Abstract
Background
Despite observations suggesting a benefit for late opening of occluded infarct-related arteries (IRA) post-myocardial infarction (MI), the Occluded Artery Trial (OAT) demonstrated no reduction in the composite of death, reinfarction and class IV heart failure (HF) over 2.9-yearmean follow-up. Follow-up was extended to determine whether late trends would favor either treatment group.
Methods and Results
OAT randomized 2201 stable patients with IRA occlusion >24hours (calendar days3-28) after MI. Severe inducible ischemia, rest angina, class III-IV HF and 3-vessel/left main disease were excluded. We conducted extended followed up of enrolled patients for an additional 3 years for the primary endpoint and angina (6-year median survivor follow up, longest 9 years, 12,234 patient-years).Rates of the primary endpoint (HR 1.06, 95% CI 0.88-1.28), fatal and nonfatal MI (HR 1.25, 95% CI 0.89-1.75), death and class IV HF were similar for PCI vs. MED groups. No interaction between baseline characteristics and treatment group on outcomes were observed. The vast majority of patients at each follow-up visit did not report angina. There was less angina in the PCI group through early in follow-up; by 3 years the between group difference was consistently <4 patients per 100 treated and not significantly different though there was a trend toward less angina in the PCI group at 3 and 5 years. The 7-year rate of PCI of the IRA during follow up was 11.1% for the PCI group compared to 14.7% for the MED group (HR 0.79, 95% CI 0.61-1.01. p=0.06).
Conclusions
Extended follow up of the OAT cohort provides robust evidence for no reduction of long-term rates of clinical events after routine PCI in stable patients with an occluded IRA and without severe inducible ischemia in the subacute phase post-MI.
Keywords: myocardial infarction, stents, trials
Background
Early reperfusion reduces mortality from acute myocardial infarction (MI). However, the role of late opening of the totally occluded infarct-related artery post MI has been controversial. Despite observational data suggesting a lower event rate for those demonstrated to have an open artery post MI and experimental studies reporting a reduction in adverse left ventricular (LV) remodeling following late reperfusion, the Occluded Artery Trial (OAT) failed to confirm the hypothesis that percutaneous coronary intervention (PCI) post MI in stable patients with a totally occluded infarct-related artery who met entry criteria on calendar days 3-28 reduced the occurrence of death, reinfarctionor hospitalization for class IV heart failure (HF) over 2.9 years mean follow-up compared with optimal medical therapy alone. There was an adverse trend in the PCI group in the secondary endpoint of nonfatal reinfarctions (p=0.08).[1] Angina was reduced in the PCI group through 3 years in the main trial. Rose angina and dyspnea were demonstrated in the quality of life substudy to have been reduced in the PCI group over 24 months with no difference in physical functioning beyond 4 months of follow up.[2] Based on event rates observed over the initial study period, it was determined that power was excellent (80-96%) to show superiority for medical therapy alone with extended follow-up. We therefore conducted an extended follow-up phase of OAT to examine long-term trends.
Methods
The design, methods and primary results of the Occluded Artery Trial have been described in detail previously.[1, 3] In brief, 2,201 patients with total occlusion of the infarct-related artery (IRA) as visualized on coronary angiography performed>24 hours (calendar days 3-28) after myocardial infarction were enrolled in the trial if they met criteria for increased risk of events based on ejection fraction (EF) <50% and/or proximal occlusion of a large vessel (supplying >25% of the left ventricular myocardium). Major exclusion criteria were severe inducible ischemia, angina at rest, class III-IV HF, and significant left main or three-vessel coronary artery disease. Stress testing was recommended prior to randomization unless there was akinesis or dyskinesis in the infarct zone and no disease remote from the IRA. The time window was based on calendar days, not hours, with Day 1 defined as the date of onset of symptoms. The minimum time from MI to qualifying angiography was therefore just over 24 hours.[4]
Patients were randomized to PCI of the occluded IRA with optimal medical therapy [PCI group] or optimal medical therapy alone [MED group]. PCI of vessels other than the IRA was permitted at the discretion of the treating physician. Patients assigned to receive PCI were to undergo the procedure within 24 hours of randomization. A stent was to be used unless placement was not possible or contraindicated. If the IRA had opened spontaneously between the time of qualifying angiography and protocol-assigned PCI, the investigators proceeded with PCI if technically feasible, provided that residual stenosis was >50%. PCI success was judged by the angiographic core laboratory, which reviewed all qualifying and procedural angiograms, as an open artery with <50% residual stenosis and TIMI flow grade 2 or 3. All patients in the trial were to receive optimal medical therapy as outlined in a Procedures Manual, and included aspirin, anticoagulation if indicated, ACE inhibitors, beta blockers and lipid lowering therapy unless such treatment was contraindicated. A thienopyridine was recommended before PCI and for 2-4 weeks for all patients undergoing stent placement. After the publication of CURE and CREDO, [5, 6] clopidogrel was recommended as part of medical therapy in general for one year.
The primary endpoint was the composite of death, reinfarction and class IV HF. The definition of reinfarction required at least two of the following: symptoms, electrocardiogram (ECG) changes and at least two-fold elevation of cardiac biomarkers. Class IV HF required admission to a hospital or a short stay unit. Primary endpoint events were confirmed by an independent Mortality and Morbidity Classification Committee (MMCC) whose members were blinded to treatment assignment. Secondary endpoints included the components of the primary composite, stroke, heart failure, revascularization, and angina, among others.[3]
The primary analysis of the trial included 2,166 patients who had been enrolled through December 2005 with an average follow up of 2.9 years. An additional 35 patients were enrolled through June 2006 during an extended period of enrollment in the nuclear viability ancillary study. All 2,201 patients are included here. Additional support was obtained from the National Heart, Lung and Blood Institute, Grant#: U01 HL062509, to extend follow up of enrolled patients by another three years and conduct detailed analyses of reinfarctions. As patients at individual sites reached the five-year mark(the maximum duration of follow up specified in the original informed consent form), consent for additional follow up or a waiver of written re-consent granted by the site IRB or Ethics Committee was obtained.
All additional follow-up was conducted by telephone. Hospital records were obtained for events. Sites no longer able to follow patients due to staffing issues were encouraged to transfer patients to the Clinical Coordinating Center in the United States or the appropriate regional coordinating center with the approval of the local Institutional Review Board or Ethics Committee. A national or local death index was used to determine vital status (and date of death) at study completion for patients who did not agree to continued follow up or who could not be contacted.
