Abstract
B1b B cells generate a novel form of memory and provide antibody mediated-protection to persisting bacterial pathogens. To understand how B1b B cells establish memory to polysaccharide antigens we studied an oligo-clonal B cell response to α 1→3-dextran (DEX) expressed on Enterobacter cloacae. B cells specific for DEX enrich in the marginal zone (MZ) and B1b B cell populations. After E. cloacae immunization, MZ B cells were responsible for the generation of initial peak DEX-specific antibody titers, whereas, DEX-specific B1b B cells expanded and played an important role in boosted production of DEX-specific antibody titers upon E. cloacae re-challenge. Cell transfer experiments demonstrate that B1b B cells possess the capacity for both robust proliferation and plasma cell differentiation, thus distinguishing themselves from MZ B cells, which uniformly commit to plasma cell differentiation. These results define B1b B cells as the principal reservoir for memory to bacterial-associated polysaccharide antigens.
Introduction
Antibody responses to T cell independent (TI) antigens expressed on bacteria and viruses play a vital role in the establishment of rapid antibody-mediated immunity. TI antigens are classified into two groups: type 1 (TI-1) such as LPS that act independently of B cell receptor (BCR) ligation and type 2 (TI-2), which are typically high molecular weight, polyvalent antigens such as polysaccharide or phospholipid components of microorganisms, on synthetic polysaccharides (1). Polysaccharides are the most extensively studied TI-2 antigens and are targets of vaccination strategies designed to boost memory and production of protective antibodies against invasive, pathogenic microorganisms such as Streptococci and Haemophilus influenzae (2).
In contrast to T-dependent antibody responses, in which B cells have the capability to generate high affinity antibodies through somatic hyper mutation, TI-2 antigens induce rapid production of low affinity antibodies, mostly of the IgM isotype (3). The inability of most TI-2 antigens to induce stable germinal centers results in minimal class switching and conventional memory B cell generation (4, 5). Antibody responses in mice to several classical TI-2 antigens are dominated by canonical B cell specificities expressing germ line immunoglobulin V gene sequences (3, 6).
T-dependent antibody responses are generated primarily from recirculating FO B cells and to lesser extent MZ and other B cell subsets, whereas, antibodies specific for TI-2 antigens are primarily produced by MZ and B1 B cell subsets (7). MZ and B1 B cell subsets display phenotypic markers of antigen-experienced B cells (8), exhibit pre-plasma cell differentiation programs (8), and are often enriched with clones with specificity for epitopes shared by TI-2 and self antigens (9-14). The localization of MZ B cells at the blood-lymphoid interface in the spleen and the ability of B1 B cells to home to mucosal sites allows them to rapidly respond to blood borne and mucosal pathogens (15-17).
Recent findings suggest that B1b B cell encounter with TI-2 antigens results in the generation of long-term clonal expansion and long-lasting antibody production independent of germinal center-induced affinity maturation and immunoglobulin isotype switching (18). These same features were also responsible for protection against the pathogens Borrelia hermsii and Streptococcus pneumonia (19, 20), findings that provide evidence that each subset contributes to antibody production at different stages of the antibody response. However the mechanisms involved in the induction of B1b B cell specific clonal expansion and the integrated responses of other B cell subsets to TI-2 antigens during the course of the antibody response are less clear.
To address these questions we examined the function of B cell subsets in a well characterized, clonally restricted antibody response to α 1→3-dextran. This polysaccharide contains antigenic epitopes present on a variety of clinically relevant organisms including the opportunistic pathogen E. cloacae (21), α 1→3 glycans expressed by dimorphic fungal pathogen Histoplasma capsulatum (22), the commensal fungus Aspergillus fumigatus (Dizon and Kearney, unpublished results), and the epiphytic bacterial species Leuconostoc mesenteroides (23). Using an Ig heavy chain transgenic mouse, which expresses large numbers of DEX-specific J558 idiotype positive B cells, and reagents to detect DEX-specific B cells in BALB/c mice, we examined the roles of MZ, FO, and B1b B cells in the DEX-specific antibody in response to E. cloacae. We demonstrate overlapping roles for MZ and B1b B cells in generating robust DEX-specific antibody titers, however, B1b B cells play a pivotal role in maintaining sustained DEX-specific antibody production and are responsible for the generation of an enhanced secondary antibody response to E. cloacae. The increased responsiveness and long-term maintenance of this population in comparison to MZ B cells strengthens the concept that B1b B cells provide memory to bacterial challenge.
Materials and Methods
Animals
VH J558 transgenic mice were generated from a VHJ558.3-DSP2.2-JH1 VDJ rearrangement amplified from genomic DNA derived from an J558 IgG3 hybridoma (Clemens and Koelsch, unpublished; NCBI accession sequence AJ002693 http://www.ncbi.nlm.nih.gov/) using the following primers: 5’-GCC ATGGGATGGAGCTGCATCTTT-3’ (anneals to the promoter) and 5’-CACGGATCCACAGGCAGCTAGCCT-3’ (anneals to the JH1 intron). The PCR fragment was cloned into the KC9 construct previously used to make VH81x transgenic mice (24) at Nco1 and BamH1 restriction sites. Transgenic mice were then generated using previously described methods (25) and backcrossed onto the C57BL/6 background for more than 10 generations. VHJ558 transgenic mice were also bred with CD19 -/- mice on a C57BL/6 background (A gift from Dr. Robert Carter, UAB, Department of Rheumatology). 8 week Female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were bred and housed in specific pathogen free facilities according to rules and regulations established by the University of Alabama animal resource program.
Immunizations
8 to 12 wk old VHJ558 transgenic and 8 week old female BALB/c mice were immunized intravenously at day 0 with 108 heat killed, paraformaldehyde fixed Enterobacter cloacae strain MK7 (21) and re-challenged intraperitoneally with the same dose at day 40 (VHJ558 TG) or day 60 (BALB/c mice). At the appropriate time point’s mice were sacrificed and blood and tissues were harvested for ELISA, FACS, and histological analysis.
Flow cytometry
FITC labeled anti-CD23, anti-Mac 1, anti-Ki67, anti-BrdU, PE-labeled anti-CD1, anti-CD5, anti-Mac 1, anti-CD44, anti-CD80, anti-CD86, anti-FASR, anti-MHCII, rat IgG2aκ, biotin labeled anti-CD23, Annexin V Cy5, 7AAD, and PE-Cy7 labeled anti-B220 were purchased from Pharmingen (San Diego, CA). APC and PE labeled anti-AA4.1 (also known as CD93) were purchased from eBioscience (San Diego, CA). Goat anti-mouse IgM Cy5 was purchased from Jackson Immunoresearch Inc. (West Grove, PA) and goat anti-mouse IgM biotin was purchased from SBA (Birmingham, AL). Streptavidin Alexa Pacific Blue was purchased from Molecular Probes (Invitrogen Corporation, Carlsbad, CA). PE anti-CD21 (clone 7G6) was described previously (15). Anti-λ (clone JC5-1), CD36 (clone MZ1), FcRL5 (clone MZ2), CD9 (clone MZ3), TD6-4 and EB3-7-2, both specific for the anti-DEX idiotype (26,30) were all generated in our laboratory and directly conjugated to Alexa 488, 647, and Pacific Blue dyes purchased from Molecular Probes (Invitrogen Corporation, Carlsbad, CA). α 1→3-dextran (gift from Dr. Allene Jeanes) was conjugated to Alexa 488 (Molecular Probes, Invitrogen Corporation, Carlsbad, CA). RS3.1 described previously (27) was conjugated to Alexa 488, 555, and 647 dyes (Molecular Probes, Invitrogen Corporation, Carlsbad, CA). All 5-7 color FACS analysis were performed on a BD LSRII flow cytometer (BD Biosciences, Mountain View, CA) and analyzed using FlowJo software (Tree Star, Inc, Ashland, OR).
