Abstract
An analytical approach for the determination of trans-resveratrol (3,5,4′-trihydroxy-trans-stilbene) and its glucuronide and sulfate conjugates in dog plasma by LC-MS/MS (without enzymatic hydrolysis of the conjugates) was validated to support pre-clinical toxicological and pharmacological studies. The approach required two independent sample extractions and consequent instrument runs. Samples for resveratrol determination were prepared by protein precipitation with acetonitrile; acetonitrile-methanol was used instead for resveratrol metabolites. Chromatographic separation was performed using a C18 column (30 × 2.0 mm) at a flow rate of 0.25 mL/min. For resveratrol the mobile phase consisted of A: 5 mM ammonium acetate in water-isopropanol (98:2, v/v) and B: methanol-isopropanol (98:2, v/v) and for metabolites the mobile phase was modified as follows: A: 0.1% (v/v) formic acid in water and B: 0.1% (v/v) formic acid in acetonitrile. Total run time was 12 min for each run with retention times of about 4-5 min for all analytes. A turbo ion spray source was used operating in negative mode for resveratrol and resveratrol sulfate and in positive mode for resveratrol glucuronide. Calibration curves were linear from 5 to 1000 ng/mL for resveratrol and its glucuronide, and 10 to 2000 ng/mL for resveratrol sulfate. Linearity was assessed using the internal standard method for resveratrol and the external standard method for the metabolites. Method accuracy was 90 to 112% of the true value for all analytes with precision of 9 %RSD or less for all validation experiments. The validated method was applied to a preclinical toxicology study in dogs after oral administration (200 to 1200 mg/kg) of the agent. Peak plasma resveratrol concentration (Cmax) for most animals was observed within 1 to 5 h of dosing, with group mean values in the 1.7 to 9.9 μg/mL (7.5 to 43 μM) range. Area under the plasma concentration-time curve (AUC) mean values for resveratrol ranged from 3.6 to 44 h*μg/mL for all study groups and were generally proportional to the dose, with no consistent statistically significant changes observed for gender or number of doses. Mean molecular-weight adjusted ratios of resveratrol metabolites to resveratrol for AUC ranged from 1 to 9 for resveratrol glucuronide and from 2 to 11 for resveratrol sulfate.
Keywords: Resveratrol, Metabolites, Dog, Plasma, Pharmacokinetics
Introduction
The polyphenolic natural product trans-resveratrol (5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol; Fig. 1; referred to hereafter as resveratrol), found in grapes, berries, peanuts and other foodstuffs, continues to be the subject of numerous studies probing into its diverse properties and potential health benefits. Literature about its varied biological activity derived from in-vivo and in-vitro studies and about its potential as a disease-preventing agent is extensive and includes cancer prevention, cardioprotection and life span prolongation [1-7]. However, in spite of the large volume of published research, complete understanding of resveratrol's fundamental properties as a therapeutic remains elusive [8-10]. For example, resveratrol's health benefits when ingested in the diet; the efficacy of supplementation for disease prevention; and its therapeutic window and toxicity at the high doses proposed as necessary for beneficial effects have yet to be fully researched. Resveratrol's low oral bioavailability and fast first-pass metabolism have been reported in species including human, mouse, rat, rabbit and pig [11-16]. Specifically, oral bioavailability was reported as 1.5% in rabbit [15] and up to 30% in rat [13]. In human studies, resveratrol plasma levels have been reported at below the limit of quantitation or low levels after oral administration of the agent [17]. Although metabolic profiles may differ, all species have in common an acceptable absorption after oral administration followed by fast conversion of the agent to glucuronide and sulfate metabolites, with plasma levels of the metabolites larger than those of the parent compound. Whether the prevalent metabolites are biologically active [8,18-19] or serve as a systemic repository of the agent through, for example, enterohepatic recirculation [14] is not completely understood. It is therefore apparent that the complete assessment of resveratrol's potential as a therapeutic should include not only the agent's pharmacokinetics but that of its prevalent conjugate metabolites as well.
Fig. 1.
Chemical structure of resveratrol
A number of references are available with validated methods for the determination of resveratrol in plasma from multiple species [14,20-25]. Additionally, resveratrol metabolite identification following oral administration of the agent has been performed for multiple species, with results consistently identifying mono-glucuronides and mono-sulfates as two of the prevalent metabolites [13-14,20,26-27]. Published methodologies for the determination of resveratrol metabolites in plasma and tissues include HPLC with UV detection [11,20-21,28-29] and tandem mass spectrometry [14,30]. With few exceptions [28-29], in these earlier methods enzymatic hydrolysis of the conjugates and indirect determination from the increases observed in the concentration of resveratrol were necessary to estimate the degree of Phase II metabolism. HPLC methods are long, approximately 25 min per run, and sample preparation is demanding, often requiring solid phase extraction (SPE) to purify the extracts and remove interferences. The indirect approach using LC-MS/MS with selective hydrolysis requires three separate extractions and its accuracy depends heavily on the selectivity of the enzymatic hydrolysis and the extent to which the reaction is carried out. A direct determination approach for resveratrol mono-sulfate by LC-MS/MS was published earlier; however, resveratrol mono-glucuronide concentrations were estimated using the parent compound calibration curve and method validation results were not provided [26].
In this work, we present a fully validated LC-MS/MS analytical approach for the determination of resveratrol and of resveratrol glucuronide and resveratrol sulfate without previous enzymatic hydrolysis to free resveratrol. The chromatographic separation of resveratrol is based on an HPLC method published earlier [20] and optimized here for throughput and performance using tandem mass spectrometry detection. This modified approach was first validated for resveratrol and later validated for the direct determination of resveratrol mono-glucuronide and mono-sulfate metabolites after additional modifications using a separate protein precipitation sample preparation and instrumental analysis. The approach was applied to examine the pharmacokinetics of resveratrol, resveratrol glucuronide and resveratrol sulfate in dogs after a 13 week oral administration of resveratrol, a study that was performed as part of the preclinical investigation of resveratrol as a candidate chemopreventive agent by the US National Cancer Institute.
