Abstract
The motor symptoms of Parkinson’s disease (PD) are due to the progressive loss of dopamine (DA) neurons in substantia nigra pars compacta (SNc). Nothing is known to slow the progression of the disease, making the identification of potential neuroprotective agents of great clinical importance. Previous studies using the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD have shown that antagonism of L-type Ca2+ channels protects SNc DA neurons. However, this was not true in a 6-hydroxydopamine (6-OHDA) model. One potential explanation for this discrepancy is that protection in the 6-OHDA model requires greater antagonism of Cav1.3 L-type Ca2+ channels thought to underlie vulnerability and this was not achievable with the low affinity dihydropyridine (DHP) antagonist used. To test this hypothesis, the DHP with the highest affinity for Cav1.3 L-type channels – isradipine – was systemically administered and then the DA toxin 6-OHDA injected intrastriatally. Twenty-five days later, neuroprotection and plasma concentration of isradipine were determined. This analysis revealed that isradipine produced a dose-dependent sparing of DA fibers and cell bodies at concentrations achievable in humans, suggesting that isradipine is a potentially viable neuroprotective agent for PD.
Keywords: isradipine, 6-OHDA, substantia nigra, neuroprotection
Introduction
PD is a slowly progressing neurodegenerative condition whose presenting motor symptoms – bradykinesia, rigidity and tremor– are attributable to the loss of DA neurons (Riederer and Wuketich, 1976). Postmortem analysis of PD patient brains has revealed a striking loss of tyrosine hydroxylase (TH) immunoreactive, DA neurons in the SNc and relative sparing of DA neurons in the neighboring ventral tegmental area (VTA) (Hirsch et al., 1988). As nothing is known to slow the progression of the disease, the identification of neuroprotective agents in PD is of considerable importance.
One potential target for neuroprotective therapies in PD is the L-type Ca2+ channel with a Cav1.3 pore-forming subunit. The rationale for targeting these channels comes from an appreciation of the potentially deleterious consequences of elevations in intracellular Ca2+ (Gleichmann and Mattson, 2010) and the recent discovery that vulnerable SNc DA neurons have an usually strong engagement of Cav1.3 L-type Ca2+ channels during autonomous pacemaking (Guzman et al., 2010; Khaliq and Bean, 2010). This influx has been shown to increase mitochondrial oxidant stress in SNc DA neurons and this stress is exacerbated in a genetic model of PD (Guzman et al., 2010). In principle, this stress should increase the sensitivity to mitochondrial toxins used to create animal models of PD. In agreement with this inference, previous work has shown that antagonizing L-type Ca2+ channels by systemic administration of the DHP nimodipine increased the resistance of SNc DA neurons to both acute and chronic challenge with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration (Chan et al., 2007; Kupsch et al., 1995; Kupsch et al., 1996). However, systemic administration of nimodipine did not protect SNc DA neurons against a challenge with 6-hydroxydopamine (6-OHDA) (Sautter et al., 1997).
The reason for this apparent discrepancy is unclear. Although both 6-OHDA and MPTP disrupt mitochondrial function, they do so through different mechanisms (Bove et al., 2005). As a consequence, it is possible that protection against 6-OHDA toxicity requires a greater reduction in Ca2+ influx through L-type channels. The efficacy of systemically administered DHPs in antagonizing Cav1.3 Ca2+ channels in SNc DA neurons depends upon their bioavailability and potency. In this regard, DHPs are heterogeneous (Eisenberg et al., 2004). Although nimodipine has good brain bioavailability (Kupsch et al., 1996), it has a relatively low affinity for Cav1.3 Ca2+ channels (Sinnegger-Brauns et al., 2009). In contrast, isradipine has much higher (>40 fold) affinity for Cav1.3 channels as well as good brain bioavailability (Bonci et al., 1998; Fitton and Benfield, 1990; Sinnegger-Brauns et al., 2009). Another relevant factor is DHP potency at Cav1.2 Ca2+ channels found in the cardiovascular system (Simuni et al., 2010). The antagonism of these channels limits the dose of DHPs that can be used for neuroprotective purposes. Theoretically, the ideal DHP for protecting SNc DA neurons would be one that was selective for Cav1.3 channels. Although none of the known DHPs have a higher affinity for Cav1.3 channels than Cav1.2 channels, the DHP that comes the closest is isradipine, which has nearly equal potency at Cav1.2 and Cav1.3 channels (Sinnegger-Brauns et al., 2009).
The studies reported were designed to test the hypothesis that isradipine would protect SNc DA neurons and fibers in a progressive model of PD created by intrastriatal injection of 6-OHDA. We found that indeed isradipine produced a dose-dependent protection in this model. Moreover, it was found that the plasma concentrations of isradipine that led to protection were within those that can be safely achieved in humans.
Methods
Animals and Surgical Procedures
Male C57BL/6 mice (6–7 weeks old and 20±1 g) were purchased from Jackson Laboratory (Bar Harbor, Maine). Animals were handled according to the guidelines established by the Northwestern University Animal Care and Use Committee, the National Institutes of Health and the Society for Neuroscience.