BrdU labeling and detection
J558 TG mice immunized with E. cloacae as described above were fed BrdU (Sigma-Aldrich, St Louis, MO) at a concentration of 0.8mg/ml in drinking water for 3 days after immunization. Staining with antibodies specific for BrdU was performed according to the manufacturer protocol’s (BD Pharmingen, San Diego, CA).
α 1→3-DEX ELISA
Sera collected from VHJ558 transgenic mice immunized with E. cloacae strain MK7 were assayed for the presence of α 1→3-dextran specific IgM antibodies using a previously established protocol (21). Briefly, serum diluted 1/1000 was incubated for 2 hrs at 37°C on 96 well EIA/RIA plates (Costar, Corning, NY) previously coated overnight at 4°C with 1 ug/ml of purified α 1→3-dextran. Plates were washed and developed with either goat anti-mouse IgM or λ AP for 2 hours at 37°C. Concentrations of α 1→3-dextran specific antibodies were quantified by ELISA using purified J558 Id+ IgM antibody as a reference standard.
Immunofluorescence Analysis of Tissue Sections
Tissue sections were processed and viewed as described previously (8). Frozen sections were stained with Moma-1 (Rat, IgG2aκ, a gift from Dr. George Kraal, VU University, Amsterdam, The Netherlands) and developed with goat anti-rat IgG Alexa 350 or 647 (Molecular Probes, Invitorgen Corporation, Carlsbad, CA), blocked with normal rat serum (Peel-Freeze, Rogers, AR), washed and then stained with TD6-4 alexa 488, which is specific for the anti-DEX idiotype, and RS3.1 Alexa 555.
Cell sorting and adoptive transfers
B cell subsets were purified using a FACS Aria flow cytometer (Becton-Dickinson, Carlsbad, CA) from the spleen or peritoneal cavity of either naïve or VHJ558 transgenic mice immunized 30 days previously with E. cloacae strain MK7. MZ (B220+ CD21 hi CD23 -) and FO (B220+ CD21 int CD23 +) B cells were sorted from näive or previously immunized VHJ558 transgenic mice with final purities ranging from 95 – 99%. B1b (CD5 – Mac 1 + B220 int) B cells were sorted from the peritoneal lavage with purities ranging from 90 – 95%. C57BL/6 recipient mice were reconstituted with 2.5 × 105 MZ, FO, or B1b B cells and then immunized IV 24 hours later with 108 E. cloacae strain MK7. The extent of the DEX specific response by donor cells was evaluated using FACS and ELISA.
Statistics
Data from three or more groups were analyzed by a one-way ANOVA test for data with normal distribution and Kruskal-Wallis test for data that did not distribute normally. Data with two groups were analyzed by a two-tailed paired t test to determine if statistically significant differences existed. Statistically significant results were determined by a p value of <0.05 (*) or <.01 (**).
RESULTS
Generation and characterization of VHJ558 transgenic mice
The J558 heavy chain transgene (TG) mice induced very efficient allelic exclusion of endogenous Ig heavy chain rearrangement in mice on a C57BL/6 background, such that >95% of the B cells in the bone marrow and spleen expressed the allotype (IgMa). A large proportion of immature B cells in the spleen and bone marrow were J558 Id+ λ+ and displayed a similar rate of turnover (data not shown) when compared to wt and J558 Id- immature B cells indicating that J558 Id+ were produced normally from the bone marrow. In VHJ558 TG mice 15 - 30% of B cells in the bone marrow, spleen, lymph nodes, and peritoneal cavity were J558 Id+ and DEX-specific (Figure 1A and data not shown).
Figure 1. J558 Id+ B cells in the spleen preferentially enrich in the splenic MZ.
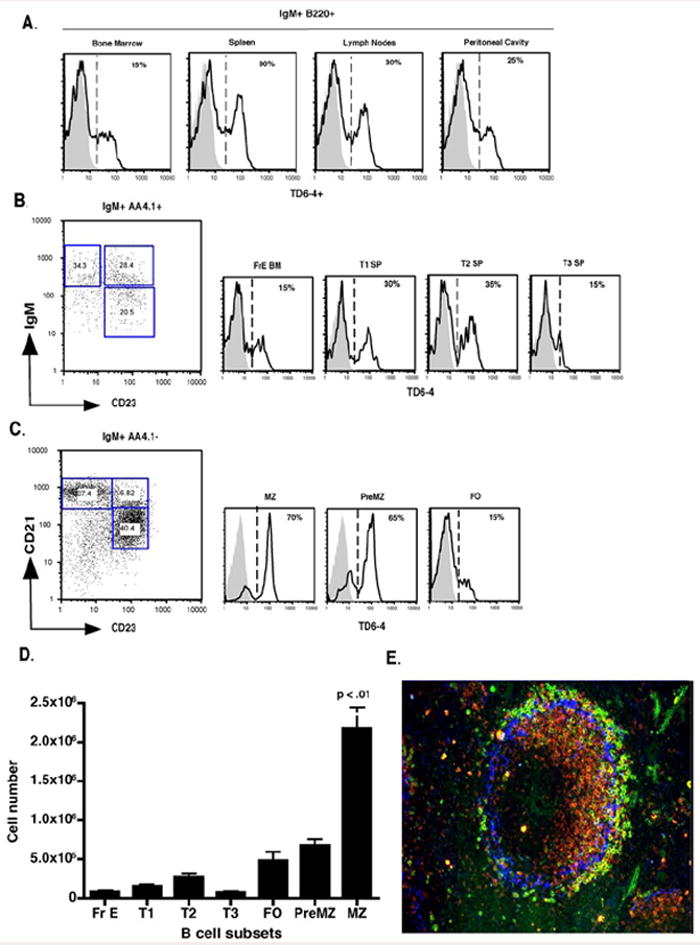
(A) FACS analysis of J558 Id expression using the anti-J558 idiotypic antibody clone TD6-4 in IgM+ B220 + bone marrow, spleen, lymph nodes, and peritoneal cavity B cells. TD6-4 staining is represented by black histograms denoting VHJ558 transgenic mice and grey histograms denoting non-transgenic littermate controls. (B) Analysis of J558 Id+ enrichment in immature bone marrow fraction E (CD21- CD23- IgM hi AA4.1 hi B220+), splenic T1 (CD21 – CD23 – IgM hi AA4.1 hi B220+), T2 (CD21 int CD23+ IgM hi AA4.1 int B220 +), T3 (CD21 int CD23+ IgM int AA4.1 lo B220+), and (C) mature MZ (CD21 hi CD23 – AA4.1- B220+), preMZ (CD21 hi CD23 hi AA4.1 – B220+), FO (CD21 int IgM int CD23+ AA4.1- B220+) B cells from spleens of VHJ558 TG (black lines) and littermate controls (grey histograms). (D) Total numbers of J558 Id+ B cells in bone marrow and splenic B cell populations. (E) Spleen sections were stained with anti-IgMa (red), anti-J558 Id (green), and anti-Moma-1 (blue). All FACS plots and splenic sections are representative of 10 – 20 mice per group.