1. Experimental
1.1. Chemicals and reagents
Resveratrol (Sochinaz SA, Vionnaz, Switzerland) was provided by the Division of Cancer Prevention, National Cancer Institute (Bethesda, MD, USA). Trans-resveratrol-3-O-β-D-glucuronide (Toronto Research Chemicals, Inc., North York, Ontario, Canada) and trans-resveratrol-3-O-sulfate (SynFine Research, Inc., Richmond Hill, Ontario, Canada) were used as reference standards for the determination of conjugated resveratrol. Trans-resveratrol-13C6 (Toronto Research Chemicals, Inc.) was used as the internal standard for the determination of resveratrol. Acetonitrile, methanol, isopropanol, ammonium acetate and formic acid (all HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA, USA). HPLC-grade water was generated using a PURELAB Ultra system from ELGA (Lowell, MA, USA) followed by filtration with a Millipore (Billerica, MA, USA) 0.25 μm filter. Blank dog plasma was obtained from Bioreclamation Inc. (Westbury, NY, USA).
1.2. Animals
Twenty male and twenty female healthy naïve beagle dogs (approximately five months old; Ridglan Farms, Inc., Mount Horeb, WI, USA) were held in quarantine for approximately 4 weeks and observed daily for survival and general health status. Prior to randomization into experimental groups, each animal underwent a detailed physical examination to ensure suitability as a test animal. Dogs were housed individually in pens in accordance with standard protocols [31]. Animal rooms were held within a temperature range of approximately 20-25°C and a humidity range of approximately 30-70%. Fluorescent lighting in the animal rooms was provided on a cycle of 12 h of light followed by 12 h of darkness. Each dog was provided with 400 g of commercial chow (Certified Canine Diet 2021C, Harlan Teklad, Madison, WI, USA) daily for a minimum of 2 h during the treatment period. Drinking water was available ad libitum by automatic watering systems.
1.3. Preparation of analytical stock and standards solutions
Stock solutions of resveratrol, trans-resveratrol-13C6, resveratrol-3-O-β-D-glucuronide and resveratrol-3-O-sulfate were prepared in methanol at 1, 0.1, 0.2 and 0.4 mg/mL concentrations, respectively, and stored at -20°C when not in use. Stock standards were prepared by further diluting the stock solutions in methanol-water (50:50, v/v) to 50, 100, 200, 500, 2000, 5000 and 10000 ng/mL for resveratrol and its glucuronide, and to 100, 200, 400, 1000, 2000, 4000, 10000 and 20000 ng/mL for resveratrol sulfate. The internal standard working solution was prepared by diluting the stock solution with acetonitrile to a concentration of 25 ng/mL.
1.4. Extractions
Plasma samples (100 μL) were transferred to 2-mL microcentrifuge plastic vials (placed in ice and protected from light) and mixed with 10 μL of stock standards (for plasma calibrators and QC samples) and 1 mL of internal standard solution in acetonitrile. The samples were vortex-mixed for 1 min, centrifuged at 4 °C in a Sorvall RC 5C Super Speed centrifuge (Thermo Fisher Scientific, Waltham, MA, USA) at 8000 ×g for 10 min to remove precipitated proteins, and the supernatant was transferred to a clean tube and dried under nitrogen flow at room temperature (about 25 °C). After evaporation was complete, the residue was reconstituted in 100 μL of methanol with five min of sonication, added to 400 μL of water, vortex-mixed and centrifuged again. The resulting supernatant was transferred to a sample vial for instrumental analysis. For the determination of conjugated resveratrol metabolites, the protein precipitation procedure was performed instead with 1 mL of acetonitrile-methanol (1:1, v/v) solution. All other steps were the same.
1.5. Instrumentation
Samples were analyzed on an API 3000 MS/MS system (Applied Biosystems/MDS Sciex, Foster City, CA, USA) equipped with an Agilent 1100 HPLC (Agilent Technologies, Wilmington, DE, USA) and run by Analyst™ 1.4.2 software.
Separation of resveratrol from plasma components was achieved using a Luna 3 μm C18(2) 100Å 30 × 2.0 mm column (Phenomenex, Torrance, CA, USA). The column was maintained at room temperature, the flow rate was 0.25 mL/min, and the injection volume was 25 μL. For the resveratrol determination method, the mobile phase consisted of A: 5 mM ammonium acetate in water-isopropanol (98:2, v/v) and B: methanol-isopropanol (98:2, v/v). The mobile phase gradient was as follows: after injection, initial conditions with Solvent A at 90% were held for 0.5 min, decreased to 5% in 3.5 min and held constant for 5 min, returning to initial conditions for another 3 min of reequilibration time. The mobile phase was changed for the analysis of resveratrol metabolites as follows: A: 0.1% (v/v) formic acid in water and B: 0.1% (v/v) formic acid in acetonitrile. All other chromatographic conditions were the same.
Retention times of resveratrol, resveratrol glucuronide and resveratrol sulfate were approximately 5.2, 4.0 and 4.9 min, respectively. Total run time was 12 min for each analytical run.