Among 192 of 212 (91%) sites with patients eligible for long term follow-up, 175 sites continued follow up and 17 transferred follow up to the clinical coordinating center or a regional coordinating center. The remaining 20 sites did not participate (total 51 surviving patients).432 patients did not provide consent; vital status was available for 198 of these patients and vital status was unavailable for the remaining 234 patients (12% of survivors to 5 years), 193 of whom had not reached a primary endpoint (102 PCI, 91 MED). Only 1.4% of patients in the trial (14 PCI, 16 MED) were lost to follow-up before the occurrence of a primary endpoint event or 12 months.
The primary endpoint for long-term follow up of the cohort remained the composite of death, reinfarction and hospitalization for class IV HF.[3] Secondary endpoints included the components of the composite in addition to cardiovascular death, class III or IV HF. Sites were requested to indicate whether a reinfarction event was procedure-related and to submit cardiac marker data. All potential reinfarction events submitted by sites that occurred at any time throughout follow up were reviewed centrally by a group of 5 investigators blinded to treatment assignment to permit classification according to the universal definition of myocardial infarction.[7] Sites were not queried routinely for cardiac marker results after revascularization in follow up or for source documents when isolated post-procedure marker elevation was reported; therefore we may not have completely captured silent PCI or coronary artery bypass grafting (CABG)-related reinfarctions defined by marker elevation as a sole criterion, or combined with EKG in the case of CABG.[7]
Sensitivity analyses were performed comparing 5-year outcomes by treatment group among those surviving patients who declined consent for follow up beyond the original study period of 5 years (n=432) as well as among those surviving patients who did provide consent for continued follow up (n=1504). In addition, 5-year outcomes were compared between patients followed at sites with better (≥80%) retention of patients in the long term follow up phase (n=1267) and among those surviving patients followed at sites with <80% retention (n=669). In these analyses, death was not included in the endpoints, because deaths before 5 years were excluded. Finally, we performed a second sensitivity analysis comparing long-term outcomes by treatment assignment among patients who did and did not consent to continued follow up, counting patients who died before 5 years as having provided consent.
Statistical Methods
Baseline characteristics were summarized as frequencies and percentages for categorical variables and as means and standard deviations for continuous variables. Comparisons by assigned treatment were performed using the Chi square/Fisher's exact test for categorical variables and the Student t-test for continuous variables. Estimates of the cumulative event rates were calculated by the Kaplan-Meier product-limit method [8, 9] and groups were compared by the log-rank test. Hazard ratios and 95% confidence intervals were calculated by Cox proportional hazards regression models.[10] Interaction tests of treatment by pre-specified baseline characteristics were performed by Cox proportional hazards regression models, including the following terms: treatment, baseline characteristic and interaction. To generate the covariate-adjusted HR, we used a Cox proportional hazards regression model with nine baseline variables for the prediction of the long term follow-up primary outcome. These variables were chosen by backward elimination and were the only variables remaining in the final model with p<0.01. A test of treatment interaction with a composite of the nine baseline variables was performed by ranking patients on the Cox predictors, forming 3 groups (tertiles) and evaluating treatment HR within each risk tertile. The 7-year event rates are presented because the number of patients followed for more than 7 years was small. Data for patients lost to follow-up were censored as of the time of the last contact. This last contact occurred at 5 years from randomization for patients who declined consent for extension of follow up. Analyses were performed according to the intention-to-treat principle, except for an as-treated analysis.
To control for the Type I error rate, it was pre-specified in the study protocol that a p-value of less than or equal to 0.01 would be considered as showing evidence of differences in secondary analysis.
SAS version 9.2(SAS Institute, Cary, NC) was used for statistical analyses.
Power considerations
The power at the end of the additional follow up, conditional on the observed event rates, was projected to be 80-96% (based on average event rates over years 4-5 or 3-5, respectively) to detect a significant difference between groups at the end of Year 8with a log-rank test (two tailed) at alpha=0.05.
Results
The addition of the 35 patients in the OAT NUC extension phase did not affect baseline characteristics, which were previously published.[1] The average age was 58.6 years. The cohort was comprised of 22% women, 21% patients with diabetes, 11% with prior history of MI, 22% with prior angina. The mean left ventricular ejection fraction was 47.7%. Q waves were noted on the index MI/ECG in 67%.The majority (83%) had single vessel coronary artery disease. The IRA was the LAD in 36%. The median time from MI to randomization was 8 days (IQR, 5-16 days); 331 (15%) were randomized ≤3 days post-MI. The median time from MI to PCI of the IRA among PCI-assigned patients was 9 days (IQR, 5-17 days).
Duration of Follow Up
Patients were followed up to 9 years; median 5.8 years[IQR, 4.5-7.1] (6.1 years[IQR, 5.0-7.4] for survivors), with total follow up of 12,234 patient-years. Extended follow up increased the number of surviving patients followed for ≥5 years from 163 to 1388 and increased the number of primary endpoint events by 148 (49%) and deaths by 132 (77%).
Primary Outcome
The primary outcome (the first occurrence of death from any cause, nonfatal myocardial infarction or class IV HF) occurred in 230 patients in the PCI group versus 219 patients in the MED group with similar seven-year cumulative event rates (see Table 1, Figure 1). In an as-treated analysis comparing the 953 patients in the PCI group in whom PCI was adjudicated to be successful with the 1070 patients in the medical therapy group who did not cross over to PCI within 30 days after randomization, there was no difference between groups for the primary endpoint [HR 1.04, 95%CI 0.86 -1.27, p=0.68].
Table 1. Primary and Secondary Outcomes.
