Abstract
In recent years, there has been a great deal of progress toward understanding the role of the striatum and dopamine in action selection. The advent of new animal models and the development of optical techniques for imaging and stimulating select neuronal populations has provided the means by which identified synapses, cells and circuits can be reliably studied. This review attempts to summarize some of the key advances in this broad area, focusing on dopaminergic modulation of intrinsic excitability and synaptic plasticity in canonical microcircuits in the striatum as well as recent work suggesting that there are neuronal assemblies within the striatum devoted to particular types of computation and possibly action selection.
Overview
The basal ganglia are part of the forebrain circuitry involved in the selection of actions (Redgrave et al., 1999, Wichmann and DeLong, 2003, Cisek and Kalaska, 2010). Diseases of the basal ganglia result in a spectrum of movement and thought disorders, ranging from those characterized by a paucity of action, such as Parkinson's disease (PD), to those producing an ‘exuberance’ of action, such as chorea and dystonia (Mink, 2003).
How the basal ganglia contribute to action selection is poorly understood. It is generally believed that the striatum evaluates competing ‘action plans’ held in cortical networks and then feeds back a recommendation to cortex (through the thalamus) based upon sensory and motivational states, as well as past experience. Dopamine (DA) plays a critical role in this process, both as a modulator of cellular excitability on a moment-to-moment basis and as a modulator of long-term changes in synaptic strength that shape network activity. The review will spend much of the space allotted to it going over how dopamine shapes the function of neurons and canonical circuits in the largest of the basal ganglia nuclei – the striatum. This nucleus receives the richest dopaminergic innervation of all the nuclei in the basal ganglia and has been the most intensively studied. However, dopamine also modulates all of the other nuclei of the basal ganglia and this influence should not be under-estimated. It won't be discussed much here only because there isn't nearly as much known about it. The review concludes with a discussion of recent work characterizing neuronal assemblies in the striatum and their possible modulation by dopamine. These neuronal assemblies, which are dynamically constructed from the canonical circuit elements discussed in the first part of the review, might be the network representation of actions.
The striatal DA innervation
A cardinal feature of the striatum is its dense DA innervation, which arises from the midbrain (Bolam et al., 2000). This dopaminergic input is critical to shaping the activity of striatal networks. This is accomplished through activation of G-protein coupled receptors (GPCRs) for dopamine (DA) receptors distributed along the dendrites and axon terminals of both principal neurons and interneurons. The principal neurons of the striatum are GABAergic spiny projection neurons (SPNs). SPNs constitute as much as 90% of the striatal neuron population. SPNs can be divided into two approximately equally-sized populations based on axonal projections. One population – direct pathway SPNs (dSPNs) – projects axons to the nuclei at the interface between the basal ganglia and the rest of the brain, whereas the other population – indirect pathway SPNs (iSPNs) – projects only to an intermediate basal ganglia nucleus, connecting only indirectly to the interface nuclei (Gerfen and Surmeier, 2011).
Another important way in which dSPNs and iSPNs differ is in their expression of DA receptors. Five G-protein coupled receptors mediate DA signaling (D1-D5). These receptors are grouped into two classes based on the G-protein to which they couple: D1, D5 (D1-like) receptors stimulate Gs and Golf proteins, whereas D2, D3, and D4 (D2-like) receptors stimulate Go and Gi proteins (Neve et al., 2004). Gs and Golf proteins stimulate adenylyl cyclase, elevating intracellular levels of cyclic adenosine monophosphate (cAMP) and activating protein kinase A (PKA). PKA has a broad array of cellular targets, including transcription factors, voltage-dependent ion channels and glutamate receptors (Svenningsson et al., 2004). Gi/o proteins target voltage-dependent ion channels through a membrane-delimited mechanism, as well as enzymes like phospholipase C (PLC) isoforms (e.g.Hernandez-Lopez et al., 2000). Gi proteins also inhibit adenylyl cyclase (Stoof and Kebabian, 1984). Another important feature of the D2-like pathway is its potent modulation by RGS (regulators of G-protein signaling) proteins that are robustly expressed in striatal neurons (Geurts et al., 2003).
All five DA receptor are expressed in the striatum, but D1 and D2 receptors are far and away the most abundant. These two receptors are largely segregated in dSPNs and iSPNs: D1 receptors are expressed by dSPNs, whereas D2 receptors are expressed by iSPNs (Gerfen et al., 1990, Surmeier et al., 1996). This dichotomy was first inferred from the effects of DA depletion on gene expression. D1 receptor agonists restored striatal SP levels and D2 receptor agonists restored ENK levels (Gerfen et al., 1990). In situ hybridization studies confirmed that D1 receptor mRNA and SP colocalized in dSPNs and D2 receptor mRNA and ENK colocalized in iSPNs. As simple as this story was, it didn't align perfectly with electrophysiological studies showing that D1 and D2 receptor agonists seemed both to have effects on individual striatal neurons (Surmeier et al., 1992). The simplest explanation for the physiological studies was that D1 and D2 receptors were co-localized in SPNs. However, the absence of any significant degree of D1 and D2 receptor co-localization was evident in single cell RT-PCR studies (Surmeier et al., 1996). Because these studies were not quantitative, the very modest extent of co-localization found in these studies could be attributable to the detection of low abundance transcripts that had little or no functional impact. This point has been driven home more recently by the development of BAC transgenics in which eGFP or Cre-recombinase are expressed under control of the D1 or D2 promoter (Gong et al., 2003). Examination of these mice has confirmed the stark segregation of D1 and D2 receptor expression in direct and iSPNs (Gertler et al., 2008, Valjent et al., 2009), confirming the original hypothesis advanced by Gerfen (Gerfen et al., 1990).
What then explains the physiological data? There are two obvious alternatives to colocalization. First, because the pharmacological tools are crude and only allow the D1- and D2-like receptors to be discriminated, it could be that SPNs express the less abundant DA receptors (D5, D3, D4). Single cell RT-PCR profiles of SPNs clearly support this view, showing that dSPNs expressed low levels of D3 receptor mRNA and iSPNs expressed low levels of D5 mRNA (Surmeier et al., 1996).
Another possibility that has gained more support in recent years is that DA receptor expressing striatal interneurons play an important role in regulating both types of SPN, leading to indirect responses to a global DA signal. There are four well-characterized classes of striatal interneuron: cholinergic interneurons, parvalbumin-expressing GABAergic interneurons, calretinin-expressing GABAergic interneurons and NPY/nitric oxide-expressing GABAergic interneurons (Tepper et al., 2008). Together, these interneurons constitute about 5-10% of all striatal neurons. One of them, the cholinergic interneuron, co-express D2 and D5 receptors (Bergson et al., 1995, Hersch et al., 1995, Yan et al., 1997) and modulate both SPN populations through muscarinic receptors (Bernard et al., 1992, Yan and Surmeier, 1996). Two other prominent interneurons, the somatostatin/neuropeptide Y (NPY) expressing GABAergic interneuron and the parvalbumin (PV) GABAergic interneuron, express D5 DA receptors (Centonze et al., 2002, Centonze et al., 2003). To complicate matters, the PV interneuron is strongly innervated by globus pallidus neurons that express D2 receptors (Bevan et al., 1998), creating a microcircuit that is influenced by both D1- and D2-class receptors (Wiltschko et al., 2010). As outlined below, there are good reasons to believe that these interneurons play important roles in regulating direct and indirect pathway activity, providing a mechanism by which a ligand for a single receptor class can have broad effects.
An additional complication is that DA terminals themselves express D2-class autoreceptors, making the interpretation of pharmacological manipulations targeting these receptors problematic.
Thus, the striatal circuitry controlling direct and indirect pathways is complex and the impact of DA on these circuits even more so. Nevertheless, in the last decade, considerable progress has been made toward understanding both. To make the review of this work more tractable, five canonical striatal microcircuits will be considered. These basic circuits are found throughout the striatum.
The corticostriatal circuit
The most basic striatal microcircuit is the one formed by glutamatergic cortical pyramidal neurons and SPNs (Bolam et al., 2000). The synapses formed by cortical pyramidal neurons are exclusively on dendritic spines of SPNs. These spines are absent from soma and the most proximal dendrites, rising to a peak density (1-2 per micron) 50-60 microns from the soma and then falling off very gradually in density to the tips of the sparsely branching dendrites (250-400 μm) (Wilson, 1994). Individual cortical axons are sparsely connected to any one SPN, typically making one or two en passant synapses (Parent and Parent, 2006). There is no obvious organization to the cortical synapses on the dendritic tree of SPNs, but this could simply be that this organization is difficult to see, as the striatum lacks the lamination characteristic of other regions where this is apparent (e.g., cerebral cortex).
New techniques are revealing the extent of differences between dSPNs and iSPNs (Lobo et al., 2006, Heiman et al., 2008, Meurers et al., 2009). The functional implications of this dichotomy are only beginning to be understood. One dichotomy that is readily interpretable is in the somatodendritic anatomy (Gertler et al., 2008). In rodents, the total dendritic length of iSPNs is significantly less that that of dSPNs (Fig. 1). This difference is due to iSPNs having on average two fewer primary dendrites than dSPNs. This difference in surface area, in the absence of any obvious difference in spine density, suggests that dSPNs receive roughly 50% more glutamatergic input than iSPNs. This also makes iSPNs more excitable, as judged by both somatic current injection and activation of nominally the same number of corticostriatal axons (Flores-Barrera et al., 2010). Thus, although SPNs have long been thought to a single type of neuron, there are clear differences between dSPNs and iSPNs.
FIGURE 1.