J558 Id+ B cells initially detected in bone marrow fraction E (FrE BM) became increasingly enriched as they traversed the splenic transitional 1, and 2 compartments before committing predominantly to preMZ and MZ B cell populations (Figure 1 B and C). Evaluation of the total numbers of J558 Id+ B cells in the bone marrow and spleen B cell subpopulations confirmed the dominance of this clone within the MZ B cell subset, which was further evidenced by their positioning in the marginal zone of the spleen (Figure 1, D and E). In the peritoneal cavity J558 Id+ B cells were enriched equally in the B1b and B2 B cell subsets and minimally within the B1a B cell subset (Figure 2A and B). Examination of the peripheral and mesenteric lymph nodes showed that the majority of J558 Id+ B cells displayed a RF B cell phenotype, however, small numbers of J558 Id+ MZ and preMZ B cells were also present in these tissues confirming previous findings indicating the existence of MZ B cells in lymph nodes (7), (Figure 2C).
Figure 2. Peripheral J558 Id+ B cell development in the peritoneal cavity and lymph nodes.
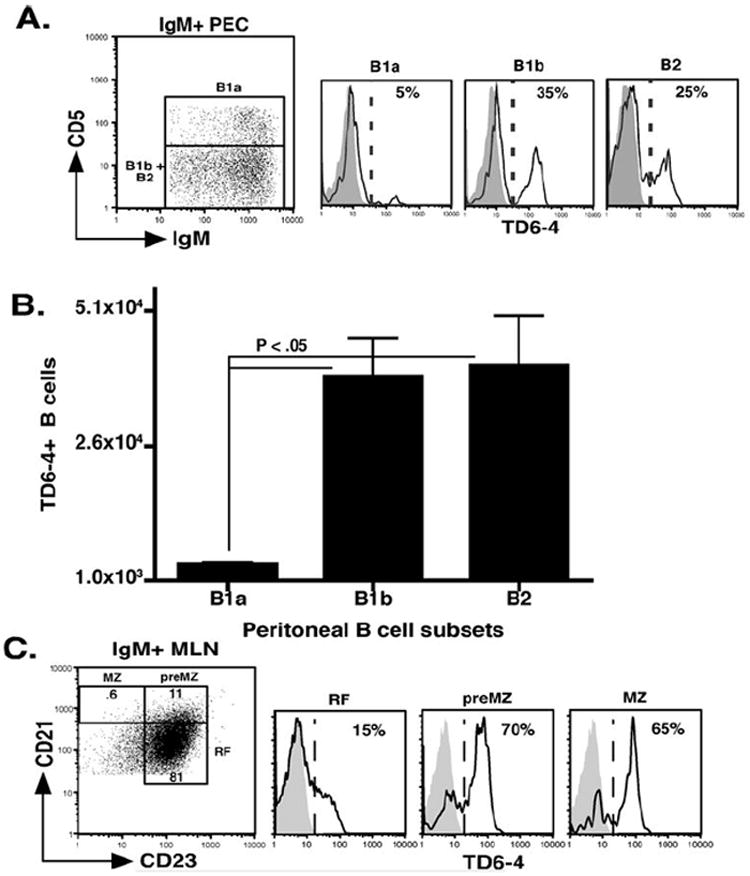
(A) FACS analysis of J558 Id+ B cell enrichment in B1a (CD5+ IgM hi Mac-1+ B220 int) B1b (CD5- IgM hi Mac-1+ B220 int) and B2 (CD5- IgM int Mac-1- B220 hi) from the peritoneal cavities of VHJ558 transgenic (black lines) and littermate controls (grey histograms). (B) Total numbers of J558 Id+ B cell subsets in the peritoneal cavity. (C) FACS analysis of J558 Id+ MZ (CD21 hi CD23- B220+ IgM hi CD9+), preMZ (CD21 hi CD23+ B220+ CD9+), and RF B cells (CD21 int CD23+ B220+ IgM int CD9-) in the mesenteric lymph nodes of VHJ558 transgenic (black lines) and littermate controls (grey histograms). (D) Total numbers of J558 Id+ B cells in the different subsets in the lymph nodes.
In summary, J558 Id+ B cells constitute a considerable fraction of B cells in VHJ558 transgenic mice and are present in all B cell compartments, yet, in the spleen, display a developmental bias for the MZ B cell population.
Development of DEX-specific B cells in normal BALB/c mice
Using a staining procedure and flow cytometry to detect infrequent clones, we detected DEX+ λ1+ B cells at a frequency of 2~5 cells/106 total splenic cells in BALB/c mice. As a control for the specificity of this analysis we tested, C57BL/6 mice, which do not make antibodies of this specificity in response to either DEX or E. cloacae challenge (28, 29), and found that they lacked detectable staining (Figure 3A and B). Sequence analysis of sorted DEX+ λ1+ B cells revealed that these cells expressed Ig heavy chain genes that give rise to the canonical J558 idiotype (Mahmoud and Kearney, manuscript in preparation) confirming the specificity of the flow cytometric assay. In adult BALB/c mice DEX+ λ1+ B cells were equally enriched within the splenic MZ and peritoneal B1b B cell subsets where these were present in similar numbers, but they were scarce in FO, B1a, and B2 B cell subsets (Figure 3C).
Figure 3. J558 Id+ B cells in BALB/c mice preferentially enrich in the MZ and B1b B cell compartments.
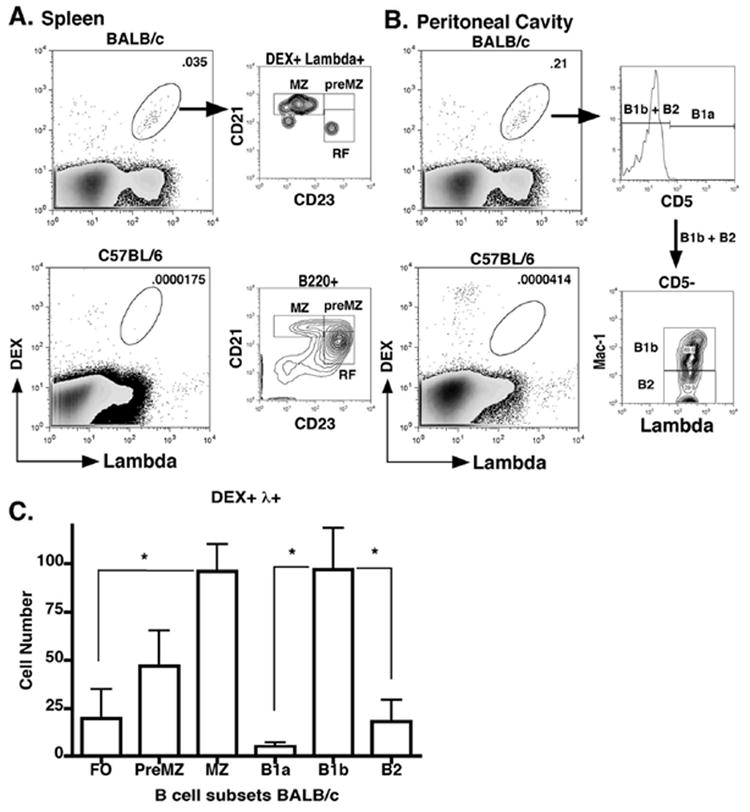
(A) The phenotypes of splenic (A) and peritoneal (B) DEX+ λ1+ B cells from pre-immune BALB/c mice were determined by flow cytometry using antibodies specific for splenic (see comment 12, reviewer 2) MZ (B220 + CD21hi CD23-), RF (B220 + CD21 int CD23+), B2 (IgM int CD5- Mac-1-), B1a (IgM hi CD5+ Mac-1+), and B1b (IgM hi CD5- Mac-1-). (C) Graph of total numbers DEX+ λ1+ B cell subsets per mouse (n=5 mice).
These findings indicate that even in normal mice the MZ and B1b B cell populations constitute major reservoirs in the spleen and peritoneal cavity for DEX-specific λ1+ B cells expressing the J558 idiotype, thus providing a significant pool of primary responders to DEX or E. cloacae challenge.