All of the experiments shown, isradipine was delivered by subcutaneous Alzet osmotic minipumps (model 2004; Alzet, Cupertino, CA). These minipumps were found to provide a more reliable, sustained elevation in plasma isradipine concentration than other strategies. Pumps were loaded with isradipine dissolved in vehicle (DMSO/PEG300) at a concentration of 3mg/kg/day. There were nine mice in isradipine and seven mice in vehicle treated group. Isradipine was continuously delivered with a flow rate of 0.25ml/hr for 28 days. Prior implantation, pumps were placed in 0.9% saline overnight at 37°C to insure immediate release of the drug. Pumps were implanted subcutaneously following manufacturer’s guidelines. Briefly, under short-term anesthesia, an incision was made between scapulas and a subcutaneous pocket was formed via blunt dissection. The infusion end of the pump was placed away from the site of incision, which was closed with wound clips. On day 3 post implantation, striatal unilateral lesion with 6-OHDA (Sigma Chemical Co., St. Louis, MO) was performed stereotaxically as following: mice were anesthetized with ketamine/xylazine mixture and then immobilized on a stereotaxic frame (Model μ 940, David Kopf Instruments, Tujunga, CA) with a Cunningham adaptor (Harvard Apparatus, USA). For injection into the dorsal striatum, a hole was drilled at 0.4 mm anterior and 1.8 mm lateral to bregma. 6-OHDA was dissolved in saline with 0.02 % ascorbic acid and injected by calibrated glass micropipette (2-000-00, Drumond Scientific Company, Broomall, PA), at a depth of 2.9 mm from dura at a rate 0.1 μl/min. The concentrations and volumes of 6-OHDA to be injected in the striatum had been determined in preliminary experiments. To maximize tissue retention of 6-OHDA, the micropipette was left in situ for another 3 min before slow retraction.
Histology and stereology
Twenty-five days after the lesion, animals were sacrificed for biochemical and anatomical analysis. For anatomical analysis, animals were perfused through the aorta, first with 0.9% NaCl and then with 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were removed and cryoprotected in 30% sucrose/phosphate buffered saline (PBS) overnight at 4°C. Serial coronal sections (30 μm) were cut throughout the striatum and the midbrain and processed for microscopic analysis. For TH immunohistochemistry, following over-night incubation of free-floating sections with primary TH antibody raised in rabbit (Affinity BioReagents, Golden, CO, USA; dilution 1:1000), sections were rinsed and incubated for one hour with biotinylated anti-rabbit IgG (1:500, Amersham, Piscataway, NJ). After rinsing in PBS, sections were incubated with ABC Elite Kit (Vector, Burlingame, CA), and processed for diaminobenzidine reaction. Sections were then mounted on gelatinized slides, left to dry overnight, dehydrated in ascending alcohol concentrations and mounted with Permount mounting medium (Fisher Scientific). Another adjacent series of midbrain sections was processed for fluorescence microscopy of TH immunohistochemistry and ethidium bromide counterstaining. Briefly, bound primary antibodies (as above) were visualized by secondary antibodies coupled to Alexa 488 (Invitrogen, Carlsbad, CA). After rinsing in PBS, sections were incubated in 0.005% ethidium bromide for 1 hour at room temperature and mounted with Vectashield (Vector). Bright field and immunofluorescence images were acquired with a Spot RT CCD video camera (Diagnostic Instruments, Sterling Heights, MI) mounted on a Nikon Eclipse 800 microscope (Nikon, Instech Co., Kanagawa, Japan) and processed with Adobe Photoshop CS4 (Adobe Systems Inc., San Jose, CA) to adjust contrast and brightness.
Estimates of the number of TH positive cells in SNc were obtained by the optical fractionator method and unbiased counting rules (West et al., 1991). The borders of the SNc at all levels in the rostro-caudal axis were defined according to McCormack (McCormack et al., 2002) with a modification. The medial border was defined by a vertical line passing through the medial tip of the cerebral peduncle and by the medial terminal nucleus of the accessory nucleus of the optic tract, when present in sections, thereby excluding the TH positive cells in the VTA. Ventral border excluded the TH positive cells in SN pars reticulata. Sampling was done using the Zeiss Axioplan 2 Imaging microscope (Cals Zeiss MicroImaging, Inc. Thornwwod, NY) with assistance from a computerized stereology system (Stereo Investigator, MicroBrightField, Colchester, VT). From each animal, SNc was delineated at 4X magnification in every third section with counting areas of 150 × 150 μm. The counting frame (80 × 80 μm) was placed randomly on the first counting area and systematically moved through all fields. The estimate of the total number of neurons was calculated according to the optical dissector formula (for more details, see Oorschot) (Oorschot, 1996). The co-efficient of error was calculated according to Gundersen and Jensen (Gundersen and Jensen, 1987), and values 0.10 were accepted. TH-positive cells were counted using a 40X objective (NA 0.95) only when the nucleus could be identified. The data are presented as percentage of the intact side.