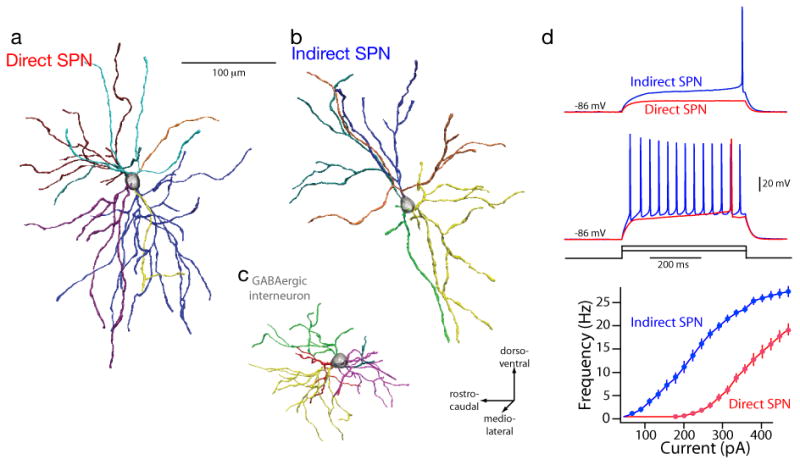
Dichotomous anatomy and physiology of direct and indirect pathway SPNs. Reconstructions of biocytin-filled direct (a) and indirect pathway (b) SPNs from P35–P45 BAC transgenic mice; a GABAergic interneuron (c) is included for comparison. The different dendrites are color coded to allow regions of overlap to be interpreted. The dendrites of SPNs were not oriented in any particular direction or plane. Intrasomatic current injection consistently revealed that indirect pathway SPNs were more excitable than direct pathway SPNs. Current amplitudes at near rheobase for each cell type were used to illustrate the differences; note that these values were remarkably consistent within a population. (d) Summary of the responses of direct and indirect pathway SPNs to a range of intrasomatic current steps; indirect pathway SPNs were more excitable over a broad range of injected currents. From Gertler et al., 2008.
The neuromodulatory effects of DA on dSPN/iSPNs parallel this dichotomy in intrinsic excitability. Both SPNs have a similar core physiological phenotype. At rest, SPNs are dominated by inwardly rectifying, Kir2 K+ channels that hold the membrane potential near the K+ equilibrium potential (∼-90 mV), far from spike threshold (Wilson, 1993, Shen et al., 2007). This is the so-called ‘down-state’. In response to excitatory glutamatergic synaptic input from the cerebral cortex, SPNs depolarize. If this input lacks spatial or temporal convergence, the constitutively open Kir2 K+ channels shunt this synaptic current, minimizing the cellular response. However, if this input is highly convergent, the glutamatergic synapses can overwhelm the Kir2 channels and promote their closure (Wilson and Kawaguchi, 1996). Closure of dendritic Kir2 K+ channels and inactivation of neighboring Kv4 (A-type) K+ channels leads to a dramatic elevation of the input impedance of SPN dendrites and a reduction in their electrotonic length, as revealed with ionic Kir2 channel blockers (Wilson, 1993, Reyes et al., 1998, Day et al., 2008). With this, the SPN somatic membrane reaches a potential near spike threshold. This ‘up-state’ can last hundreds of milliseconds. It is during this up-state that SPNs spike. These spikes are typically not correlated with the transition to the up-state, suggesting that they are driven by an independent synaptic input (Stern et al., 1998).
DA modulates the glutamatergic synapses responsible for the transition to the up-state and the ion channels controlling spiking. The qualitative features of the modulation depend upon which DA receptor is being stimulated. As originally hypothesized on the basis of changes in gene expression induced by DA depletion (Gerfen et al., 1990), D2 receptor signaling impedes the up-state transition and diminishes up-state spiking in indirect pathways SPNs, whereas D1 receptor signaling does precisely the opposite in dSPNs (Surmeier et al., 2007). The responses to suprathreshold corticostriatal stimulation, which elicit plateau potentials similar to in vivo up-states, are modulated by D1 and D2 receptor agonists in the same way (Flores-Barrera et al., 2011). How this happens is still being unraveled, but some elements of the formula have been defined. In the somatic region where spikes are generated, activation of D2 receptor signaling reduces inward, depolarizing currents through Cav1 (L-type) Ca2+ channels and Nav1 Na+ channels, while increasing outward, hyperpolarizing K+ channel currents (Surmeier et al., 1992, Kitai and Surmeier, 1993, Greif et al., 1995, Schiffmann et al., 1998, Hernandez-Lopez et al., 2000, Olson et al., 2005). D2 receptor stimulation also decreases dendritic Ca2+ entry through voltage-dependent channels (Day et al., 2008, Flores-Barrera et al., 2010, Higley and Sabatini, 2010a).
Complementing these alterations in the gating of voltage-dependent ion channels is a reduction in glutamatergic signaling. Although it is not clear whether they are located pre- or postsynaptically (Yin and Lovinger, 2006), D2 receptor stimulation diminishes presynaptic release of glutamate by cortical afferents (Flores-Hernández et al., 1997, Bamford et al., 2004). In large part, this modulation depends upon the production of endocannabinoids and activation of presynaptic CB1 receptors (see below). Activation of D2 receptors also has been reported to decrease AMPA receptor currents in SPNs (Cepeda et al., 1993, Hernandez-Echeagaray et al., 2004). This could be accomplished by D2 receptor triggered dephosphorylation of GluR1 subunits, which should promote trafficking of AMPA receptors out of the synaptic membrane (Hakansson et al., 2006). However, recent work using glutamate uncaging on proximal dendritic spines failed to find any acute effect of D2 receptor stimulation on AMPA receptor currents (Higley and Sabatini, 2010b). What Higley and Sabatini did find was that D2 receptor activation decreased NMDA receptor currents, in keeping with the general pattern of D2 receptor effects.
D1 receptor signaling has almost diametrically opposing effects on dSPNs. D1 receptor stimulation and PKA activation increases somatic Cav1 L-type Ca2+ channel currents and decreases somatic K+ currents (Kitai and Surmeier, 1993, Surmeier et al., 1995, Galarraga et al., 1997, Gao et al., 1997, Hernandez-Lopez et al., 1997). In addition, D1 receptor signaling decreases the opening of Cav2 Ca2+ channels that control activation of Ca2+-dependent, small conductance K+ (SK) channels that slow repetitive spiking in SPNs (Surmeier et al., 1995, Vilchis et al., 2000). All three of these effects serve to increase spiking of dSPNs with somatic depolarization. The only apparently incongruent modulation is the one that was described first showing D1 receptor signaling decreased, excitatory Na+ currents (Calabresi et al., 1987). This observation was confirmed by voltage clamp work showing that D1 receptor signaling reduces Na+ channel availability without altering the voltage-dependence of fast activation or inactivation (Surmeier et al., 1992). Subsequent work has shown that PKA phosphorylation of the pore-forming subunit of the Na+ channel promotes activity dependent entry into a non-conducting, slow inactivated state that can be reversed only by membrane hyperpolarization (Carr et al., 2003, Chen et al., 2006). It is likely that the D1 receptor modulation is mediated by phosphorylation of somatic Nav1.1 channels, as axon initial segment Nav1.6 channels are not efficiently phosphorylated by PKA (Scheuer and Catterall, 2006) and Na+ channels do not extend any significant distance into the dendrites of SPNs (Day et al., 2008). Given this restricted site of action and its time and voltage-dependence, it's reasonable to hypothesize that this effect simply acts as a brake on the spike-promoting effects of D1 receptors on Ca2+ and K+ channels (as well as glutamergic signaling described below).
A number of studies suggest that D1 receptor signaling has positive effects on AMPA and NMDA receptor function and trafficking, in contrast to D2 receptors. For example, D1 receptor activation of PKA enhances surface expression of both AMPA and NMDA receptors (Snyder et al., 2000, Sun et al., 2005, Hallett et al., 2006). The precise mechanisms underlying the trafficking remains unknown but the tyrosine kinase Fyn and the protein phosphatase STEP (striatal-enriched-phosphatase) appear to be important regulators of surface expression of glutamate receptors (Braithwaite et al., 2006). Trafficking and localization might also be affected by a direct interaction between D1 and NMDA receptors (Dunah et al., 2000, Lee et al., 2002, Scott et al., 2006). Rapid D1 receptor effects on glutamate receptor gating have been more difficult to see physiologically. Although PKA phosphorylation of the NR1 subunit is capable of enhancing NMDA receptor currents (Blank et al., 1997), the presence of this modulation in SPNs is controversial. In neurons where the engagement of dendritic voltage-dependent ion channels has been minimized by dialyzing the cytoplasm with cesium ions, D1 receptor agonists have little or no discernible effect on AMPA or NMDA receptor mediated currents (Nicola and Malenka, 1998). However, in SPNs where this has not been done, D1 receptor stimulation appears to rapidly enhance currents evoked by NMDA receptor stimulation (Cepeda et al., 1993). The difference between these results suggests that the effect of D1 receptors on NMDA receptor currents is largely indirect and mediated by voltage-dependent dendritic channels that are taken out of play by more effectively clamping dendritic voltage. Indeed, blocking Cav1.3 L-type Ca2+ channels, which open in the same voltage range as NMDA receptors attenuates the D1 receptor mediated enhancement of NMDA receptor currents (Liu et al., 2004).
In healthy rodents, the ambient level of extracellular striatal DA is thought to be in the low nanomolar range (reflecting the basal pacemaking of DA neurons innervating the striatum), rising into near micromolar ranges with burst spiking of DA neurons (Bass et al., 2010). Because D2 receptors exist primarily in a high affinity state in the striatum, ambient, nanomolar concentrations of DA are sufficient to tonically inhibit iSPNs, and perhaps, several types of interneuron. Conversely, transient elevation of DA into the micromolar range activates low affinity D1 receptors on dSPNs, increasing their excitability. Hence, it appears that under basal conditions, the intrinsic differences in excitability of dSPNs and iSPNs (described above) are counterbalanced by a tonic D2 receptor inhibition of iSPNs. This will tend to balance the responsiveness of direct and indirect pathways to cortical signals - which is a key feature of classical models of basal ganglia function (Albin et al., 1989). This also suggests that any variation in extracellular DA created by drugs or disease states, like Parkinson's disease, might disrupt this balance (see below).
