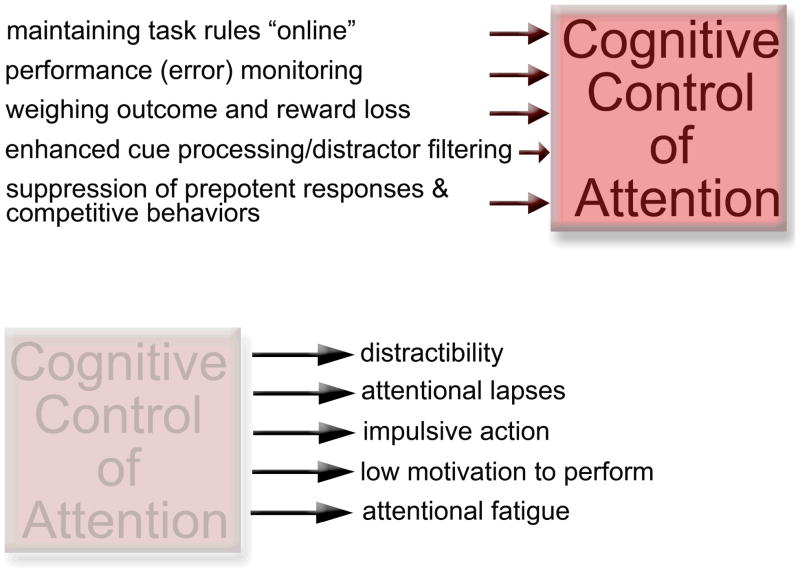
Figure 1.
Synopsis of the mental operations that constitute the construct “cognitive control of attention” (top) and of the consequences of weakened top-down control (symbolized by the transparent box) for attentional performance (bottom).
Deficits in “top-down” or “executive” control have also been conceptualized as an overarching cause of the attentional impairments and the impulsivity of patients with ADHD (e.g., Corkum & Siegel, 1993; Gorenstein, Mammato, & Sandy, 1989). Distractibility, lapses and fluctuations of attentional performance may not represent secondary consequences of primary impairments in response inhibition and impulsivity (e.g., Oosterlaan & Sergeant, 1998). Rather, inattention and impulsivity in ADHD are hypothesized to reflect a deficient executive, prefrontal control system (Castellanos, Sonuga-Barke, Milham, & Tannock, 2006; see Fig. 1).
Motivational deficits are also present in patients with ADHD or schizophrenia. The relationships between motivational and attentional symptoms are poorly understood (e.g., Cubillo, Halari, Smith, Taylor, & Rubia, 2011; Douglas & Parry, 1994; Gorissen, Sanz, & Schmand, 2005). Abnormalities in the perception and processing of rewards, including a preference for immediate reward (Luman, Oosterlaan, & Sergeant, 2005; Sonuga-Barke, 2002; Volkow, et al., 2010) may contribute to, but also result from, poor cognitive control mechanisms (Haenlein & Caul, 1987).
Consequences of poor attentional control: distractibility and lapses, impulsivity, low motivation to perform and attentional fatigue
“Top-down”, “executive” or “cognitive” control of attention concerns a set of broadly defined mental mechanisms which act collectively to sustain attentional performance, particularly in response to challenges such as distractors. These mechanisms include; (1) maintaining task-rules, including switching rules, and behavioral goals in working memory (or “online”); (2) performance monitoring, specifically error monitoring; (3) weighing reward and reward loss against levels of motivation; (4) recruiting mechanisms, such as enhanced cue processing and distractor filtering, to combat performance decline; and (5) suppression of prepotent responses and competitive behaviors (Figure 1; e.g., Baluch & Itti, 2010; E. K. Miller & Cohen, 2001; Pessoa, Rossi, Japee, Desimone, & Ungerleider, 2009; Rossi, Pessoa, Desimone, & Ungerleider, 2009; M. Sarter, Gehring, & Kozak, 2006; Woods & Sarter, 2010). As will be described next, a weakening of these mechanisms are hypothesized to cause a wide spectrum of impairments in attention.
Distractibility and lapses
Distractibility is a cognitive construct that describes the increased probability for erroneous detection of (irrelevant) stimuli. Posner and colleagues (Posner, Snyder, & Davidson, 1980) defined detection as “…the entry of information concerning the presence of a signal into a system that allows the subject to report the existence of the signal by an arbitrary response indicated by the experiment”. Erroneous detections can be triggered by salient stimuli or stimuli with unique features (often called “feature singletons”). Furthermore, weak top-down control of attention to the search field, object group or scene is a fundamental prerequisite for committing false detections (e.g., Theeuwes & Godijn, 2002).
Neurophysiological studies demonstrated that erroneous detection begins with the representation of a non-target cue in primary sensory regions (e.g., Roelfsema & Spekreijse, 2001). As will be discussed further below, such representation may not be sufficient for (false) detection. Likewise, weak signals may evoke an orienting response but they are not necessarily detected. The efficacy with which cues are amplified and distractors are filtered indicate the strength of attentional control processes. Cue amplification and distractor filtering are less effective if the demands on cognitive control are already high and if there is competition among stimuli (e.g., Lavie, 2005). fMRI studies have generated evidence in support of both cue amplification and distractor filtering as main mechanisms activated to combat distractibility (Polk, Drake, Jonides, Smith, & Smith, 2008). In the presence of explicitly defined distractors, as is the case with most laboratory tasks, evidence seems to favor neuronal mechanisms acting to enhance the processing of target cues (Nieuwenhuis & Yeung, 2005; Weissman, Warner, & Woldorff, 2004).
There are conceptual overlaps between the nature of, and the attentional mechanisms underlying, attentional lapses and increases in distractibility. Lapses may involve erroneous detection but more typically refer to failures to detect and errors of action, such as erroneous repeats of responses, unintended responses, and omissions that reflect detection failures (as opposed to loss of motivation; e.g., Reason, 1984). The primary source for such lapses are failures to keep the task-rules “online”, to stay-on-task (see Manly, Robertson, Galloway, & Hawkins, 1999), to resist attentional distraction (e.g., Leber, 2010), and to suppress competitive behaviors, the latter leading to unintended responses. Imaging (Eichele, et al., 2008; Weissman, Roberts, Visscher, & Woldorff, 2006) and neurophysiological (O’Connell, et al., 2009) studies in humans, as well as our real-time recordings of prefrontal cholinergic activity in rats (Parikh, Kozak, Martinez, & Sarter, 2007) demonstrated that such lapses can be predicted as early as 20–30 sec prior to their occurrence, by measuring neuronal markers indicating insufficient suppression of task-irrelevant neuronal activity and decreases in task-relevant brain activity.