Total Number of Events for full long term follow-up – 7-year life table rates
| PCI (n=1101) |
MED (n=1100) |
||||||
|---|---|---|---|---|---|---|---|
| n | 7yr rate | n | 7yr rate | p-value | HR | 95%CI | |
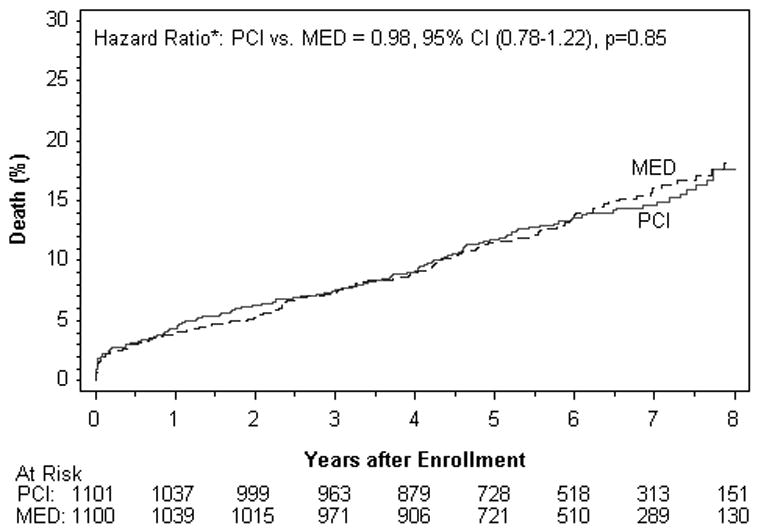
| Death | 150 | 14.6 | 153 | 16.0 | 0.85 | 0.98 | 0.78-1.22 |
| CV Death | 77 | 8.0 | 81 | 8.0 | 0.76 | 0.95 | 0.70-1.30 |
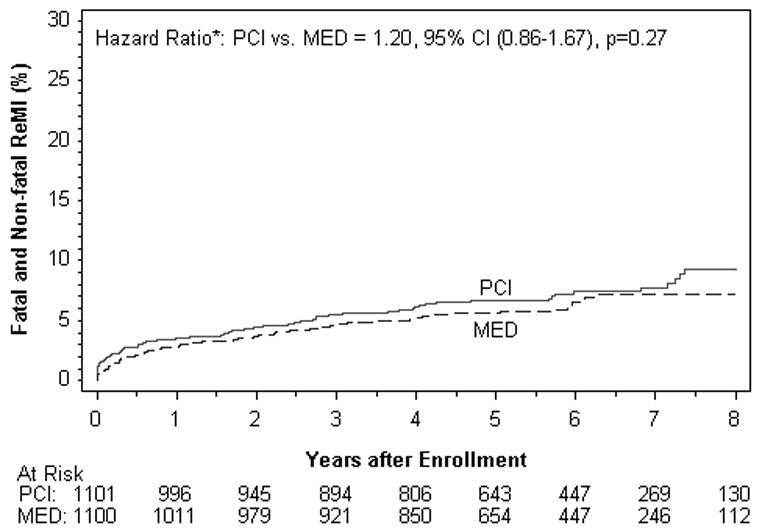
| Fatal or Non Fatal MI | 77 | 7.7 | 65 | 7.2 | 0.27 | 1.20 | 0.86-1.67 |
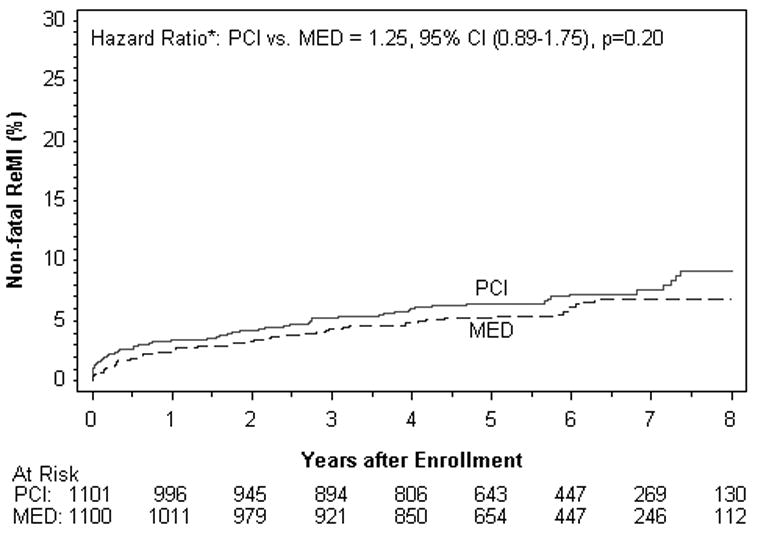
| Non Fatal MI | 75 | 7.5 | 61 | 6.8 | 0.20 | 1.25 | 0.89-1.75 |
| Death or MI | 212 | 20.6 | 199 | 21.3 | 0.44 | 1.08 | 0.89-1.31 |
| HF IV | 51 | 5.2 | 53 | 5.4 | 0.87 | 0.97 | 0.66-1.42 |
| HF III-IV | 76 | 7.4 | 74 | 7.5 | 0.86 | 1.03 | 0.75-1.42 |
| Death, MI, HF IV* | 230 | 22.3 | 219 | 22.9 | 0.51 | 1.06 | 0.88-1.28 |
| Death, MI, HF III-IV | 249 | 23.8 | 235 | 24.4 | 0.43 | 1.07 | 0.90-1.28 |
| Revascularization** | 212 | 21.3 | 252 | 25.6 | 0.03 | 0.81 | 0.68-0.98 |
| Universal definition MI | 95 | 9.4 | 74 | 8.0 | 0.08 | 1.31 | 0.97-1.77 |
| Death, Universal definition MI, HF IV | 236 | 22.9 | 221 | 23.1 | 0.37 | 1.09 | 0.91-1.31 |
Primary Outcome
Excluding Protocol PCI
CV = cardiovascular; HF = heart failure; MI = myocardial infarction
Figure 1.
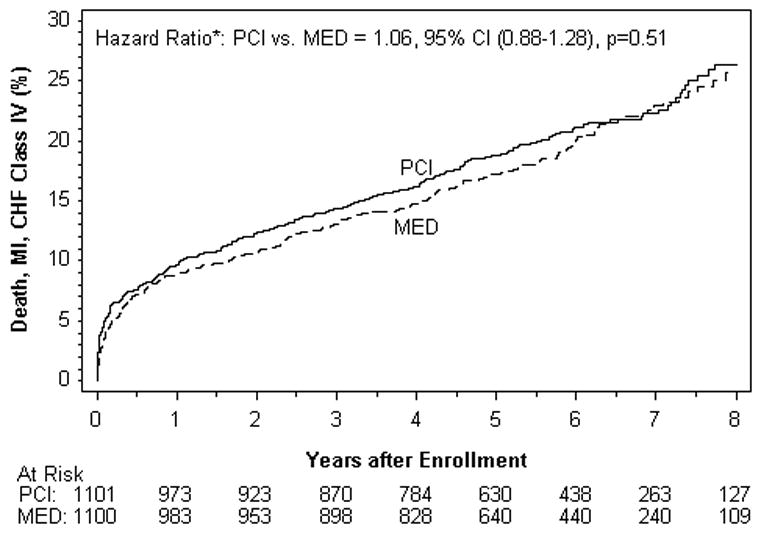
Kaplan–Meier Curves for the Primary End Point, According to Intention-to-Treat Analysis.