Although intuitively appealing, this simple model fails to take into account DA regulation of striatal interneurons. What is known about this important component of the striatal network is discussed briefly below.
As important as the impact of DA is on the moment-to-moment activity of the corticostriatal network, it also has important part to play in regulating long-term changes in synaptic strength. There are several recent reviews that cover this ground (Surmeier et al., 2007, Kreitzer and Malenka, 2008, Wickens, 2009, Gerfen and Surmeier, 2011), so only the major points bearing on the dichotomy between direct and indirect pathways will be discussed here. At the outset, it must be acknowledged that nearly all of what has been described as corticostriatal synaptic plasticity is truly glutamatergic synaptic plasticity, as corticostriatal and thalamostriatal glutamatergic synapses have not been reliably distinguished. With that caveat in mind, we'll follow the convention and assume that what has been described applies to the corticostriatal synapse formed on a dendritic spine.
The best characterized form of SPN synaptic plasticity is long-term depression (LTD). Unlike the situation at many other synapses, striatal LTD induction requires pairing of postsynaptic depolarization with moderate to high frequency afferent stimulation at physiological temperatures (Lovinger et al., 1993, Kreitzer and Malenka, 2005). Typically for the induction to be successful, postsynaptic L-type calcium channels and mGluR5 receptors need to be co-activated. Both L-type calcium channels and mGluR5 receptors found near glutamatergic synapses on MSN spines, making them capable of responding to local synaptic events (Testa et al., 1994, Carter and Sabatini, 2004, Day et al., 2006, Carter et al., 2007). The induction of LTD requires the postsynaptic generation of endocannabinoids (ECs) (Gerdeman et al., 2002). ECs diffuse retrogradely to activate presynaptic CB1 receptors and decrease glutamate release probability. Both of the abundant striatal ECs – anandamide and 2-arachidonylglycerol (2-AG) – are involved in the metabolic mechanisms responsible for EC production (Giuffrida et al., 1999, Gao et al., 2010, Lerner et al., 2010, Tanimura et al., 2010). A key question about the induction of striatal LTD is whether activation of D2 receptors is necessary. Activation of D2 receptors is a potent stimulus for anandamide production (Giuffrida et al., 1999). However, recent work showing the sufficiency of L-type channel opening in EC-dependent LTD (Adermark and Lovinger, 2007), makes it clear that D2 receptors play a modulatory – not obligatory – role. The real issue is the role of D2 receptors in LTD induction using synaptic stimulation. Studies have consistently found that in iSPNs, D2 receptor activation is necessary (Wang et al., 2006, Kreitzer and Malenka, 2007, Shen et al., 2008). This could be due to the need to suppress A2a adenosine receptor signaling that could impede efficient EC synthesis and LTD induction (Fuxe et al., 2007, Shen et al., 2008). Indeed, Lerner et al. demonstrate quite convincingly that antagonism of A2a receptors promotes EC-dependent LTD induction in iSPNs (Lerner et al., 2010).
The question then is can EC-dependent LTD be induced in dSPNs that do not express D2 receptors? When a minimal local stimulation paradigm is used, LTD does not appear to be induced in these SPNs (Kreitzer and Malenka, 2007, Shen et al., 2008). However, using macroelectrode stimulation, EC-dependent LTD is readily inducible in identified dSPNs (Wang et al., 2006), consistent with the high probability of SPN induction seen in previous work (Calabresi et al., 2007). In this circumstance, LTD induction was dependent upon D2 receptors. But how? There are a couple of possibilities. One is that D2 receptor stimulation reduces DA release through a presynaptic mechanism, preferentially reducing stimulation of D1 receptors that oppose the induction of LTD in direct pathway neurons (Shen et al., 2008). The other possibility is that for LTD to be induced in dSPNs, acetylcholine release and postsynaptic M1 muscarinic receptor signaling must fall (Wang et al., 2006). Recent work by Tozzi et al. has confirmed this earlier inference and extended it to highlight the interaction between A2a and D2 receptors in the regulation of cholinergic interneuron activity.
Long-term potentiation (LTP) at glutamatergic synapses is less well characterized because it is more difficult to induce in the in vitro preparations typically used to study plasticity. Most of the work describing LTP at glutamatergic synapses has been done with sharp electrodes (either in vivo or in vitro), not with patch clamp electrodes in brain slices that afford greater experimental control and definition of the cellular and molecular determinants of induction. Previous studies have argued that LTP induced in SPNs by pairing HFS of glutamatergic inputs and postsynaptic depolarization depends upon co-activation of D1 and NMDA receptors (Calabresi et al., 2007). The involvement of NMDA receptors in LTP induction is clear. The necessity of D1 receptors is another matter. If this were the case, there would be no LTP in iSPNs – an unlikely situation. Again, the advent of BAC transgenic mice has provided an invaluable tool for unraveling this puzzle. In dSPNs, the induction of LTP at glutamatergic synapses is dependent upon D1 DA receptors (Pawlak and Kerr, 2008, Shen et al., 2008). However, this is not the case in iSPNs (Flajolet et al., 2008, Shen et al., 2008). In them, LTP induction required activation of A2a adenosine receptors. These receptors are robustly expressed in the indirect pathway and have a very similar intracellular signaling linkage to that of D1 receptors; that is, they positively couple to adenylyl cyclase and protein kinase A (PKA). Acting through PKA, D1 and A2a receptor activation leads to the phosphorylation of DARPP-32 and a variety of other signaling molecules, including MAPKs, linked to synaptic plasticity (Svenningsson et al., 2004, Sweatt, 2004).
In addition to the convergence of PKA and NMDA receptor signaling (presumably through CaMKII), the induction of corticostriatal LTP also requires activation of receptor tyrosine kinases. Both fibroblast growth factor (FGF) and brain derived neurotrophic factor (BDNF) receptors have been implicated in corticostriatal LTP induction (Flajolet et al., 2008, Jia et al., 2010). How these receptor tyrosine kinases interact with the other obligate signaling pathways involved in LTP induction and whether they are operative at all glutamatergic synapses remains to be determined.
Although most of the induction protocols for synaptic plasticity that have been used to study striatal plasticity are heroic, involving sustained, strong depolarization and/or high frequency synaptic stimulation that induces dendritic depolarization, they do make the necessity of postsynaptic depolarization clear. In a more physiologically relevant setting, what types of depolarization are likely to gate induction? One possibility is that spikes generated in the axon initial segment (AIS) propagate into dendritic regions where synapses are formed. Recent work has shown that STDP is present in SPNs (Fino et al., 2005, Pawlak and Kerr, 2008, Shen et al., 2008). But there are reasons to believe that this type of plasticity is relevant for only a subset of the synapses formed on SPNs. SPN dendrites are several hundred microns long, thin and modestly branched. Their initial 20-30 microns are largely devoid of spines and glutamatergic synapses. Glutamatergic synapse and spine density peaks near 50 microns from the soma and then modestly declines with distance (Wilson, 2004). Because of their geometry and ion channel expression, AIS generated spikes rapidly decline in amplitude as they invade SPN dendrites (as judged by their ability to open voltage-dependent calcium channels), producing only a modest depolarization 80-100 microns from the soma. This is less than half the way to the dendritic tips (Day et al., 2008), arguing that a large portion of the synaptic surface area is not normally accessible to somatic feedback about the outcome of aggregate synaptic activity. High frequency, repetitive somatic spiking improves dendritic invasion, but distal (>100 microns) synapses remain relatively inaccessible, particularly in dSPNs (Day et al., 2008).
In the more distal dendritic regions, what controls plasticity? The situation in SPNs might be very similar to that found in deep layer pyramidal neurons where somatically generated bAPs do not invade the apical dendritic tuft (Golding et al., 2002). In this region, convergent synaptic stimulation is capable of producing a local calcium spike or plateau potential that produces a strong enough depolarization to open NMDA receptors and promote plasticity. Calcium imaging using 2PLSM has shown that there is robust expression of both low threshold Cav3 and Cav1 Ca2+ channels – in addition to NMDA receptors – in SPN dendrites (Carter and Sabatini, 2004, Day et al., 2006, Carter et al., 2007), a result that has been confirmed using cell-type specific gene profiling (Day et al., 2006). If distal dendrites are capable of regenerative activity, they could also be important to the induction of plasticity in SPNs. SPN dendrites do appear to be active in some circumstances (Kerr and Plenz, 2002, Vergara et al., 2003, Carter and Sabatini, 2004, Kerr and Plenz, 2004, Flores-Barrera et al., 2010) and this activity does not appear to arise from the somatic and proximal dendrites of adult SPNs (Wilson and Kawaguchi, 1996). Moreover, from this latter study, it is clear that SPNs are not bistable in the absence of synaptic stimulation.
One possible scenario is that distal SPN dendrites are bistable when NMDA receptors are active during synaptic stimulation. If this is the case, spatial convergence of glutamatergic inputs onto a distal dendrite could induce a local plateau potential capable of pulling the rest of the cell into depolarized state – like an up-state. Doing so would collapse the electrotonic structure of SPNs (Wilson, 1992), enhancing the impact of excitatory input anywhere on the dendrite. The lack of temporal correlation between up-state transitions and EPSP-driven spike generation (Stern et al., 1998) is consistent with this scenario. Indeed, recent work by our group has shown that distal dendrites of both types of SPN are capable of regenerative activity (Plotkin et al., 2011). Using two photon uncaging of glutamate (2PLUG) (Carter and Sabatini, 2004, Higley and Sabatini, 2010b) to probe specific dendritic regions of identified SPNs, it was found that about halfway from the base to the tip of a SPN dendrite, 2PLUG at 10-15 adjacent spines can evoke long lasting plateau potentials that are virtually identical to up-states, at least from the standpoint of somatic membrane potential. These up-states were dependent upon an interaction between NMDA receptors and voltage-dependent Ca2+ channels (Cav3, Cav2.3). The ability of distal, but not proximal, dendrites to generate these plateau potentials appears to reflect their elevated expression of Cav3 channels their increased input resistance (due to dendritic tapering).