The optical density of the TH positive fibers in the striatum was determined from five coronal sections from each animal, throughout the whole rostro-caudal extension of the striatum (AP anatomical levels: +1.1 to −1.7 mm, with respect to bregma, according to Franklin and Paxinos) (Franklin and Paxinos, 1997). Fiber densities were measured in digitalized images using the NIH ImageJ 1.42 version. The data are presented as percentage of the intact side, which was defined as 100%.
Biochemical analysis of isradipine concentration
For determination of isradipine in the plasma, 1.0 ml of blood was drawn from anesthetized mice directly from the heart and incubated at room temperature in heparin-coated vials for 30 min. After centrifugation, supernatants were collected, transferred to capped polypropylene test tubes, and stored at −80°C until the day of the assay. All samples were wrapped in aluminum foil during the procedure. Blood levels of isradipine were measured by liquid chromatography-tandem mass spectrometry. In brief, 10 μL of a methanolic 100 ng/mL nifedipine (internal standard) solution and 0.1 mL of a plasma sample were mixed in a polypropylene tube and centrifuged. The sorbent of each well of an Oasis HLB 30 μm (30 mg) μElution Plate (Waters Chromatography, Milford, Massachusetts) was conditioned with 0.5 mL of methanol and 0.5 mL of water, the prepared sample was applied to the conditioned sorbent, and vacuum was applied. The sorbent was washed with 0.5 mL of 5% methanol in water. Samples were eluted with 0.2 mL of methanol, dried under vacuum, reconstituted with 100 μL of mobile phase, and eluted isocratically from a Synergi 4 μ Max-RP 80A column (50 × 2.0 mm, Phenomenex, Torrance, California), with a mobile phase consisting of water containing 0.1% formic acid:acetonitrile containing 0.1% trifluoroacetic acid (30:70, vol:vol) at a flow rate of 0.125 mL/min. The API 3000 LC-MS/MS system (Applied Biosystems, Foster City, California) was operated with its electrospray source in the positive ionization mode. Mass-to charge ratios of the precursor-to-product ion reactions monitored were 347.2 → 315.1 for nifedipine and 372.1 → 312.2 for isradipine. Concentrations were calculated by comparing isradipine/nifedipine peak area ratios to those of a standard curve prepared in blank plasma. The plasma isradipine standard curve was linear for isradipine concentrations from 0.5 to 500 ng/mL with inter-assay coefficients of variation of less than 10%.
Statistical Analysis
Data analyses were done with IGOR Pro 6.0 (WaveMetrics), Graphpad Prism 5.0 (GraphPad Software) and MATLAB (MathWorks). Non-normal distributions were assumed for all data sets that were analyzed with nonparametric tests Kruskal–Wallis ANOVA and Mann-Whitney. Probability threshold for statistical significance was P<0.05. A nonlinear regression model was used to fit dose-response data with a modified Hill equation model using MATLAB; coefficients were constrained to integers between 1 and 4.
Modeling DHP interaction with Cav1.3 Ca2+ channels: The assumptions and methods used in this calculation have recently been described in detail elsewhere (Surmeier et al., 2010). The model was implemented in MATLAB using modulated receptor model originally proposed by Bean (Bean, 1989) using estimates of the isradipine and nifedipine KDs generated by Sinegger-Brauns et al. (Sinnegger-Brauns et al., 2009)
Results
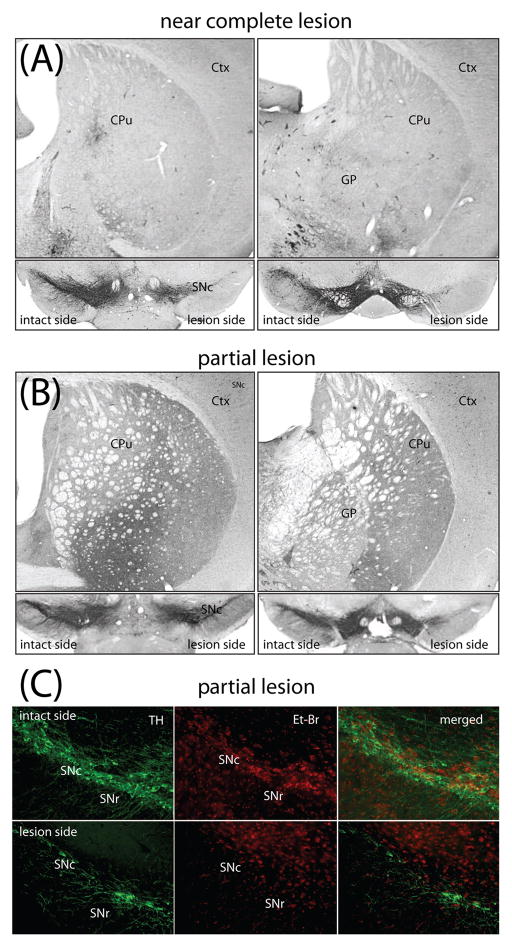
In preliminary experiments, unilateral striatal lesions were made with decreasing doses of 6-OHDA to determine a dose that would produce roughly a 60% loss of SNc DA neurons 3–4 weeks after injection. Injection of 3.5 μg 6-OHDA induced a complete loss of TH positive fibers in the whole dorsal part of the striatum (caudate-putamen) and near a complete loss of TH positive DA cell bodies in SNc (Figure 1A). In contrast, 2.5 μg of 6-OHDA only partially lesioned SNc DA neurons (Figure 1B). To ensure that the loss of TH immunoreactivity was a reflection of cell loss and not phenotypic downregulation (Sauer and Oertel, 1994), coronal sections of the mesencephalon were counterstained with ethidium bromide. TH and ethidium bromide staining were tightly correlated in sections of the ventral midbrain (Fig. 1C), arguing that TH immunoreactivity 3–4 weeks after lesioning was a reliable index of neuronal survival.