Impulsivity
In the specific context of attentional task performance, impulsivity first concerns the frequent and compulsive disengagement from task performance (often termed, motor impulsivity). This form of impulsivity involves readily activated sets of complex yet fixed action patterns, such as grooming, exploration, locomotion, climbing, and fidgeting, all of which interfere with task performance. Deficits in the processing of time (Castellanos, et al., 2006) may foster the frequent execution of these motor programs.
Second, impulsivity concerns the failure to cancel or inhibit a specific behavioral response and, depending on task demands, to inhibit one response to execute a different, correct response. This more specific form of impulsivity often involves attentional reorientation and repositioning that is not controlled by task stimuli (impulsive action; Winstanley, Eagle, & Robbins, 2006; Winstanley, Olausson, Taylor, & Jentsch, 2010). A range of cognitive mechanisms may increase the propensity for such impulsive responses, including a weak “online” representation of the task rules. Both forms of impulsivity can be conceptualized as consequences of cognitive control deficits (e.g., Nigg, 2003). Weak cognitive control diminishes the capacity to suppress prepotent responding and competitive behaviors, and to sustain attention to the source of target cues and maintaining task-rules in working memory.
It is important to note that although symptoms of impulsivity and hyperactivity often are lumped together to describe the hyperkinetic component of ADHD, the performance of patients in structured tasks is not necessarily contaminated by impulsive and hyperkinetic responsivity. Rather, response latencies and frequencies of ADHD patients may remain below those of control subjects (Carte, Nigg, & Hinshaw, 1996; Casey, et al., 1997) and thus more generally reflect poor cognitive control (see also Oosterlaan & Sergeant, 1998; Suskauer, et al., 2008). Similar to the demonstration of hyperactivity in animal models, requiring the absence of structured and behavior-constraining demands on performance (see, e.g., the absence of hyperkinetic symptoms in rats treated with an escalating dosing regimen of amphetamine and performing an attention task; Kozak, et al., 2007; Martinez, Parikh, & Sarter, 2005), it appears that demonstration of the hyperkinetic symptoms in patients is fostered by the absence of cognitive demands and controlled task performance.
Low motivation for attention/attentional fatigue
The bidirectional, intricate relationships between motivational processes and levels of attentional performance (e.g., Engelmann & Pessoa, 2007; Mischel & Ebbesen, 1970; Savine & Braver, 2010; Small, et al., 2005; St. Peters, Demeter, Lustig, Bruno, & Sarter, 2011) challenge the determination of individual mechanisms responsible for controlling performance changes (see also Frith, 2001). Indeed, attentional control and reward mechanisms have been suspected to remain conceptually, experimentally, and neuronally inseparable (Gottlieb & Balan, 2010; Maunsell, 2004). For example, a decrease in the value of a reward weakens the impact of unrewarded misses or false alarms and thus may evoke an only limited recruitment of processes for recovering response accuracy, yielding further performance decline. Importantly, however, reward perception is not merely a function of the subject’s degree of motivational saturation but is also influenced top-down. For example, prolonged sustained attention is associated with increasing demands on top-down control to suppress switching to alternative or competitive behaviors; such increases in “task difficulty” result in a discounting of the value of trial-based reward (“effort discounting”; Botvinick, Huffstetler, & McGuire, 2009; Croxson, Walton, O’Reilly, Behrens, & Rushworth, 2009). Thus, poor or exhausted attentional control mechanisms and associated effort discounting interact to further impair attentional performance, eventually generating performance levels that correspond with those expected from subjects with generally low levels of motivation (e.g., Sykes, Douglas, & Morgenstern, 1973). Omitting a majority of trials or even disengaging from task may rarely indicate motivational saturation, specifically in experiments with humans who typically perform for negligible rewards. Rather, low motivation to perform in attention tasks is a consequence of weakened attentional control and thus may be better termed “attentional fatigue” (e.g., Lim, et al., 2010). Challenges that tax top-down control mechanisms, such as the presentation of distractors, are particularly effective in evoking periods of attentional fatigue (e.g., Demeter, Sarter, & Lustig, 2008).
Tonic and phasic cholinergic mediation of attention
Below we will review the evidence indicating that the tonic component of cortical cholinergic neurotransmission modulates cortical circuitry and the efficacy of attention as a function of the demands on top-down control. The circuitry model describing the regulation and function of the cortical, specifically prefrontal cholinergic input system postulates that a branch of the cholinergic system is tonically active (changes over tens of seconds to several minutes) and modulates primarily, but likely not exclusively, the glutamatergic terminals of afferent arising from the mediodorsal thalamic nucleus (Hasselmo & Sarter, 2011). As will be further detailed below, this cholinergic activity modulates the prefrontal representation of cue salience and thereby, necessarily but not sufficiently, cue detection and attentional performance.
Cholinergic activity modulates glutamate released from thalamic inputs primarily by stimulating α4β2* nicotinic acetylcholine receptors (nAChRs) that are expressed at the terminals of these thalamic afferents (Howe, et al., 2010; Lambe, Picciotto, & Aghajanian, 2003; Parikh, Ji, Decker, & Sarter, 2010; Parikh, Man, Decker, & Sarter, 2008; M. Sarter, Parikh, & Howe, 2009). Separate from this tonic component of the cortical cholinergic input system, local cortical circuitry, including glutamate release events from thalamic afferents, generates a brief cholinergic release event (“transient”; scale of seconds; Figure 2). In attentional task-performing animals, this cholinergic transient is necessary for a cue to be detected or, in other words, for the animal to score a hit (Parikh, et al., 2007; Parikh & Sarter, 2008). The probability for such cholinergic transients to occur is modulated indirectly by the tonic component of cholinergic neurotransmission and its effects on cue-evoked glutamate release. Additional neuronal mechanisms, including GABAergic interneurons, also influence the generation of cholinergic transients (e.g., Berry, et al., 2011).
