Summary
Estrogens regulate body weight and reproduction primarily through actions on estrogen receptor-α (ERα). However, ERα-expressing cells mediating these effects are not identified. We demonstrate that brain-specific deletion of ERα in female mice causes abdominal obesity stemming from both hyperphagia and hypometabolism. Hypometabolism and abdominal obesity, but not hyperphagia, are recapitulated in female mice lacking ERα in hypothalamic steroidogenic factor-1 (SF1) neurons. In contrast, deletion of ERα in hypothalamic pro-opiomelanocortin (POMC) neurons leads to hyperphagia, without directly influencing energy expenditure or fat distribution. Further, simultaneous deletion of ERα from both SF1 and POMC neurons causes hypometabolism, hyperphagia and increased visceral adiposity. Additionally, female mice lacking ERα in SF1 neurons develop anovulation and infertility, while POMC-specific deletion of ERα inhibits negative feedback regulation of estrogens and impairs fertility in females. These results indicate that estrogens act on distinct hypothalamic ERα neurons to regulate different aspects of energy homeostasis and reproduction.
Introduction
Ovarian estrogens exert important anti-obesity effects in women and female mammals. Lower levels of estrogens in postmenopausal women or in ovariectomized (OVX) animals are associated with obesity (Carr, 2003; Rogers et al., 2009). Estradiol-17β replacement in rodents prevents OVX-induced obesity by decreasing food intake and increasing energy expenditure (Gao et al., 2007). Hormone replacement therapy reverses the progression of obesity and metabolic dysfunctions in postmenopausal women (Wren, 2009). However, current hormone replacement therapy is often associated with increased prevalence of heart disease and breast cancer (Billeci et al., 2008). Because estrogens have both positive and negative effects on disease progression which are likely mediated by estrogen receptors (ERs) expressed in a variety of tissues, identification of the critical ERs and their site of actions is imperative in order to develop selective estrogen-based therapies which can selectively treat diseases associated with obesity.
Effects of estrogens on energy balance are primarily mediated by estrogen receptor-α (ERα), as women or female mice with mutations in the ERα gene display hyperadiposity (Heine et al., 2000; Okura et al., 2003), characteristically seen in postmenopausal women and OVX animals. However, the critical ERa sites that mediate estrogenic effects on energy homeostasis have not been identified. In the present study, we generated genetic mouse models with ERα selectively deleted in the central nervous system (CNS), in hypothalamic steroidogenic factor-1 (SF1) neurons, in pro-opiomelanocortin (POMC) neurons, or in both SF1 and POMC neurons, respectively. These models allowed us to identify ERα neuronal populations that regulate food intake, energy expenditure, fat distribution, and reproduction.
Results
Loss of CNS ERα impairs multiple aspects of energy homeostasis
Validation
To determine if CNS ERα is required for body weight control, we crossed mice carrying loxP-flanked ERα alleles (ERαlox/lox) to the Nestin-Cre transgenic mice. These crosses produced mice lacking ERα in most of brain regions (ERαlox/lox/Nestin-Cre) and their control littermates (ERαlox/lox). Using immunohistochemistry, we demonstrated almost complete absence of ERα in the hypothalamus (and other brain regions) in the ERαlox/lox/Nestin-Cre mice (Supple Fig. 1).
Increased body weight, adiposity, and visceral fat distribution
Compared to controls, both male and female ERαlox/lox/Nestin-Cre mice displayed significant increases in body weight (Fig. 1A and 1B). Further characterizations in female mice revealed that increases in body weight were mainly reflected by increased body fat mass (Fig. 1C). We further demonstrated that ERαlox/lox/Nestin-Cre female mice had significantly higher visceral fat distribution (% of the whole body fat), but lower subcutaneous fat distribution (Fig. 1D). These data indicate that CNS ERα is required to regulate body weight, adiposity and fat distribution.