This discovery has fundamental implications for how the striatal circuit is wired and controlled by corticostriatal signals. Rather than requiring hundreds or thousands of temporally convergent inputs that are spatially distributed across the dendritic tree of SPNs, up-state transitions require only a handful of temporally and spatially convergent inputs onto a single dendrite. Obviously, this changes the types of cortical signal SPNs will be sensitive to and dramatically increases SPN pattern recognition capacity (e.g.,Poirazi and Mel, 2001). Because regenerative events engage NMDA receptors that are central to LTP induction (and synapse stability), this capacity will promote the clustering of co-active synapses in dendritic microdomains (10-20 micron stretches of dendrite). Because the dendritic plateaus are regulated by D1, D2 and A2a receptor signaling in the expected way, they provide a means by which afferent activity alone can induce changes in the strength of distal synapse, complementing how plasticity is induced in the proximal dendrites. Unpublished work by our group using 2PLUG stimulation of distal dendritic sites has confirmed this hypothesis, showing in iSPNs that co-activation of NMDA, A2a and TrkB receptors leads to potentiation of a subset of distal synapses.
In vivo studies of striatal synaptic plasticity have provided an important counterpoint to the perspectives based upon reduced in vitro preparations. The pioneering work of Charpier and Deniau (Charpier and Deniau, 1997, Charpier et al., 1999) demonstrated that with more intact input, LTP was readily inducible in SPNs. More recently, it has been shown that the sign of synaptic plasticity in SPNs is influenced by anesthetic and presumably the degree of cortical synchronization in corticostriatal projections (Stoetzner et al., 2010). In particular, in barbiturate-anesthetized rats, 5Hz stimulation of motor cortex evokes LTP in the striatum, but in awake animals the same stimulation induced LTD. A challenge facing the field is how to bridge these observations. Because glutamatergic connections are sparse, it is virtually impossible to reliably stimulate a collection of synapses onto a particular SPN dendrite with an electrode in a brain slice. Optogenetic techniques might provide a feasible alternative strategy, but these approaches are not well-suited to high frequency stimulation of axons. It also will be critical to determine the roles of proximal and distal dendritic synaptic input in the regulation of this activity. At present, nothing is known about the origins or properties of these two types of input. For example, it is not clear whether they originate from the same cortical and thalamic ensembles.
The feedback striatal circuit
SPNs have a richly branching recurrent axon collateral that arborizes in the neighborhood of its parent cell body (Kawaguchi et al., 1989). This feedback could provide the substrate for lateral inhibition (Groves, 1983) and has figured prominently in several models of striatal processing (Beiser et al., 1997). However, the functional significance of this feedback circuit has been controversial. In large measure, this is because the synapses formed by recurrent collaterals are largely onto distal dendrites of other SPNs (Wilson and Groves, 1980, Bolam et al., 1983), making their physiological effects difficult to see with a somatic electrode (Jaeger et al., 1994). Using paired patch clamp recordings from neighboring SPNs, it has been possible to more reliably see the effects of collateral activation (Czubayko and Plenz, 2002, Tunstall et al., 2002, Koos et al., 2004, Taverna et al., 2008), but the percentage of synaptically connected neighbors has been small (∼10-15%) in randomly selected SPNs in brain slices. Using D1 and D2 BAC transgenic mice to direct sampling, it was found that although iSPNs project to both themselves and direct pathway SPN dSPNs, direct pathway SPN dSPNs connect essentially only with other direct pathway SPN dSPNs (Taverna et al., 2008). The percentage of SPNs showing demonstrable connectivity doubled when sampling was not random. DA modulates GABA release at recurrent collateral synapses. D2 receptor stimulation decreases GABA release, whereas D1 receptor stimulation increases release (Guzman et al., 2003, Tecuapetla et al., 2007, 2009). Given that D2 receptors should be active at basal extracellular DA levels, the feedback circuit of the indirect pathway should be depressed, disinhibiting dendritic integration. A transient elevation in striatal DA should preferentially activate D1 receptors, leading to enhanced GABA release at collaterals formed between direct pathway SPN dSPNs, enhancing lateral inhibition of dendritic integration. Because D1 receptor stimulation enhances the somatodendritic excitability of dSPNs, the collateral modulation should in principle promote the type of population sculpting typically envisioned for lateral inhibition (Beiser et al., 1997). Conversely, a transient drop in striatal DA should enhance GABA release at collaterals formed by the iSPNs. Because this drop elevates the somatodendritic excitability of indirect pathways SPNs, the collateral modulation should promote a similar type of population sculpting, but one favoring a subset of strongly activated indirect pathway SPN iSPNs (Tecuapetla et al., 2009). The critical gap in this scenario is the lack of compelling data about how dendritic GABAergic synapses shape synaptic integration in SPNs, although this gap is beginning to be filled (Flores-Barrera et al., 2010).
The feedforward corticostriatal circuit
Fast-spiking (FS), PV GABAergic interneurons receive a prominent glutamatergic input from cortical pyramidal neurons and, in turn, convey this activity through perisomatic synapses to both dSPNs and iSPNs (Kita, 1993, Bennett and Bolam, 1994, Koos and Tepper, 1999, Gittis et al., 2010, Planert et al., 2010), thus being strategically located to control neuronal spiking. This feed-forward inhibition is thought to contribute to action selection by suppressing SPN activity in circuits associated with unwanted actions (Kita et al., 1990, Parthasarathy and Graybiel, 1997, Gage et al., 2010). In the striatum and other circuits, FS-PV neurons contribute to fast gamma oscillations (Whittington and Traub, 2003, Berke, 2009), which in the motor system are pro-kinetic, making movements easier (Hutchison et al., 2004). Although both types of SPN are targeted in this circuit, paired recordings in BAC mice have found some preferential connectivity of FS interneurons with dSPNs (Gittis et al., 2010), although this has not been seen in all studies (Planert et al., 2010).
One of the major projections to FS interneurons originates from GPe neurons that are preferentially controlled by iSPNs (Rajakumar et al., 1994, Spooren et al., 1996, Bevan et al., 1998). This feedback loop complements the one formed by collateral projections of SPNs. D2 receptor agonists depress the GABAergic inputs to FS interneurons (Bracci et al., 2002, Centonze et al., 2003, Sciamanna et al., 2009, Gage et al., 2010), which are presumably derived in large measure from GPe neurons which express D2–class receptors (Hoover and Marshall, 2004). This dual control of the feedback by D2 receptors in indirect pathway and GPe neurons suggests that its function is attenuated by basal DA, but capable of rapid facilitation if DA levels fall. This suppression of the GABAergic feedback circuit complements the elevation in FS interneuron excitability mediated by postsynaptic D5 receptors.
Somatostatin (SOM)/neuropeptide Y (NPY)/nitric oxide synthanse (NOS) GABAergic interneurons also form another, less well studied, part of the feedforward corticostriatal circuit (Tepper et al., 2008, Tepper et al., 2010). Because these interneurons respond to intrasomatic current injection with persistent plateau potentials and rebound, low threshold spikes, they are often referred to as PLTS interneurons (Kawaguchi, 1993, Tepper et al., 2010). If these interneurons are like the SOM expressing, Martinotti interneurons of cortex (Wang et al., 2004), their innervation of distal dendrites could make it difficult to accurately judge their importance using somatic electrodes, as with SPN recurrent collaterals. What should allow a more accurate estimation of their functional role is to examine the effect on synaptic integration. An intriguing study by Flores-Barrera et al. (2010) found that cortical stimulation produced a shunting inhibition in iSPNs that reduced summation of concurrent EPSPs, whereas the same stimulation produced a weaker response in dSPNs that was often depolarizing. It is tantalizing to hypothesize, particularly in light of the work of Gittis et al. and Planert et al. described above, that these responses to cortical stimulation were a consequence of engagement of SOM/NPY/NOS interneurons contacting distal dendrites of SPNs. Because these interneurons express D5 receptors, this regulation should be influenced by DA (Centonze et al., 2002).
The feedforward thalamostriatal circuit
The other major glutamatergic projection to the striatum originates in the thalamus (Smith et al., 2004). The synapses formed by this projection are found both on dendritic shafts and spine heads, in the same regions as those formed by the corticostriatal projection. In contrast to the corticostriatal synapses, those formed by thalamic axons have a high release probability, making them well-suited to signaling transient events (Ding et al., 2008). Another major target of this projection is the cholinergic interneuron. Like the corticostriatal feedforward circuit involving FS interneurons, the thalamostriatal projection makes a feedforward connection to SPNs through cholinergic interneurons (Ding et al., 2010). With a burst of thalamic activity like that seen after presentation of a salient stimulus, cholinergic interneurons exhibit a burst-pause pattern of activity that enhances the somatic excitability of both SPNs (Perez-Rosello et al., 2005, Pisani et al., 2007), but preferentially enhances the dendritic excitability of iSPNs by decreasing Kir2 K+ channel opening (Shen et al., 2007). As noted above, reducing Kir2 K+ channel opening increases dendritic input resistance and the ability to sum corticostriatal inputs. The role of DA in regulating the thalamostriatal projection to SPNs has not been systematically explored. However, DA potently modulates the activity of cholinergic interneurons through D2 and D5 receptors. D2 receptors suppress the ongoing pacemaking activity of interneurons (Maurice et al., 2004) and diminished acetylcholine release (DeBoer et al., 1996, Ding et al., 2006). D5 receptors complement this modulation by enhancing GABAergic responsiveness (Yan and Surmeier, 1997, Deng et al., 2007).