Reinfarction events are those confirmed to meet the OAT definition (see methods).
Risk factors for the primary endpoint on multivariate analysis included age [HR 1.14 per 10 years older, p=0.006]; history of diabetes at study entry [HR 1.70, p<0.0001]; lower eGFR [HR 1.08 per 10 ml/min/1.73 m2 decrease, p=0.002); lower EF at baseline [HR 1.34 per 10 percentage points lower, p<0.001]; history of cerebrovascular disease [HR 1.81, p=0.001], history of PCI before study entry [HR 1.70, p=0.002];shorter time from qualifying MI to randomization [HR=1.03 p<0.0001]; history of peripheral vascular disease [HR 1.74, p=0.002] and HF at baseline [HR 1.41, p=0.0006], which was defined as one or more of the following: history of HF prior to randomization, rales on examination, S3 gallop on examination, Highest Killip class >1 during index MI prior to randomization, Highest NYHA class >I prior to index MI, or NYHA Class II at randomization.
Secondary Outcomes (Table 1, Figure 2)
Figure 2.
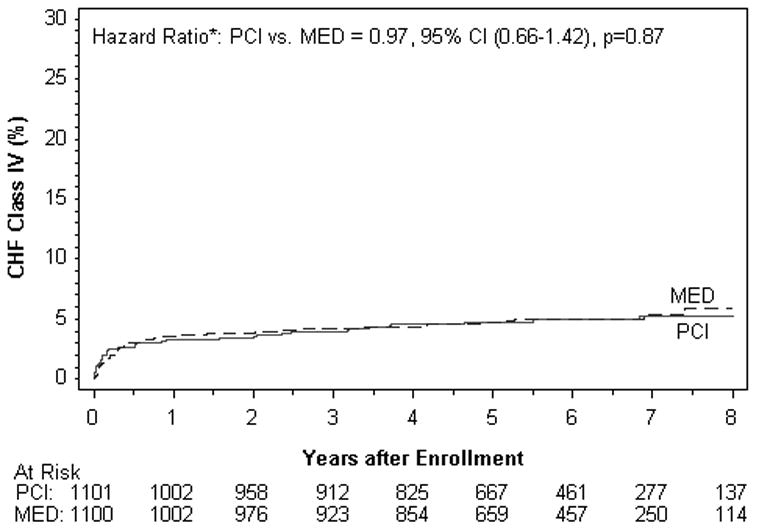
Kaplan–Meier Curves for the Secondary End Points, According to the Intention-to-Treat Analysis.
Figure 2A. Death (%).
Figure 2B. Fatal and Nonfatal Reinfarction (%).
Reinfarction events are those confirmed to meet the OAT definition (see methods).
Figure 2C. Nonfatal Reinfarction (%).
Reinfarction events are those confirmed to meet the OAT definition (see methods).
Figure 2D. Class IV Heart Failure (%).
There were no differences between treatment groups in the individual endpoints of death, reinfarction, class IV HF, cardiovascular death, class III-IV HF, or any of the other composite endpoints examined in the intent to treat or as-treated analysis. Among the reinfarctions, there were only 7 peri-PCI reinfarction events, one of which occurred in the MED group. All 7 met symptom and/or ECG criteria plus elevation of CK-MB to >3× the upper limit of normal. When the universal definition of MI was used, a total of 169site determined MI events were confirmed compared to 142 that met OAT criteria. The reinfarction rates according to the universal definition of myocardial infarction were similar for PCI vs. MED (HR 1.31, 95% CI 0.97-1.77 p=0.08) as was true for the composite of death, reinfarction by the universal definition and class IV HF (HR 1.09, 95% CI 0.91-1.31 p=0.37).
There was a trend toward a lower rate in the PCI group of PCI other than that specified by the protocol. Non-protocol PCI was performed during follow up in 179 patients assigned to PCI vs. 218 in MED, p=0.03; see Figure 3A. Coronary artery bypass grafting was performed in 47 patients in each group during follow up. The reason for non-protocol revascularization was new MI or unstable angina in 43% of cases, with no difference between treatment groups (p=0.24). Among 397 non-protocol PCIs, 152 (38%) did not include PCI of the IRA. There was a trend toward less PCI of the IRA during follow up in the PCI group, but this difference was not statistically significant (HR 0.79, 95% CI 0.61-1.01, p=0.06, figure 3B). Among 135 non-protocol PCIs performed on the IRA over the follow up period in the MED group, 22 occurred after a primary endpoint event.
Figure 3.