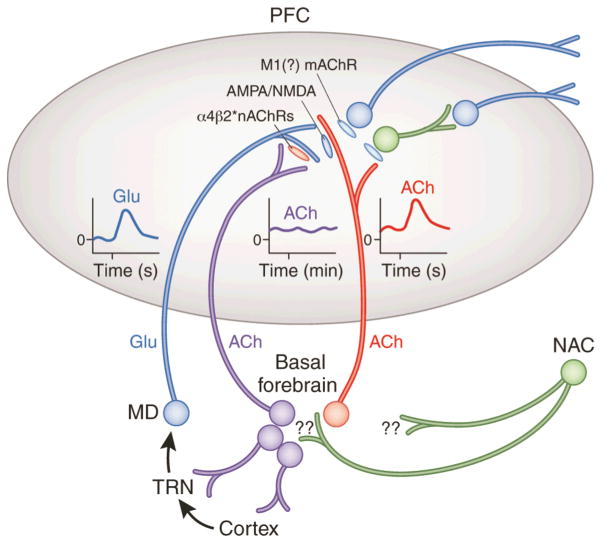
Figure 2.
Circuitry model describing the main components of the prefrontal cortex (PFC) circuitry mediating signal detection and processing mode shifts. The model combines evidence with parsimonious assumptions required to explain electrochemical and attentional performance data (see main text for details). The glutamatergic (GLU) inputs to the PFC, originating from the mediodorsal thalamic nucleus (MD) “import” a preattentionally processed representation of the signal into the PFC (see text for definition). MD neurons are part of a network that includes the thalamic reticular nucleus (TRN) and its topographic afferents from sensory cortical regions. The cue-evoked glutamatergic transient (see insert) generates a cholinergic transient (see insert), via stimulation of ionotropic presynaptic glutamate receptors (Parikh, et al., 2010; Parikh, et al., 2008). This cholinergic transient mediates the actual detection process or, depending on the task, a processing mode shift that fosters detection (see main text). Prefrontal output neuron activity is stimulated by ACh primarily via muscarinic (m)AChRs, thereby organizing the behavioral responses that indicate successful detection.
The terminals of the MD inputs to the PFC are equipped with α4β2* nAChRs. Cholinergic stimulation of these receptors is thought to vary over minutes, refecting a tonic component of cholinergic neurotransmission (see elevated release illustrated by the insert). nAChR agonists enhance detection performance primarily by positively modulating GLU release from these terminals, thereby augmenting the amplitudes of the cholinergic transients (Howe, et al., 2010; Parikh, et al., 2010). This model therefore proposes two separate roles for cholinergic inputs, mediated via separate populations of cholinergic neurons. A rather tonically active cholinergic input modulates glutamate release from MD neurons which, in turn, target the terminals of a separate group of cholinergic neurons, generating the transients that enhance attentional orienting and cue detection. Reproduced, with permission of Nature Publishing Group, from Hasselmo and Sarter (2011; p. 58).
Measuring tonic cholinergic activity versus cholinergic transients
A methodological corollary of this scheme concerns the methods used to measure and to differentiate between tonic cholinergic neurotransmission and cholinergic transients (microdialysis versus enzyme-coated microelectrodes/amperometry). Of particular importance in this context is evidence indicating that measures of tonic levels of cholinergic activity, measured by using microdialysis and collection periods over several minutes, do not merely represent integrated transients as measured by amperometry. First, manipulations that reduce the probability of transients in animals performing a sustained attention task (SAT; described below), such as distractor presentation, elevate tonic levels of cholinergic neurotransmission (below). Second, drugs that enhance cue detection and thus produce more cholinergic transients at the same time reduce levels of tonic cholinergic activity (Paolone, Howe, Gopalakrishnan, Decker, & Sarter, 2010). Both examples indicate opposite effects on tonic cholinergic activity and the number of cholinergic transients, rejecting the hypothesis that dialysates collected over minutes merely indicate the integration of second-based transients. The reasons why microdialysis appears to be optimized for detecting tonic levels of acetylcholine (ACh) that are not contaminated by transients are not fully understood but may be due in part to the formation of a glial-derived diffusion barrier that encapsulated the probe (Jaquins-Gerstl & Michael, 2009).
Attentional performance-associated tonic cholinergic activity and the nature of cholinergic top-down control
Augmented increases in tonic cholinergic activity
The evidence reviewed in this section has largely been obtained from experiments in which ACh release was measured while animals performed a sustained attention task (SAT), including a version during which a distractor (dSAT) was presented during several blocks of trials. Briefly, this task consists of a random sequence of unpredictable signal events or blanks following which two (species-specific) manipulanda become available for a restricted time. The subject reports the prior presence or absence of a signal by operating one or the other manipulanda (retractable levers in rats, keys in humans, retractable nose ports (“MICARPS”; St Peters, Cherian, Bradshaw, & Sarter, 2011) in mice). Rewarded responses are hits and correct rejections, respectively, while misses and false alarms are not rewarded and trigger a variable intertrial interval. Task versions have been developed and validated for testing mice, rats and humans (for details see Demeter, Hernandez-Garcia, Sarter, & Lustig, 2011; Demeter, et al., 2008; McGaughy & Sarter, 1995; Nuechterlein, et al., 2009; St Peters, et al., 2011).
Early studies on the effects of selective removal of the cortical cholinergic input system demonstrated that such lesions resulted in the robust, permanent and selective impairments in the detection of signals, while sparing response accuracy in non-signal trials (McGaughy, Kaiser, & Sarter, 1996; McGaughy & Sarter, 1998). Although this evidence established the necessity of the cortical cholinergic input system for cue detection, such lesions disrupt all modes of cholinergic neurotransmission and thus effects cannot be attributed solely to the absence of tonic cholinergic activity.
SAT performance has been frequently demonstrated to increase levels of ACh release, in prefrontal, anterior and posterior parietal regions. Performance of various procedures controlling for effects of lever pressing rate, reward rate, and sensory effects of stimuli consistently indicated that such increases in release are not reproduced in the absence of demands on sustained attention (Arnold, Burk, Hodgson, Sarter, & Bruno, 2002; Himmelheber, Sarter, & Bruno, 1997, 2000, 2001; Kozak, Bruno, & Sarter, 2006; Kozak, et al., 2007). However, while SAT performance reliably increases ACh release by 100–140% (over basal levels), release levels were not found to correlate with measures of performance. In the past, the considerable variability of measures of ACh release in performing animals was speculated to prevent the demonstration of such relationships. This view was rejected by more recent studies that attributed a different cognitive function to levels of ACh release associated with attentional performance.