A turbo ion spray interface was used as the ion source, operating in negative ion mode for the resveratrol determination and with polarity switch for the metabolites determination (negative ion mode for resveratrol sulfate and positive mode for resveratrol glucuronide). Acquisition was performed in multiple reaction monitoring (MRM) mode using m/z 227 ([M-H]-) → 185 (loss of 42 (C2H2O) [32]; 233 ([M-H]-) → 191 (loss of 42 (C2H2O); 405 ([M+H]+) → 229 (resveratrol, [M+H]+); and 307 ([M-H]-) → 227 (resveratrol, [M-H]-) at unit resolution for resveratrol, resveratrol-13C6, resveratrol glucuronide and resveratrol sulfate, respectively. Ion spray voltage and collision energy were -3000 and -30 V, 4500 and 24V, and -4500 and -24 V for resveratrol, resveratrol glucuronide and resveratrol sulfate, respectively. The collision gas was nitrogen; the ion spray temperature was 450 °C; and dwell time was 200 ms.
1.6. Method validation
Method validation was performed following the FDA's Guidance for Industry: Bioanalytical Method Validation [33]. Selectivity, linearity, precision, accuracy, recovery and stability were used to assess assay performance.
Selectivity was assessed by analyzing extract from six individual animals for the presence of analytical interferences and comparing the results to those obtained from spiking blank plasma sources with the analytes at the lowest limit of quantitation (LLOQ; 5 ng/mL for resveratrol and resveratrol glucuronide, 10 ng/mL for resveratrol sulfate).
Linearity was assessed using the internal standard method for resveratrol and the external standard method for its conjugated metabolites and up to eight calibrators prepared with blank dog plasma and analyte concentrations in the 5 to 1000 ng/mL (resveratrol and its glucuronide) and 10 to 2000 ng/mL (resveratrol sulfate) ranges. Curves were built from peak areas ratios to the internal standard (resveratrol) or peak areas (resveratrol metabolites) using least-squares linear regression with a (1/x2) weighting factor that was chosen based on goodness-of-fit criteria, including coefficient of determination (r2), the back-calculated concentration of individual calibrators, and minimization of the intercept value.
Precision and accuracy were determined from three validation runs with dog plasma quality control (QC) samples (n = 6) spiked at the LLOQ (5 ng/mL) and at low (12 ng/mL), mid (400 ng/mL) and high (800 ng/mL) concentrations for resveratrol and its glucuronide and corresponding concentrations of 10, 24, 800 and 1600 ng/mL, respectively, for resveratrol sulfate. Within-run precision and accuracy were assessed from the results from a single day, while between-run precision and accuracy were determined from the results from the three validation runs on different days. Dog plasma extraction recovery of resveratrol and metabolites was determined by comparison of peak area results of dog plasma QC samples (n= 6) to peak area results of extracted blank dog plasma samples spiked post extraction with analyte concentrations at the same levels. For matrix effect evaluation, the peak areas were also compared to those from the corresponding standards prepared in the reconstitution solvent.
Bench-top stability was determined by analyzing low and high level QC samples for the analyte concentrations after 4 and 24 h of storage at ambient temperature and comparing the results to those obtained from freshly prepared samples. The 24 h stability experiment was repeated for resveratrol under refrigerated conditions (approximately 4 °C). Similar experiments were performed for the long-term stability evaluation of the analytes in dog plasma after 50 days storage at -70 °C. Freeze-thaw stability of the low and high level QC samples was determined at -70 °C and over three cycles. Stability was also determined for low and high level QC samples that were extracted and stored in the instrument autosampler under refrigerated conditions (approximately 4 °C); sample extracts were injected on the LC-MS/MS instrument and the concentrations determined after approximately 6 days of storage using freshly prepared calibrators.
To evaluate the impact of sample dilution on the method's accuracy and precision, six replicate QC samples were prepared in blank dog plasma at 4000 ng/mL (resveratrol and its glucuronide) and 8000 ng/mL (resveratrol sulfate) and diluted 5-fold with additional blank plasma prior to analysis.
1.7. Application to a pre-clinical toxicological study
Resveratrol was administered to beagle dogs to evaluate the toxicity and pharmacokinetics of the agent following daily oral administration for 91 consecutive days. Animals were randomly assigned to a control or one of three treatment groups at the end of the quarantine period using a computerized body weight stratification procedure that produced similar group mean body weight values. Body weights for the animals assigned to the study ranged from 5 to 10 kg. The study groups, each consisting of four male and four female dogs, received capsules with neat doses of resveratrol at 0, 200, 600 and 1200 mg/kg (control, low, mid and high groups, respectively).
Blood samples for determination of plasma levels of resveratrol, resveratrol glucuronide and resveratrol sulfate were collected from all treated animals after the first dose and during the last week (week 13) of the study. Blood samples (approximately 3 mL) were collected from the jugular or cephalic vein into Vacutainer tubes (Fisher Scientific, Pittsburgh, PA, USA) containing EDTA at 9 time points (0, 0.25, 0.5, 1, 2, 4, 8, 12 and 24 h post-dose). Tubes were inverted several times to mix; placed on ice until centrifuged to separate plasma (within 1 h); transferred into storage tubes (0.5 mL); and stored frozen (approximately -70°C) until analyzed.
1.8. Pharmacokinetics analysis
Plasma concentration-time profiles of resveratrol, resveratrol glucuronide and resveratrol sulfate for individual animals were analyzed using a noncompartmental model for extravascular administration with WinNonlin® Professional Edition software, Version 5.0.1 (Pharsight Corporation, Mountain View, CA, USA). Pharmacokinetic parameters reported for all target analytes included: time to maximum plasma concentration (Tmax), peak plasma concentration (Cmax), area under the plasma concentration-time curve (AUC), and elimination half-life (t½). Clearance (CL/F) and apparent volume of distribution (Vz/F) were calculated only for resveratrol. AUC from time zero to the last measured concentration (AUC0-t) was estimated by the linear trapezoidal rule up to Cmax, followed by the log trapezoidal rule for the remainder of the curve. AUC extrapolated to infinity (AUC0-∞) is defined as AUC0-t + Ct /λ z, where λz is the disposition rate constant estimated using log-linear regression during the terminal elimination phase and Ct is the last measureable plasma concentration. Statistical comparisons of the data to assess changes due to dose level, gender, or study day were performed using Systat software (version 10.2). Data was analyzed via analysis of variance (ANOVA) followed, as necessary, by the post hoc Tukey's test (inter-group comparison) or via t-test (Day 1 vs. Week 13 and male vs. female); p≤0.05 (all comparisons); data was log-transformed (with the exception of t1/2 and Tmax); Cmax and AUC parameters were normalized to the body surface area dose (i.e., mg resveratrol/m2) prior to log-transformation [34].