Fig 1.
Near complete lesion and partial nigrostriatal lesion induced with different 6-OHDA doses. (A) Unilateral intrastriatal injection of 3.5 μg 6-OHDA caused a near complete elimination of TH-immunoreactive fibers throughout the rostro-caudal axis of lateral striatum (A) and a near complete loss of SNc DA cells (B). SNc is shown in coronal sections at different levels of mouse midbrain in rostro-caudal order. (C) Fluorescence microphotographs of TH positive neurons counterstained with ethidium bromide in coronal sections through the midbrain of mice injected with 2.5 μg 6-OHDA. On the side contralateral to the lesion (intact side) the SNc contained a normal number of large neurons with dendrites positive for TH fluorescence. Ethidium bromide counterstaining overlaped with TH positive neurons. Ipsilateral to the injection site (lesion side) there were few viable neurons labeled with TH and ethidium bromide fluorescence. Ctx - cortex, CPu-caudate putamen (striatum), GP-globus pallidus, VTA-ventral tegmental area, SNc – substantia nigra pars compacta, SNr - substantia nigra pars reticulata, TH – tyrosine hydroxylase, Et-Br - ethidium bromide.
Twenty-five days after unilateral intrastriatal injections of 2.5 μg of 6-OHDA, mice were sacrificed, their blood collected and the concentration of isradipine in the plasma samples determined using LC-MS/MS. Unexpectedly, with nominally the same amount of isradipine being delivered by the subcutaneous Alzet osmotic minipumps, plasma concentration varied considerably (2.6–46.7 ng/ml). The source of this variability was not determined, but was most likely due to variation in pump performance based upon examination of the pump contents after animal sacrifice. Inadvertently, this provided us with information about the relationship between plasma isradipine concentration and protection.
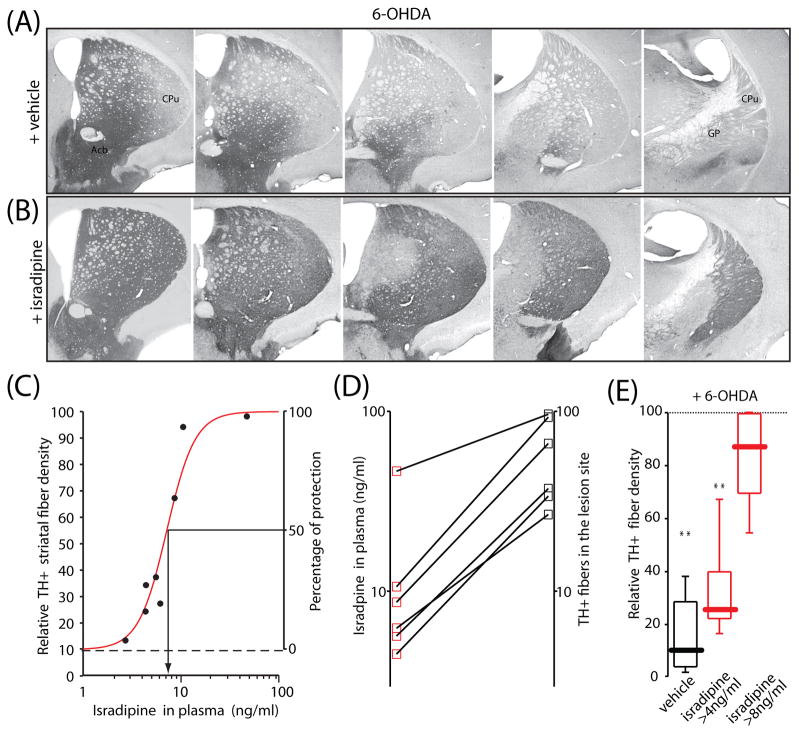
The extent of terminal fiber loss induced by intrastriatal 6-OHDA lesions was assessed by TH-immunohistochemistry in serial coronal sections throughout the rostro-caudal extent of the striatum. A quantitative measure of striatal TH fiber density was obtained by optical density measurements at 5 defined levels. By comparison with striatal TH density on the intact side of the brain, there was extensive loss of TH positive fibers on the lesion side in the vehicle-pretreated mice (> 90% loss, n=8) (Fig. 2A). Pre-treatment with isradipine significantly reduced the 6-OHDA induced fiber loss (Fig. 2B). Plots of plasma isradipine concentration and TH fiber density were well-fit with the sum of a constant factor (TH fiber density with no isradipine) and a Hill function having an IC50=7.2 ng/ml (19 nM) and a slope factor of 3 (Fig. 2 C). Plots of the relationship between plasma isradipine concentration and protection in individual mice also consistently showed a dose-dependence (Fig. 2D).