In 2006, Kozak and colleagues observed that in animals performing the SAT, blockade of NMDA receptors in the basal forebrain resulted in impairments in detection performance that dose-dependently stabilized and recovered towards the end of the session (and about 30 min after infusions). During this late period, performance-associated increases in ACh release were found to be augmented over regular SAT performance-associated increases in ACh release, reaching over 200% over baseline in the last collection interval (Kozak, et al., 2006). This finding formed the basis for the hypothesis that the degree of cognitive control of attention taxed by task conditions, not levels of performance, are correlated with levels of ACh release (M. Sarter, et al., 2006). In other words, the greater the demands for cognitive control mechanisms the greater the tonic cholinergic modulation of cortical circuitry.
This hypothesis was tested in a recent study in which SAT performing rats were exposed to a distractor in the 2nd and 3rd out of a total of 5 blocks of 8-min trials. Performance during the distractor (houselights falling on-off at 0.5 Hz) period was impaired and, depending on the individual measures of performance, plateaued or began to recover during the second distractor period. ACh release was highest during the two distractor periods, peaking at about 180% over baseline (and compared with 90–100% over baseline and in the absence of the distractor). Most importantly, release levels were significantly correlated with performance, with less severe distractor effects on performance associated with higher release levels (St. Peters, et al., 2011). Using a measure that collapses signal and non-signal trial performance into one score (SAT score, ranging from zero {random response selection} to 1 {perfect performance}), better dSAT performance by one unit of the SAT score (0.1) was associated with an additional 38% increase in ACh release (see Figure 1 in St. Peters, et al., 2011).
To stabilize and recover performance in the presence of a distractor, subjects are required to enhance the processing cues, filter distractors, suppress the perhaps impulsive tendency to disengage from task, and to maintain motivation to perform (note that omission remained low in the experiment described above). Presumably, these mechanisms are activated by the onset of the distractor and the loss of reward early into the first distractor period (as the animals commit significantly more misses and false alarms). Augmented levels of tonic cholinergic activity are hypothesized to represent a main mechanism via which attentional control mechanisms are recruited and their efficacy is increased.
Relationship between increases in cholinergic activity and fMRI-based activity measures
Using arterial spin labeling (ASL) fMRI, dSAT performance of healthy humans was correlated with increases in activity in the (right) middle frontal gyrus (Demeter, et al., 2011). This correlation was orthogonal to that observed between the severity of the distractor effect and cholinergic activity (St. Peters, et al., 2011). Specifically, in humans, greater activity was correlated with more severe effects of the distractor on performance. In animals, higher levels of cholinergic neurotransmission predicted better residual performance (above). We hypothesize, therefore, that higher levels of cholinergic activity optimizes prefrontal circuitry, by reducing neuronal noise patterns and network fluctuations (e.g., Cohen & Maunsell, 2009; Leber, 2010), and thereby reduces demands on metabolic supply and consumption of this region.
Mesolimbic control of augmented tonic cholinergic activity
The “top in top-down control” (B. T. Miller & D’Esposito, 2005) has been attributed to the prefrontal regions (Pessoa, et al., 2009; Rossi, et al., 2009). However, exactly what information recruits top-down control mechanisms, and via what neuronal circuitries and mechanisms, has remained largely unclear. Research on such mechanisms has focused on demonstrating altered neuronal processing in extra-prefrontal cortical regions as a result of prefrontal manipulations, or on studying the time course of the transfer of information from prefrontal to extra-prefrontal regions. Medial prefrontal regions calculate prediction errors and are thought to initiate corrective action by recruiting dopaminergic mesolimbic regions to modify performance (e.g., Modirrousta & Fellows, 2008; Rutledge, Dean, Caplin, & Glimcher, 2010; Schultz, 2006; S. F. Taylor, et al., 2006). Thus, the loss of rewards early in the distractor block, and perhaps also the perception of distractors per se, may be sufficient to activate prefrontal efferent systems and attention-supporting neuronal mechanisms to maintain and recover performance under challenging conditions.
The prefrontal cortex provides direct glutamatergic feedback to the basal forebrain and, indirectly, also via projections to mesolimbic regions, including the nucleus accumbens (NAC), ventral tegmentum and the amygdala. All these mesolimbic regions project to the cholinergic basal forebrain (see Figure 1 in M Sarter & Lustig, 2009; Zaborszky, 2002; Zaborszky, Buhl, Pobalashingham, Bjaalie, & Nadasdy, 2005; Zaborszky & Cullinan, 1992, 1996; Zaborszky, Cullinan, & Luine, 1993; Zaborszky, Gaykema, Swanson, & Cullinan, 1997; Zaborszky, Heimer, Eckenstein, & Leranth, 1986; Zaborszky, Leranth, & Heimer, 1984; Zaborszky, Pang, Somogyi, Nadasdy, & Kallo, 1999). Given the evidence indicating that the NAC processes information about instrumental effort (e.g., Farrar, et al., 2008; e.g., Font, et al., 2008; e.g., Mingote, et al., 2008), we investigated the possibility that NAC-basal forebrain interactions contribute to tonic cholinergic activity, specifically in situations taxing attentional control.
NAC neuronal activity is necessary to demonstrate performance-associated increases in cortical cholinergic neurotransmission (Neigh, Arnold, Rabenstein, Sarter, & Bruno, 2004). Furthermore, stimulation of ionotropic glutamate receptors in the NAC shell selectively stimulates ACh release in prefrontal regions (Zmarowski, Sarter, & Bruno, 2005, 2007). Thus we hypothesized that such stimulation benefits the attentional performance specifically in the presence of a distractor.
SAT performing animals were equipped with guide cannula to allow the remote infusion of NMDA into the NAC while performing the SAT. Following a first block of undisturbed performance, NMDA was infused into the NAC, shell or core, and either SAT continued or the distractor was presented following another 8-min block of regular performance. NAC infusions did not affect SAT performance. Furthermore, performance in the presence of the distractor did not benefit from infusions into the core of the NAC. In contrast, infusions into the NAC shell improved the performance in the presence of a distractor. This improvement reached a level that was statically similar to the performance seen in the absence of the distractor (and of infusions). Furthermore, we also demonstrated that the beneficial effects of NMDA NAC shell infusions required the presence of cholinergic projections to the PFC (St. Peters, et al., 2011).