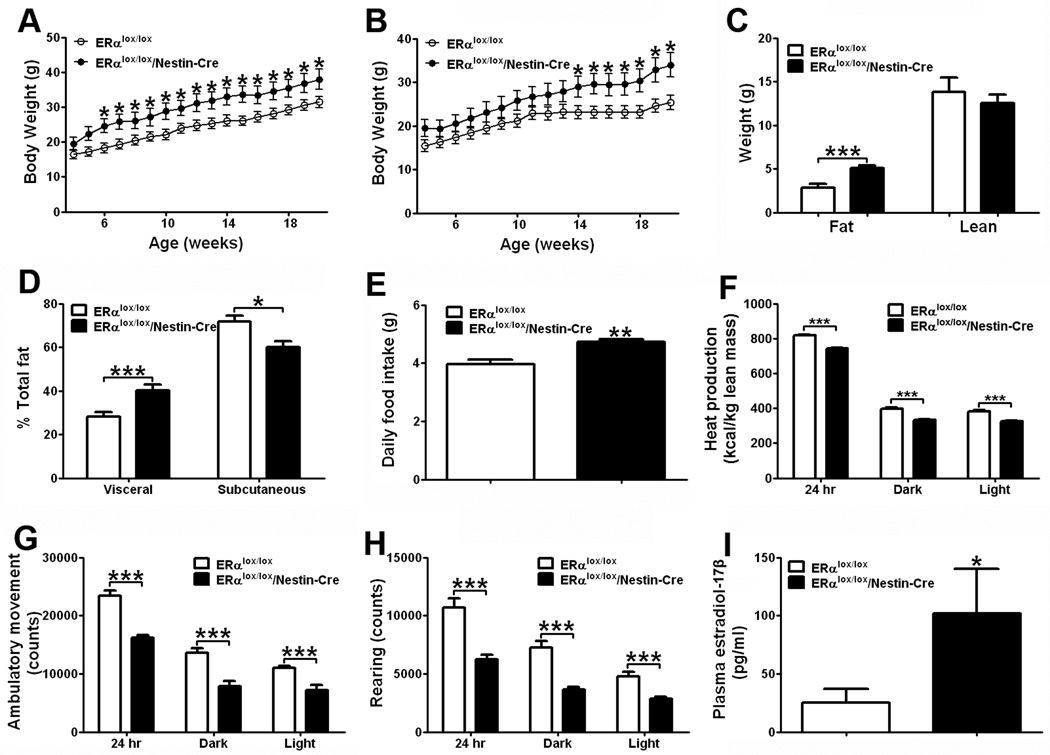
Figure 1.
CNS ERα regulates energy homeostasis. (A) Weekly body weight in male mice weaned on regular chow (n=8/genotype). (B) Weekly body weight in female mice weaned on regular chow (n=8/genotype). (C) Body composition in 15-week old female mice fed with regular chow (n=8/genotype). (D) Relative fat distribution in the visceral and subcutaneous depots in 7-week old female mice fed with regular chow (n=8 or 10/genotype). (E) Daily food intake in 7-week old female mice fed with regular chow (n=8 or10/genotype). (F–H) Chow-fed female mice (n=8 or 10/genotype) were acclimated to the TSE metabolic chambers. Average heat production (F), ambulatory movements (G) and rearing activities (H) during 24 hr, 12 hr dark cycle and 12 hr light cycle. Note: mice used in (F–H) were 12-week old littermates, and had comparable body weight (ERαlox/lox: 22.8±1.5 vs ERαlox/lox/Nestin-Cre: 27.2±2.5, P>0.05) and lean mass (ERαlox/lox: 13.80±1.72 vs ERαlox/lox/Nestin-Cre: 12.50±0.98, P>0.05), but different fat mass (ERαlox/lox: 2.80±0.45 vs ERαlox/lox/Nestin-Cre: 5.10±0.27, P<0.001). (I) Plasma estradiol-17β at diestrus in 7-week old female mice fed with regular chow (n=8 or 10/genotype). Note: mice used in (D), (E) and (I) had different total fat mass (ERαlox/lox: 3.53±0.48 vs ERαlox/lox/Nestin-Cre: 6.01±1.67, P<0.05), but comparable lean mass (ERαlox/lox: 13.51±0.21 vs ERαlox/lox/Nestin-Cre: 14.10±0.76 (P>0.05). Data are presented as mean ± SEM, and * P<0.05 and ***P<0.001 between ERαlox/lox/Nestin-Cre mice and ERαlox/lox mice.
Hyperphagia, and decreased energy expenditure and physical activity
ERαlox/lox/Nestin-Cre mice displayed hyperphagia (Fig. 1E) and decreased heat production (Fig. 1F). The lower energy expenditure may be partly caused by decreased physical activity, as the ambulatory movements and rearing activities in ERαlox/lox/Nestin- Cre mice were significantly reduced (Fig. 1G and 1H). These results demonstrate that CNS ERα is required to mediate estrogenic effects on feeding, energy expenditure, and physical activity.