2. Method validation results
2.1. Specificity
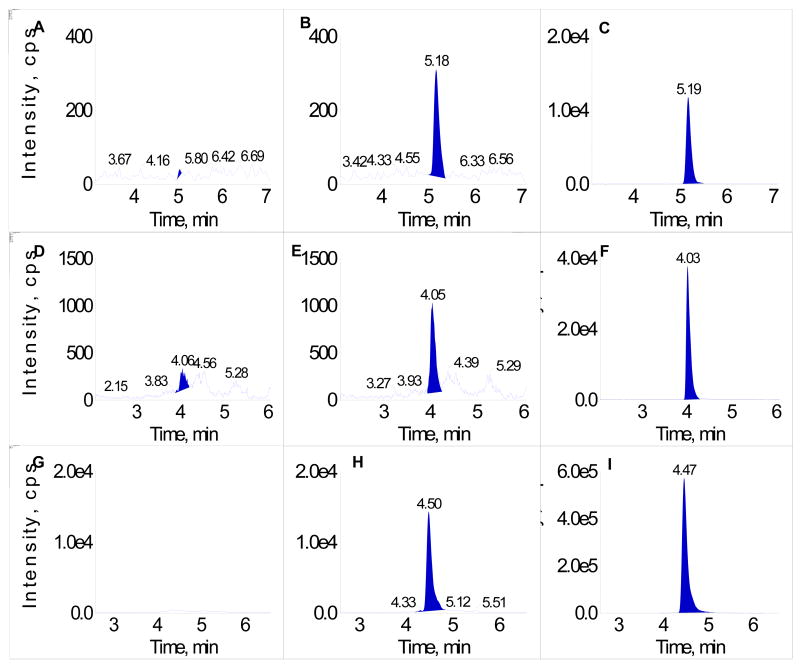
No significant peaks interfering with the quantitation of resveratrol and metabolites were detected in the chromatograms of blank dog plasma at the method's LLOQ for the three analytes. Fig. 2 shows representative chromatograms of blank dog plasma extract, LLOQ (5 ng/mL or 10 ng/mL for resveratrol sulfate), and a 200 ng/mL calibrator (400 ng/mL for resveratrol sulfate) in dog plasma for each of the analytes.
Fig. 2.
A. Resveratrol – blank dog plasma; B. Resveratrol – calibrator at the LLOQ (5 ng/mL) in dog plasma; C. Resveratrol – calibrator at 200 ng/mL in dog plasma; D. Resveratrol glucuronide – blank dog plasma; E. Resveratrol glucuronide – calibrator at the LLOQ (5 ng/mL) in dog plasma; F. Resveratrol glucuronide – calibrator at 200 ng/mL in dog plasma; G. Resveratrol sulfate – blank dog plasma; H. Resveratrol sulfate – calibrator at the LLOQ (10 ng/mL) in dog plasma; I. Resveratrol sulfate – calibrator at 400 ng/mL in dog plasma
2.2. Calibration curves
Calibration curves were linear from 5 to 1000 ng/mL (resveratrol and glucuronide) and 10 to 2000 ng/mL (resveratrol sulfate). Calibrators that fell outside the range of 85 to 115% were not used to calculate the standard curve (two instances for the 200 ng/mL calibrator for resveratrol glucuronide). Mean r2 values were 0.995 or greater for all analytes. Representative regression equations for the calibration curves were y = 0.0365 x + 0.0031, y = 1780 x + 876 and y = 6340 x + 1380 for resveratrol, resveratrol glucuronide and resveratrol sulfate, respectively. The mean accuracy (% of true value) of individual calibrators used to determine the calibration curves ranged from 97 to 103%, 93 to 112% (106% without the 200 ng/mL calibrator), and 97 to 108% of the true value for resveratrol, resveratrol glucuronide and resveratrol sulfate, respectively. Between-run precision [% relative standard deviation (RSD)] for the back-calculated calibrator concentrations ranged from 1 to 8% for the three analytes.
2.3. Precision and accuracy
Precision and accuracy experiment results are presented in Table 1. For all QC samples and analytes the method precision was 9 %RSD or less. Accuracy was within 15% of the true value for all determinations.
Table 1. Within-run and between-run precision and accuracy for QC dog plasma samples.
