Abstract
Pain is common among opioid dependent patients, yet pharmacologic strategies are limited. The aim of this study was to explore whether escitalopram, a selective serotonin reuptake inhibitor, was associated with reductions in pain. The study used longitudinal data from a randomized, controlled trial that evaluated the effects of escitalopram on treatment retention in patients with depressive symptoms who were initiating buprenorphine/naloxone for treatment of opioid dependence. Participants were randomized to take escitalopram 10mg or placebo daily. Changes in pain severity, pain interference and depression were assessed at 1, 2 and 3 months visits using the Visual Analog Scale, Brief Pain Inventory and the Beck Depression Inventory II, respectively. Fixed-effects estimator for panel regression models were used to assess the effects of intervention on changes in outcomes over time. Additional models were estimated to explore whether the intervention effect was mediated by within-person changes in depression. In this sample of 147 adults, we found that participants randomized to escitalopram had significantly larger reductions on both pain severity (b = −14.34, t = −2.66, p < .01) and pain interference (b = −1.20, t = −2.23, p < .05) between baseline and follow-up. After adjusting for within-subject changes in depression, the estimated effects of escitalopram on pain severity and pain interference were virtually identical to the unadjusted effects. In summary, this study of opioid-dependent patients with depressive symptoms found that treatment with escitalopram was associated with clinically meaningful reductions in pain severity and pain interference during the first three months of therapy.
Keywords: Pain, opioid dependence, anti-depressant, escitalopram
1. Introduction
Pain is common in opioid dependent patients. Among methadone treated patients, estimates of chronic pain prevalence range between 37-61% [3,14,21]. Several hypothetical pathways may lead to the co-existence of pain and opioid dependence, including depression [2], co-morbidities such as HIV [10] or HCV [24], and opioid-induced hyperalgesia [8]. Management of pain in opioid-dependent patients is a clinical challenge given concerns for opioid abuse and misuse among individuals with prior substance use disorders [20]. Yet unresolved pain may be a risk factor for relapse among patients whose pain is not fully treated [15]. Some small studies have suggested that buprenorphine/naloxone may be associated with improved pain in opioid dependent patients with chronic pain [5,16]. However, a study of opioid dependent patients who were treated with methadone did not find overall changes in pain level at one year [13]. Alternative, non-opioid pharmacologic therapies are needed to address pain in opioid dependent populations.
Antidepressants may constitute an appealing option for treating pain in opioid dependent patients because of the frequent co-existence of depression in this population [22]. Systematic reviews and clinical guidelines support the use of antidepressants as pharmacotherapy for chronic pain conditions such as lower back pain [7], fibromyalgia [27] and neuropathy [23], with the bulk of research to date being focused on use of tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors (SNRIs). The use of selective serotonin reuptake inhibitors (SSRIs) for chronic pain conditions has been less well studied. Escitalopram belongs to a class of newer SSRIs. It is the S-antiomer of the SSRI citalopram, which has been shown to be responsible for the drug’s pharmacologic effect. Two small studies have reported escitalopram to be effective in treating pain in the setting of polyneuropathy [19] and lower back pain [17]. No studies have evaluated the effects of escitalopram on pain in opioid dependent populations.
We recently completed a clinical trial of escitalopram for the treatment of depressive symptoms to reduce treatment drop-out in opioid-dependent persons initiating buprenorphine [26]. This secondary analysis was undertaken to examine the effects of escitalopram on pain severity and pain interference in that sample.
2. Methods
2.1 Study Sample and Design
This study used longitudinal data from a randomized, controlled trial that evaluated whether treatment with escitalopram increased treatment retention among opioid dependent patients with depressive symptoms who were initiating buprenorphine/naloxone [26]. Participants were recruited through community advertising, physician referrals and word-of-mouth. Study inclusion criteria included: age 18-65, a DSM-IV diagnosis of opioid dependence, a score on the Modified Hamilton Depression Revised Scale (MHDRS) greater than 14 [18], the absence of significant suicidal ideation, willingness and ability to complete a 3-month treatment with buprenorphine, no history of severe mental illness (bipolar disorder, schizophrenia, schizo-affective, or paranoid disorder), no currently prescribed medications for depression (participants were not excluded if they were taking a tricyclic anti-depressant for pain), and the ability to complete the study assessment in English. The study was approved by the Rhode Island Hospital and Butler Hospital Institutional Review Boards.