Elevated plasma estradiol-17β
Notably, we found that circulating estradiol- 17β was significantly elevated in ERαlox/lox/Nestin-Cre mice (Fig. 1I). Our observations that mice lacking ERα only in the CNS develop obesity despite the elevated levels of estradiol- 17β in the circulation suggest that compared to ERα expressed in the peripheral tissues, CNS ERα appears to play more predominant roles in the regulation of energy balance.
Loss of ERα in VMH SF1 neurons affects energy expenditure and fat distribution
Validation
The ventromedial hypothalamic nucleus (VMH) is important for regulation of body weight (King, 2006). We hypothesized that ERα in the VMH is required to mediate the estrogenic effects on energy balance. Because steroidogenic factor-1 (SF1), a transcription factor, is expressed exclusively in the VMH within the brain (Ikeda et al., 1995), we used a SF1-Cre transgenic mice (line 7) (Dhillon et al., 2006) to generate mice lacking ERα in the VMH (ERαlox/lox/SF1-Cre). In control (SF1-Cre/rosa26GFP) mice, 48.3±5.7% of ERαneurons in the VMH co-express SF1 and 12.0±1.9% of SF1 neurons express ERα. In ERαlox/lox/SF1-Cre/rosa26GFP mice, the majority of ERα was selectively deleted from the VMH SF1 neurons (Supple Fig. 2). Importantly, we found that the numbers of SF1neurons in ERαlox/lox/SF1-Cre/rosa26GFP mice and in control mice were comparable (Supple Fig. 2), suggesting that deletion of ERα did not cause loss of SF1 population in the VMH.
Increased body weight, adiposity, and visceral fat distribution
When fed on regular chow, male ERαlox/lox/SF1-Cre and control (ERαlox/lox) littermates had comparable body weight and body composition (Fig. 2A and 2B). The chow-fed ERαlox/lox/SF1-Cre females had modest but significant increases in body weight (Fig. 2C) and trended increases in both fat mass and lean mass (Fig. 2D). When fed with HFD, male ERαlox/lox/SF1-Cre and control mice showed comparable body weight (Fig. 2E) and fat/lean mass (Fig. 2F). On the other hand, HFD-fed ERαlox/lox/SF1-Cre females had significant increases in body weight (Fig. 2G), which were reflected by increases in both fat mass and lean mass (Fig. 2H). Collectively, these results indicate that ERα expressed by SF1 cells is required to maintain body weight homeostasis in females, but not in males.
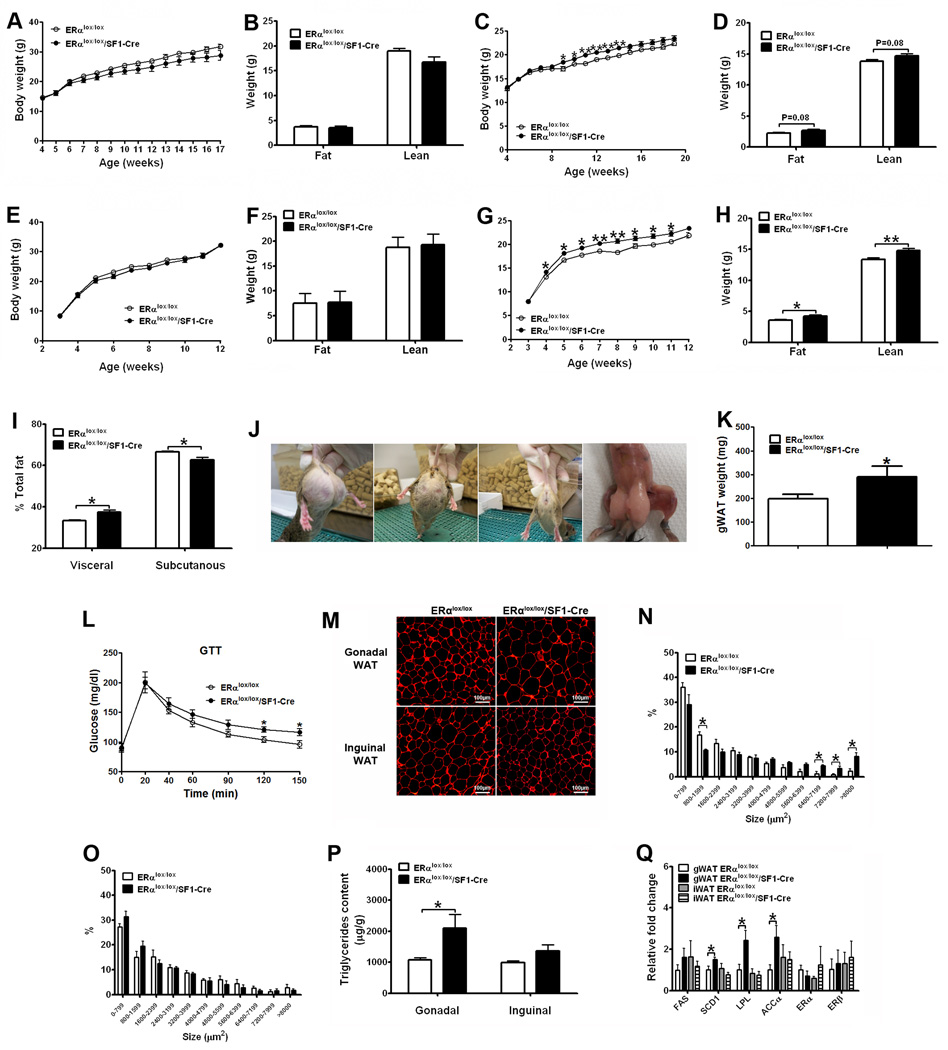
Figure 2.