DEX specific antibody response to E. cloacae
To determine the kinetics of DEX-specific antibody production we immunized BALB/c mice with heat killed, paraformaldehyde fixed E. cloacae strain MK7 expressing DEX and determined the titers of anti-DEX antibodies at 0, 7, 14, 28, 60 and 150 days post immunization. DEX-specific antibody peaked at 7 days followed by long-term production of lower titers for up to 150 days after immunization (Figure 4A). When these mice were re-immunized at 60 days after the primary immunization there was a 5-fold increase in peak antibody titers compared to the peak of the primary response at 7 days (Figure 4A).
Figure 4. DEX-specific antibody response in BALB/c mice.
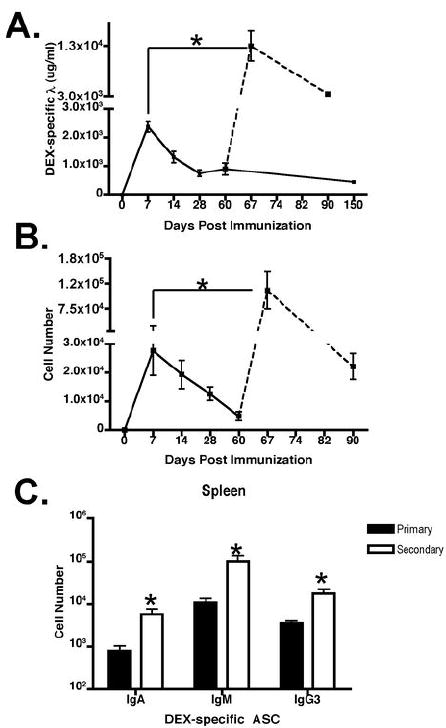
(A) DEX-specific ELISA was used to determine serum DEX-specific Igλ antibody titers from BALB/c mice immunized with E. cloacae in the primary (solid lines) and secondary response (dashed lines). The amount of DEX-specific antibodies in the serum were displayed as micrograms/milliliter or ug/ml. (B) Total DEX-specific Igλ secreting cells in the primary (solid lines) and secondary (dashed lines) was determined by ELISpot and (C) other immunoglobulin heavy chain isotype-expressing DEX specific ASC’s in BALB/c mice immunized with E. cloacae were determined at day 7 (black bars) for the primary peak and day 67 (white bars) for the secondary peak.
To correlate the observed enhanced secondary serum anti-DEX response with AFC production we performed ELISpot analysis for total DEX-specific AFCs at days 0, 7, 14, 28, 60, 67 and 90 days post primary and secondary immunizations. Splenic DEX-specific ASC’s peaked at day 7 and then declined to reach stable numbers of >7000 ASC’s per mouse at 60 days (Figure 4B), mirroring the trend in serum DEX-specific antibody titers (Figure 4A). After re-challenge there was a 5-fold increase in DEX-specific ASC’s (Figure 4B) compared to the primary response. Primary and secondary ASC responses were dominated by IgM followed by the IgG3, and IgA isotype; no other IgG subclasses were detectable (Figure 4C and data not shown). The numbers of IgM, IgG3, and IgA DEX-specific ASC’s at the peak of the secondary response were increased compared to numbers detected in the primary (Figure 4C).
Taken together, these findings show that the primary serum anti-DEX antibody response shows the expected early peak, but this is followed by a long-lasting phase of low level DEX-specific antibody production. Re-challenge with E. cloacae results in an enhanced DEX-specific antibody response, indicative of a memory response, which is dominated by IgM isotype-specific antibodies, but also a significant IgG3 and IgA component.
Stable expansion and slow turnover of DEX-specific J558 Id+ B1b B cells in E. cloacae immunized TG and BALB/c mice
The above data demonstrate that there is a significant boost in anti-DEX antibody titers following re-challenge with E. cloacae. To analyze the cellular changes associated with this response, we determined the numbers, location, and phenotypes of DEX-responding J558 Id+ B cells after immunization of TG and BALB/c mice. In TG mice, splenic J558 Id+ MZ B cells were rapidly recruited into the plasmablast pool, generating high titers of anti-DEX antibodies (Figure 5A and B). At 21 days after primary immunization J558 Id+ MZ B cell numbers had recovered to pre-immune levels, consistent with the re-colonization of the MZ (Figure 5A and data not shown). In the peritoneal cavity J558 Id+ B1b B cells expanded beginning at 3 days post E. cloacae immunization and were maintained as a stable population 10-fold higher than their initial numbers, throughout the primary response (Figure 5C). J558 Id+ Mac-1+ B cells displaying an increased size, granularity and lower levels of B220, all features of peritoneal B1b B cells, were present in the circulation in increased numbers beginning at 3 days and persisting for at least 28 days after immunization (Figure 5D) indicating that these cells re-circulate.
Figure 5. Characterization of J558 Id+ B cell subsets in TG mice post E. cloacae immunization.
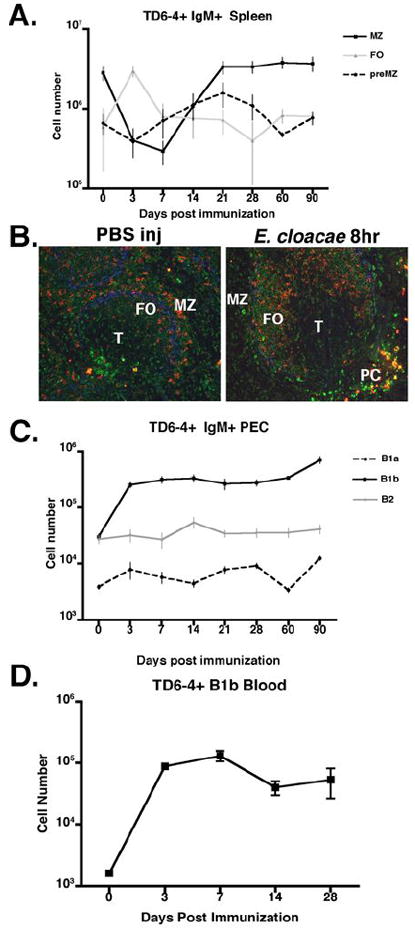
(A) Numbers of J558 Id+ MZ (CD21hi CD23- B220+), preMZ (CD21hi CD23+ B220+), and FO (CD21int CD23+ B220+) B cells at 0, 7, 24, 21, 28, 60, and 90 days in J558 TG mice immunized with E. cloacae. (B) Rapid migration of J558 Id+ MZ B cells to the T cell border and differentiation into plasma blasts 8 hours post E. cloacae immunization. Anti-J558 idiotype (clone TD6-4, red), anti-IgMa (green), and anti-Moma-1 (blue). (C) Numbers of J558 Id+ B cell subsets in the peritoneal cavity post immunization. (D) Numbers of J558 Id+ B1b B cells in the blood of TG mice at 0, 3, 7, 14, and 28 days after E. cloacae immunization.
DEX+ λ+ B cells bearing the J558 idiotype were rapidly depleted from the MZ of BALB/c mice immunized with E. cloacae. This depletion persisted for at least 28 days before the MZ was reconstituted at day 60 (Figure 6A). This slower recovery of DEX-specific MZ B cells in BALB/c mice highlights differences in the development of these cells in TG and normal BALB/c mice immunized with E. cloacae. During the course of the primary and secondary responses the majority of splenic DEX-specific B cells displayed increased size, granularity, low levels of B220, CD21, CD23, high IgM expression and an intermediate level of Mac-1 (Figure 6B) consistent with the phenotype of a B1b B cell population. In addition there was a significant fraction of CD138+ DEX-specific plasma blasts present in the spleen throughout the antibody response in agreement with the persistence of DEX-specific ASC’s (Figure 6B and 4B). In the peritoneal cavity there was a 200-fold expansion of DEX-specific B1b B cells that persisted throughout the primary response (Figure 6C and D). Re-challenge of both TG and BALB/c mice induced a further 5-fold expansion of these DEX-specific B1b B cells in comparison to the numbers present 7 days after primary challenge (Figure 6D and data not shown).