Cholinergic interneurons also might enhance FS interneuron GABAergic input to SPNs, as FS interneurons express nicotinic receptors (Koos and Tepper, 2002, Sullivan et al., 2008, Xiao et al., 2009b). As described above, FS interneurons potently inhibit SPNs. However, a direct demonstration of a functional connection between cholinergic interneurons and FS interneurons is lacking (Tepper et al., 2010). Interestingly, a recurrent GABAergic input to cholinergic interneurons does not depend upon nicotinic receptors (Sullivan et al., 2008), suggesting that there are alternative networks containing GABAergic neurons that might influence SPNs.
Striatal cell assemblies
How are these canonical microcircuits assembled to control striatal target structures, like the GP during action selection? This is a daunting question. Hebb created a theoretical framework in which this question could be approached over 60 years ago (Hebb, 1949, Harris, 2005). He posited that long-term synaptic plasticity is the initial stage of a series of processes that store information by shaping the connectivity of brain microcircuits into cell-assemblies (CAs). CAs have been envisioned as the building blocks of sensory and motor representations (Huyck, 2001, Harris et al., 2003, Harris, 2005, Dragoi and Buzsaki, 2006, Pastalkova et al., 2008).
However, demonstrating the existence of CAs in living tissue has not been trivial. To date, the evidence that CAs exist is indirect and based upon the assumption that experience-dependent correlations in spiking reflect the formation of CAs. For example, correlations between the spiking of an individual neuron (or small group of neurons) and field potentials (reflecting aggregate neural activity in a region) is taken as evidence of CAs (e.g., Sakurai, 1996, Berke et al., 2004, Costa et al., 2006, Ballion et al., 2009). Experiments using voltage-sensitive dyes also suggest that groups of neuron can be co-activated in stereotyped ways under certain conditions, indicating the presence of CAs (Grinvald et al., 2003, Grinvald, 2005). Another manifestation of CAs is thought to be the existence of recurring patterns of activity in a population of neurons in response to a single stereotyped input. This recurring, patterned activity is thought to be shaped by activity-dependent, long-term synaptic plasticity of the sort described above (Abeles et al., 2004, Grinvald, 2005, Harris, 2005).
Correlated or synchronized spiking among neurons has been observed in many nuclei (e.g., Petersen and Sakmann, 2000, Doupe et al., 2004, Carrillo-Reid et al., 2008, Li et al., 2010). In some cases, synchronization is on the millisecond timescale (Diesmann et al., 1999, Leger et al., 2005, Robbe et al., 2006). However, in most cases, synchrony is on a slower timescale, leading to some ‘jitter’ in spike timing (Calvin and Stevens, 1968, Shadlen and Newsome, 1994, Grinvald et al., 2003, Kostal et al., 2007). The correlation in spiking is envisioned to arise from activity being propagated through the network in a particular, patterned way – a way that is determined by the elements in the network and the strength of the synaptic connections between them (Grinvald et al., 2003, Doupe et al., 2004, Carrillo-Reid et al., 2008, Pastalkova et al., 2008, Uhlhaas et al., 2009). The strength of the synaptic connections, which dictate the correlations in activity, is hypothesized to be sculpted by experience through the mechanisms of LTP/LTD.
Recurring, patterned activity also has been seen, albeit less frequently (Hebb, 1949, Cossart et al., 2003, Grinvald et al., 2003, Abeles et al., 2004, Grinvald, 2005). These repeated, closed loop activity patterns much arise from networks that have re-entrant or cyclical architecture (Hebb, 1949, Harris et al., 2003, Sporns and Kötter, 2004). Recurrent, patterned activity, also called “phase sequences”, have been observed in cortical circuits (Tsodyks et al., 1999); this kind of activity is hypothesized to be a substrate of working memory (Hebb, 1949, Pastalkova et al., 2008). These types of circuit have the property of compositionality (Hammer, 2003). That is, they possess a “syntax” that allows reconfiguration, resulting in the expansion or contraction of the number of participating neurons (Carrillo-Reid et al., 2009). Compositionality implies that the network contains a modularity that allows small groups of neuron (modules) to be brought into or removed from an active circuit (Bienenstock and Geman, 1995, Hammer, 2003, Abeles et al., 2004). In the case of the striatum, these modules might be the canonical microcircuits described above. Neuromodulators, like DA, that shape synaptic transmission and its integration, could serve to control the excitability of these canonical modules and the compositional properties of striatal CAs (Jáidar et al., 2010). For example, on a short time scale, DA modulation of dendritic excitability and up-state transitions could dictate which SPNs participate in a CA at any one point in time. On a longer time scale, DA modulation of corticostriatal synaptic strength through LTP/LTD mechanisms could determine the SPNs participating in a particular CA.
Are there striatal CAs capable of recurrent, closed loop activity?
From a theoretical standpoint, the striatal network has many of the canonical features required to generate this kind of activity.
First, as described above, synaptic connections within the striatum is capable of undergoing experience-dependent, long-term changes in strength, as required of a Hebbian CA. Although plasticity at the corticostriatal glutamatergic synapse formed with SPNs is the best studied, similar sorts of plasticity exist at other synaptic connections within the striatum (Adermark and Lovinger, 2009, Rueda-Orozco et al., 2009), suggesting that the mechanisms are in place to allow experience to shape connectivity and CA composition.
Second, as outlined above (the feedback striatal circuit) there are recurrent collateral connections between SPNs. This type of connection is necessary to establish neuronal pools, or aggregates, whose synchronous activity underlie network states (Sporns et al., 2002, Grillner, 2006). Whether recurrent collaterals subserve this function in the striatum is controversial, but this might simply be a consequence of our reliance upon a weak outcome measure (e.g., somatic IPSPs) that is not really indicative of their function in an intact network. An indication that this is the case comes from recent work showing that their blockade abolishes the reverberation of network states in the striatum (Carrillo-Reid et al., 2008). Moreover, weakening these connections by DA deprivation (Taverna et al., 2008, Tecuapetla et al., 2009) tends to abolish microcircuit dynamics by leaving a single dominant network state that traps most active neurons (Jáidar et al., 2010).
Third, the striatum possesses strong canonical feedforward and feedback networks involving interneurons (Sporns et al., 2002, Grillner, 2006). Activity in interneurons can be recorded in several network states and they appear to be capable of synchronizing SPNs (Fig. 2) (Carrillo-Reid et al., 2008). As noted above, DA (and, hence, experience) modulates the activity of interneurons and their connections. Reverberation, or repetitive activity patterns could shape network activity in way that enhances the ability of a particular cortical or thalamic input to activate a CA and to reduce variability in the temporal pattern of CA activity; this synaptic sculpting might also reduce the ‘completeness’ of the input pattern needed to activate a CA. A similar scenario has been postulated in the cortex (Feldmeyer et al., 2005, Yoshimura and Callaway, 2005, Yoshimura et al., 2005, Feldmeyer et al., 2006).
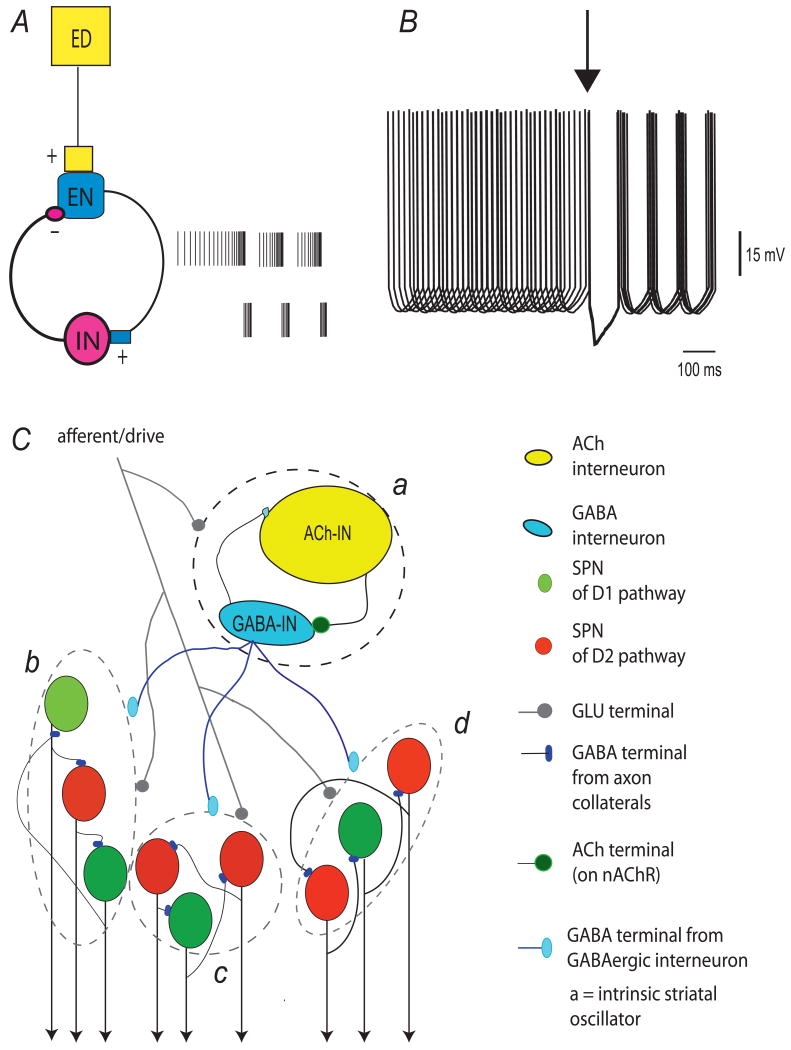
FIGURE 2.