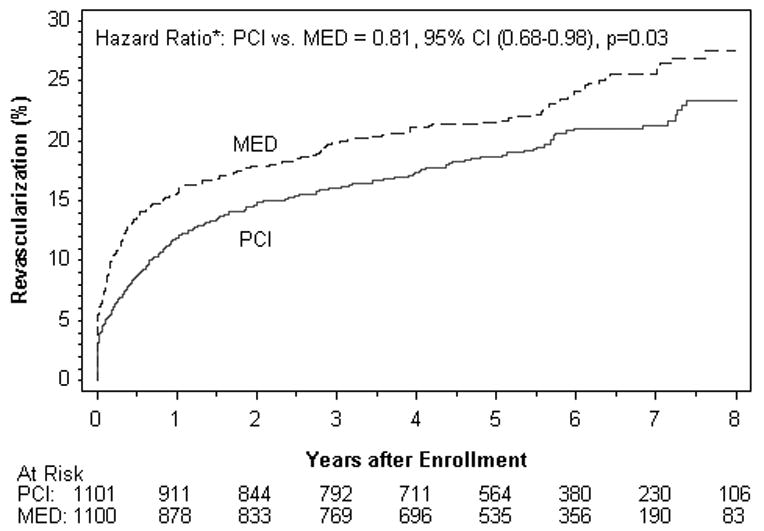
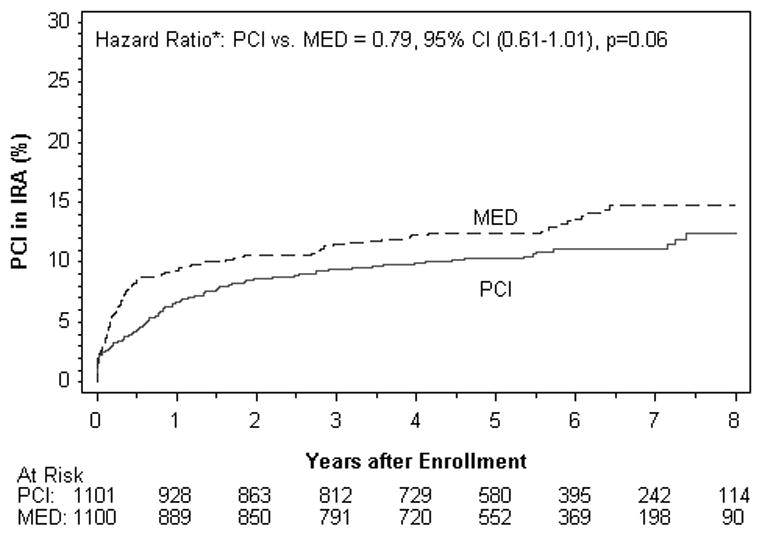
Kaplan-Meier curves for non-protocol revascularization by treatment assignment.
Figure 3A. Non-protocol PCI of any vessel (IRA or non-IRA, percutaneous or surgical).
Figure 3B. Non-protocol PCI of the IRA by treatment assignment (all indications)
Among 135 non-protocol PCIs performed on the IRA over the follow up period in the MED group, 22 occurred after a primary endpoint event.
Angina (Table 2) and Functional Status
Table 2.
Angina as reported at each follow up visit, by treatment group.
| PCI | MED | p-value | |||
|
|
|
|
|||
| n | % | n | % | ||
| 4 months | 192/1030 | 18.6 | 261/1042 | 25.0 | 0.0004 |
| 1 year | 165/1010 | 16.3 | 219/1006 | 21.8 | 0.002 |
| 2 years | 132/952 | 13.9 | 165/948 | 17.4 | 0.03 |
| 3 years | 88/865 | 10.2 | 117/877 | 13.3 | 0.04 |
| 4 years | 98/768 | 12.8 | 106/790 | 13.4 | 0.70 |
| 5 years | 73/671 | 10.9 | 97/662 | 14.7 | 0.04 |
| 6 years | 43/431 | 10.0 | 58/437 | 13.3 | 0.13 |
| 7 years | 26/277 | 9.4 | 29/253 | 11.5 | 0.43 |
| 8 years | 16/153 | 10.5 | 10/132 | 7.6 | 0.40 |
At each time point of follow up, the vast majority of patients did not report angina. There was less angina in the PCI group through 1 year; thereafter the between group difference was consistently <4 patients per 100 treated and not significantly different, though there was a trend toward less angina in the PCI group at 2,3 and 5 years (see Table). The relationship over time between revascularization and angina was complex; most patients with angina did not undergo revascularization, and some patients developed angina after revascularization.[11] There were no differences between treatment groups in the presence of heart failure symptoms or New York Heart Association (NYHA) functional class at any time during follow up (data not shown).
Medication use during follow up
Use of cardiovascular medications did not differ between treatment groups at any follow up visit with the exception of clopidogrel, which was used more commonly in the PCI group through the 12 month visit. After this time, use was similar between treatment groups at approximately 10-12%. Use of aspirin, beta blockers, lipid lowering agents and angiotensin converting enzyme inhibitors was high throughout follow up.
Subgroup analysis
There was no interaction between any baseline patient characteristic and treatment assignment on the primary endpoint. There was also no interaction between treatment assignment and risk defined as a continuous measure (p=0.81) nor risk tertile (p=0.71) (Figure 4).
Figure 4.
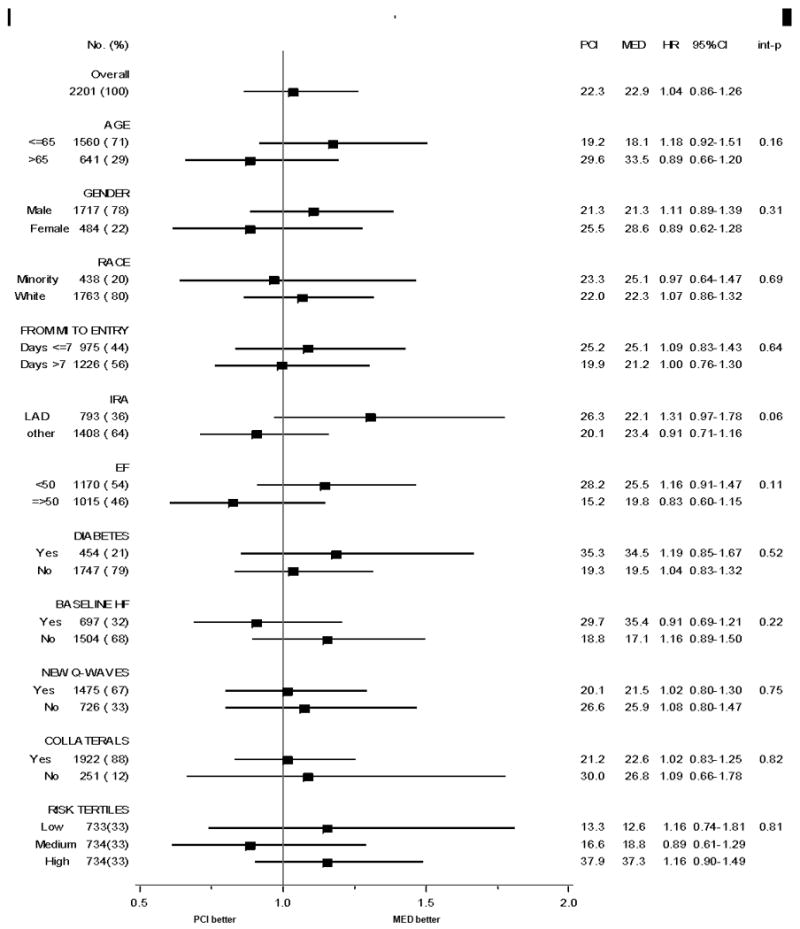
Subgroup analysis of 7-year primary endpoint rate by treatment assignment, with interaction testing.