Between November 2006 and May 2009, 932 individuals were screened by telephone, and of those, 394 callers appeared eligible for the study and were invited for an in-person screening visit. Of the 226 who attended this visit, 147 fully met criteria and agreed to enroll the parent study. Of the 147, 72 were randomized to the intervention with escitalopram and 75 were randomized to placebo. In the intervention arm 48 completed the week 12 study visit and in the placebo arm 42 completed the week 12 study visit. The reason for loss to follow-up was drop-out from buprenorphine treatment.
Participants who enrolled in the study completed a baseline interview which included questions on pain, and were randomized to take the study medication escitalopram 10mg or placebo daily. Double-blinding regarding the medication group was maintained throughout the study period for all research staff; the off-site compounding pharmacist kept the key to the blind. Study medication and placebo were provided in identical capsule form. Approximately five days after beginning the study medication, participants returned to the research office for buprenorphine (buprenorphine/naloxone) induction. Dose adjustments were based on previous opioid use, craving and reported symptoms. In general, buprenorphine doses ranging from 12-24 mg/day were required for stabilization. At follow-up interviews at 1, 2 and 3 months post-enrollment, participants were again asked about their pain.
2.2 Measures
The primary outcomes measured were pain severity and pain interference in the past week. Pain severity was measured using the visual analogue scale (VAS) [6]. Participants were asked to rate their pain by placing a mark on a 100 mm non-hatched line which was marked at one end as “No pain” and “Pain as bad as you can imagine” on the other end. Pain interference was assessed using the mean of the 7-item subscale from the Brief Pain Inventory Short Form (BPI) [9]. This subscale measures pain interference in different domains such as sleep, work and relationships, rating each item from “0” (pain does not interfere) to “10” (pain completely interferes). Other measures included age, gender, race/ethnicity, educational status, primary illicit opioid used, withdrawal pain (number of days experienced in the past week) and depressive symptoms. Depressive symptoms were measured using the Beck Depression Inventory II (BDI II) [4].
2.3 Analytical Methods
Descriptive statistics are presented to summarize the characteristics of the study cohort. T-tests and Pearson 2-tests are presented to compare intervention arms on a range of background characteristics and indicators of study attrition. We used graphical methods to describe the pattern of pain severity and pain interference observed over time. We used the fixed-effects estimator [25] for panel regression models to statistically analyze change in pain over time, and to assess the effects of intervention on change in these outcomes over time. The fixed-effects estimator uses only within subject variability and effectively controls between subject heterogeneity on all time-invariant characteristics [1]. A potential limitation is that the effect of time-constant between subject characteristics (e.g., gender, ethnicity) on outcomes cannot be estimated. However, the fixed-effects estimator effectively controls for all unmeasured between subject differences that do not change over time [1]. All confidence interval estimates and tests of significance were based on the robust standard error estimators as implemented in Stata 10.1 [25]. The effect of time-invariant characteristics (treatment assignment) on change in the outcome over time can be estimated as the predictor by time interaction.
Our analysis proceeded in stages. We first estimated unconditional growth models to evaluate the change process. We used the likelihood-ratio difference in chi-square test to compare the linear growth model to an unconstrained time model estimating separate parameters for each follow-up using 3 dummy variables. Examination of results suggested a more parsimonious parameterization in which time was represented by a single dummy variable coded 0 if baseline and 1 if follow-up. Conditional growth models that included the treatment by time interaction were specified to estimate the effects of intervention on change in pain severity and pain interference. Additional fixed-effects regression models were estimated to test the hypothesis that the effect of escitalopram on pain was mediated by within person changes in depression.
3. Results
In our sample of 147 adults, participants averaged 37.5 (± 9.9) years of age and the majority were male (76%)(Table 1). Most participants (80.1%) were non-Hispanic Caucasian, 4.9% were African-American, 9.6% were Hispanic, and 5.5% were of other racial or ethnic origins. Ninety-three (63.7%) said heroin was their opiate of choice. The mean BDI II score at baseline was 29.4 (± 9.7). The majority (85%) of participants would be classified as having moderate to severe depression [4]. Using the Structured Clinical Interview for DSM Disorders (SCID) [12], 51% met criteria for current major depression and 4% for dysthymia. The majority (122/83%) reported some pain in the past week, and of those with pain, 48% had pain that was chronic ( 6 months). Among participants who reported pain, the most common sites were back pain (66%), joint pain (48%) and muscle pain (38%). Over 90% of participants were located for at least 1 follow-up, and there was no evidence of significant between group differences with respect to attrition at any time point (Table 1). Intervention groups also did not differ significantly with respect to other background characteristics and pain. There was no evidence of systematic differences with respect to study attrition.