ERα expressed by SF1 neurons regulates body weight and fat distribution. (A) Weekly body weight in male mice weaned on regular chow (n=6 or 12/genotype). (B) Body composition in 11-week old male mice fed with regular chow (n=6 or 12/genotype). (C) Weekly body weight in female mice weaned on regular chow (n=22 or 25/genotype). (D) Body composition in 11-week old female mice fed with regular chow (n=22 or 25/genotype). (E) Weekly body weight in male mice weaned on HFD (n=1 1 or 21/genotype). (F) Body composition in 12-week old male mice fed with HFD (n=11 or 21/genotype). (G) Weekly body weight in female mice weaned on HFD (n=10 or 15/genotype). (H) Body composition in 8-week old female mice fed with HFD (n=10 or 15/genotype). (I) Relative fat distribution in the visceral and subcutaneous depots in 8-week old female mice fed with HFD (n=5/genotype). (J) Photographs of two ERαlox/lox/SF1-Cre female mice (the first and second from the left) and one ERαlox/lox female (the third from the left); the photograph of the carcass of one ERαlox/lox/SF1-Cre female (the fourth from the left; note that the abdominal wall of the mouse was intact). (K) Weight of gonadal WAT in 6-week old female mice fed with regular chow (n=6 or 9/genotype). (L) Glucose tolerance test in 10-week chow-fed female mice (i.p. 1 g/kg glucose, n=8 or 11/genotype). (M) Representative photomicrographs of H&E staining of gonadal WAT and inguinal WAT from 16-week old HFD-fed females. (N–O) Cell size in gonadal WAT (N) and inguinal WAT (O) from 16-week old HFD-fed females (n=3 or 4/genotype). (Q) Messenger RNA levels in WAT from 16-week old HFD-fed females (n=6/genotype). Data are presented as mean ± SEM, and * P<0.05 and **P<0.01 between ERαlox/lox/SF1-Cre mice and ERαlox/lox mice.
ERαlox/lox/SF1-Cre females displayed significantly higher visceral fat distribution but lower subcutaneous fat distribution than controls (Fig. 2I). Interestingly, 30% of adult ERαlox/lox/SF1-Cre females had a massive accumulation of gonadal adipose tissue as demonstrated by appearance (Fig. 2J) and weight (Fig. 2K). Although it is unclear why the gonadal fat pads in subsets of mutant mice form this peculiar shape, this phenomenon is consistent with the abdominal obese phenotype. Given that increased visceral fat is commonly associated with impaired glucose homeostasis (Lafontan and Girard, 2008), we performed glucose tolerance tests, and found that ERαlox/lox/SF1-Cre females were glucose intolerant (Fig. 2L). Collectively, these results indicate that ERα in SF1 neurons is required for the regulation of fat distribution. Loss of ERα in SF1 neurons leads to abdominal obesity, which subsequently causes glucose dysregulation.