Figure 6. Expansion of DEX-specific λ+ B1b B cells in the spleen and peritoneal cavity of BALB/c mice post E. cloacae immunization.
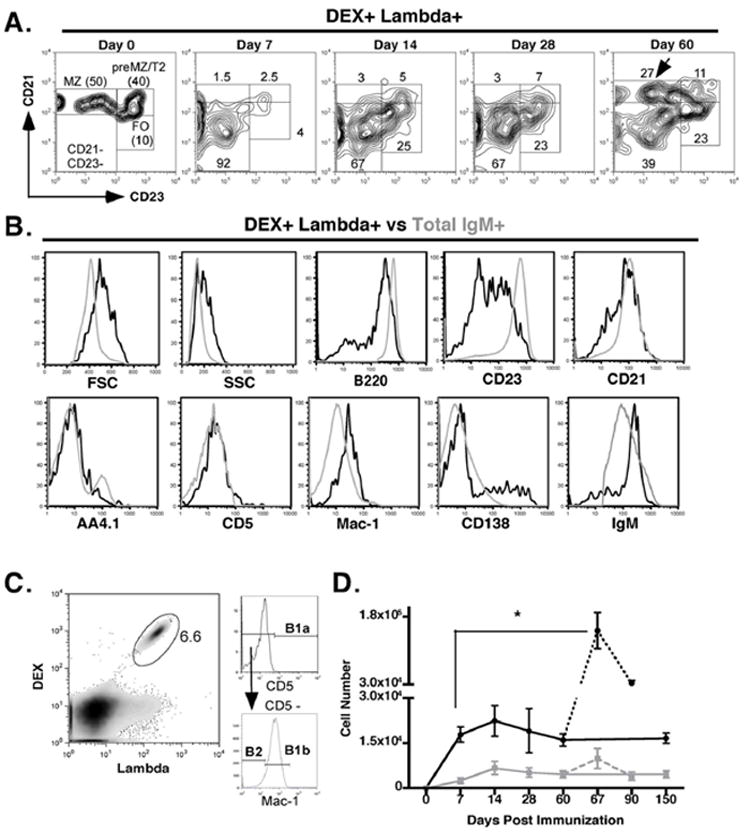
(A) DEX-specific λ+ MZ B cells are depleted in BALB/c mice after E. cloacae immunization. CD21 and CD23 expression on splenic DEX+ λ+ B cells from BALB/c mice at 0, 7, 14, 28, and 60 days post E. cloacae immunization. (B) Phenotype of total splenic DEX+ λ+ B cells (black histogram) and total B cells (grey histogram) from BALB/c immunized with E. cloacae. Representative of profiles of total splenic DEX+ λ+ B cells of seen in BALB/c mice at 14, 28, and 60 post E. cloacae immunization. (C) After immunization the majority of DEX+ λ+ B cells in the peritoneal cavity display a B1b B cell phenotype. Representative FACS plot gating on peritoneal DEX+ λ+ B cells and displaying expression of CD5 and Mac-1. (D) Peritoneal DEX+ λ+ B1b cells undergo a population expansion upon both primary and secondary challenge with E. cloacae. Numbers of peritoneal DEX+ λ+ B1b (black line) and B2 B cells (grey line) were calculated throughout the primary response (days 0 – 150; solid lines) and secondary response (days 67 – 90; dashed lines).
We next used BrdU labeling to determine the lifespan and proliferation patterns of B cell subsets in the ongoing DEX-specific antibody response in TG mice (Figure 7A). After three days of continuous BrdU administration following E. cloacae immunization, all J558 Id+ B cell subsets in the spleen and peritoneal cavity were labeled with BrdU. After the 3-day pulse and chase, total numbers of J558 Id+ FO, and preMZ splenic B cells labeled with BrdU decreased slowly with time until at 90 days >90% of these cells were BrdU- (Figure 7B and C). In contrast BrdU labeling of J558 Id+ MZ B cells steadily increased until their numbers peaked at day 21 and were maintained thereafter representing 30% of the total J558 Id+ MZ B cells at day 90 (Figure 7D). Despite the high frequency of BrdU+ J558 Id+ B cells in the MZ 90 days after immunization, these cells displayed no distinctive phenotypes compared to J558 Id+ MZ B cells from naive TG mice (data not shown). The majority of J558 Id+ B1b B cells in the peritoneal cavity were BrdU labeled (Figure 7E) and they displayed a much slower rate of turnover compared to the other B cell subsets. Analysis of turnover in the peritoneal B1b B cell population revealed no significant levels of apoptosis or cell division throughout the antibody response beginning 1 week after E. cloacae challenge, indicating that turnover in this population likely occurred outside of the peritoneal cavity (data not shown). J558 Id+ B1b B cells from immunized TG mice displayed minimal phenotypic differences when compared to J558 Id+ B1b B cells from naïve TG mice, with the exception of increased expression of CD80 (data not shown), suggesting that these cells are not phenotypically distinct from their naïve counterparts.
Figure 7. Analysis of J558 Id+ B cell turnover in mature B cell subsets of TG mice post E. cloacae immunization.
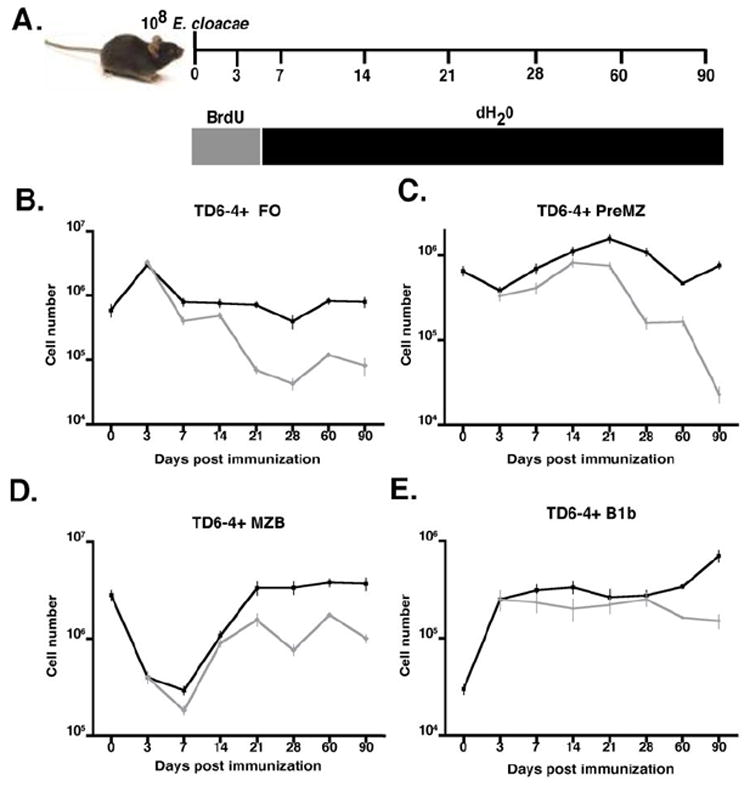
(A) Diagram of BrdU labeling regimen. Mice were immunized intravenously at day 0 with 108 E. cloacae and given BrdU at a concentration of .8 mg/ml in the drinking water for 3 days after immunization. Incorporation of BrdU in J558 Id+ splenic B cell subsets was evaluated. (B-E) Numbers of total J558 Id+ B cells in the different subsets (black lines) were compared to numbers of BrdU+ J558 Id+ cells in the spleen (B) FO, (C) preMZ, (D) MZ, and in the peritoneal cavity (E) B1b B cell subsets (grey lines) in TG mice pulsed for 3 days with BrdU post E. cloacae immunization.