| Nominal Concentrationa (ng/mL) |
Method Precision and Accuracy (%)b, c | |||
|---|---|---|---|---|
| Resveratrol | Resveratrol Glucuronide | Resveratrol Sulfate | ||
| 5 or 10 (LLOQ) |
Within-Run | 98.2, 101, 105 | 103, 98.2, 109 | 100, 115, 109 |
| %RSD | 4.8, 6.2, 6.2 | 3.0, 5.8, 4.3 | 3.8, 3.5, 3.3 | |
| Between-Run | 101 | 103 | 108 | |
| %RSD | 6.1 | 6.0 | 6.8 | |
|
| ||||
| 12 or 24 (Low QC) |
Within-Run | 91.9, 93.2, 98.5 | 97.4, 89.7, 96.5 | 92.9, 97.6, 95.5 |
| %RSD | 6.5, 2.7, 9.1 | 1.0, 1.9, 3.2 | 1.7, 3.1, 4.7 | |
| Between-Run | 94.3 | 94.5 | 95.1 | |
| %RSD | 7.2 | 4.4 | 3.7 | |
|
| ||||
| 400 or 800 (Mid QC) |
Within-Run | 101, 102, 105 | 112, 107, 111 | 108, 104, 105 |
| %RSD | 2.5, 1.7, 5.8 | 1.8, 1.7, 4.1 | 2.4, 5.4, 1.4 | |
| Between-Run | 102 | 110 | 106 | |
| %RSD | 4.0 | 3.3 | 3.6 | |
|
| ||||
| 800 or 1600 (High QC) |
Within-Run | 104, 103, 106 | 109, 105, 112 | 110, 106, 102 |
| %RSD | 4.3, 2.3, 6.9 | 1.3, 3.6, 5.2 | 1.7, 5.0, 4.5 | |
| Between-Run | 104 | 109 | 106 | |
| %RSD | 4.8 | 4.3 | 4.7 | |
QC dog plasma samples were prepared at 5, 12, 400 and 800 ng/mL for resveratrol and its glucuronide and 10, 24, 800 and 1600 ng/mL for resveratrol sulfate.
For all experiments, the number of QC samples (n) was 6 and 18 for individual validation runs (within-run) and between-run, respectively.
Accuracy expressed as % of true value, precision as % relative standard deviation (RSD).
2.4. Recovery
Average recovery for the low, mid and high QC replicate samples was 95, 98 and 90; 74, 77 and 73; and 83, 81 and 78% for resveratrol, resveratrol glucuronide, and resveratrol sulfate, respectively. Precision ranged from 4 to 7, 2 to 9 and 3 to 9% RSD for resveratrol, resveratrol glucuronide, and resveratrol sulfate, respectively. Comparison of the peak areas of the above experiments to those from the analytes in the reconstitution solvent did not show any significant matrix ion suppression.
2.5. Lower limit of quantitation
Results for the LLOQ determination experiments are included in Table 1. For samples prepared at the LLOQ, method precision was 7 %RSD or less for all analytes. Accuracy was within 15% of the true value for all determinations.
2.6. Stability studies
The experiments designed to evaluate the bench-top stability of the target analytes in dog plasma after 4 or 24 h at room temperature had recoveries within ±10% of their nominal concentration for all analytes and concentrations, with the exception of resveratrol after 24 h (the low and high QC samples degraded to 65 and 49%, respectively, of their initial concentrations) and resveratrol glucuronide (high QC, 4 h time period, 113% recovery). Recovery of resveratrol in dog plasma improved under refrigerated conditions, at 96 and 95%, respectively, of the initial concentrations for the low and high QC samples after 24 h. The changes after three freeze-thaw cycles for the low and high QC samples were within ±10% of their nominal concentrations. The changes after six days of storage of the QC sample extracts in the instrument refrigerated autosampler were within ±10% of their nominal concentrations. No degradation was observed for resveratrol or metabolites in dog plasma after 50 days storage at -70 °C. Results for all stability experiments performed are presented in Table 2.
Table 2. Stability assessment in dog plasma and dog plasma extracts.
| Nominal Concentrationa (ng/mL) - Time |
Mean Measured (SD) Recovery (% of true value) | ||
|---|---|---|---|
| Resveratrol | Resveratrol Glucuronide | Resveratrol Sulfate | |
|
|
|
||
| Plasma Bench-Top Stability (Room Temperature) | |||
|
|
|||
| 12 or 24 - 4 hours | 92.4 (2.6) | 98.5 (1.8) | 96.3 (6.6) |
| 12 or 24 - 24 hours | 65.1 (4.4) | 94.1 (1.6) | 102 (5.6) |
| 800 or 1600 - 4 hours | 93.9 (3.3) | 113 (2.2) | 92.3 (3.4) |
| 800 or 1600 - 24 hours | 49.0 (2.9) | 106 (1.7) | 95.2 (2.8) |
|
| |||
| Plasma Refrigerated Stability (4 °C) | |||
|
|
|||
| 12 or 24 - 24 hours | 95.5 (1.0) | -b | -b |
| 800 or 1600 - 24 hours | 95.2 (2.2) | -b | -b |
|
| |||
| Plasma Freeze-Thaw Stability (3 Cycles)c | |||
|
|
|||
| 12 or 24 | 97.6 (10) | 109 (1.5) | 99.9 (6.0) |
| 800 or 1600 | 102 (3.4) | 105 (2.7) | 90.7 (8.0) |
|
| |||
| Plasma Extract Autosampler Stability (4 °C) | |||
|
|
|||
| 12 or 24 - 6 days | 103 (9.3) | 99.4 (5.8) | 102 (12) |
| 800 or 1600 - 6 days | 106 (4.4) | 95.4 (4.6) | 97.9 (1.8) |
|
| |||
| Plasma Long Term Stability (-70 °C) | |||
|
|
|||
| 12 or 24 - 50 days | 101 (17) | 100 (2.0) | 105 (15) |
| 800 or 1600 - 50 days | 100 (2.9) | 105 (16) | 107 (2.7) |
QC dog plasma samples (n = 6) prepared at 12 and 800 ng/mL for resveratrol and its glucuronide and 24 and 1600 ng/mL for resveratrol sulfate.
Experiment not performed for the indicated target analyte.
Plasma samples were kept frozen at -70 °C.
2.7. Sample dilution
For the 6-replicate set of QC dog plasma samples prepared at 4000 or 8000 ng/mL and diluted 5-fold before analysis, accuracy was 98.3, 101 and 106% of the target value, with a precision of 2, 3 and 1% RSD for resveratrol, resveratrol glucuronide and resveratrol sulfate, respectively.