Fig 2.
Isradipine pre-treatment reduced 6-OHDA induced neurotoxicity at the striatal level. Representative microphotographs showing TH immunoreactive fibers in the striatum after unilateral 6-OHDA injection in mice pretreated with (A) vehicle, and with (B) isradipine (plasma concentration 10.4 ng/ml). Approximately equally spaced, five coronal sections of the left striatum are shown. Protection afforded by isradipine was evident in all rostro-caudal levels. (C) The curve shows a nonlinear regression of relative TH+ striatal fiber density and isradipine plasma concentration with a Hill coefficient of 3 and an IC50=7.2 ng/ml. Starting point of the curve is at the median of TH positive fibers in the vehicle group (median=9.7, n=7). (D) Protective effect of isradipine at the striatal level was dose-dependent as shown from 6 mice. (E) Box-plots summarizing the relative percent TH+ fiber density in the vehicle (n=8), and isradipine pretreated group (n=7). Isradipine significantly reduced toxic effect of 6-OHDA in the striatum. Statistical significance in all plots is shown by asterisks and determined using non-parametric test (Kruskal-Wallis ANOVA). Probability (P) threshold for statistical significance was 0.05. Cpu – caudate-putamen, GP-globus pallidus, Acb – nucleus accumbens
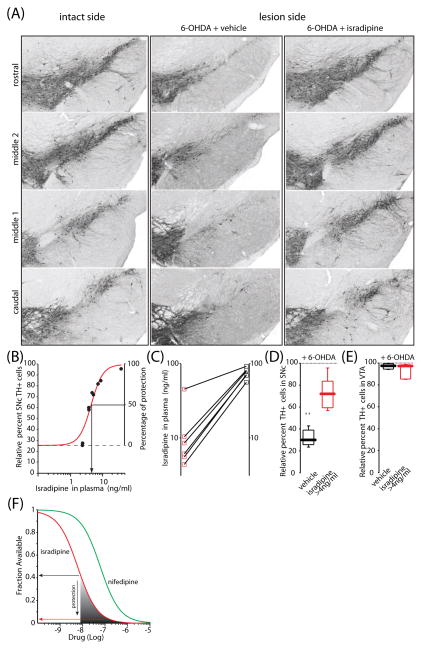
The number of surviving SNc DA neurons was determined by stereological analysis using the optical fractionator principle. To minimize variability in the immunohistochemical staining between samples, the number of surviving neurons was expressed as a percentage of those present on the unlesioned SNc (Kirik et al., 1998). As with striatal TH fibers, isradipine afforded significant protection against 6-OHDA. Protection was seen at all rostro-caudal levels of the SNc (Fig. 3A). As with fibers, the effect of isradipine on cell bodies was dose-dependent. Plots of plasma isradipine concentration and TH cell number were well-fit with the sum of a constant factor (TH cell number with no isradipine) and a Hill function having an IC50=5.0 ng/ml (13 nM) and a slope factor of 3 (Fig. 3B). Plots of the relationship between plasma isradipine concentration and protection in individual mice also consistently revealed a dose-dependence (Fig. 3C).
Fig 3.
Isradipine pre-treatment increased the number of surviving SNc DA cells after 6-OHDA induced degeneration. (A) Microphotographs of coronal sections through the ventral mesencephalon showing TH positive cells on the intact side, 6-OHDA injected group (column “6-OHDA+ vehicle”), and 6-OHDA injected group pretreated with isradipine (column “6-OHDA + isradipine”). Sections show four different levels of the SNc. Note increased number of TH positive cells in isradipine pretreated group (plasma concentration 10.4 ng/ml). (B) The curve shows a nonlinear regression of relative TH+ cells and isradipine plasma concentration with a Hill coefficient of 3 and an IC50=5.0 ng/ml. (C) Protective effect of isradipine at the nigral level was dose-dependent as shown from 6 mice. (D) Box-plots summarizing the relative percent TH positive cells in the vehicle (n=8), and isradipine pretreated group (n=7). Isradipine significantly reduced toxic effect of 6-OHDA at the level of DA cell bodies. Statistical significance in all plots is shown by asterisks and determined using non-parametric test (Mann-Whitney test). Probability (P) threshold for statistical significance was 0.05. (E) Box-plots summarizing the relative percent of TH positive cells in the ventral tegemental area (VTA) after striatal 6-OHDA injection in both vehicle and isradipine pretreated animals (no significant difference is found). (F) Plot of the fraction of L-type calcium channels as a function of isradipine concentration (red line) and of lower affinity antagonist nifedipine (green line) at −60mV. Roughly 60% of Cav1.3 channels are antagonized by ~3 ng/ml of isradipine (~8 nM) which is the threshold for protection against 6-OHDA. Almost complete protection of cell bodies is achieved by 50 ng/ml (~135 nM) of isradipine that antagonizes 95% or more of channels.