These findings demonstrate the unique ability of DEX-specific B1b B cells bearing the J558 idiotype to undergo stable expansion, in the peritoneal cavity where they slowly turnover and re-circulate, in TG and BALB/c mice immunized with E. cloacae.
Contributions of MZ, FO, and B1b B cells to the anti-DEX antibody response
To further delineate the relative contributions of DEX-specific J558 Id+ MZ, FO, and B1b B cells to the anti-DEX antibody response, splenic MZ, FO, and peritoneal B1b B cells sorted from either naïve or E. cloacae immunized VHJ558 transgenic mice (data not shown) were transferred into intact C57BL/6 non-DEX responsive recipients. One day after transfer, the recipients were immunized IV with 108 E. cloacae and the ability of each subset to generate a DEX-specific antibody response was evaluated 3 days later by examining the proportions of DEX-specific CD138+ B cells in the spleen and the titers of DEX-specific antibodies in the serum.
J558 Id+ B cells were detectable in E. cloacae immunized recipient mice reconstituted with naïve or antigen experienced MZ, FO, and B1b B cells (Figure 8A). Transferred MZ and B1b B cells displayed the largest J558 Id+ B cell expansion while FO B cells displayed only a modest increase. B1b B cells from antigen-experienced mice displayed the greatest capacity for J558 Id+ B cell expansion in comparison to all other transferred subsets (Figure 8B). By normalizing numbers of J558 Id+ B cells to those present in the initial innocula, B1b B cells from E. cloacae primed TG mice expanded >7-fold, whereas transferred FO, MZ, and naïve B1b B cells expanded only 2.5-5 fold (Figure 8C). Three days after E. cloacae immunization MZ B cells transferred from either naïve or convalescent TG mice gave rise to J558 Id+ B cells that were uniformly positive for CD138 in the recipient, whereas less than half of the FO and B1b B cells from both naïve and convalescent TG mice expressed CD138 (Figure 8D). Analysis of DEX-specific antibody titers in recipient mice 3 days after E. cloacae immunization revealed that MZ and B1b B cells, but not the FO B cells, were capable of rapidly generating substantial DEX-specific antibody titers (Figure 8E). In parallel with their superior proliferative capacity, the primed B1b B cells generated higher DEX-specific antibody titers than those produced from naive B1b. By contrast, prior antigen experience had no effect on antibody titers generated from the MZ B cells. These results indicate that MZ and B1b B cells from both naïve and antigen-experienced mice generate high titers of DEX-specific antibody in response to E. cloacae upon transfer into C57BL/6 recipients. However, B1b B cells from convalescent mice possess an increased capacity for clonal expansion compared to the other B cell subsets resulting in increased numbers of DEX-specific ASC’s and accompanying antibody titers.
Figure 8. Role of splenic MZ, FO, and peritoneal, B1b B cells in DEX specific antibody response.
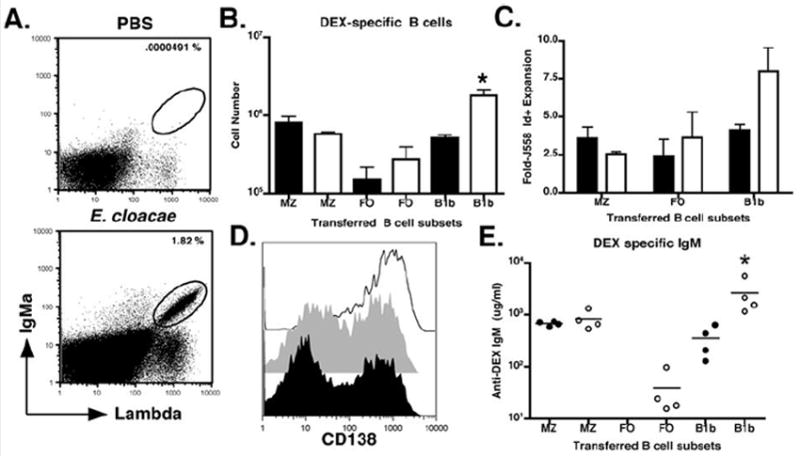
(A) Representative FACS profile of B6 mice reconstituted with either splenic MZ, FO, or peritoneal B1b B cells from naïve or E. cloacae primed VHJ558 TG mice. Mice were immunized with E. cloacae 24 hours after reconstitution with each B cell population. (B) Numbers of IgMa+ λ1+ B cells recovered from the spleens of B6 recipient mice reconstituted with naïve MZ (91 +/- 13% TD6-4+), FO (25 +/- 10% TD6-4+), B1b (50 +/- 15% TD6-4+) (black) or antigen experienced (white) MZ (90 +/- 15 TD6-4+), FO (30 +/- 10% TD6-4+), or B1b B cells (90 +/- 10% TD6-4+) 3 days after E. cloacae immunization. (C) To calculate the fold expansion, numbers of J558 Id+ B cells in recipient mice 3 days after E. cloacae immunization. Numbers of J558 Id+ B cells derived from naïve (black) and antigen experienced (white) MZ, FO, and B1b reconstituted recipients immunized with E. cloacae were calculated and divided by the initial numbers of the corresponding populations (D) CD138 expression by IgMa+ λ1+ B cells recovered from B6 recipient mice reconstituted with naïve or antigen experienced MZ (black line), FO (grey histogram), and B1b (black histogram) B cell subsets 3 days post E. cloacae immunization. (E) DEX specific IgMa antibody titers in recipient mice at 3 days post E. cloacae immunization for primary (closed circles) and secondary (open circles) DEX-specific antibody production. The amount of DEX-specific antibodies in the serum were displayed as micrograms/milliliter or ug/ml. All FACS plots and data (mean +/- SEM) are representative of 3 independent experiments containing 3 mice per group.
Although the above experiments give us a clearer picture of functional differences between each of the mature B cell subsets and confirm the importance of both MZ and B1b B cells as first responders, the ability of the FO B cells to differentiate into plasmablasts suggested that they can also contribute to anti-DEX antibody production, but, in later phases of the response. To determine the contribution of FO B cells to E. cloacae immunization in vivo we crossed VHJ558 transgenic mice with FO B sufficient, MZ-B deficient CD19 -/- mice. As expected development of J558 Id+ B cells in VHJ558 TG × CD19-/- mice was impaired beginning with a diminished splenic AA4.1 T2 B cell population, which accompanied the highly impaired development of mature J558 Id+ MZ and B1b B cells. By contrast, J558 Id+ FO B cell development was only moderately impeded (Figure 9A-C). Upon immunization of J558 TG × CD19 -/- mice with E. cloacae the total numbers of J558 Id+ B cells in the spleen (Figure 9D) and peritoneal cavity (Figure 9E) increased by 10 and 200 fold respectively. After immunization the majority of J558 Id+ B cells in the spleen expressed AA4.1 and Mac-1 and in the peritoneal cavity, most displayed a B1b B cell phenotype (Data not shown). Peak DEX-specific antibody titers 3 days after E. cloacae immunization in transgenic mice correlated with the presence or absence of MZ B cells in WT and CD19 -/- TG respectively, implicating MZ B cells in the initial peak antibody response (Figure 9F). However, beginning at 7 days post immunization DEX-specific antibody titers were similar between wt and CD19 -/- TG mice further suggesting a major role for B1b B cells and not MZ or FO B cells in the development of DEX-specific antibodies that occurs after the initial peak response, which is normally derived from DEX-specific MZ B cells.