3. Pharmacokinetics results
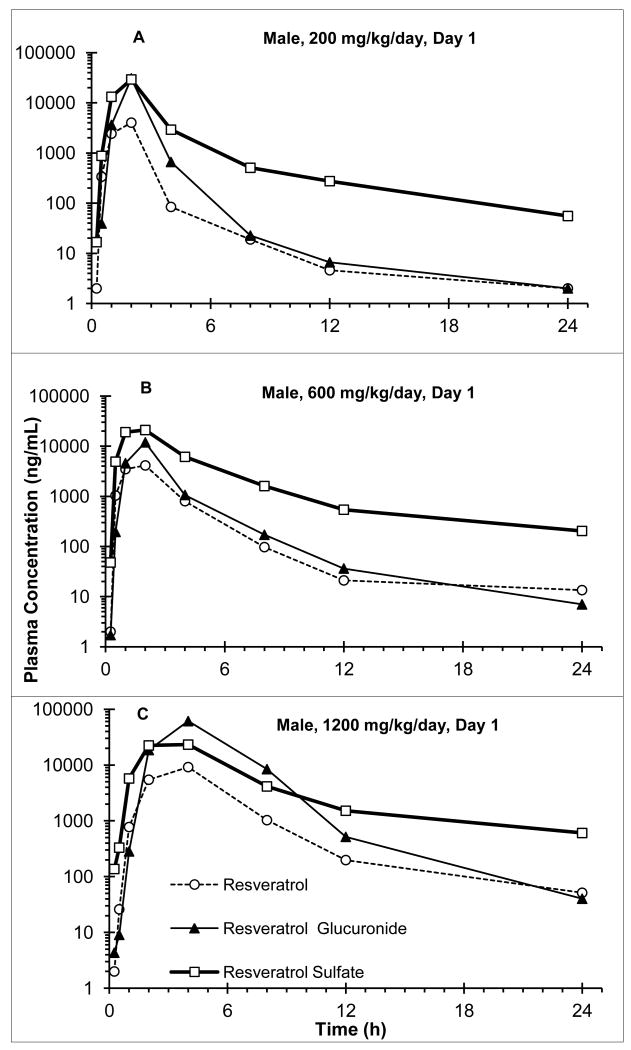
Representative individual animal plasma concentrations (ng/mL) of resveratrol and its metabolites are presented graphically for males and females from the low, mid and high dosing groups in Fig. 3A, B and C, respectively. Group mean value ranges for resveratrol, resveratrol glucuronide and resveratrol sulfate are summarized in Table 3.
Fig 3.
Representative plasma concentration-time curve for resveratrol, resveratrol glucuronide and resveratrol sulfate; A. male dog, 200 mg/kg/day group, Day 1; B. male dog, 600 mg/kg/day group, Day 1; C. male dog; 1200 mg/kg/day group, Day 1
Table 3. Summary of Pharmacokinetic Results – Group Mean Value Ranges.
| Resveratrol Dose (mg/kg/day) | t1/2 (h) | Tmax (h) | Cmax (μg/mL) | AUC a (h*μg/mL) | AUC Ratio b | Vz/F c (L/kg) | CL/F c (L/h/kg) |
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Resveratrol | |||||||
|
|
|||||||
| 200 | 2-4 | 1-2 | 1.7-2.6 | 3.6-5.0 | 1 | 120-280 | 41-58 |
| 600 | 3-4 | 3-4 | 3.7-9.0 | 13-30 | 1 | 120-300 | 22-51 |
| 1200 | 3-4 | 3-5 | 5.1-9.9 | 21-44 | 1 | 130-440 | 28-110 |
|
|
|||||||
| Resveratrol Glucuronide | |||||||
|
|
|||||||
| 200 | 1-5 | 2-3 | 3.8-12 | 7.2-17 | 1-2 | - | - |
| 600 | 2-3 | 2-5 | 19-110 | 48-260 | 2-5 | - | - |
| 1200 | 2-3 | 4-7 | 40-210 | 160-560 | 3-9 | - | - |
|
|
|||||||
| Resveratrol Sulfate | |||||||
|
|
|||||||
| 200 | 4-8 | 2-3 | 17-23 | 40-58 | 8-11 | - | - |
| 600 | 4-7 | 2-3 | 20-28 | 83-120 | 2-7 | - | - |
| 1200 | 4-9 | 2-5 | 18-30 | 76-170 | 2-4 | - | - |
AUC = AUC0-∞ for Day 1and AUC0-24hr for Week 13
AUC ratio was calculated as (AUC/MW)metabolite/(AUC/MW)resveratrol, where MW=molecular weight: 228, 404 and 308 for resveratrol, resveratrol glucuronide and resveratrol sulfate, respectively.
Vz/F and CL/F were not determined for resveratrol metabolites.
3.1. Resveratrol results
Following oral administration, Cmax for most animals was observed within 1 to 5 h of dosing; with group mean values in the 1.7 to 9.9 μg/mL (7.5 to 43 μM) range. Statistical comparisons indicated proportional increases with the dose; however, no other consistent statistically significant changes were observed. Although Tmax appeared to shift to larger values with the dose, no consistent statistically significant changes were observed related to dose, gender, or study day. Mean t1/2 values for the different study groups were similar (ranging from 2 to 4 h). No statistically significant changes were observed for t1/2 related to dose level, gender, or study day. AUC mean values ranged from 3.6 to 44 h*μg/mL for all study groups and were proportional to the dose. Vz/F mean values ranged from 120 to 440 L/kg; CL/F mean values ranged from 22 to 110 L/h/kg. No consistent statistically significant changes were observed for Vz/F or CL/F.
3.2. Metabolites results
Resveratrol metabolized rapidly to resveratrol glucuronide and resveratrol sulfate, producing similar plasma concentration time profiles; no significant changes were apparent for Tmax between resveratrol and its metabolites.