We further characterized the white adipose tissue (WAT) in these mutant females. We found that the average adipocyte size of gonadal WAT was larger in ERαlox/lox/SF1-Cre mice (Fig. 2M and 2N). Importantly, compared to controls, ERαlox/lox/SF1-Cre females stored more energy in the gonadal WAT, demonstrated by significantly increased triglyceride content (normalized by fat weight) (Fig. 2P). Consistently, mRNA levels of genes promoting lipogenesis and triglyceride storage, including stearoyl-CoA desaturase-1 (SCD1), lipoprotein lipase (LPL) and acetyl-CoA carboxylase α (ACCα), were significantly elevated in gonadal WAT of ERαlox/lox/SF1-Cre females (Fig. 2Q). These findings indicate that ERα in SF1 neurons is required for the regulation of energy partitioning in the gonadal WAT. On the other hand, the inguinal WAT of ERαlox/lox/SF1-Cre mice and controls had comparable adipocyte size (Fig. 2M and 2O), triglyceride content (Fig. 2P), and gene expression profile (Fig. 2Q). No significant difference was observed in the expression of fatty acid synthase (FAS), ERα, and ERβ in all the fat pads (Fig. 2Q).
Decreased energy expenditure
The increased body weight and adiposity in ERαlox/lox/SF1-Cre females was not due to differences in energy intake, because young or adult female ERαlox/lox/SF1-Cre and control mice consumed comparable levels of calories (Fig. 3A). In contrast, ERαlox/lox/SF1-Cre females were hypometabolic, as demonstrated by significant decreases in heat production (Fig. 3B). Components of total energy expenditure include energy required for physical activities, basal metabolism and diet-induced thermogenesis (Castaneda et al., 2005). In particular, ERαlox/lox/SF1-Cre females showed normal ambulatory movements and rearing activities (Fig. 3C and 3D), indicating that ERα in SF1 neurons is not required to regulate physical activity. We further assessed the basal metabolic rate by measuring the minimal heat production during the light cycle (Kaiyala et al., 2010), and found that ERαlox/lox/SF1-Cre females had significant reductions in basal metabolic rate (Fig. 3E). To assessed diet-induced thermogenesis, we monitored energy expenditure in response to a fasting-re-feeding paradigm. The increased heat production in ERαlox/lox/SF1-Cre mice was significantly reduced (Fig. 3F and 3G). Thus, our results suggest that ERα expressed by VMH SF1 neurons is required to regulate basal metabolic rate and to mediate appropriate thermogenic responses to feeding.
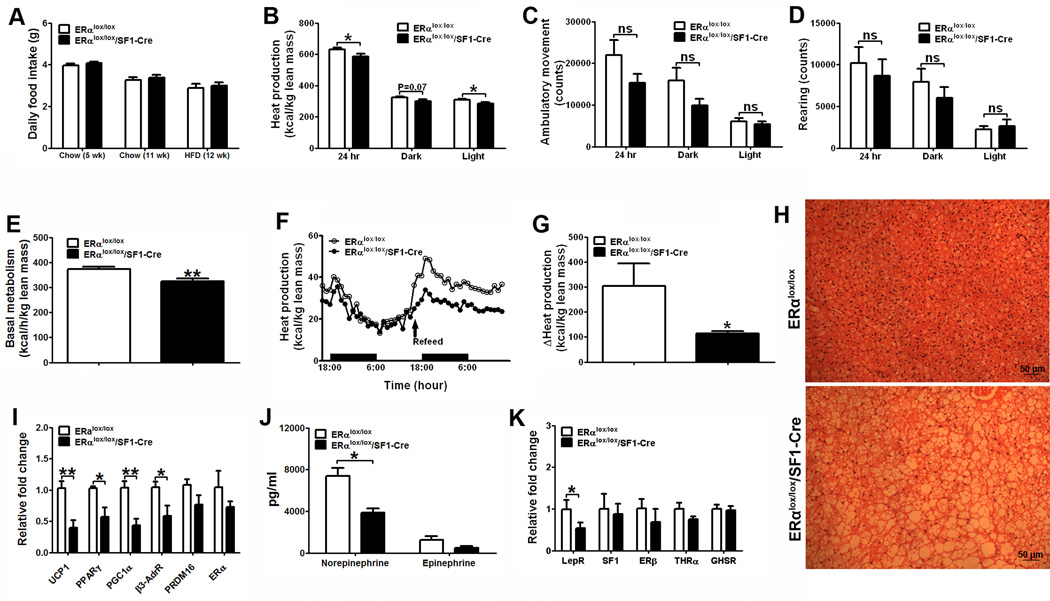
Figure 3.