The hazard ratio (PCI vs.MED) for the primary endpoint was 0.99 (95% CI, 0.62-1.56) for 331 patients enrolled within 3 days of MI onset and 1.05 (0.86-1.30) among 1870 patients enrolled >3 days from MI onset, interaction p=0.80.
The hazard ratio (PCI vs.MED) for the primary endpoint for the subset of patients with proximal LAD occlusion (n=271) was 1.58 (95% CI 0.93.2.69).
The hazard ratio (PCI vs. MED) for the primary endpoint was 1.08 (95% CI 0.78-1.49) among 449 patients with EF <40% and 1.00 (95% CI 0.79-1.27) among 1736 patients with EF ≥40%.
Baseline HF was defined as one or more of the following: history of HF prior to randomization, rales on examination, S3 gallop on examination, Highest Killip class > 1 during index MI prior to randomization, Highest NYHA class > I prior to index MI, or NYHA Class II at randomization.
Reinfarction events are those confirmed to meet the OAT definition (see methods).
The hazard ratio (PCI vs. MED) for the primary endpoint was 1.08 (95% CI 0.60-1.94) for 240 patients who had ischemia a pre-randomization stress test, 1.13 (95% CI 0.64-1.99) for 358 patients with no ischemia and 1.04 (95% CI 0.85-1.29) for 1603 patients who did not have a pre-randomization stress test. Stress testing was required unless there was akinesis or dyskinesis of the infarct zone.
Sensitivity Analysis
No differences were observed in the primary outcome or any secondary outcomes between treatment groups among patients who did or did not consent to follow up after 5 years, whether or not deaths before 5 years were included in the analysis. Similarly, there were no differences in the primary outcome or any secondary outcomes by treatment groups among patients followed at sites with higher (≥80%) or lower (<80%) rates of obtaining consent from patients for continued follow-up. Women were less likely than men to consent to continued follow up (p=0.01) but older (>65 years) and younger patients were equally likely to consent. There were no differences by treatment group in outcomes when sites were divided according to numbers of patients enrolled. There was also no difference in treatment effect on the primary endpoint or any secondary endpoint after covariate adjustment.
Effect of type of stent on outcomes
During the period 2003-2006 (after FDA approval of drug-eluting stents in the US), 393 patients who were assigned to PCI received a bare metal stent (BMS) to the IRA and 79 patients received a drug-eluting stent (DES). There was no difference in the primary outcome in these patients based on the type of stent implanted (6-year event rate 20.4 for DES vs.18.9 for BMS, HR 1.20, 95% CI 0.68-2.1, p=0.53). Rates of nonfatal reinfarction(HR 2.12 for DES vs.BMS, 95% CI 0.93-4.83, p=0.07) and death or reinfarction(HR 1.11, 95% CI 0.61-2.01, p=0.74) were also similar between groups based on type of stent used.
Discussion
Additional follow up and accrual of 194 additional events in the Occluded Artery Trial cohort with over 12,000 total patient-years provides robust evidence that there was no long term benefit to a strategy of routine PCI of the totally occluded IRA in patients who were clinically stable in the early post-MI period.
The large majority of stents placed during protocol PCI were bare-metal stents and in most patients, clopidogrel was stopped by the four-month visit. Though we cannot exclude the possibility that a longer duration of clopidogrel use in PCI-assigned patients would have resulted in a lower rate of stent-related reinfarction events, we believe this should not have led to an advantage of assignment to PCI or MED because the rates of non-stent-related (types 1-3) MI were similar between groups and the large majority of reinfarction events were spontaneous, as determined when OAT MI events were classified according to the universal definition of MI.[12] In fact, greater use of clopidogrel in the PCI group might have been expected to lead to a lower rate of reinfarction in that group, based on the known benefit of clopidogrel post ACS.[6, 13]
Additional follow-up did not unmask any effect of routine PCI on the outcomes of heart failure or mortality. It had been hypothesized that the apparent attenuation of remodeling associated with assignment to the PCI group in a subset of patients in the TOSCA-2 angiographic ancillary study over the year following randomization [14] might result in diverging heart failure rates later in follow up. This hypothesis is disproved by the data.
It should be noted that there was no difference by treatment assignment in one-year change in EF, an indicator of viable myocardium at baseline, among 389 patients who had serial measurements of LV function within the nuclear viability and angiographic ancillary studies to OAT;[14, 15] EF improved from baseline to one year in 66% of these patients, and by ≥5 points in 73% of those. In addition, there was no difference in one-year change in LV volume in the nuclear viability ancillary study of OAT, which was smaller than the angiographic study but had complete volume data.[15] The viability study confirmed that most OAT patients (70%) had at least moderately retained viability in the infarct zone and that PCI did not affect EF or volume changes compared to MED among patients with viability. This finding is consistent with the results of the multicenter Surgical Treatment for Ischemic Heart Failure, which found no interaction between the effect of revascularization on death or cardiovascular hospitalization whether viability was or was not present at baseline, in 601 patients with LVEF ≤35%.[16] In contrast, prior studies which reported an association between viability and improvement in EF and outcome with revascularization were observational and did not have a randomized comparator group either treated with an initial strategy of medical therapy alone.[17-21]
Analyses of subgroups, including those at highest risk, and as treated analyses of those with successful PCI and MED-assigned patients who did not receive PCI of the IRA, as well as by type of stent received yielded findings remarkably consistent with the primary analysis.
The early significant benefit of assignment to PCI on the prevalence of angina was not durable at most long term follow up time points, although there was a trend toward less angina at 5 years with an angina free difference between treatment groups of less than 4 per 100. Angina was reported in a minority of patients in follow up. The likelihood of angina decreased over time in both groups with only 10-15% reporting angina in years 3-7. We have previously shown that revascularization outside of the OAT protocol was not the reason for loss of the early benefit on angina in the PCI group and that there was no difference between treatment groups in the indication for non-protocol revascularization.[11] In addition, treatment with medical therapy was less expensive than PCI in OAT with a marginal quality of life difference between groups in very early follow up and no difference in quality of life thereafter.[2] Rose angina and dyspnea were reported in a minority of patients in the quality of life substudy of OAT; both symptoms were less common in the PCI arm through 24 months.[2] These results suggest that revascularization should be used selectively for management of angina in patients with persistent total occlusion of the infarct artery as were enrolled in this trial.