These findings indicate that the NAC, presumably based on its afferents from prefrontal and other mesolimbic regions and its GABAergic projections to cholinergic cells of the basal forebrain (above), represents a major component of the efferent projection systems of the prefrontal cortex that mediates top-down effects. NAC-basal forebrain interactions are activated in situations requiring the enhanced cognitive control of attentional performance. The effects of NAC NMDA infusions suggest that activation of NAC circuitry is sufficient to attenuate the detrimental performance effects of distractors.
The effects of NAC stimulation on dSAT performance cannot be interpreted in simple terms of enhanced motivation. First, such stimulation did not improve SAT performance (no distractor). Second, the distractor did not robustly increase the number of errors of omission and NAC NMDA receptor stimulation did not affect omission rates. Thus, NMDA infusions did not simply increase the animals’ instrumental effort. An important third and related argument concerns the finding the NAC NMDA receptor stimulation selectively benefited the animals’ detection rate. This finding is consistent with the hypothesis that augmented cholinergic activity benefits dSAT performance specifically by enhancing the neuronal mechanisms that mediate the likelihood for signal detection. These mechanisms are described next.
Tonic cholinergic enhancement of thalamic glutamatergic representation of signals
As already mentioned, neuropharmacological experiments involving mice lacking nAChR subtypes demonstrated that cholinergic activity modulates the activity of mediodorsal thalamic (MD) glutamatergic afferents via α4β2* nAChRs expressed at these neurons’ terminals (references above; see also Guillem, et al., 2011; Lambe, et al., 2003).
Thalamic glutamatergic projections are thought to “import” a pre-attentional representation of the cue into the prefrontal detection circuit. For a signal to be detected it first needs to undergo sensory analysis and coding, and be identified as a component of a group of stimuli that are potential targets for attention (“coarse categories”; Logan, 1992; Treisman, Vieira, & Hayes, 1992). The neuronal circuitry that generates such preattentional representation of stimuli includes the projections from sensory regions to the thalamic reticular nucleus and reticular projections to the MD (e.g., Guillery, Feig, & Lozsadi, 1998; Pinault, 2004).
Consistent with this hypothesis, amperometric recordings of signal-evoked glutamatergic release events in the middle layers of the PFC of SAT performing animals indicate that all signals, irrespective of subsequent detection, evoke glutamatergic transients. Furthermore, the amplitude of such transients codes signal salience, but only in well-performing animals. During poor performance periods glutamatergic signal amplitudes are attenuated and salience-coding is lost. This findings indicate that these cue-evoked glutamate release events are modulated (Howe & Sarter, 2010).
Stimulation of α4β2* nAChRs is hypothesized to augment signal-evoked glutamatergic transients and was demonstrated, as predicted by this hypothesis, to enhance the detection of cues (Howe, et al., 2010). Larger glutamatergic transients are more likely to evoke a cholinergic transient and thus mediate detection (Parikh, et al., 2008). Note again that, in contrast to glutamatergic transients, cholinergic transients are not evoked by signals that are missed, and unlike glutamatergic transients, they are not modulated in graded manner (Figure 2).
Tonic cholinergic control of attention
Collectively, and in somewhat simplified terms, this evidence suggests the following general scenario. During attentional performance, presentation of a distractor causes misses and false alarms and thus reward loss. This loss is computed in prefrontal regions and triggers recruitment of prefrontal efferents to mesolimbic regions which converge primary on NAC outputs to the basal forebrain, increasing tonic cholinergic activity of corticopetal projections. As a result of such upregulation of tonic cholinergic activity, the preattentional representation of signals is amplified and thus these signals are more likely to be detected. Clearly, this simplified description obscures numerous key issues that remain to be addressed, including the possibility that top-down effects on cholinergic activity manifest cortex-wide and may also involve cortico-cortical mechanisms (Nelson, Sarter, & Bruno, 2005).
Animals exhibiting poor attentional control
The neuronal origin of poor cognitive control of attention remains not well understood. We are in need of animal models that are characterized by low tonic cholinergic activity and poor cognitive control, to study the development of attentional symptoms and their escalating contributions to other psychiatric symptom clusters. Preliminary evidence from our studies suggests that rats with these characteristics are present in outbred populations and can readily be identified by assessing their propensity to approach a stimulus associated with reward delivery. Animals classified as “sign-trackers” (Flagel, Akil, & Robinson, 2009; Flagel, Watson, Akil, & Robinson, 2008; Flagel, Watson, Robinson, & Akil, 2007; Lovic, Saunders, Yager, & Robinson, 2011; Saunders & Robinson, 2010, 2011) exhibit a high frequency of periods of extremely poor SAT performance, approaching random response selection. Impulsive action, that is, the inability to inhibit responding to the incorrect lever should animals be positioned in front of that lever as it is extended, represents a substantial source for these poor periods of performance (Paolone, Angelakos, Meyer, Robinson, & Sarter, 2011). Importantly, these animals are able to periodically perform as well as their consistently well-performing counterparts (“goal-trackers”); therefore, their impairments cannot simply be accounted for by assuming more fundamental deficits in sensory coding or bottom-up attentional processes.
Circuitry-derived cholinergic treatment of poor attentional control
Our current understanding of the role of tonic cholinergic activity in the control of attention and the underlying neuronal circuitry suggests a rather straightforward therapeutic approach to benefit poor cognitive control of attention. Stimulation of α4β2* nAChRs is hypothesized to specifically improve the detection rate of subjects performing attention tasks (Howe, et al., 2010; McGaughy, Decker, & Sarter, 1999; Mohler, et al., 2010; K. Taylor, Decker, Sarter, & Parikh, 2011). We have also began to explain why acetylcholinesterase inhibitors and nicotine are not as effective as α4β2* nAChR agonists. These reasons include the excessive extra-synaptic availability of ACh and the resulting presynaptic inhibition resulting from high ACh levels that is caused by esterase inhibitors, and the stimulation of the α7 nAChR resulting from both such inhibitors and the administration of nicotine (for details see Howe, et al., 2010; M.S arter, Lustig, & Taylor, 2011).