Table 1.
ARISE Baseline Characteristics by Intervention (n = 147).
| Placebo (n = 75) Mean (SD) or Number (%) |
Escitalopram (n = 72) Mean (SD) or Number (%) |
p-value | |
|---|---|---|---|
| Age (Yrs) | 36.8 (±9.8) | 38.3 (±9.6) | 0.39 |
| Education (Yrs) | 12.5 (±1.8) | 11.9 (±1.6) | 0.06 |
| Ethnicity | |||
| Caucasian | 60 (81.1%) | 57 (79.2%) | |
| African-American | 3 (4.0%) | 4 (5.6%) | 0.98 |
| Hispanic | 7 (9.5%) | 7 (9.7%) | |
| Other | 4 (5.4%) | 4 (5.6%) | |
| Male | 57 (76.0%) | 55 (76.4%) | 0.90 |
| Heroin User | 44 (59.5%) | 49 (68.1%) | 0.28 |
| Baseline Pain Type | |||
| None | 17 (22.7%) | 8 (11.1%) | .125 |
| Pain, Not Chronic | 22 (29.3%) | 29 (40.3%) | |
| Chronic Pain | 36 (48.0%) | 35 (48.6% | |
| Past Week # Days with Pain Due to Withdrawal |
3.3 (±2.7) | 2.9 (±2.6) | 0.35 |
| BDI Score | 28.6 (±9.8) | 28.3 (±9.6) | 0.83 |
| VAS | 44.6 (±31.8) | 54.1 (±31.8) | 0.08 |
| BPI | 3.6 (±3.0) | 4.5 (±2.8) | 0.06 |
| Observed at 1 month | 64 (85.3%) | 64 (88.9%) | 0.52 |
| Observed at 2 months | 51 (68.0%) | 53 (73.6%) | 0.46 |
| Observed at 3 months | 48 (64.0%) | 42 (58.3%) | 0.48 |
| ≥ 1 Follow-Up | 69 (92.0%) | 69 (95.8%) | 0.33 |
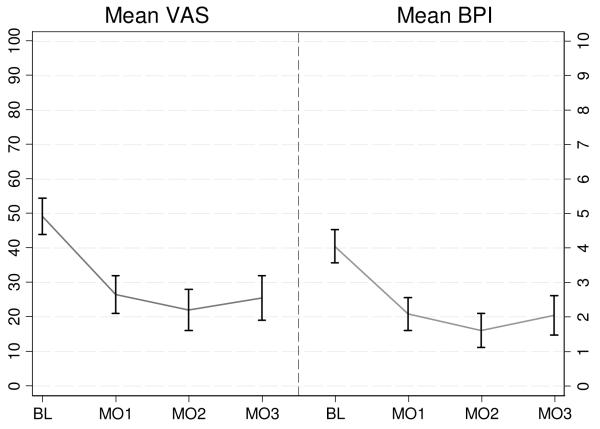
In the full cohort, mean VAS and BPI scores declined from baseline to 1-month, and stayed relatively constant at months 2 and 3 (Figure 1). Statistical comparisons of mean VAS and BPI scores across the 1-, 2-, and 3-month follow-up visits were not significant (i.e., p-value >.10) for all pairwise comparisons. Because differences in mean VAS and BPI scores across follow-up assessments were substantively small and not statistically significant we also estimated a more parsimonious model constraining the outcome means to be equal across all follow-up assessments. Likelihood ratio chi-square difference tests indicated that the more complex unconstrained time model did not fit the data significantly better than the simpler parameterization of time for either the VAS (LR2=1.41, df=2, p=0.50) or BPI (LR2=3.54, df=2, p=0.17), therefore we used this parameterization for the subsequent analysis.
Figure 1.
Change in Pain Severity (VAS) and Pain Interference (BPI) Baseline to 3-Months.