The results presented here apply only to patients who would be eligible for the trial, that is, patients with persistent total occlusion of the IRA >24 hours after myocardial infarction who were clinically stable, without rest angina or severe inducible ischemia, class III-IV HF, or significant left main or three-vessel coronary artery disease. The majority of patients had single vessel CAD. This study has several limitations. Approximately 20% of patients surviving to 5 years did not consent to additional follow up. However, vital status was available for 46% of these patients. Clopidogrel was not continued in all patients for one year. Outcomes in patients with recent STEMI treated with PCI or medical therapy may be significantly improved with the more potent platelet receptor ADP antagonist prasugrel.[22] The 2009 ACC/AHA guidelines include a class I recommendation for thienopyridines for at least one year in patients who have undergone stenting with a DES and “for a minimum or 1 month and ideally up to 12 months” for patients who have undergone stenting with a BMS.[23] Prolonged (one-year) treatment with thienopyridines was not required in OAT and was not utilized in 85% of patients. Although most reinfarctions occurred after one year, the extent to which this affected the results of the trial cannot be determined. Routine DES use might have been more effective in reducing angina, which was uncommon in follow up; only 8% of PCI patients in OAT received DES.[24]
Current ACC/AHA guidelines which include a recommendation against routine PCI of the totally occluded IRA “greater than 24 hours after STEMI in asymptomatic patients with one- or two-vessel disease if they are hemodynamically and electrically stable and do not have evidence of severe ischemia”,[25] based on OAT and other studies.[26] Analysis of the ACC NCDR database[27] shows no meaningful reduction in the use of PCI for patients who appear to meet OAT entry criteria. A misperception regarding the literature on PCI post MI may be contributing to this. PCI for total occlusions appears to convert a stable state to a state that includes a risk of symptomatic re-occlusion. We believe this is a different pathophysiologic scenario than that which exists in patients with stenotic but patent infarct-related arteries for which a risk of symptomatic re-occlusion exists independent of PCI. These two very different types of patients have been inappropriately combined in a meta-analysis.[28] The results of this meta-analysis may have blunted the expected effect of new guideline recommendations on clinical practice. The present publication presents more robust data with far longer follow up and confirmsno improvement in clinical outcomes with routine PCI for total occlusions in stable post MI patients. As noted, these results apply to patients with at least moderately preserved viability.[15] In light of excess cost in patients assigned to routine PCI in OAT,[2] these findings should now influence this practice pattern.
In conclusion, robust long term data confirm that there is no benefit on cardiovascular events associated with a routine strategy of PCI in stable patients with persistent total occlusion of the IRA, 1-2 vessel CAD and the absence of severe inducible ischemia in the subacute phase after myocardial infarction.
Clinical Summary.
Manuscript ID: CIRCULATIONAHA/2011/041749
A substantial proportion of patients with MI do not receive early reperfusion e.g., due to late presentation. Persistent total occlusion of the infarct-related artery (IRA) is a marker of subsequent risk. Despite observational data suggesting a benefit for late opening of occluded IRAs post-MI, the Occluded Artery Trial (OAT) demonstrated no reduction in the composite of death, reinfarction and class IV heart failure (HF) over approximately 3-year mean follow-up. OAT randomized 2201 stable patients with total IRA occlusion >24 hours (calendar days 3-28) after MI. Severe inducible ischemia, rest angina, class III-IV HF and 3-vessel/left main disease were excluded. Follow-up was extended to determine whether late trends would favor either treatment group for the primary endpoint and angina (6-year median survivor follow-up, longest 9 years). Rates of the primary endpoint, reinfarction, death and class IV HF were similar for PCI vs. MED groups. No interaction between baseline characteristics and treatment group on outcomes was observed, including for those at highest risk. The vast majority of patients at each follow-up visit did not report angina. There was less angina in the PCI group through early follow-up; by 3 years the between-group difference was <4 per 100,and did not reach statistical significance. Additional follow-up of the Occluded Artery Trial cohort with >12,000 total patient-years provides robust evidence for no long-term reduction in clinical events with a strategy of routine PCI of the totally occluded IRA in clinically stable patients in the subacute phase post-MI.
Acknowledgments
In preparation of this report, data management and statistical analyses were conducted by the Data Coordinating Center with oversight by Drs. Hochman, Reynolds and Lamas, and Ms. Forman, who had full access to the data and vouch for the accuracy of the data and analysis. The authors thank Zubin Dastur, MS, MPH, for his contributions as project manager, Arline Roberts RN for her contributions as senior research nurse coordinator, and Anna Yick for assistance in the preparation of the manuscript.
Source of funding and support: The NHLBI funded and oversaw the conduct of this trial. The project described was supported by Award Numbers U01HL062509 and U01HL062511 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Footnotes
Disclosure of potential conflicts of interest: None.
Copyright Transfer Agreement: We would like the Journal to acknowledge that Authors retains the right to provide a copy of the final manuscript to the NIH upon acceptance for Journal publication, for public archiving in Pub Med Central as soon as possible but no later than 12 months after publication by Journal.