To generate a rough estimate of the proportion of Cav1.3 Ca2+ channels antagonized at protective concentrations of isradipine, a model of receptor binding was used. The model was based upon the modulated receptor hypothesis originally proposed by Bean (Bean, 1989) for DHP binding to L-type channels. Bean showed that the affinity of DHPs for L-type channels is voltage-dependent, increasing with depolarization. This model has recently been adapted to compare isradipine and nifedipine (a lower potency antagonist) binding to Cav1.3 channels in SNc DA neurons, assuming an average membrane potential of −60 mV (Surmeier et al., 2010). What this relationship suggests is that at the threshold for protection against 6-OHDA (~ 3 ng/ml, ~8 nM isradipine), about 60% of the Cav1.3 Ca2+ channels are antagonized in SNc DA neurons (Fig. 3F, grey shading). At higher isradipine concentrations, the fraction of the Cav1.3 channels available falls and protection increases. Based upon the plateauing of the dose-response curve for cell bodies near 50 ng/ml (~135 nM), almost complete protection of cell bodies appears to require antagonizing roughly 95% of the Cav1.3 channels (Fig. 3F, red line). This level of channel antagonism by nifedipine or nimodipine (which has a similar affinity for Cav1.3 channels (Bonci et al., 1998)) would appear to require at least an order of magnitude higher concentration than isradipine because of the lower potency of these DHPs.
Discussion
The main finding of this study was that in mice, systemically administration of the DHP L-type calcium channel antagonist isradipine protected striatal dopaminergic terminals and parent SNc cell bodies against intrastriatal injection of the toxin 6-OHDA (2.5 μg). In the absence of isradipine, this insult decreased striatal TH fiber density by ~90% and dopaminergic neurons in the SNc by ~70% within 25 days. The protection afforded by isradipine in this model of PD was evident at all levels of the striatum and mesencephalon. Neuroprotection was dose-dependent, with an estimated plasma isradipine IC50 of 13 nM for cell bodies and 19 nM for terminals. These plasma concentrations are in the range achieved in humans with doses of isradipine within those approved for the treatment of hypertension (Ganz et al., 2005; Shenfield et al., 1990), suggesting that isradipine could be a viable neuroprotective agent in early stage PD.
DHPs are neuroprotective in the 6-OHDA model
Previous studies have shown that in both primates and mice, DHP antagonism of L-type calcium channels protects the cell bodies of SNc dopaminergic neurons against MPTP, another commonly used dopaminergic neuron toxin (Chan et al., 2007; Kupsch et al., 1995; Kupsch et al., 1996). The earlier of the studies used the DHP nimodipine, which has a roughly 40 fold lower affinity than isradipine for Cav1.3 L-type calcium channels (Huber et al., 2000; Sinnegger-Brauns et al., 2009). These channels, rather than the more commonly found channels with a Cav1.2 subunit, have been implicated in pacemaking and vulnerability of SNc DA neurons to toxins (Chan et al., 2007). The difference in DHP potency at Cav1.3 channels could explain the failure of these earlier studies to see protection of striatal dopaminergic terminals, even though cell bodies were protected. In our hands, the estimated IC50 for protection of cell bodies (~10 nM) was lower than that for protection of striatal dopaminergic terminals (~20 nM). Based upon our modeling, these isradipine concentrations will achieve roughly 60% and 80% antagonism of Cav1.3 channels respectively in pacemaking SNc DA neurons. The difference in the sensitivity of the two regions to isradipine could be interpreted in two ways. One interpretation is that terminals are more sensitive to toxins than cell bodies, demanding more complete antagonism of L-type channels to achieve protection. The other is that terminals are more hyperpolarized than cell bodies, diminishing the ability of DHPs to antagonize L-type channels (Bean, 1984). In either case, the reported failure of nimodipine to protect dopaminergic terminals against MPTP could simply be a consequence of the difficulty in achieving an adequate antagonism of Cav1.3 channels with this relatively low potency DHP, rather than the lack of Cav1.3 channel involvement in terminal degeneration.
The same caveat applies to an earlier study reporting that nimodipine did not protect either dopaminergic terminals (as measured by striatal DA levels) or cell bodies following intrastriatal injection of 6-OHDA (Sautter et al., 1997). Although isradipine failed to protect against near total 6-OHDA lesions in our hands (data not shown), this was not the basis for the discrepancy, as their lesions were very comparable to ours. Rather, it is more likely that the apparent discrepancy was due to the reliance upon nimodipine. At the plasma concentrations estimated in the Sautter et al. study (~70 nM), nimodipine will antagonize fewer Cav1.3 channels (~50%) than isradipine at the concentrations found to be protective in our study (Fig. 3B).
Why are DHPs neuroprotective?