The predictive validity of this preclinical evidence is indicated by the results from clinical trials showing that α4β2* nAChR agonists improve the attention of adult (Apostol, et al., 2011; Wilens, et al., 1999; Wilens, Verlinden, Adler, Wozniak, & West, 2006) but not pediatric (Wilens, et al., 2011) patients with ADHD. These effects of α4β2* nAChR agonists have been considered promising given the limited cognitive benefits of psychostimulants in adult ADHD patients (reviewed in Wilens & Decker, 2007). In the present context, the failure of a α4β2* nAChR agonist to robustly improve attention in pediatric ADHD patients may be speculated to be consistent with our hypothesis that such compounds specifically enhance the cognitive control of attention. Given that cognitive control mechanisms continue to mature into the third decade of life (e.g., Andrews-Hanna, et al., 2011), it seems likely that different cognitive mechanisms underlie the attentional impairments and impulsivity of pediatric versus adult patients with ADHD. As the field focused on describing overlapping symptom and associated brain activity and describing adult ADHD as continual disorder (e.g., Cubillo, et al., 2011), little appears to be known about the cognitive mechanisms which potentially differentiate between pediatric and adult ADHD. If it the attentional symptoms of adult ADHD were a more direct result of weak cognitive control of attention than is the case in pediatric patients, α4β2* nAChR agonists would be expected to benefit primarily the adult version of the disorder.
The preclinical evidence also suggests that it is worthwhile to assess α4β2* nAChR agonists as adjunct treatment for improving the cognitive symptoms of patients with schizophrenia (M. Sarter, et al., 2011). However, there is presently little knowledge about the putative interactions between the effects of α4β2* nAChR agonists, antipsychotic drugs and high smoking rates; these issues require study to predict the usefulness of such adjunct treatment trials (K. Taylor, et al., 2011).
CONCLUSIONS
This review postulates the following main hypotheses: (1) Distractibility, impulsivity, attentional lapses, low motivation to perform attention tasks and attentional fatigue all are consequences of poor cognitive control of attention. (2) The tonic component of the cortical cholinergic projection systems modulates cue detection processes as a function of the degree of top-down control. (3) Prefrontal-mesolimbic circuitry, specifically the NAC projections to the cholinergic basal forebrain, contribute to the activation of the cholinergic system in situations characterized by demands on cognitive control of attention. (4) Stimulation of α4β2* nAChRs mimics and presumably amplifies the tonic cholinergic modulation of cortical circuitry and thus benefits attentional control mechanisms.
While our understanding of the regulation and function of cholinergic activity has evolved and departed from traditional descriptions of this neuronal system as a unitary, reticular, arousal-mediating group of neurons, our knowledge of the temporal dynamics of the multiple modes of cholinergic activity remains rudimentary. Furthermore, our understanding of the neuronal causes of poor attentional control, specifically in disorders, is largely undeveloped. Circuitry models such as shown in Figure 2 will evolve rapidly and undoubtedly become hugely more complex. Such models provide a framework for studying the neuronal mechanisms contributing to poor attentional control, they explain the limitations of traditional cholinomimetic treatment strategies (see also M. Sarter, et al., 2011) and they suggest treatments that have already been demonstrated to be effective inpatients with poor attentional control (Apostol, et al., 2011).
Acknowledgments
Our research was supported by PHS Grants MH080332 and MH086530 (MS).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS One. 2011;6(6):e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol G, Abi-Saab W, Kratochvil CJ, Adler LA, Robieson WZ, Gault LM, et al. Efficacy and safety of the novel alpha(4)beta (2) neuronal nicotinic receptor partial agonist ABT-089 in adults with attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled crossover study. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2393-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114(2):451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Baluch F, Itti L. Training top-down attention improves performance on a triple-conjunction search task. PLoS One. 2010;5(2):e9127. doi: 10.1371/journal.pone.0009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, St Peters M, Urumek N, Gritton H, Mirza N, Sarter M. Selective GABA inverse agonist RO4938581 modulates nicotine-evoked transients in prefrontal cortex. Paper presented at the Society for Neuroscience Annual Meeting.2011. [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens. Cogn Affect Behav Neurosci. 2009;9(1):16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carte ET, Nigg JT, Hinshaw SP. Neuropsychological functioning, motor speed, and language processing in boys with and without ADHD. J Abnorm Child Psychol. 1996;24(4):481–498. doi: 10.1007/BF01441570. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12(12):1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collerton D, Perry E, McKeith I. Why people see things that are not there: a novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav Brain Sci. 2005;28(6):737–757. doi: 10.1017/S0140525X05000130. [DOI] [PubMed] [Google Scholar]
- Corkum PV, Siegel LS. Is the Continuous Performance Task a valuable research tool for use with children with Attention-Deficit-Hyperactivity Disorder? J Child Psychol Psychiatry. 1993;34(7):1217–1239. doi: 10.1111/j.1469-7610.1993.tb01784.x. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29(14):4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2011 doi: 10.1016/j.cortex.2011.04.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Demeter E, Hernandez-Garcia L, Sarter M, Lustig C. Challenges to attention: a continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. Neuroimage. 2011;54(2):1518–1529. doi: 10.1016/j.neuroimage.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22(6):787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas VI, Parry PA. Effects of reward and nonreward on frustration and attention in attention deficit disorder. J Abnorm Child Psychol. 1994;22(3):281–302. doi: 10.1007/BF02168075. [DOI] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci U S A. 2008;105(16):6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B, Weinberger DR, Suter JC, Goldberg TE. Continuous performance test and schizophrenia: a test of stimulus-response compatibility, working memory, response readiness, or none of the above? Am J Psychiatry. 2000;157(5):772–780. doi: 10.1176/appi.ajp.157.5.772. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Pessoa L. Motivation sharpens exogenous spatial attention. Emotion. 2007;7(3):668–674. doi: 10.1037/1528-3542.7.3.668. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Font L, Pereira M, Mingote S, Bunce JG, Chrobak JJ, et al. Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience. 2008;152(2):321–330. doi: 10.1016/j.neuroscience.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186(1):48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191(3):599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Font L, Mingote S, Farrar AM, Pereira M, Worden L, Stopper C, et al. Intra-accumbens injections of the adenosine A2A agonist CGS 21680 affect effort-related choice behavior in rats. Psychopharmacology (Berl) 2008;199(4):515–526. doi: 10.1007/s00213-008-1174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. A framework for studying the neural basis of attention. Neuropsychologia. 2001;39(12):1367–1371. doi: 10.1016/s0028-3932(01)00124-5. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Mammato CA, Sandy JM. Performance of inattentive-overactive children on selected measures of prefrontal-type function. J Clin Psychol. 1989;45(4):619–632. doi: 10.1002/1097-4679(198907)45:4<619::aid-jclp2270450419>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Gorissen M, Sanz JC, Schmand B. Effort and cognition in schizophrenia patients. Schizophr Res. 2005;78(2–3):199–208. doi: 10.1016/j.schres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Balan P. Attention as a decision in information space. Trends Cogn Sci. 2010;14(6):240–248. doi: 10.1016/j.tics.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, et al. Nicotinic acetylcholine receptor beta2 subunits in the medial prefrontal cortex control attention. Science. 2011;333(6044):888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Lozsadi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21(1):28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- Haenlein M, Caul WF. Attention deficit disorder with hyperactivity: a specific hypothesis of reward dysfunction. J Am Acad Child Adolesc Psychiatry. 1987;26(3):356–362. doi: 10.1097/00004583-198705000-00014. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36(1):52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Operant performance and cortical acetylcholine release: role of response rate, reward density, and non-contingent stimuli. Cogn Brain Res. 1997;6(1):23–36. doi: 10.1016/s0926-6410(97)00014-1. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Cogn Brain Res. 2000;9(3):313–325. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. The effects of manipulations of attentional demand on cortical acetylcholine release. Cogn Brain Res. 2001;12(3):353–370. doi: 10.1016/s0926-6410(01)00064-7. [DOI] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35(6):1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Sarter M. Prefrontal; glutamatergic-cholinergic interactions for attention: glutamatergic coding of signal salience as a function of performance levels. In: Westerink B, Clinckers R, Smolders S, Sarre S, Michotte Y, editors. Monitoring molecules in neuroscience. Brussels: Vrije Universiteit Brussels; 2010. pp. 57–59. [Google Scholar]
- Jaquins-Gerstl A, Michael AC. Comparison of the brain penetration injury associated with microdialysis and voltammetry. J Neurosci Methods. 2009;183(2):127–135. doi: 10.1016/j.jneumeth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16(1):9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Kozak R, Martinez V, Young D, Brown H, Bruno JP, Sarter M. Toward a neuro-cognitive animal model of the cognitive symptoms of schizophrenia: disruption of cortical cholinergic neurotransmission following repeated amphetamine exposure in attentional task-performing, but not non-performing, rats. Neuropsychopharmacology. 2007;32(10):2074–2086. doi: 10.1038/sj.npp.1301352. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Clinical Psychiatry. New York: Macmillan; 1912. [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28(2):216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Leber AB. Neural predictors of within-subject fluctuations in attentional control. J Neurosci. 2010;30(34):11458–11465. doi: 10.1523/JNEUROSCI.0809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36(1):316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage. 2010;49(4):3426–3435. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. Attention and preattention in theories of automaticity. Am J Psychol. 1992;105(2):317–339. [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223(2):255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Ford JM, Sarter M, Lustig C. CNTRICS final biomarker selection: control of attention. Schizophrenia Bulletin. 2011 doi: 10.1093/schbul/sbr065. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64(1):34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Manly T, Robertson IH, Galloway M, Hawkins K. The absent mind: further investigations of sustained attention to response. Neuropsychologia. 1999;37(6):661–670. doi: 10.1016/s0028-3932(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Martinez V, Parikh V, Sarter M. Sensitized attentional performance and Fos-immunoreactive cholinergic neurons in the basal forebrain of amphetamine-pretreated rats. Biol Psychiatry. 2005;57(10):1138–1146. doi: 10.1016/j.biopsych.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Maunsell JH. Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci. 2004;8(6):261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology (Berl) 1999;144(2):175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110(2):247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995;117(3):340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: effects of physostigmine and FG 7142. Behav Neurosci. 1998;112(6):1519–1525. doi: 10.1037//0735-7044.112.6.1519. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in ealry schizophrenia. British Journal of Medical Psychology. 1961;34:103–117. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Miller BT, D’Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48(4):535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, et al. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci. 2008;28(36):9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Ebbesen EB. Attention in delay gratification. Journal of Personality and Social Psychology. 1970;16:329–337. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci. 2008;28(51):14000–14005. doi: 10.1523/JNEUROSCI.4450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler EG, Franklin SR, Rueter LE, Fox GB, Decker MW, Browman KE. ABT-594 improves performance in the 5-choice serial reaction time task under conditions of increased difficulty, sub-chronic dosing, and in poorly-performing subjects. Pharmacol Biochem Behav. 2010;95(2):146–157. doi: 10.1016/j.pbb.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Arnold HM, Rabenstein RL, Sarter M, Bruno JP. Neuronal activity in the nucleus accumbens is necessary for performance-related increases in cortical acetylcholine release. Neuroscience. 2004;123(3):635–645. doi: 10.1016/j.neuroscience.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132(2):347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N. Neural mechanisms of attention and control: losing our inhibitions? Nat Neurosci. 2005;8(12):1631–1633. doi: 10.1038/nn1205-1631. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Response inhibition and disruptive behaviors: toward a multiprocess conception of etiological heterogeneity for ADHD combined type and conduct disorder early-onset type. Ann N Y Acad Sci. 2003;1008:170–182. doi: 10.1196/annals.1301.018. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M. CNTRICS final task selection: control of attention. Schizophr Bull. 2009;35(1):182–196. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Robertson IH, Bellgrove MA, Foxe JJ, Kelly SP. Uncovering the neural signature of lapsing attention: electrophysiological signals predict errors up to 20 s before they occur. J Neurosci. 2009;29(26):8604–8611. doi: 10.1523/JNEUROSCI.5967-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterlaan J, Sergeant JA. Response inhibition and response re-engagement in attention-deficit/hyperactivity disorder, disruptive, anxious and normal children. Behav Brain Res. 1998;94(1):33–43. doi: 10.1016/s0166-4328(97)00167-8. [DOI] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Poor and unstable sustained attentional performance in sign-trackers: an animal model of poor top-down cognitive control of attention. Paper presented at the ACNP 50th Annual Meeting.2011. [Google Scholar]