ERα expressed by SF1 neurons regulates energy expenditure. (A) Daily food intake in 5-week old female mice fed wit regular chow (n=7 or 23/genotype), 11-week old female mice fed with regular chow (n=9/genotype) and in 12-week old female mice fed with HFDHFD (n=12/genotype). (B–E) Chow-fed female mice were acclimated to the TSE metabolic chambers (n=11 or 12/genotype). Average heat production (B), ambulatory movements (C), rearing activities (D) during 24 hr, 12 hr dark cycle and 12 hr light cycle, and basal metabolism (E). Note: mice used in (B–E) were 20-week old littermates, and had comparable body weight (ERαlox/lox: 20.26±0.47 vs ERαlox/lox/SF1-Cre: 21.31±0.53, P>0.05), fat mass (ERαlox/lox: 3.37±0.26 vs ERαlox/lox/SF1-Cre: 4.10±0.45, P>0.05), and lean mass (ERαlox/lox: 15.45±0.45 vs ERαlox/lox/SF1-Cre: 15.97±0.34, P>0.05). (F–G) HFD-fed female mice were acclimated to the TSE metabolic chambers. HFD was removed from the TSE metabolic chambers for 24 hrs, followed by re-feeding for 24 hrs. Temporal responses in heat production (F) of female mice during fasting and HFD-re-feeding were monitored. (G) Increases in heat production after HFD re-feeding were calculated by subtracting heat production during 24 hr fasting from heat production during 24 hr re-feeding. Note: mice used in (F–G) were 12-week old littermates, and had comparable body weight (ERαlox/lox: 20.75±0.39 vs ERαlox/lox/SF1-Cre: 21.05±0.65, P>0.05), but different fat mass (ERαlox/lox: 3.55±0.19 vs ERαlox/lox/SF1-Cre: 4.23±0.22, P<0.05) and lean mass (ERαlox/lox: 13.55±0.32 vs ERαlox/lox/SF1-Cre: 14.77±0.34, P<0.05). (H) Representative photomicrographs of H&E staining of BAT from 16-week old HFD-fed females. (I) Messenger RNA levels in BAT from 16-week old HFD-fed females (n=6/genotype). (J) Plasma norepinephrine and epinephrine in chow-fed females (n=6/genotype). (K) Messenger RNA levels in micro-dissected VMH from 16-week old HFD-fed females (n=6/genotype). Data are presented as mean ± SEM, and * P<0.05, **P<0.01 and *** P<0.001 between ERαlox/lox/SF1-Cre mice and ERαlox/lox mice.
Impaired BAT thermogenesis
Brown adipose tissue (BAT) plays crucial roles in mediating thermogenesis (Dulloo, 2002). Although the weight of BAT was not different between genotypes (ERαlox/lox: 86.67±7.68 mg vs ERαlox/lox/SF1-Cre: 97.86±7.16 mg, P>0.05, N=12 or 15/genotype), histological analyses revealed a large amount of lipid deposition in the ERαlox/lox/SF1-Cre BAT (Fig. 3H). Consistently, UCP1 mRNA levels in the ERαlox/lox/SF1-Cre BAT were significantly reduced (Fig. 3I). In addition, mRNA levels of peroxisome proliferator-activated receptor γ (PPARγ), PPARγ co-activator-1α (PGC-1α) and β3 adrenergic receptor, factors known to stimulate UCP1 expression (Seale et al., 2007), were significantly lower in ERαlox/lox/SF1-Cre BAT (Fig. 3I). The levels of PRDM16 and ERα in BAT were not altered (Fig. 3I). Taken together, these findings suggest that reduced ERα signals in VMH SF1 neurons led to a reduction in thermogenic functions of BAT by suppressing UCP1 expression.
Decreased sympathetic outflow
ERαlox/lox/SF1-Cre females had significantly lower plasma norepinephrine levels than controls; plasma epinephrine levels were not significantly different (Fig. 3J). These findings suggest that ERα in VMH SF1 neurons is required to maintain normal central sympathetic outflow to the peripheral tissues. The decreased sympathetic tone in ERαlox/lox/SF1-Cre mice could contribute to decreased BAT thermogenesis and the increased lipid accumulation in gonadal WAT.