Figure 9. B1b B cells contribute to the continuous phase of DEX specific antibody production in CD19 -/- mice.
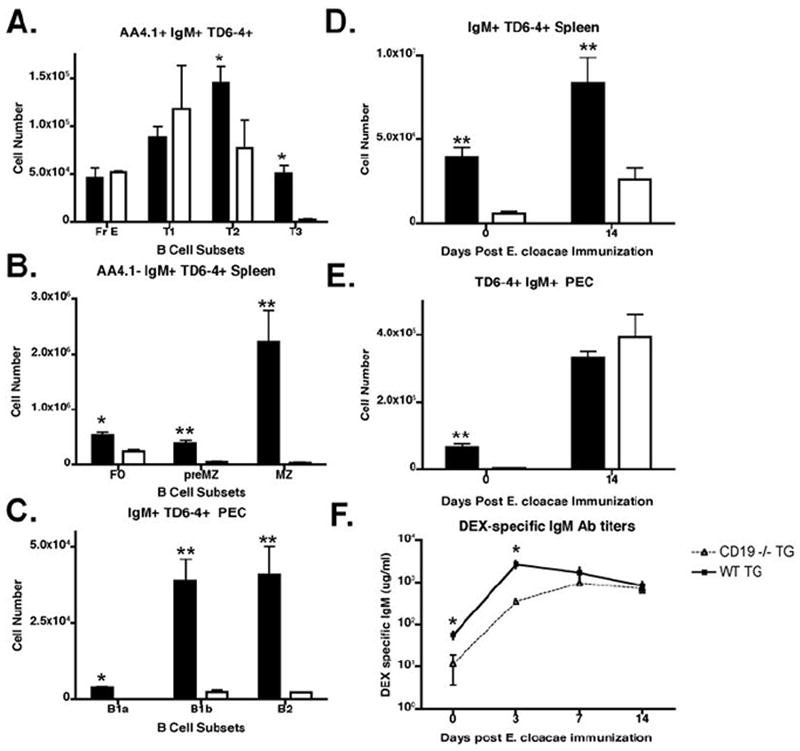
(A). CD19 -/- mice express normal numbers of J558 Id+ FO B cells, but lack all other mature J558 Id+ B cell subsets. Numbers of J558 Id+ B cells in the different subsets in naïve VHJ558 TG CD19 -/- (white bar) and naïve VHJ558 TG (black bar) from the bone marrow, spleen, and peritoneal cavity were calculated using reagents specific for each subset as described in figures 1 and 2. (B) E. cloacae challenge generates an expansion of J558 Id+ splenic and peritoneal B cells in CD19-/- transgenic mice. Total numbers of J558 Id+ B cells in the spleen (B) and peritoneal cavity (C) of VHJ558 TG CD19 -/- (white bar) and naïve VHJ558 TG (black bar) were calculated on day 0 and 14 post immunization with E. cloacae. (D) E. cloacae challenged CD19-/- transgenic mice mount a normal DEX-specific antibody response 1 week after challenge. DEX specific IgM antibody titers from either VHJ558 TG CD19 -/- or VHJ558 TG mice immunized with E. cloacae.
Discussion
The goal of this study was to investigate whether the bacterial polysaccharide α 1→3-dextran (DEX) could induce the formation of B cell memory and characterize this process at a clonal level. We chose α 1→3-dextran as our model antigen for several reasons: (i) This glucan or related epitopes are present on the surface of a variety of microorganisms of clinical relevance (21, 22), (Dizon and Kearney, unpublished observations), (ii) the availability of transgenic mice and clonal specific-reagents to dissect both the antibody response and follow clonal B cell behavior in both TG and BALB/c mice (26), and (iii) the ability to immunize with whole bacteria and/or purified bacterial polysaccharide (21) provided us with a system to investigate the induction of polysaccharide specific memory.
The antibody response to type 2 T-cell independent antigens has been extensively studied in the past, but until recently these studies focused on the acute phases of the antibody response to purified polysaccharides rather than to whole organisms. Here we examined the extent of the primary DEX-specific antibody response to E. cloacae over a 5-month period and after a re-challenge at day 60. Our results show, as previously demonstrated that peak titers of DEX-specific serum antibody in a primary response occurred during the first week (30), but there was continued production of anti-DEX antibodies for at least 5 more months after the primary immunization. Additionally re-challenge with E. cloacae after 60 days resulted in a 5-fold increase in serum anti-DEX antibody when compared to the peak primary response occurring 7 days after immunization. This re-call response could also be induced within a shorter time frame by 2 successive E. cloacae immunizations 1 week apart (data not shown) indicating that the population of B cells responsible for generating enhanced production of anti-DEX antibodies is established early after immunization. These results demonstrate that bacterial challenge is capable of generating long-term polysaccharide specific antibody production and an enhanced secondary antibody response, which is a defining characteristic of B cell memory.
The ability to visualize B cell behavior at a clonal level in both TG and BALB/c provided us with the opportunity to track J558 Id+ B cell fate during the antibody response to E. cloacae and to investigate the contributions of each population to DEX-specific antibody production in both primary and secondary antibody responses. Similar to studies with S. pneumonia (15), Borrelia burgdoferi (31), and NP-Ficoll (32), MZ B cells played an important role in the initial peak antibody response to DEX, whereas, J558 Id+ FO B cells provided only a minor contribution. Evaluation of B cell turnover in the spleen revealed no evidence for the existence of memory within the MZ and FO B cell populations, which were slow to recover after primary immunization. The depletion of polysaccharide specific B cells from the marginal zone may pose serious problems in dealing with recurring bacterial infections (31, 33), however, the ability of B1b B cells to both expand and differentiate into plasmablasts (18) provides a constant source of plasma cell precursors which can maintain antibody production (19, 20). In TG and BALB/c mice J558 Id+ B1b B cells are present in both the spleen and peritoneal cavity for the duration of the DEX-specific antibody response, similar to the findings in studies with Borrelia hermsii and NP-Ficoll immunized mice (18, 19).
B1b B cells have been shown in these mouse models to possess a unique capacity to confer protection against clinically relevant pathogenic organisms through their production of protective polysaccharide specific antibodies (19, 20). In our study we have found that DEX-specific B1b B cells are stably expanded in both TG and BALB/c mice for the entire period of observation after immunization with either E. cloacae or purified DEX. These B cells are maintained at a stable level for the duration of the response. The presence of DEX-specific B1b B cells in the blood, bone marrow, and spleen of E. cloacae challenged TG and BALB/c mice indicate that they re-circulate to secondary lymphoid organs to be recruited into DEX-specific plasmablast compartments responsible for production of anti-DEX antibodies. The ability of B1 B cells to migrate into the peripheral lymphoid tissues after antigen or TLR-mediated activation has been documented previously (34) and in a recent finding indicating that B1a B cells possess a similar capacity for memory to Francisella tularensis LPS, providing protection against lethal challenge (35). Similar studies with NP-Ficoll as a model synthetic antigen (18), have shown that B1b B cells are able to undergo successive NP-Ficoll induced expansions thus avoiding clonal exhaustion and thereby maintaining antibody production to NP. This cellular expansion not only allows for maintenance of their stable population size throughout the antibody response, but also allows these cells to generate a larger plasmablast pool upon re-challenge resulting in enhanced secondary responses. It is well known that both B1a and B1b B cells maintain themselves through homeostatic expansion (36) and it is possible that this poorly understood property may account for their memory functions in TI antibody responses.