Resveratrol glucuronide
Tmax mean values (2 to 7 h) were similar to those observed for resveratrol and also appeared to increase with the dose. Mean Cmax values were in the 3.8 to 210 μg/mL range and statistical comparisons indicated proportional increases with the dose. Mean t1/2 values were comparable to those for resveratrol and did not change significantly among the study groups. AUC mean values ranged from 7.2 to 560 h*μg/mL for all study groups and were mostly proportional to the dose.
Resveratrol sulfate
Tmax mean values for resveratrol sulfate were similar to those observed for resveratrol. Mean Cmax values were in the 17 to 30 μg/mL range and did not change significantly among the study groups. Mean t1/2 values ranged from 4 to 9 h and were, in general, larger than those for resveratrol. AUC mean values ranged from 40 to 170 hr*μg/mL for all study groups and although in general they increased with the dose, the changes were not proportional for Day 1 and less apparent for Week 13.
Mean molecular-weight adjusted ratios of resveratrol metabolites to resveratrol for AUC ranged from 1 to 9 for resveratrol glucuronide and from 2 to 11 for resveratrol sulfate. Although the AUC ratio for resveratrol glucuronide appeared to increase with the dose, the changes were not statistically significant. The statistical comparisons for resveratrol sulfate AUC ratios indicated a general decrease with the dose.
4. Discussion and Conclusions
4.1. Method development
Initial attempts in our laboratory to use the resveratrol plasma extraction and chromatographic conditions for the conjugated metabolites analysis were unsuccessful due to poor reproducibility. Recovery for resveratrol sulfate improved from about 45% to approximately 75% with the use of acetonitrile-methanol for the extractions; although still not optimal, this proved consistent and reproducible. In addition, we found better method performance for resveratrol glucuronide with the mass spectrometer operating in positive mode and with the addition of formic acid in the mobile phase. A reference standard for resveratrol di-sulfate, another conjugated metabolite found in multiple species, was not commercially available at the time of the study, thus the method could not be validated for this metabolite.
4.2. Assay performance
As presented, the determination of resveratrol and target metabolites required two independent sample extractions and consequent instrument runs. Although the approach worked acceptably, the assay throughput could be substantially improved by combining the sample preparation and instrumental analysis into a single method for all analytes; in our work we could not find the right analysis conditions to achieve this. The utilization of deuterated analogs of the Phase II metabolites as internal standards, not commercially available at the time of the study, could further improve the method's performance. Additionally, if resveratrol di-sulfate reference material were to become available, the method could also be validated for its determination.
4.3. Pharmacokinetics of resveratrol in dogs
The plasma concentration individual results and concomitant concentration-time profiles for this study contained several irregularities based on expected pharmacokinetics. Some animals showed occasional delayed absorption, while others presented sudden increases in plasma concentrations approximately 8 h after oral administration, with significant prolongation of the terminal elimination half-life. This last observation is consistent with results from similar studies with resveratrol in other species and is attributed to enterohepatic recirculation [13-14]. The limited number of blood collection time points in the 8 to 24 h post-dose period for this study did not allow the modeling and estimation of the two apparent elimination phase rate constants believed to exist in the pharmacokinetic profile of resveratrol. Therefore, the pharmacokinetic parameters presented correspond to the estimation of the average elimination rate constant as permitted by the experimental data.
There were no consistent statistically significant changes in the Cmax or AUC group mean values between Day 1 and Week 13 that would indicate evidence of induction or accumulation for either resveratrol or its metabolites. Similarly, statistical comparisons of Cmax or AUC group mean values did not provide consistent evidence of differences among experimental groups due to gender. For resveratrol and resveratrol glucuronide, statistical comparison of AUC data indicated proportional increases with the dose for Day 1; however, for Week 13 AUC increases lacked proportionality to the dose. For resveratrol sulfate, changes for AUC on Day 1 were not proportional to the dose increases, while at Week 13, AUC changes were proportional to the dose changes, with one exception [low vs. high (females)].
Statistical comparison of mean AUC resveratrol glucuronide values normalized to resveratrol AUC values did not provide evidence of differences for the study groups. However, similar statistical comparisons performed for resveratrol sulfate indicated significant decreases relative to resveratrol AUC values with the dose for both Day1 and Week 13.
Details of the toxicological observations for this dog study are presented elsewhere [35].
4.4. Comparison of resveratrol pharmacokinetics in dog, human and rat studies
Resveratrol pharmacokinetic results from experiments with a single oral dose in humans at 42 and 83 mg/kg (1500 and 3100 mg/m2; assuming 60 kg subjects) [36] and rats at 50 and 150 mg/kg (300 and 900 mg/m2) [13] as compared to our dog study at 200 to 1200 mg/kg (4000 to 24000 mg/m2) are as follows:
Mean Tmax values in human and rat groups were 1.5 and 1.0 h, respectively, which compares well with 1.8 and 1.1 h (male and female, respectively) for our 200 mg/kg dog dose group, although increased to 3-5 h for the higher dose groups. Dose-normalized Cmax mean values were 6.4 and 6.5, 1.5 and 5.6, and 4.3 to 13 ng/mL*kg/mg for human, rat and dog, respectively.
Mean t1/2 values were 4.2 and 8.5 h for human, 12 and 3.6 h for rat and 1.9 and 3.8 h for dog. Dose-normalized AUC mean values were 19 and 16, 27 and 17, and 20 to 37 h*ng/mL*kg/mg for human, rat and dog, respectively.
Mean AUC ratios of resveratrol glucuronide to resveratrol were 13 and 14 (sum of glucuronide isomers), 8.6 and 8.8, and 1.8 to 3.4 for human, rat and dog, respectively. Similarly, the AUC ratios for resveratrol sulfate were 22 and 23, 9.3 and 18, and 2.9 to 11 for human, rat and dog, respectively. Although comparable, these results seem to indicate more extensive conjugation of the agent in humans.