*Error bars denote 95% confidence interval estimates of the mean.
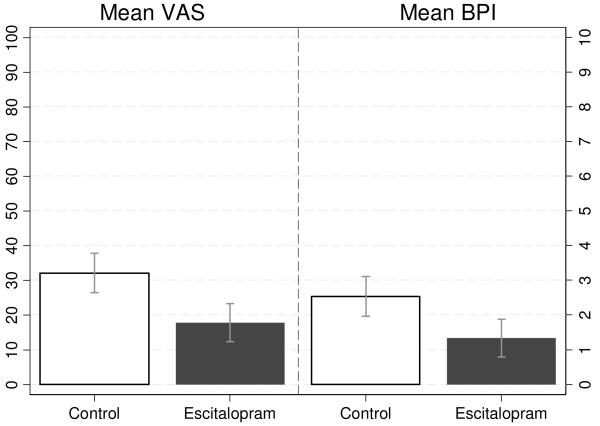
Table 2, Model 1 gives the results of fixed-effects regression estimating the effect of intervention on VAS and BPI at follow-up. The coefficient for time gives the estimated change in outcomes between baseline and follow-up in the placebo group among whom mean VAS and BPI scores decreased by 16.8 (t=−4.50, p<.01) and 1.15 (t=−3.69, p<.01) points, respectively, between baseline and follow-up. The “treatment by time” coefficient represents the effect of escitalopram relative to placebo. Compared to those receiving placebo, participants who were randomized to escitalopram had 14.34 (t = −2.66, p < .01) and 1.20 ( t = −2.23, p < .05) larger mean reductions in VAS and BPI, respectively. Figure 2 gives the expected follow-up mean VAS and BPI scores by treatment arm, as estimated by the fixed-effects model. Mean VAS scores a follow-up were estimated to be 32.1 (5% CI: 26.4-37.8) and 17.7 (95% CI: 12.2-23.2) among those randomized to placebo and escitalopram, respectively. Predicted mean BPI scores were 2.53 (95%CI: 1.96-3.10) for those receiving placebo and 1.33 (95% CI: 0.79-1.87) in the escitalopram arm.
Table 2.
Fixed-Effects Regression Models Estimating the Effect of Escitalopram on Pain Severity and Pain Interference, Without and With Adjustment for Depression
| PAIN SEVERITY | PAIN INTERFERENCE | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
|
β
(SE) |
β
(SE) |
β
(SE) |
β
(SE) |
|
| Time a | −16.82** (3.74) |
−8.37 (4.50) |
−1.48** (0.40) |
−0.16 (0.51) |
| Treatment by Time b | −14.34** (5.39) |
−14.37** (5.34) |
−1.20* (0.54) |
−1.20* (0.52) |
| BDI (Time-Varying) | NA | 0.55* (0.22) |
NA | 0.09** (0.02) |
| Intercept | 48.89 | 33.47 | 4.01 | 1.60 |
p < .05
p < .01
Coefficient gives the expected change in pain between baseline and follow-up visits for participants randomized to placebo.
Relative to controls this coefficient gives the expected mean change between baseline and follow-up visits for participants randomized to escitalopram.
Figure 2.
Predicted Follow-Up Mean VAS and BPI Scores by Intervention Group. *
*Predicted values were estimated using the fixed-effects estimator (see Table 2 – Model 1); error bars denote 95% confidence interval estimates of the predicted mean.
Since changes in depression represent a plausible mechanism through which the effect of escitalopram on pain may be mediated, we also estimated a model that included BDI II as a time-varying covariate (Model 2 in Table 2). If changes in depression mediated the effect of escitalopram on pain we would have expected that the effect of escitalopram on pain would be attenuated. We found no support for the mediation hypothesis. After adjusting for within-subject changes in depression, the estimated effect of escitalopram on pain severity (b = −14.37, t = −2.69, p < .01) and pain interference (b = −1.20, t = −2.30, p < .05) were virtually identical to the unadjusted effects reported in Model 1. However, within-subject change in BDI was associated significantly with within subject change in VAS pain severity (t = 2.52, p < .05) and BPI pain interference (t = 4.25, p < .01). A within-subject increase of 1 point on the BDI was associated with a .55 point increase in mean VAS and a .09 point increase in mean BPI scores across time.