Schematic summary of a hypothetical mechanism for activating spontaneous dynamics in striatal cell assemblies as related with the canonical striatal microcircuit. A. A basic oscillatory mechanism capable of generating recurrent firing patterns. An excitatory drive or input (ED: yelow box) is capable to activate selected fast-ligand gated synapses, e.g., previously potentiated by LTP, in a pool of excitatory neurons (EN, blue symbol) according to intensity/concentration. EN are connected to a pool of inhibitory neurons (IN, pink symbol) through fast-ligand gated synapses such that when active they immediately excite the pool of IN which in turn transiently inhibits the pool of EN. The basic mechanism for recurrent firing depicted is almost counterphase. However, according to variables such as synaptic force and intrinsic membrane currents, phase locking may have phase variances. This basic oscillator has been described in various places, e.g., the subthalamic-pallidum loop (Bevan et al., 2002), the reticular-relay thalamic loop (Steriade, 1999), etc. Recurrent firing may be on as long as ED is on, or else, if pacemaking properties of the neurons that conform the oscillator are turned on (Grillner, 2006). Loops between neurons pools that are mutually connected through fast-ligand gated synapses in the striatal microcircuit can be though as conformed by cholinergic interneurons connecting to GABAergic interneurons through nicotinic ACh receptors (nAChR)(Koos and Tepper, 2002). Some interneuron classes connect back to the cholinergic interneurons through fast-ligand gated GABAergic synapses (Fino and Venance, 2011). B. A basic mechanism to synchronize pools of SPNs through the oscillator. Irregular or regular firing unsynchronized neurons, or silent neurons, may receive a strong inhibitory input from a pool of inhibitory interneurons which is known as making a mesh united by electrical and chemical synapses. A strong inhibition resets the network so that when leaving the IPSP many recipient neurons fire in synchrony (Baufreton et al., 2009). An alternative mechanism arises from the glutamate co-release of cholinergic interneurons (Higley et al., 2011). C. Explaining recurrent and alternating network states in the striatal microcircuit. a) Represents the intrinsic oscillator made up by the cholinergic interneurons and any of several classes of GABAergic interneurons. These interneurons may exhibit pacemaking properties according to context. Dashed circle indicates that the excitatory drive may impinge on many of the neuron pools represented by the symbols. Afferent inputs or a continuous excitatory drive turn on these pools according to intensity and location. b-d) Dashed lines enclose represented pools of SPNs (10-40 neurons) recruited by the oscillator as explained in B. These pools have mixtures of direct and iSPNs (unpublished) which are interconnected according to certain rules (Taverna et al., 2008). The synchronized firing of these neuron pools underlie the networks states (peaks of spontaneous synchronization) that alternate their activity in regular and fixed patterns such as reverberating cycles of activity. That is the three pools represented may alternate their activity in a fixed sequence. They are not anatomically separated as in the scheme, but most probably intermingled (Carrillo-Reid et al., 2008). Alternation of activity among the pools may be explained either by a sequential activation of oscillators, or more likely, to a recovery variable that stops synchronized activity within a pool. This recovery variable is under study but may emerge from intrinsic properties, e.g., repolarizing K+-currents (see above) and inhibitory interconnections among SPNs. In any case the sequence of network states that show a reverberating cycle is orchestrated by GABAergic interneurons, that unlike SPNs, are active during each network state (Carrillo-Reid et al., 2008).
Fourth, the striatum possesses autonomously active, pacemaking interneurons (Grillner et al., 2005b) (Fig. 2A). Pacemaking is one way to establish recurrent CA activity (Grillner 2006) (Fig. 2B). As noted above, striatal cholinergic interneurons are autonomous pacemakers, but other GABAergic interneurons might also possess this property (Koos and Tepper, 1999, Berke et al., 2004, Ding et al., 2010, Tepper et al., 2010) (Fig. 2C). The reciprocal connections between cholinergic and GABAergic interneuron pools (Koos and Tepper, 2002, Sullivan et al., 2008, Fino and Venance, 2011) might create sustained network activity (Fig. 2A). For this to be the case, fast, ligand-gated excitatory synapses must exist at one of the synapse in the network. Nicotinic ACh receptors found on FS interneurons fit this bill (Koos and Tepper, 2002, Xiao et al., 2009a) and other types of fast receptor might be present at cholinergic synapses (Sullivan et al., 2008). Thus, the striatum has the circuit elements necessary to sustain autonomous network activity. It should be noted that autonomous recurrent activity can also be generated by networks were the connections are ‘hard-wired’ or innate. These networks have been called Central Pattern Generators (CPGs) (Brown, 1911, Grillner, 2006). In contrast to CPGs, the connections which underlie autonomous activity in CAs are thought to be “acquired” through experience-dependent synaptic plasticity (Grillner et al., 1981, Guertin and Hounsgaard, 1998).
Although much remains to be done, there is growing evidence that these four canonical elements of the striatum result in to correlated and recurrent CA activity in the striatum. For example, robust recurrent bursting has been seen in the striatum, both in vivo (Herrling et al., 1983) and in vitro (Vergara et al., 2003), following elevation of extracellular NMDA concentration to increase network excitability; the same manipulation (unilateral NMDA administration) induces contralateral turning behavior, establishing a link between recurrent bursting and a motor representation (Ossowska and Wolfarth, 1995). The ability of an unstructured ‘drive’, like elevation of extracellular NMDA concentration, to induce patterned striatal activity is strong evidence of CAs. More recently, by using a combination of electrophysiology and dynamic calcium imaging, which allows the activity of dozens of cells to be monitored simultaneously, we have recently demonstrated that SPNs display correlated spiking in response to a stereotyped input and even display recurrent patterns of activity (Carrillo-Reid et al., 2008). These recurrent patterns of activity followed Hamiltonian or Eulerian trajectories, excluding the possibility that this behavior was fortuitous or random. Furthermore, ex vivo interrogation of striatal tissue from over-trained rodents show clear signs of CA creation (Yin et al., 2009).
Thus, there are good reasons to believe that the canonical circuit elements needed to create CAs are present in the striatum and that striatal CAs can be activated under certain circumstances. What role these CAs play in the striatum remains to be determined. It has been hypothesized that both CPG-like circuits controlling innate movements, like postures and facial expressions, (Ekman et al., 1992, Grillner et al., 2005a) and CAs controlling learned voluntary movements and habits (Graybiel, 2008, Obeso et al., 2008) co-exist within the striatum (or basal ganglia). Testing this conjecture will require methods that allow the activity of CAs to be mapped over time and with changes in experience; it will also be necessary to selectively activate CAs while monitoring behavioral outcomes. Although this experimental situation seemed distant only a few years ago, optical approaches that allow the activity in large collections of neurons to be monitored and the stimulation of collections of neuron based upon molecular phenotype or connectivity are rapidly bringing it within reach.
Highlights.
Dopamine regulates striatal excitability in a cell-type specific manner.
Dopamine modulates synaptic plasticity in different ways.
We review the main types of canonical striatal microcircuits.
Cell assemblies might underlie canonical microcircuits and action selection.
We discuss how striatal cell assemblies might be regulated by dopamine.
Acknowledgments
This work was supported by grants from the NIH (R01 34696), Falk Trust and Hartman Foundation to DJS and CONACyT=154131 to JB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeles M, Hayon G, Lehmann D. Modeling compositionality by dynamic binding of synfire chains. J Comput Neurosci. 2004;17:179–201. doi: 10.1023/B:JCNS.0000037682.18051.5f. [DOI] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Frequency-dependent inversion of net striatal output by endocannabinoid-dependent plasticity at different synaptic inputs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:1375–1380. doi: 10.1523/JNEUROSCI.3842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, P JB. The functional anatomy of basal ganglia disorders. Trends in neurosciences. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Ballion B, Frenois F, Zold CL, Chetrit J, Murer MG, Gonon F. D2 receptor stimulation, but not D1, restores striatal equilibrium in a rat model of Parkinsonism. Neurobiology of disease. 2009;35:376–384. doi: 10.1016/j.nbd.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Vance ZB, Sullivan RP, Bonin KD, Budygin EA. Optogenetic control of striatal dopamine release in rats. Journal of neurochemistry. 2010;114:1344–1352. doi: 10.1111/j.1471-4159.2010.06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Kirkham E, Atherton JF, Menard A, Magill PJ, Bolam JP, Bevan MD. Sparse but selective and potent synaptic transmission from the globus pallidus to the subthalamic nucleus. J Neurophysiol. 2009;102:532–545. doi: 10.1152/jn.00305.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiser DG, Hua SE, Houk JC. Network models of the basal ganglia. Current opinion in neurobiology. 1997;7:185–190. doi: 10.1016/s0959-4388(97)80006-2. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994;62:707–719. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur J Neurosci. 2009;30:849–859. doi: 10.1111/j.1460-9568.2009.06843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends in neurosciences. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Bienenstock E, Geman S. Compositionality. In: Arbib MA, editor. The Handbook of Brain Theory and Neural Networks. Cambridge, MA: The MIT press; 1995. pp. 223–226. [Google Scholar]
- Blank T, Nijholt I, Teichert U, Kugler H, Behrsing H, Fienberg A, Greengard P, Spiess J. The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14859–14864. doi: 10.1073/pnas.94.26.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Somogyi P, Takagi H, Fodor I, Smith AD. Localization of substance P-like immunoreactivity in neurons and nerve terminals in the neostriatum of the rat: a correlated light and electron microscopic study. J Neurocytol. 1983;12:325–344. doi: 10.1007/BF01148468. [DOI] [PubMed] [Google Scholar]
- Bracci E, Centonze D, Bernardi G, Calabresi P. Dopamine excites fast-spiking interneurons in the striatum. J Neurophysiol. 2002;87:2190–2194. doi: 10.1152/jn.00754.2001. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: one STEP at a time. Trends in neurosciences. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TG. The Intrinsic Factors in the Act of Progression in the Mammal. Proceedings of the Royal Society of London Series B, Containing Papers of a Biological Character. 1911;84:308–319. [Google Scholar]