All of the toxins commonly used to kill SNc dopaminergic neurons and produce a parkinsonian state, including 6-OHDA, are thought to target mitochondria (Przedborski et al., 2004). Following toxin administration, signs of mitochondrial oxidant stress rise, leading ultimately to the engagement of signaling pathways underlying apoptosis (Mochizuki et al., 1996; Tatton and Kish, 1997; Vila et al., 2001; Vila and Przedborski, 2003). Commonly, manipulations that lower mitochondrial oxidant stress diminish the sensitivity to these toxins (Chan et al., 2007)
Recent work by our group has shown that Ca2+ entry through L-type channels elevates mitochondrial oxidant stress in SNc DA neurons (Guzman et al., 2010). The oxidant stress appears to be a consequence of the metabolic burden created by the need to remove Ca2+ from the cytosolic space by adenosine triphosphate dependent mechanisms. This basal oxidant stress should elevate the sensitivity of SNc DA neurons to mitochondrial toxins. Hence, antagonizing L-type channels should be neuroprotective in toxin-based PD models.
Although it was not explicitly examined in our study, antagonizing L-type Ca2+ channels should diminish the impact of toxins even after they have been administered as long as the interval between the events is relatively short. If the interval between toxin and isradipine administration is stretched to a week, no protection is evident (Schuster et al., 2009).
Are DHPs of potential therapeutic value for early stage PD?
The broader goal of this study was to determine whether there was pre-clinical support for the proposition that isradipine has neuroprotective potential in PD. As outlined above, previous experimental studies in rodent and primate MPTP models support this proposition, but the evidence that DHPs were protective in the 6-OHDA model was lacking. As these are both models of PD, this discrepancy was puzzling. The studies reported here have resolved this issue, pointing to the potency of the antagonist used in the previous studies and the degree of Cav1.3 Ca2+ channel antagonism as critical to protection.
Another key point of our study was a determination of the plasma concentration range in which protection was achieved. DHPs, including isradipine, are approved for human use for the treatment of hypertension (Nelson et al., 1986). In humans, pharmacokinetic analysis has shown ~2 ng/ml (~5 nM) plasma concentrations of isradipine with 5 mg/day (Park et al., 2009), rising to ~8 ng/ml (22 nM) with 10 mg/day (Clifton et al., 1988). These are clearly within the range achieving protection in our studies. At 30 mg/day, the plasma concentration of isradipine rises to around 40 nM – a concentration that should achieve robust protection of SNc dopaminergic cell bodies and terminals (Johnson et al., 2005). As isradipine is lipophilic and effectively crosses the blood-brain barrier, its concentration at SNc dopaminergic neuron membranes could be even higher (Anekonda et al., 2011; Kupsch et al., 1996; Uchida et al., 2000).
The obvious limitation in these studies is that they use a toxin model of PD. Although these models mimic the parkinsonian phenotype, they have limited predictive validity as none of the agents shown to be protective in these models have proven to be unequivocally protective in humans. Nevertheless, it is reasonable to assume that a drug with neuroprotective value in PD will be protective in these models.
Another line of evidence supporting the potential value of isradipine comes from epidemiological studies. DHPs have been used for years to treat hypertension, establishing a database that can be queried. There are three recently published studies that have pursued this question. One small study failed to find any alteration in PD risk with use of Ca2+ channel antagonists (Simon et al., 2010); however, as Ca2+ channel antagonists used to treat hypertension are very heterogeneous in channel specificity and pharmacokinetics, the failure to distinguish between them is a major limitation, particularly in a study of this size. In contrast, two larger, more focused studies have found that DHPs significantly reduce the risk of PD (Becker et al., 2008; Ritz et al., 2010). The latter study of a large Danish population revealed that protection was conferred only by DHPs that crossed the blood-brain barrier, and in this group the effect was substantial (~30% reduction in risk) (Ritz et al., 2010). Given the relatively short (2 year) treatment required for inclusion in this study and the relatively low potency of most DHPs at Cav1.3 channels (as discussed above), this is a striking outcome.
Conclusion
The studies presented show that systemic administration of isradipine is capable of protecting striatal dopaminergic terminals and SNc dopaminergic cell bodies against a slow, progressive insult created by intrastriatal injection of 6-OHDA. The effect of isradipine was dose-dependent spanning a range of plasma concentrations achievable within humans at doses that are approved for the treatment of hypertension. These data add to a growing pre-clinical and epidemiological collection of studies supporting the potential value of isradipine as neuroprotective agent in early stage PD.
Research Highlights.
Using a progressive model of Parkinson’s disease created by intrastriatal 6-hydroxydopamine, the neuroprotective potential of the L-type Ca2+ channel antagonist isradipine was tested.
Isradipine is dihydropyridine approved for human use in the treatment of hypertension.
Systemic administration of isradipine protected both striatal dopaminergic terminals and parent cell bodies.
Protection was dose-dependent and afforded by plasma concentrations of isradipine achievable in humans within FDA guidelines.