4. Discussion
This study of opioid dependent patients with depressive symptoms found that treatment with escitalopram resulted in significantly decreased pain severity and interference over time. Adjusting for within subject changes in depression scores did not impact the effects of escitalopram, suggesting the analgesic properties of escitalopram were independent of its anti-depressant effects. This is the first study of which we are aware that demonstrates an association between the anti-depressant escitalopram and improved general pain. A small randomized, controlled cross-over study of patients with painful peripheral neuropathy found that escitalopram was associated with significantly lower pain ratings, and that the treatment effect was concentrated among patients with hyperalgesia [19]. Another randomized, controlled trial compared treatment with escitalopram to duloxetine (which is FDA approved for treatment of diabetic neuropathy, fibromyalgia and chronic musculoskeletal pain) in patients with chronic lower back pain, and found it to be equally efficacious for reducing pain [17]. Although our results demonstrated statistical significance, it is also important to assess clinical significance. In our study, the intervention escitalopram was associated with a 14 point decrease beyond what was observed with placebo, which reflects a 29% improvement from the baseline mean of 49. Recent consensus guidelines suggest that 10-20% and 30% decreases in numeric pain intensity represent minimally and moderately important changes in pain, respectively [11]. Therefore, our results suggest that escitalopram is associated with improvement in pain severity that exceeds minimal clinical importance. The same guidelines suggest that a change of 1 point of the BPI interference scale should be considered the threshold for clinically important change in pain interference. Using this criterion, our results, which show a 1.2 point change associated with escitalopram, also suggest a clinically significant effect on pain interference.
The effects of escitalopram on pain in this study did not appear to depend on the drug’s anti-depressant effect. This was observed by adjusting for within subject changes in depression in the second model. Depression scores did not differ significantly between the placebo and escitalopram over the course of the study (possibly related to the relatively low dose of escitalopram) [26]. This underscores the conclusion that the escitalopram’s effect on reducing pain in this study was independent of the drug’s anti-depressant effect. However, the study also demonstrated that there were improvements in depressive symptoms over time in both placebo and intervention arms that were associated with reductions in pain. This reinforces the close relationship between pain and depression [2] which is relevant since depressive symptoms are highly prevalent in this population [22]. It is also of interest to note that pain decreased in the placebo group during the study period. This could be due to placebo effect, the effects of buprenorphine, resolution of withdrawal pain, or (likely) some combination of all factors. Nonetheless, additional pain relief was observed in the escitalopram arm.
This study has numerous limitations and strengths. The study is based on secondary analysis of data; however, it makes use of a randomized, controlled study design with a blinded intervention. At baseline, the intervention group had slightly higher (though non-significant) mean VAS and BPI severity scores, but this should not influence the study’s main findings, which focus on the effects of the intervention on change in VAS and BPI over time. The study had a relatively short follow-up time of 3 months. It is unknown if reductions in pain associated with escitalopram are sustained beyond this period. The dose of escitalopram used in this study was relatively low (10mg). It is possible that larger reductions in pain might have occurred with use of a higher dose. The study is focused on a relatively specific patient population, namely opioid dependent patients initiating buprenorphine with depressive symptoms, which may limit the generalizability of the findings. However, there are studies that support escitalopram’s effectiveness treating pain in non-opioid dependent populations [19,17]. Furthermore, opioid dependent patients are disproportionately impacted by pain (yet rarely included in clinical trials), and alternatives to narcotic medications are greatly needed for this population.
In summary, this study of opioid-dependent patients with depressive symptoms found that treatment with escitalopram was associated with a reduction in pain severity and pain interference during the first three months of buprenorphine therapy. Treatment with escitalopram was associated with a nearly 30% reduction in pain severity after one month compared to control, and its analgesic effect appeared to be independent from any anti-depressant effect. More research is needed on the use of non-narcotic medications such as SSRIs to treat pain in opioid dependent populations.
Acknowledgements
This study was funded by the National Institute on Drug Abuse DA 022207, Clinical Trial # NCT 00475878. Dr. Stein is a recipient of a NIDA Mid-Career Award DA 000512. Dr. Tsui is a recipient of a NIDA Career Development Award DA 027367.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest related to this study.