It has been demonstrated previously that antibody responses to TI antigens often involve cooperation between MZ and B1 B cell subsets (15), even though the same clone is segregated into both MZ and B1b B cell compartments. The compartmentalization of MZ and B1b B cell functions was revealed upon breeding TG mice onto a CD19 -/- background. In these mice the deficiency in J558 Id+ MZ B cells impaired the initial peak antibody response, however, B1b B cell expansion occurred in J558 CD19 -/- mice and normal DEX-specific antibody levels were achieved later in the response as suggested previously (20).
Appreciation of the roles of TI-2 specific antibodies in protective immunity has revealed novel functions of B cell subsets in providing a unique form of memory and lasting antibody production. Existing models focus on the protective influences of TI-2 specific B1b B cells during the course of infection with pathogenic microorganisms (19, 20). Here we have investigated this phenomenon at a clonal level and demonstrated in detail the establishment of memory to DEX arrayed on E. cloacae. This clonal approach has identified an unappreciated proliferative capacity of B1b B cells, which allow them to mediate long lasting antibody production and, at the same to establish a form of memory associated with clonal expansion and long-term maintenance of DEX-specific B1b B cells. The ability of these cells to proliferate in response to antigen re-challenge thus expanding their numbers, allows them to generate increased numbers of plasmablasts, thus increasing anti-DEX serum antibody titers. This study clarifies the mechanism of B1b B cell mediated-memory induction and provides a useful model to characterize the roles of the BCR, antigen, and TLR signals in the generation of B1b B cell memory to polysaccharides.
Acknowledgments
The authors gratefully acknowledge the invaluable technical help of Lisa Jia and Jeffrey Sides. We acknowledge the helpful critical comments of Drs. Peter Burrows, Tamer Mahmoud and Nicholas Kin.
This work was supported by research funds from the National Institutes of Health grant RO1AI014782-32. This work is part of the dissertation research conducted by Jeremy B. Foote who is a predoctoral student in the Microbiology Graduate Program, The University of Alabama at Birmingham, Birmingham, AL 35294.
References
- 1.Zandvoort A, Timens W. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin Exp Immunol. 2002;130:4–11. doi: 10.1046/j.1365-2249.2002.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesinski GB, Westerink MA. Vaccines against polysaccharide antigens. Curr Drug Targets Infect Disord. 2001;1:325–334. doi: 10.2174/1568005014605964. [DOI] [PubMed] [Google Scholar]
- 3.Stohrer R, Kearney J. Ontogeny of B cell precursors responding to alpha 1-greater than 3 dextran in BALB/c mice. J Immunol. 1984;133:2323–2326. [PubMed] [Google Scholar]
- 4.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiens GD, Brown M, Rittenberg MB. Repertoire shift in the humoral response to phosphocholine-keyhole limpet hemocyanin: VH somatic mutation in germinal center B cells impairs T15 Ig function. J Immunol. 2003;170:5095–5102. doi: 10.4049/jimmunol.170.10.5095. [DOI] [PubMed] [Google Scholar]
- 6.Claflin JL, Davie JM. Clonal nature of the immune response to phosphorylcholine (PC). V. Cross-idiotypic specificity among heavy chains of murine anti-PC antibodies and PC-binding myeloma proteins. J Exp Med. 1975;141:1073–1083. doi: 10.1084/jem.141.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- 8.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- 9.Shaw PX, Goodyear CS, Chang MK, Witztum JL, Silverman GJ. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J Immunol. 2003;170:6151–6157. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 10.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J Exp Med. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23:297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Mandik-Nayak L, Racz J, Sleckman BP, Allen PM. Autoreactive marginal zone B cells are spontaneously activated but lymph node B cells require T cell help. J Exp Med. 2006;203:1985–1998. doi: 10.1084/jem.20060701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanayama N, Cascalho M, Ohmori H. Analysis of marginal zone B cell development in the mouse with limited B cell diversity: role of the antigen receptor signals in the recruitment of B cells to the marginal zone. J Immunol. 2005;174:1438–1445. doi: 10.4049/jimmunol.174.3.1438. [DOI] [PubMed] [Google Scholar]
- 15.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 16.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 18.Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci U S A. 2006;103:5905–5910. doi: 10.1073/pnas.0601502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Kearney JF, McCarthy MT, Stohrer R, Benjamin WH, Jr, Briles DE. Induction of germ-line anti-alpha 1-3 dextran antibody responses in mice by members of the Enterobacteriaceae family. J Immunol. 1985;135:3468–3472. [PubMed] [Google Scholar]
- 22.Rappleye CA, Eissenberg LG, Goldman WE. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci U S A. 2007;104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilham CA, Alexander BH, Jeanes A. Heterogeneity in dextran preparations. Arch Biochem Biophys. 1955;59:61–75. doi: 10.1016/0003-9861(55)90463-x. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Martin F, Chen X, Kearney JF. Development of VH81X transgene-bearing B cells in fetus and adult: sites for expansion and deletion in conventional and CD5/B1 cells. Int Immunol. 1997;9:493–505. doi: 10.1093/intimm/9.4.493. [DOI] [PubMed] [Google Scholar]
- 26.Kearney JF, Pollok BA, Stohrer R. Analysis of idiotypic heterogeneity in the anti-alpha 1-3 dextran and anti-phosphorylcholine responses using monoclonal anti-idiotype antibodies. Ann N Y Acad Sci. 1983;418:151–170. doi: 10.1111/j.1749-6632.1983.tb18063.x. [DOI] [PubMed] [Google Scholar]
- 27.Martin F, Won WJ, Kearney JF. Generation of the germline peripheral B cell repertoire: VH81X-lambda B cells are unable to complete all developmental programs. J Immunol. 1998;160:3748–3758. [PubMed] [Google Scholar]
- 28.Blomberg B, Geckeler WR, Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972;177:178–180. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- 29.Riblet R, Blomberg B, Weigert M, Lieberman R, Taylor BA, Potter M. Genetics of mouse antibodies. I. Linkage of the dextran response locus, VH-DEX, to allotype. Eur J Immunol. 1975;5:775–777. doi: 10.1002/eji.1830051109. [DOI] [PubMed] [Google Scholar]
- 30.Stohrer R, Lee MC, Kearney JF. Analysis of the anti-alpha 1 leads to 3 dextran response with monoclonal anti-idiotype antibodies. J Immunol. 1983;131:1375–1379. [PubMed] [Google Scholar]
- 31.Belperron AA, Dailey CM, Booth CJ, Bockenstedt LK. Marginal zone B-cell depletion impairs murine host defense against Borrelia burgdorferi infection. Infect Immun. 2007;75:3354–3360. doi: 10.1128/IAI.00422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 33.Kumar H, Belperron A, Barthold SW, Bockenstedt LK. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J Immunol. 2000;165:4797–4801. doi: 10.4049/jimmunol.165.9.4797. [DOI] [PubMed] [Google Scholar]
- 34.Ghosn EE, Yang Y, Tung J, Herzenberg LA, Herzenberg LA. CD11b expression distinguishes sequential stages of peritoneal B-1 development. Proc Natl Acad Sci U S A. 2008;105:5195–5200. doi: 10.1073/pnas.0712350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole LE, Yang Y, Elkins KL, Fernandez ET, Qureshi N, Shlomchik MJ, Herzenberg LA, Herzenberg LA, Vogel SN. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci U S A. 2009;106:4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kantor AB, Stall AM, Adams S, Watanabe K, Herzenberg LA. De novo development and self-replenishment of B cells. Int Immunol. 1995;7:55–68. doi: 10.1093/intimm/7.1.55. [DOI] [PubMed] [Google Scholar]