- Calabresi P, Mercuri N, Stanzione P, Stefani A, Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987;20:757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends in neurosciences. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Calvin WH, Stevens CF. Synaptic noise and other sources of randomness in motoneuron interspike intervals. Journal of neurophysiology. 1968;31:574–587. doi: 10.1152/jn.1968.31.4.574. [DOI] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Carrillo-Reid L, Tecuapetla F, Ibanez-Sandoval O, Hernandez-Cruz A, Galarraga E, Bargas J. Activation of the cholinergic system endows compositional properties to striatal cell assemblies. Journal of neurophysiology. 2009;101:737–749. doi: 10.1152/jn.90975.2008. [DOI] [PubMed] [Google Scholar]
- Carrillo-Reid L, Tecuapetla F, Tapia D, Hernandez-Cruz A, Galarraga E, Drucker-Colin R, Bargas J. Encoding network states by striatal cell assemblies. Journal of neurophysiology. 2008;99:1435–1450. doi: 10.1152/jn.01131.2007. [DOI] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Carter AG, Soler-Llavina GJ, Sabatini BL. Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:8967–8977. doi: 10.1523/JNEUROSCI.2798-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Bracci E, Pisani A, Gubellini P, Bernardi G, Calabresi P. Activation of dopamine D1-like receptors excites LTS interneurons of the striatum. Eur J Neurosci. 2002;15:2049–2052. doi: 10.1046/j.1460-9568.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Mahon S, Deniau JM. In vivo induction of striatal long-term potentiation by low-frequency stimulation of the cerebral cortex. Neuroscience. 1999;91:1209–1222. doi: 10.1016/s0306-4522(98)00719-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annual review of neuroscience. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Cossart R, Aronov D, Yuste R. Attractor dynamics of network UP states in the neocortex. Nature. 2003;423:283–288. doi: 10.1038/nature01614. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Czubayko U, Plenz D. Fast synaptic transmission between striatal spiny projection neurons. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15764–15769. doi: 10.1073/pnas.242428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nature neuroscience. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer P, Heeringa MJ, Abercrombie ED. Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur J Pharmacol. 1996;317:257–262. doi: 10.1016/s0014-2999(96)00761-3. [DOI] [PubMed] [Google Scholar]
- Deng P, Zhang Y, Xu ZC. Involvement of I(h) in dopamine modulation of tonic firing in striatal cholinergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3148–3156. doi: 10.1523/JNEUROSCI.5535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesmann M, Gewaltig MO, Aertsen A. Stable propagation of synchronous spiking in cortical neural networks. Nature. 1999;402:529–533. doi: 10.1038/990101. [DOI] [PubMed] [Google Scholar]
- Ding J, Guzman JN, Tkatch T, Chen S, Goldberg JA, Ebert PJ, Levitt P, Wilson CJ, Hamm HE, Surmeier DJ. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nature neuroscience. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Solis MM, Kimpo R, Boettiger CA. Cellular, Circuit, and Synaptic Mechanisms in Song Learning. Annals of the New York Academy of Sciences. 2004;1016:495–523. doi: 10.1196/annals.1298.035. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, Standaert DG. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson's disease. Mol Pharmacol. 2000;57:342–352. [PubMed] [Google Scholar]
- Ekman P, Rolls ET, Perrett DI, Ellis HD. Facial Expressions of Emotion: An Old Controversy and New Findings [and Discussion] Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1992;335:63–69. doi: 10.1098/rstb.1992.0008. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J, Sakmann B. Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. The Journal of physiology. 2006;575:583–602. doi: 10.1113/jphysiol.2006.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Roth A, Sakmann B. Monosynaptic connections between pairs of spiny stellate cells in layer 4 and pyramidal cells in layer 5A indicate that lemniscal and paralemniscal afferent pathways converge in the infragranular somatosensory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3423–3431. doi: 10.1523/JNEUROSCI.5227-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci. 2005;25:11279–11287. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Venance L. Spike-timing dependent plasticity in striatal interneurons. Neuropharmacol. 2011;60:780–788. doi: 10.1016/j.neuropharm.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, Wallach I, Nairn AC, Surmeier DJ, Greengard P. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nature neuroscience. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Barrera E, Vizcarra- Chacón BJ, Bargas J, Tapia D, Galarraga E. Dopaminergic modula-tion of corticostriatal responses in medium spiny projection neurons from direct and indirect pathways. Front Sys Neurosci. 2011;5:15. doi: 10.3389/fnsys.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Barrera E, Vizcarra-Chacon BJ, Tapia D, Bargas J, Galarraga E. Different corticostriatal integration in spiny projection neurons from direct and indirect pathways. Frontiers in systems neuroscience. 2010;4:15. doi: 10.3389/fnsys.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Hernández J, Galarraga E, Bargas J. Dopamine selects glutamatergic excitatory inputs to the neostriatum. Synapse. 1997;25:185–195. doi: 10.1002/(SICI)1098-2396(199702)25:2<185::AID-SYN9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron. 2010;67:466–479. doi: 10.1016/j.neuron.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarraga E, Hernandez-Lopez S, Reyes A, Barral J, Bargas J. Dopamine facilitates striatal EPSPs through an L-type Ca2+ conductance. Neuroreport. 1997;8:2183–2186. doi: 10.1097/00001756-199707070-00019. [DOI] [PubMed] [Google Scholar]
- Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P. Loss of Retrograde Endocannabinoid Signaling and Reduced Adult Neurogenesis in Diacylglycerol Lipase Knock-out Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nature neuroscience. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual review of neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts M, Maloteaux JM, Hermans E. Altered expression of regulators of G-protein signaling (RGS) mRNAs in the striatum of rats undergoing dopamine depletion. Biochem Pharmacol. 2003;66:1163–1170. doi: 10.1016/s0006-2952(03)00447-7. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2223–2234. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nature neuroscience. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annual review of neuroscience. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Greif GJ, Lin YJ, Liu JC, Freedman JE. Dopamine-modulated potassium channels on rat striatal neurons: specific activation and cellular expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:4533–4544. doi: 10.1523/JNEUROSCI.15-06-04533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs--roles for the striatum and pallidum. Trends in neurosciences. 2005a;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Grillner S, Markram H, De Schutter E, Silberberg G, LeBeau FE. Microcircuits in action--from CPGs to neocortex. Trends in neurosciences. 2005b;28:525–533. doi: 10.1016/j.tins.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Grillner S, McClellan A, Sigvardt K, Wallen P, Wilen M. Activation of NMDA-receptors elicits “fictive locomotion” in lamprey spinal cord in vitro. Acta Physiol Scand. 1981;113:549–551. doi: 10.1111/j.1748-1716.1981.tb06937.x. [DOI] [PubMed] [Google Scholar]
- Grinvald A. Imaging input and output dynamics of neocortical networks in vivo: exciting times ahead. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14125–14126. doi: 10.1073/pnas.0506755102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A, Arieli A, Tsodyks M, Kenet T. Neuronal assemblies: single cortical neurons are obedient members of a huge orchestra. Biopolymers. 2003;68:422–436. doi: 10.1002/bip.10273. [DOI] [PubMed] [Google Scholar]
- Groves PM. A theory of the functional organization of the neostriatum and the neostriatal control of voluntary movement. Brain Res. 1983;286:109–132. doi: 10.1016/0165-0173(83)90011-5. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. NMDA-Induced intrinsic voltage oscillations depend on L-type calcium channels in spinal motoneurons of adult turtles. Journal of neurophysiology. 1998;80:3380–3382. doi: 10.1152/jn.1998.80.6.3380. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Hernandez A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J. Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:8931–8940. doi: 10.1523/JNEUROSCI.23-26-08931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson K, Galdi S, Hendrick J, Snyder G, Greengard P, Fisone G. Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. Journal of neurochemistry. 2006;96:482–488. doi: 10.1111/j.1471-4159.2005.03558.x. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer B. Compositionality in neural systems. In: Arbib MA, editor. The Handbook of Brain Theory and Neural Networks. Cambridge, MA: The MIT Press; 2003. pp. 244–248. [Google Scholar]
- Harris KD. Neural signatures of cell assembly organization. Nat Rev Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior; a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Echeagaray E, Starling AJ, Cepeda C, Levine MS. Modulation of AMPA currents by D2 dopamine receptors in striatal medium-sized spiny neurons: are dendrites necessary? Eur J Neurosci. 2004;19:2455–2463. doi: 10.1111/j.0953-816X.2004.03344.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+-conductance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrling PL, Morris R, Salt TE. Effects of excitatory amino acids and their antagonists on membrane and action potentials of cat caudate neurones. The Journal of physiology. 1983;339:207–222. doi: 10.1113/jphysiol.1983.sp014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PloS one. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nature neuroscience. 2010a;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010b;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Marshall JF. Molecular, chemical, and anatomical characterization of globus pallidus dopamine D2 receptor mRNA-containing neurons. Synapse. 2004;52:100–113. doi: 10.1002/syn.20007. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9240–9243. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyck CR. Cell assemblies as an intermediate level model of cognition. In: Wermter S, et al., editors. Emerging Neural Architectures Based on Neuroscience. Berlin: Springer; 2001. pp. 383–397. [Google Scholar]
- Jaeger D, Kita H, Wilson CJ. Surround inhibition among projection neurons is weak or nonexistent in the rat neostriatum. J Neurophysiol. 1994;72:2555–2558. doi: 10.1152/jn.1994.72.5.2555. [DOI] [PubMed] [Google Scholar]
- Jáidar O, Carrillo-Reid L, Hernández A, Drucker-Colín R, Bargas J, Hernández-Cruz A. Dynamics of the Parkinsonian striatal microcircuit: entrainment into a dominant network state. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11326–11336. doi: 10.1523/JNEUROSCI.1380-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Gall CM, Lynch G. Presynaptic BDNF promotes postsynaptic long-term potentiation in the dorsal striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:14440–14445. doi: 10.1523/JNEUROSCI.3310-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Dendritic calcium encodes striatal neuron output during up-states. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1499–1512. doi: 10.1523/JNEUROSCI.22-05-01499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Action potential timing determines dendritic calcium during striatal up-states. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:877–885. doi: 10.1523/JNEUROSCI.4475-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H. GABAergic circuits of the striatum. Progress in brain research. 1993;99:51–72. doi: 10.1016/s0079-6123(08)61338-2. [DOI] [PubMed] [Google Scholar]
- Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res. 1990;536:1–15. doi: 10.1016/0006-8993(90)90002-s. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Surmeier DJ. Cholinergic and dopaminergic modulation of potassium conductances in neostriatal neurons. Adv Neurol. 1993;60:40–52. [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nature neuroscience. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostal L, Lansky P, Rospars JP. Neuronal coding and spiking randomness. The European journal of neuroscience. 2007;26:2693–2701. doi: 10.1111/j.1460-9568.2007.05880.x. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Leger JF, Stern EA, Aertsen A, Heck D. Synaptic Integration in Rat Frontal Cortex Shaped by Network Activity. Journal of neurophysiology. 2005;93:281–293. doi: 10.1152/jn.00067.2003. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Horne EA, Stella N, Kreitzer AC. Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2160–2164. doi: 10.1523/JNEUROSCI.5844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ouyang G, Usami A, Ikegaya Y, Sik A. Scale-free topology of the CA3 hippocampal network: a novel method to analyze functional neuronal assemblies. Biophys J. 2010;98:1733–1741. doi: 10.1016/j.bpj.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, DeFazio RA, Espinosa-Jeffrey A, Cepeda C, de Vellis J, Levine MS. Calcium modulates dopamine potentiation of N-methyl-D-aspartate responses: electrophysiological and imaging evidence. J Neurosci Res. 2004;76:315–322. doi: 10.1002/jnr.20079. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nature neuroscience. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol. 1993;70:1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurers BH, Dziewczapolski G, Shi T, Bittner A, Kamme F, Shults CW. Dopamine depletion induces distinct compensatory gene expression changes in DARPP-32 signal transduction cascades of striatonigral and striatopallidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6828–6839. doi: 10.1523/JNEUROSCI.5310-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Archives of neurology. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Modulation of synaptic transmission by dopamine and norepinephrine in ventral but not dorsal striatum. J Neurophysiol. 1998;79:1768–1776. doi: 10.1152/jn.1998.79.4.1768. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord. 2008;23 3:S548–559. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowska K, Wolfarth S. Stimulation of glutamate receptors in the intermediate/caudal striatum induces contralateral turning. European journal of pharmacology. 1995;273:89–97. doi: 10.1016/0014-2999(94)00671-s. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. The Journal of comparative neurology. 2006;496:202–213. doi: 10.1002/cne.20925. [DOI] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rosello T, Figueroa A, Salgado H, Vilchis C, Tecuapetla F, Guzman JN, Galarraga E, Bargas J. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol. 2005;93:2507–2519. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Sakmann B. The excitatory neuronal network of rat layer 4 barrel cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7579–7586. doi: 10.1523/JNEUROSCI.20-20-07579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends in neurosciences. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Planert H, Szydlowski SN, Hjorth JJ, Grillner S, Silberberg G. Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:3499–3507. doi: 10.1523/JNEUROSCI.5139-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin JL, Day M, Surmeier DJ. Synaptically driven state transitions in distal dendrites of striatal spiny neurons. Nature neuroscience. 2011;14:881–888. doi: 10.1038/nn.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29:779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Rajakumar N, Elisevich K, Flumerfelt BA. The pallidostriatal projection in the rat: a recurrent inhibitory loop? Brain Res. 1994;651:332–336. doi: 10.1016/0006-8993(94)90714-5. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Reyes A, Galarraga E, Flores-Hernández J, Tapia D, Bargas J. Passive properties of neostriatal neurons during potassium conductance blockade. Exp Brain Res. 1998;120:70–84. doi: 10.1007/s002210050379. [DOI] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nature neuroscience. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Rueda-Orozco PE, Mendoza E, Hernandez R, Aceves JJ, Ibanez-Sandoval O, Galarraga E, Bargas J. Diversity in long-term synaptic plasticity at inhibitory synapses of striatal spiny neurons. Learn Mem. 2009;16:474–478. doi: 10.1101/lm.1439909. [DOI] [PubMed] [Google Scholar]
- Sakurai Y. Hippocampal and neocortical cell assemblies encode memory processes for different types of stimuli in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:2809–2819. doi: 10.1523/JNEUROSCI.16-08-02809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer T, Catterall WA. Control of neuronal excitability by phosphorylation and dephosphorylation of sodium channels. Biochemical Society transactions. 2006;34:1299–1302. doi: 10.1042/BST0341299. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Desdouits F, Menu R, Greengard P, Vincent JD, Vanderhaeghen JJ, Girault JA. Modulation of the voltage-gated sodium current in rat striatal neurons by DARPP-32, an inhibitor of protein phosphatase. Eur J Neurosci. 1998;10:1312–1320. doi: 10.1046/j.1460-9568.1998.00142.x. [DOI] [PubMed] [Google Scholar]
- Sciamanna G, Bonsi P, Tassone A, Cuomo D, Tscherter A, Viscomi MT, Martella G, Sharma N, Bernardi G, Standaert DG, Pisani A. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia. Neurobiology of disease. 2009;34:133–145. doi: 10.1016/j.nbd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L, Zelenin S, Malmersjo S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:762–767. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Noise, neural codes and cortical organization. Current opinion in neurobiology. 1994;4:569–579. doi: 10.1016/0959-4388(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nature neuroscience. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends in neurosciences. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren WP, Lynd-Balta E, Mitchell S, Haber SN. Ventral pallidostriatal pathway in the monkey: evidence for modulation of basal ganglia circuits. The Journal of comparative neurology. 1996;370:295–312. doi: 10.1002/(SICI)1096-9861(19960701)370:3<295::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Sporns O, Kötter R. Motifs in brain networks. PLoS Biol. 2004;2:e369. doi: 10.1371/journal.pbio.0020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Edelman GM. Theoretical neuroanatomy and the connectivity of the cerebral cortex. Behav Brain Res. 2002;135:69–74. doi: 10.1016/s0166-4328(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends in neurosciences. 1999;22:337–345. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature. 1998;394:475–478. doi: 10.1038/28848. [DOI] [PubMed] [Google Scholar]
- Stoetzner CR, Pettibone JR, Berke JD. State-dependent plasticity of the corticostriatal pathway. Neuroscience. 2010;165:1013–1018. doi: 10.1016/j.neuroscience.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Two dopamine receptors: biochemistry, physiology and pharmacology. Life sciences. 1984;35:2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Chen H, Morikawa H. Recurrent inhibitory network among striatal cholinergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8682–8690. doi: 10.1523/JNEUROSCI.2411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends in neurosciences. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annual review of pharmacology and toxicology. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Current opinion in neurobiology. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Carrillo-Reid L, Bargas J, Galarraga E. Dopaminergic modulation of short term synaptic plasticity at striatal inhibitory synapses. PNAS USA. 2007;104:10258–10263. doi: 10.1073/pnas.0703813104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Koós T, Tepper JM, Kabbani N, Yeckel MF. Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8977–8990. doi: 10.1523/JNEUROSCI.6145-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koós T, Ibáñez-Sandoval O. Heterogeneity and diversity of striatal GABAergic interneurons. Frontiers in neuroanatomy. 2010;4:150. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Wilson CJ, Koos T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain research reviews. 2008;58:272–281. doi: 10.1016/j.brainresrev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol. 2002;88:1263–1269. doi: 10.1152/jn.2002.88.3.1263. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends in neurosciences. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Vergara R, Rick C, Hernandez-Lopez S, Laville JA, Guzman JN, Galarraga E, Surmeier DJ, Bargas J. Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. The Journal of physiology. 2003;553:169–182. doi: 10.1113/jphysiol.2003.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchis C, Bargas J, Ayala GX, Galvan E, Galarraga E. Ca2+ channels that activate Ca2+-dependent K+ currents in neostriatal neurons. Neuroscience. 2000;95:745–752. doi: 10.1016/s0306-4522(99)00493-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. The Journal of physiology. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional neuroanatomy of the basal ganglia in Parkinson's disease. Adv Neurol. 2003;91:9–18. [PubMed] [Google Scholar]
- Wickens JR. Synaptic plasticity in the basal ganglia. Behavioural Brain Research. 2009;199:119–128. doi: 10.1016/j.bbr.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Dendritic Morphology, Inward Rectification and the Functional Properties of Neostriatal Neurons. In: McKenna T, Davis J, Zornetzer SF, editors. Single Neuron Computation. San Diego: Academic Press; 1992. pp. 141–171. [Google Scholar]
- Wilson CJ. The generation of natural firing patterns in neostriatal neurons. Progress in brain research. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Understanding the neostriatal microcircuitry: high-voltage electron microscopy. Microsc Res Tech. 1994;29:368–380. doi: 10.1002/jemt.1070290507. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Basal ganglia. In: Shepherd GM, editor. The synaptic organization of the brain. Vol. 5. Oxford: Oxford UP; 2004. pp. 361–414. [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. The Journal of comparative neurology. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko AB, Pettibone JR, Berke JD. Opposite effects of stimulant and antipsychotic drugs on striatal fast-spiking interneurons. Neuropsychopharmacology. 2010;35:1261–1270. doi: 10.1038/npp.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM, Lester HA. Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. J Neurosci. 2009a;29:12428–12439. doi: 10.1523/JNEUROSCI.2939-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM, Lester HA. Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009b;29:12428–12439. doi: 10.1523/JNEUROSCI.2939-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19:1115–1126. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature neuroscience. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nature neuroscience. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]