References
- 1.Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, Forman S, Ruzyllo W, Maggioni AP, White H, Sadowski Z, Carvalho AC, Rankin JM, Renkin JP, Steg PG, Mascette AM, Sopko G, Pfisterer ME, Leor J, Fridrich V, Mark DB, Knatterud GL Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–2407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mark DB, Pan W, Clapp-Channing NE, Anstrom KJ, Ross JR, Fox RS, Devlin GP, Martin CE, Adlbrecht C, Cowper PA, Ray LD, Cohen EA, Lamas GA, Hochman JS Occluded Artery Trial Investigators. Quality of Life after Late Invasive Therapy for Occluded Arteries. N Engl J Med. 2009;360:774–83. doi: 10.1056/NEJMoa0805151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochman JS, Lamas GA, Knatterud GL, Buller CE, Dzavik V, Mark DB, Reynolds HR, White HD Occluded Artery Trial Research Group. Design and methodology of the Occluded Artery Trial (OAT) Am Heart J. 2005;150:627–642. doi: 10.1016/j.ahj.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Menon V, Pearte CA, Buller CE, Steg PG, Forman SA, White HD, Marino PN, Katritsis DG, Caramori P, Lasevitch R, Loboz-Grudzien K, Zurakowski A, Lamas GA, Hochman JS. Lack of benefit from percutaneous intervention of persistently occluded infarct arteries after the acute phase of myocardial infarction is time independent: insights from Occluded Artery Trial. Eur Heart J. 2009;30:183–91. doi: 10.1093/eurheartj/ehn486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinhubl SR, Berger PB, Mann JT, 3rd, Fry ET, DeLago A, Wilmer C, Topol EJ CREDO Investigators. Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 7.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan E, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 9.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 11.Devlin G, Reynolds HR, Mark DB, Rankin JM, Carvalho AC, Vozzi C, Sopko G, Caramori P, Dzavik V, Ragosta M, Forman SA, Lamas GA, Hochman JS. Loss of short-term symptomatic benefit in patients with an occluded infarct artery is unrelated to non-protocol revascularization: results from the Occluded Artery Trial (OAT) Am Heart J. 2011;161:84–90. doi: 10.1016/j.ahj.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White HD, Reynolds HR, Carvalho AC, Liu L, Pearte CA, Dzavik V, Kruk M, Steg GP, Lamas GA, Hochman JS. Predictors of reinfarction following PCI or medical management using the universal definition in patients with total occlusion after myocardial infarction: results from OAT long term follow up. ESC. 2011 doi: 10.1016/j.ahj.2012.01.016. Abstract #87914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–21. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 14.Dzavik V, Buller CE, Lamas GA, Rankin JM, Mancini GB, Cantor WJ, Carere RJ, Ross JR, Atchison D, Forman S, Thomas B, Buszman P, Vozzi C, Glanz A, Cohen EA, Meciar P, Devlin G, Mascette A, Sopko G, Knatterud GL, Hochman JS TOSCA-2 Investigators. Randomized trial of percutaneous coronary intervention for subacute infarct-related coronary artery occlusion to achieve long-term patency and improve ventricular function: the Total Occlusion Study of Canada (TOSCA)-2 trial. Circulation. 2006;114:2449–2457. doi: 10.1161/CIRCULATIONAHA.106.669432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Udelson JE, Pearte CA, Kimmelstiel CD, Kruk M, Kufera JA, Forman SA, Teresinska A, Bychowiec B, Marin-Neto JA, Höchtl T, Cohen EA, Caramori P, Busz-Papiez B, Adlbrecht C, Sadowski ZP, Ruzyllo W, Kinan DJ, Lamas GA, Hochman JS. The Occluded Artery Trial (OAT) Viability Ancillary Study (OAT-NUC): Influence of infarct zone viability on left ventricular remodeling after percutaneous coronary intervention vs. optimal medical therapy alone. Am Heart J. 2011;161:611–621. doi: 10.1016/j.ahj.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne-Nickens P, Drozdz J, Farsky PS, Feldman AM, Doenst T, Michler RE, Berman DS, Nicolau JC, Pellikka PA, Wrobel K, Alotti N, Asch FM, Favaloro LE, She L, Velazquez EJ, Jones RH, Panza JA STICH Trial Investigators. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–25. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuocolo A, Petretta M, Nicolai E, Pace L, Bonaduce D, Salvatore M, Trimarco B. Successful coronary revascularization improves prognosis in patients with previous myocardial infarction and evidence of viable myocardium at thallium-201 imaging. Eur J Nucl Med. 1998;25:60–68. doi: 10.1007/s002590050195. [DOI] [PubMed] [Google Scholar]
- 18.Anselmi M, Golia G, Cicoira M, Tinto M, Nitti MT, Trappolin R, Rossi A, Zanolla L, Marino P, Zardini P. Prognostic value of detection of myocardial viability using low-dose dobutamine echocardiography in infarcted patients. Am J Cardiol. 1998;81:21G–28G. doi: 10.1016/s0002-9149(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 19.Sawada SG, Dasgupta S, Nguyen J, Lane KA, Gradus-Pizlo I, Mahenthiran J, Feigenbaum H. Effect of revascularization on long-term survival in patients with ischemic left ventricular dysfunction and a wide range of viability. Am J Cardiol. 2010;106:187–192. doi: 10.1016/j.amjcard.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 20.He ZX, Yang MF, Liu XJ, Shi RF, Gao RL, Hu SS, Wu QY, Yang YJ, Chen JL. Association of myocardial viability on nitrate-augmented technetium-99m hexakis-2-methoxylisobutyl isonitrilemyocardial tomography and intermediate-term outcome in patients with prior myocardial infarction and left ventricular dysfunction. Am J Cardiol. 2003;92:696–699. doi: 10.1016/s0002-9149(03)00827-0. [DOI] [PubMed] [Google Scholar]
- 21.Selvanayagam JB, Kardos A, Francis JM, Wiesmann F, Petersen SE, Taggart DP, Neubauer S. Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation. 2004;110(12):1535–41. doi: 10.1161/01.CIR.0000142045.22628.74. [DOI] [PubMed] [Google Scholar]
- 22.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 23.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 24.Freixa X, F S, Rankin JM, Buller CE, Cantor WJ, Ruzyllo W, Lamas GA, Hochman JS, Dzavík V. 6-Year Outcomes of Patients Treated with DES, BMS, or Medical Therapy in the OAT Trial. J Am Coll Cardiol. 2011;57:E1833. [Google Scholar]
- 25.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Jr, Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 26.Ioannidis JP, Katritsis DG. Percutaneous coronary intervention for late reperfusion after myocardial infarction in stable patients. Am Heart J. 2007;154:1065–1071. doi: 10.1016/j.ahj.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 27.Deyell MW, Buller CE, Miller LH, Wang TY, Dai D, Lamas GA, Srinivas VS, Hochman JS. Impact of Clinical Trial Results and National Recommendations for Coronary Intervention of Occluded Infarct Arteries after Myocardial Infarction on Clinical Practice. Arch Intern Med. 2011 doi: 10.1001/archinternmed.2011.315. Published online July 21,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dzavik V, Steg PG, Barton B, Lamas G, Hochman JS. A meta-analysis that misses the mark. J Am Coll Cardiol. 2008;52:578–580. doi: 10.1016/j.jacc.2008.04.046. [DOI] [PubMed] [Google Scholar]