Acknowledgments
This work was supported by grants from the MJFF, Hartman Foundation and the National Institutes of Health (NS047085). We thank Nicholas Schwarz, Karen Saporito, Sasha Ulrich and Michael Avram for help with LC-MS/MS analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anekonda TS, et al. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol Dis. 2011;41:62–70. doi: 10.1016/j.nbd.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984;81:6388–92. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. Multiple types of calcium channels in heart muscle and neurons. Modulation by drugs and neurotransmitters. Ann N Y Acad Sci. 1989;560:334–45. doi: 10.1111/j.1749-6632.1989.tb24113.x. [DOI] [PubMed] [Google Scholar]
- Becker C, et al. Use of antihypertensives and the risk of Parkinson disease. Neurology. 2008;70:1438–44. doi: 10.1212/01.wnl.0000303818.38960.44. [DOI] [PubMed] [Google Scholar]
- Bonci A, et al. L-Type calcium channels mediate a slow excitatory synaptic transmission in rat midbrain dopaminergic neurons. J Neurosci. 1998;18:6693–703. doi: 10.1523/JNEUROSCI.18-17-06693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove J, et al. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–94. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–6. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Clifton GD, et al. The pharmacokinetics of oral isradipine in normal volunteers. J Clin Pharmacol. 1988;28:36–42. doi: 10.1002/j.1552-4604.1988.tb03098.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, et al. Calcium channel blockers: an update. Am J Med. 2004;116:35–43. doi: 10.1016/j.amjmed.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Fitton A, Benfield P. Isradipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cardiovascular disease. Drugs. 1990;40:31–74. doi: 10.2165/00003495-199040010-00004. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Ganz M, et al. Comparison of blood pressure control with amlodipine and controlled-release isradipine: an open-label, drug substitution study. J Clin Hypertens (Greenwich) 2005;7:27–31. doi: 10.1111/j.1524-6175.2005.04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichmann M, Mattson MP. Neuronal Calcium Homeostasis and Dysregulation. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Guzman JN, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010 doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, et al. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334:345–8. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Huber I, et al. Conserved Ca2+-antagonist-binding properties and putative folding structure of a recombinant high-affinity dihydropyridine-binding domain. Biochem J. 2000;347(Pt 3):829–36. [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, et al. Kinetic and cardiovascular comparison of immediate-release isradipine and sustained-release isradipine among non-treatment-seeking, cocaine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:15–20. doi: 10.1016/j.pnpbp.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Bean BP. Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci. 2010;30:7401–13. doi: 10.1523/JNEUROSCI.0143-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, et al. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–77. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kupsch A, et al. Pretreatment with nimodipine prevents MPTP-induced neurotoxicity at the nigral, but not at the striatal level in mice. Neuroreport. 1995;6:621–5. doi: 10.1097/00001756-199503000-00009. [DOI] [PubMed] [Google Scholar]
- Kupsch A, et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res. 1996;741:185–96. doi: 10.1016/s0006-8993(96)00917-1. [DOI] [PubMed] [Google Scholar]
- McCormack AL, et al. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–27. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, et al. Histochemical detection of apoptosis in Parkinson’s disease. J Neurol Sci. 1996;137:120–3. doi: 10.1016/0022-510x(95)00336-z. [DOI] [PubMed] [Google Scholar]
- Nelson EB, et al. Antihypertensive activity of isradipine in humans: a new dihydropyridine calcium channel antagonist. Clin Pharmacol Ther. 1986;40:694–7. doi: 10.1038/clpt.1986.246. [DOI] [PubMed] [Google Scholar]
- Oorschot DE. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J Comp Neurol. 1996;366:580–99. doi: 10.1002/(SICI)1096-9861(19960318)366:4<580::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Park JH, et al. Quantification of isradipine in human plasma using LC-MS/MS for pharmacokinetic and bioequivalence study. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:59–64. doi: 10.1016/j.jchromb.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Przedborski S, et al. MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J Bioenerg Biomembr. 2004;36:375–9. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson’s disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38:277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- Ritz B, et al. L-type calcium channel blockers and Parkinson disease in Denmark. Ann Neurol. 2010;67:600–6. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–15. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Sautter J, et al. Degeneration of pre-labelled nigral neurons induced by intrastriatal 6-hydroxydopamine in the rat: behavioural and biochemical changes and pretreatment with the calcium-entry blocker nimodipine. Exp Brain Res. 1997;117:111–9. doi: 10.1007/s002210050204. [DOI] [PubMed] [Google Scholar]
- Schuster S, et al. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol Psychiatry. 2009;65:518–26. doi: 10.1016/j.biopsych.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Shenfield GM, et al. The pharmokinetics of isradipine in hypertensive subjects. Eur J Clin Pharmacol. 1990;38:209–11. doi: 10.1007/BF00265988. [DOI] [PubMed] [Google Scholar]
- Simon KC, et al. Calcium channel blocker use and risk of Parkinson’s disease. Mov Disord. 2010;25:1818–22. doi: 10.1002/mds.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simuni T, et al. Tolerability of isradipine in early Parkinson’s disease: A pilot dose escalation study. Mov Disord. 2010 doi: 10.1002/mds.23308. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, et al. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol. 2009;75:407–14. doi: 10.1124/mol.108.049981. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, et al. The origins of oxidant stress in Parkinson’s disease and therapeutic strategies. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–48. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Uchida S, et al. In vivo specific binding characteristics and pharmacokinetics of a 1,4-dihydropyridine calcium channel antagonist in the senescent mouse brain. Pharm Res. 2000;17:844–50. doi: 10.1023/a:1007512426420. [DOI] [PubMed] [Google Scholar]
- Vila M, et al. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:2837–42. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:365–75. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- West MJ, et al. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]