Highlights.
Plasma method validated for resveratrol metabolites without enzymatic hydrolysis
Peak resveratrol plasma concentration in dogs observed within 1 to 5 h after oral dosing
Resveratrol was rapidly metabolized to glucuronide and sulfate
AUC ratios of resveratrol metabolites to resveratrol ranged from 1 to 11
Acknowledgments
This research was supported by contract N01-CN-43304 (HHSN261200433004C) from the Division of Cancer Prevention, National Cancer Institute. The authors thank Heidi Kreuzer for assistance with the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 2.Brisdelli F, D'Andrea G, Bozzi A. Resveratrol: a natural polyphenol with multiple chemopreventive properties. Curr Drug Metab. 2009;10:530–546. doi: 10.2174/138920009789375423. [DOI] [PubMed] [Google Scholar]
- 3.Das DK. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interventions. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Marques FZ, Markus MA, Morris BJ. Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol. 2009;41:2125–2128. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 6.Pezzuto JM. Resveratrol as an inhibitor of carcinogenesis. Pharm Biol. 2008;46:443–573. [Google Scholar]
- 7.Pirola L, Frojdo S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian L, Youssef S, Bhattacharya S, Kenealey J, Polans AS, van Ginkel PR. Resveratrol: challenges in translation to the clinic - a critical discussion. Clin Cancer Res. 2010;16:5942–5948. doi: 10.1158/1078-0432.CCR-10-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gescher AJ, Steward WP. Relationship between mechanisms, bioavailability, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomark Prev. 2003;12:953–957. [PubMed] [Google Scholar]
- 10.Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 11.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 12.Sale S, Verschoyle RD, Boocock D, Jones DJL, Wisher N, Ruparelia KC, Potter GA, Farmer PB, Steward WP, Gescher AJ. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans-3,4,5,4′-tetramethoxystilbene. Br J Cancer. 2004;90:736–744. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2010 doi: 10.1007/s00280-010-1525-4. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 15.Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med. 2002;33:387–398. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 16.Azorín-Ortuño M, Yañes-Gascón MJ, Pallarés FJ, Vallejo F, Larrosa M, García-Conesa MT, Tomás-Barberán F, Espín JC. Pharmacokinetic study of trans-resveratrol in adult pigs. J Agric Food Chem. 2010;58:11165–11171. doi: 10.1021/jf102799m. [DOI] [PubMed] [Google Scholar]
- 17.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino J, Park EJ, Kondratyuk TP, Marler L, Pezzuto JM, van Breemen RB, Mo S, Li Y, Cushman M. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem. 2010;53:5033–5043. doi: 10.1021/jm100274c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calamini B, Ratia K, Malkowski MG, Cuendet M, Pezzuto JM, Santarsiero BD, Mesecar AD. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem J. 2010;429:273–282. doi: 10.1042/BJ20091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boocock DJ, Patel KR, Faust GES, Normolle DP, Marczylo TH, Crowell JA, Brenner DE, Booth TD, Gescher A, Steward WP. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J Chromatogr B. 2007;848:182–187. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juan ME, Maijó M, Planas JM. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J Pharm Biomed Anal. 2010;51:391–398. doi: 10.1016/j.jpba.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53:S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 23.Juan ME, Lamuela-Raventós RM, de la Torre-Boronat MC, Planas JM. Determination of trans-resveratrol in plasma by HPLC. Anal Chem. 1999;71:747–750. doi: 10.1021/ac9808831. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, He H, Wang G, Yang B, Ren W, Ma L, Yu Q. Stereospecific determination of cis-and trans-resveratrol in rat plasma by HPLC: application to pharmacokinetic studies. Biomed Chromatogr. 2007;21:257–265. doi: 10.1002/bmc.747. [DOI] [PubMed] [Google Scholar]
- 25.Urpí-Sardà M, Jáuregui O, Lamuela-Raventós RM, Jaeger W, Miksits M, Covas MI, Andres-Lacueva C. Uptake of diet resveratrol into the human low-density lipoprotein. Identification and quantification of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometry. Anal Chem. 2005;77:3149–3144. doi: 10.1021/ac0484272. [DOI] [PubMed] [Google Scholar]
- 26.Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19:1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]
- 27.Burkon A, Somoza V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides - two novel resveratrol metabolites in human plasma. Mol Nutr Food Res. 2008;52:549–557. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- 28.Wenzel E, Soldo T, Erbersdobler H, Somoza V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol Nutr Food Res. 2005;49:482–494. doi: 10.1002/mnfr.200500003. [DOI] [PubMed] [Google Scholar]
- 29.Patel KR, Brown VA, Jones DJL, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, Brown K. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Teng Z, Zhang Y, Huan M, Zhou S. High performance liquid chromatography-tandem mass spectrometric determination of resveratrol and its metabolites in rat tissues. Anal Lett. 2010;43:557–569. [Google Scholar]
- 31.Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and U.S. Department of Agriculture Animal Welfare Standards (Title 9, Code of Federal Regulations, Part 3, 1991 Revision).
- 32.Wang D, Hang T, Wu C, Liu W. Identification of the major metabolites of resveratrol in rat urine by HPLC-MS/MS. J Chromatogr B. 2005;829:97–106. doi: 10.1016/j.jchromb.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), May 2001.
- 34.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison to toxicity of anticancer agents in mouse, rat, hamster, dog, monkey and man. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 35.Johnson WD, Morrissey RL, Usborne AL, Kapetanovic I, Crowell JA, Muzzio M, McCormick DL. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem Toxicol. 2011 doi: 10.1016/j.fct.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boocock DJ, Faust GES, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]