Decreased expression of leptin receptors
Messenger RNA of leptin receptors was significantly reduced in the VMH of ERαlox/lox/SF1-Cre females (Fig. 3K). These findings support the possibility that ERα signals in SF1 neurons are required to maintain normal leptin sensitivity by regulating transcription of leptin receptors. No significant changes were found in the mRNA levels of SF1, ERβ, thyroid hormone receptor-α (THRα) and growth hormone secretagogue receptor (GHSR, ghrelin receptors) in the VMH of ERαlox/lox/SF1-Cre females (Fig. 3K).
Responses in the pituitary, adrenal gland and gonads
In addition to the VMH in the brain, SF1 cells are also found in the pituitary, adrenal gland and gonads (Zhao et al., 2001). Therefore, ERα may be deleted in these endocrine organs which may potentially confound the metabolic phenotypes. We found that ERα mRNA levels were comparable in these organs from the two genotypes (Supple Fig.3A). These results suggest that ERα may not be expressed in SF1 cells in these peripheral organs in wildtype mice, and therefore SF1-Cre-induced recombination did not affect ERα expression. Alternatively, the loss of ERα from these peripheral SF1 cells, if any, may be compensated by elevated ERα expressed by non-SF1 cells in the same organs. We further examined the impact of the possible ERα deletion on the functions of these organs. We found that there were no significant changes in the plasma estradiol-17β, corticosterone, T3/T4, progesterone, FSH or LH (Supple Table 1). No major abnormalities were observed in the morphology of the pituitary and adrenal gland (Supple Fig. 3B). However, ERαlox/lox/SF1-Cre ovaries mice showed increased number of antral follicles and lack of corpus luteum (Supple Fig. 3B), consistent with our findings that ERαlox/lox/SF1-Cre females were infertile (data not shown). Nevertheless, because the hormones secreted from the pituitary, adrenal gland and ovary were not significantly altered, it is unlikely that the metabolic phenotypes outlined above are due to the possible deletion of ERα in these peripheral organs, although this possibility cannot be fully excluded.
Loss of ERα in POMC neurons directly affects feeding and negative feedback
Validation
Pro-opiomelanocortin (POMC) neurons secrete α-melanocyte-stimulating hormone (α-MSH) to reduce food intake and increase energy expenditure (Morton et al., 2006). Estrogens activate POMC neurons (Gao et al., 2007; Malyala et al., 2008). However, the physiological significance of ERα expressed by POMC neurons has not been directly tested. We crossed ERαlox/lox mice to the POMC-Cre transgenic mice to generate mice lacking ERα in POMC neurons (ERαlox/lox/POMC-Cre). In control (POMC-Cre/rosa26GFP) mice, 21.5±2.5% of POMC neurons in the ARH and 20.9±4.8% of POMC neurons in the NTS co-expressed ERα (Supple Fig. 4). In ERαlox/lox/POMC-Cre/rosa26GFP mice, only 1.9±0.4% of POMC neurons in the ARH and none in the NTS co-expressed ERα, confirming that the majority of ERα was selectively deleted from POMC neurons (Supple Fig. 4). Importantly, deletion of ERα from POMC neurons did not cause loss of POMC neurons (Supple Fig. 4).
Increased body weight
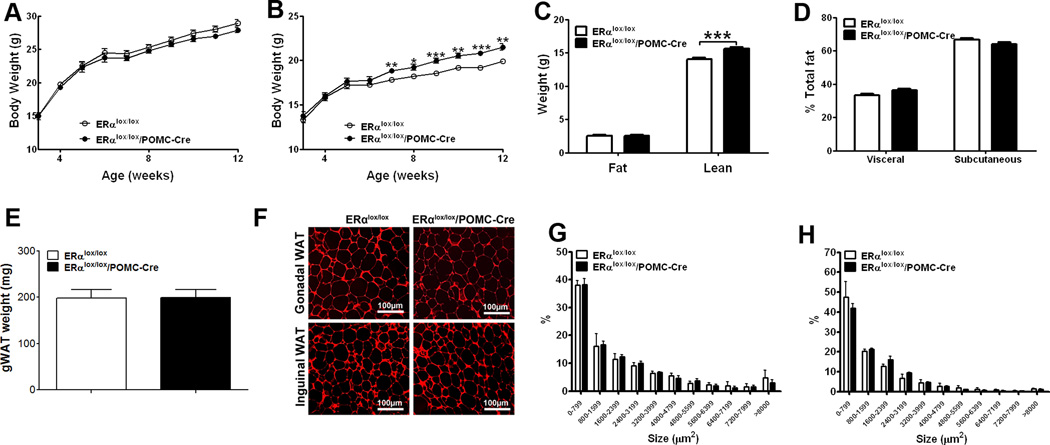
Male ERαlox/lox/POMC-Cre and control (ERαlox/lox) littermates showed comparable body weight (Fig. 4A). On the other hand, ERαlox/lox/POMC-Cre females had significant increases in body weight (Fig. 4B), mainly reflected by increases in lean mass (Fig. 4C). No significant difference were observed in the visceral/subcutaneous fat distribution (Fig. 4D), the weight of gonadal fat pads (Fig. 4E) and the adipocyte size in gonadal and inguinal WAT (Fig. 4F-4H) between the ERαlox/lox/POMC-Cre females and controls.