- Paolone G, Howe WM, Gopalakrishnan M, Decker MW, Sarter M. Regulation and function of the tonic component of cortical acetylcholine release. In: Westerink B, Clinckers R, Smolders S, Sarre S, Michotte Y, editors. Monitoring molecules in neuroscience. Brussels: Vrije Universiteit Brussels; 2010. pp. 363–365. [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30(9):3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56(1):141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci. 2008;28(14):3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Rossi A, Japee S, Desimone R, Ungerleider LG. Attentional control during the transient updating of cue information. Brain Res. 2009;1247:149–158. doi: 10.1016/j.brainres.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Rev. 2004;46(1):1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Polk TA, Drake RM, Jonides JJ, Smith MR, Smith EE. Attention enhances the neural processing of relevant features and suppresses the processing of irrelevant features in humans: a functional magnetic resonance imaging study of the Stroop task. J Neurosci. 2008;28(51):13786–13792. doi: 10.1523/JNEUROSCI.1026-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109(2):160–174. [PubMed] [Google Scholar]
- Reason J. Lapses of attention in everyday life. In: Parasuraman R, editor. Varieties of attention. New York: Academic Press; 1984. pp. 515–549. [Google Scholar]
- Roelfsema PR, Spekreijse H. The representation of erroneously perceived stimuli in the primary visual cortex. Neuron. 2001;31(5):853–863. doi: 10.1016/s0896-6273(01)00408-1. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Exp Brain Res. 2009;192(3):489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge RB, Dean M, Caplin A, Glimcher PW. Testing the reward prediction error hypothesis with an axiomatic model. J Neurosci. 2010;30(40):13525–13536. doi: 10.1523/JNEUROSCI.1747-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51(2):145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C. Attention and memory. In: Squire L, editor. New Encyclopedia of Neuroscience. Vol. 1. Oxford: Academic Press; 2009. pp. 639–645. [Google Scholar]
- Sarter M, Lustig C, Taylor SF. Cholinergic contributions to the cognitive symptoms of schizophrenia and the viability of cholinergic treatments. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2010.12.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78(7):658–667. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67(8):730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36(8):1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Braver TS. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. J Neurosci. 2010;30(31):10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P. Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. J Neuropsychiatry Clin Neurosci. 2005;17(3):391–398. doi: 10.1176/jnp.17.3.391. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cereb Cortex. 2005;15(12):1855–1865. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Interval length and time-use by children with AD/HD: a comparison of four models. J Abnorm Child Psychol. 2002;30(3):257–264. doi: 10.1023/a:1015154829796. [DOI] [PubMed] [Google Scholar]
- St Peters M, Cherian AK, Bradshaw M, Sarter M. Sustained attention in mice: Expanding the translational utility of the SAT by incorporating the Michigan Controlled Access Response Port (MICARP) Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.08.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. Journal of Neuroscience. 2011;31(26):9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB, et al. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J Cogn Neurosci. 2008;20(3):478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes DH, Douglas VI, Morgenstern G. Sustained attention in hyperactive children. J Child Psychol Psychiatry. 1973;14(3):213–220. doi: 10.1111/j.1469-7610.1973.tb01189.x. [DOI] [PubMed] [Google Scholar]
- Taylor K, Decker MW, Sarter M, Parikh V. Viability of a4b2* nAchRs as a target for treating the cognitive sympotms of schizophrenia in the presence of chronic nicotine and risperidone. Paper presented at the Society for Neuroscience Annual Meeting.2011. [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, et al. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006;26(15):4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J, Godijn R. Irrelevant singletons capture attention: evidence from inhibition of return. Perception & Psychophysics. 2002;64:764–770. doi: 10.3758/bf03194743. [DOI] [PubMed] [Google Scholar]
- Treisman A, Vieira A, Hayes A. Automaticity and preattentive processing. Am J Psychol. 1992;105(2):341–362. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. The neural mechanisms for minimizing cross-modal distraction. J Neurosci. 2004;24(48):10941–10949. doi: 10.1523/JNEUROSCI.3669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, et al. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156(12):1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochem Pharmacol. 2007;74(8):1212–1223. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Gault LM, Childress A, Kratochvil CJ, Bensman L, Hall CM, et al. Safety and efficacy of ABT-089 in pediatric attention-deficit/hyperactivity disorder: results from two randomized placebo-controlled clinical trials. J Am Acad Child Adolesc Psychiatry. 2011;50(1):73–84. e71. doi: 10.1016/j.jaac.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: results of a pilot study. Biol Psychiatry. 2006;59(11):1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26(4):379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34(8):1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DD, Sarter NB. Capturing the dynamics of atention control from individual to distributed systems: the shape of models to come. Theoretical Issues in Ergonomic Science. 2010;11:7–28. [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Prog Brain Res. 2002;136:359–372. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Buhl DL, Pobalashingham S, Bjaalie JG, Nadasdy Z. Three-dimensional chemoarchitecture of the basal forebrain: spatially specific association of cholinergic and calcium binding protein-containing neurons. Neuroscience. 2005;136(3):697–713. doi: 10.1016/j.neuroscience.2005.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE. Projections from the nucleus accumbens to cholinergic neurons of the ventral pallidum: a correlated light and electron microscopic double-immunolabeling study in rat. Brain Res. 1992;570(1–2):92–101. doi: 10.1016/0006-8993(92)90568-t. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE. Direct catecholaminergic-cholinergic interactions in the basal forebrain. I. Dopamine-beta-hydroxylase- and tyrosine hydroxylase input to cholinergic neurons. J Comp Neurol. 1996;374(4):535–554. doi: 10.1002/(SICI)1096-9861(19961028)374:4<535::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE, Luine VN. Catecholaminergic-cholinergic interaction in the basal forebrain. Prog Brain Res. 1993;98:31–49. doi: 10.1016/s0079-6123(08)62379-1. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79(4):1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Heimer L, Eckenstein F, Leranth C. GABAergic input to cholinergic forebrain neurons: an ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol. 1986;250(3):282–295. doi: 10.1002/cne.902500303. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Leranth C, Heimer L. Ultrastructural evidence of amygdalofugal axons terminating on cholinergic cells of the rostral forebrain. Neurosci Lett. 1984;52(3):219–225. doi: 10.1016/0304-3940(84)90165-4. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann N Y Acad Sci. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Zmarowski A, Sarter M, Bruno JP. NMDA and dopamine interactions in the nucleus accumbens modulate cortical acetylcholine release. Eur J Neurosci. 2005;22(7):1731–1740. doi: 10.1111/j.1460-9568.2005.04333.x. [DOI] [PubMed] [Google Scholar]
- Zmarowski A, Sarter M, Bruno JP. Glutamate receptors in nucleus accumbens mediate regionally selective increases in cortical acetylcholine release. Synapse. 2007;61(3):115–123. doi: 10.1002/syn.20354. [DOI] [PubMed] [Google Scholar]