References
- [1].Allison PD. Fixed Effects Regression Methods for Longitudinal Data using SAS. SAS Institute, Inc.; Cary, NC: 2005. [Google Scholar]
- [2].Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- [3].Barry DT, Beitel M, Garnet B, Joshi D, Rosenblum A, Schottenfeld RS. Relations among psychopathology, substance use, and physical pain experiences in methadone-maintained patients. J Clin Psychiatry. 2009;70:1213–8. doi: 10.4088/JCP.08m04367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beck AT, Steer RA, Brown GK. Manual for the BDI-II. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- [5].Blondell RD, Ashrafioun L, Dambra CM, Foschio EM, Zielinski AL, Salcedo DM. A Clinical Trial Comparing Tapering Doses of Buprenorphine with Steady Doses for Chronic Pain and Co-existent Opioid Addiction. J Addict Med. 4:140–146. doi: 10.1097/ADM.0b013e3181ba895d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- [7].Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505–14. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- [8].Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–96. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- [9].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- [10].Dobalian A, Tsao JC, Duncan RP. Pain and the use of outpatient services among persons with HIV: results from a nationally representative survey. Med Care. 2004;42:129–38. doi: 10.1097/01.mlr.0000108744.45327.d4. [DOI] [PubMed] [Google Scholar]
- [11].Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- [12].First M, Spitzer R, Williams J, Gibbons M. Structured clinical interview for DSM-IV - Patient version. NY State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- [13].Ilgen MA, Trafton JA, Humphreys K. Response to methadone maintenance treatment of opiate dependent patients with and without significant pain. Drug Alcohol Depend. 2006;82:187–93. doi: 10.1016/j.drugalcdep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- [14].Jamison RN, Kauffman J, Katz NP. Characteristics of methadone maintenance patients with chronic pain. J Pain Symptom Manage. 2000;19:53–62. doi: 10.1016/s0885-3924(99)00144-x. [DOI] [PubMed] [Google Scholar]
- [15].Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102:752–60. doi: 10.1111/j.1360-0443.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- [16].Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12:379–84. doi: 10.1097/01.mjt.0000160935.62883.ff. [DOI] [PubMed] [Google Scholar]
- [17].Mazza M, Mazza O, Pazzaglia C, Padua L, Mazza S. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049–52. doi: 10.1517/14656561003730413. [DOI] [PubMed] [Google Scholar]
- [18].Miller I, Bishop S, Norman W, Maddever H. The modified Hamilton rating scale for depression: reliability and validity. Psych Res. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- [19].Otto M, Bach FW, Jensen TS, Brosen K, Sindrup SH. Escitalopram in painful polyneuropathy: a randomized, placebo-controlled, cross-over trial. Pain. 2008;139:275–83. doi: 10.1016/j.pain.2008.04.012. [DOI] [PubMed] [Google Scholar]
- [20].Passik SD. Issues in long-term opioid therapy: unmet needs, risks, and solutions. Mayo Clin Proc. 2009;84:593–601. doi: 10.1016/S0025-6196(11)60748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289:2370–8. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- [22].Rounsaville BJ, Weissman MM, Crits-Christoph K, Wilber C, Kleber H. Diagnosis and symptoms of depression in opiate addicts. Course and relationship to treatment outcome. Arch Gen Psychiatry. 1982;39:151–6. doi: 10.1001/archpsyc.1982.04290020021004. [DOI] [PubMed] [Google Scholar]
- [23].Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. Dec;81(12):1372–3. doi: 10.1136/jnnp.2008.144964. [DOI] [PubMed] [Google Scholar]
- [24].Silberbogen AK, Janke EA, Hebenstreit C. A closer look at pain and hepatitis C: preliminary data from a veteran population. J Rehabil Res Dev. 2007;44:231–44. doi: 10.1682/jrrd.2006.05.0053. [DOI] [PubMed] [Google Scholar]
- [25].StataCorp . Stata Statistical Software: Release 10.1. StataCorp LP; College Station, TX: 2010. [Google Scholar]
- [26].Stein MD, Herman DS, Kettavong M, Cioe PA, Friedmann PD, Tellioglu T, Anderson BJ. Antidepressant treatment does not improve buprenorphine retention among opioid-dependent persons. J Subst Abuse Treat. 2010;39:157–66. doi: 10.1016/j.jsat.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Uceyler N, Hauser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59:1279–98. doi: 10.1002/art.24000. [DOI] [PubMed] [Google Scholar]