Figure 4.
Deletion of ERα in POMC neurons leads to increased body weight and lean mass. (A) Weekly body weight in male mice weaned on regular chow (n=33 or 34/genotype). (B) Weekly body weight in female mice weaned on regular chow (n=25 or 32/genotype). (C) Body composition in 11-week old female mice fed with regular chow (n=25 or 32/genotype). (D) Relative fat distribution in the visceral and subcutaneous depots in 18-week old female mice fed with regular chow (n=5/genotype). (E) Weight of gonadal WAT in 6-week old female mice fed with regular chow (n=8 or 9/genotype). (F) Representative photomicrographs of H&E staining of gonadal WAT and inguinal WAT from 5-month old chow-fed females. (G–H) Cell size in gonadal WAT (G) and inguinal WAT (h) from 5-month old chow-fed females (n=3/genotype). Data are presented as mean ± SEM, and * P<0.05, **P<0.01 and ***P<0.001 between ERαlox/lox/POMC-Cre mice and ERαlox/lox mice.
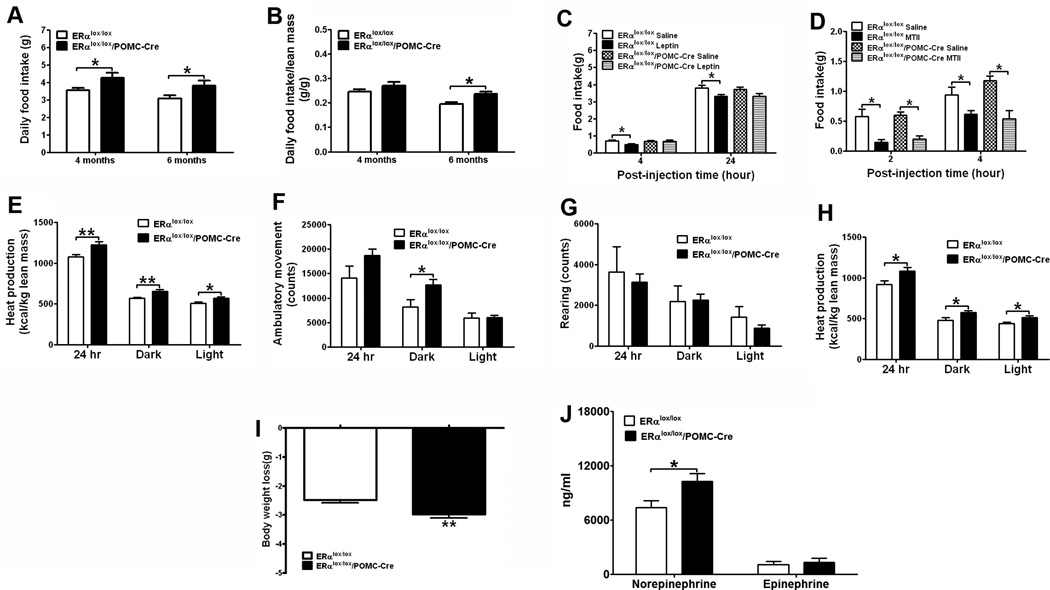
Hyperphagia and blunted anorexigenic responses to leptin
ERαlox/lox/POMC-Cre females displayed a chronic hyperphagia. This is demonstrated by significant increases in food intake in chow-fed ERαlox/lox/POMC-Cre females at 4 and 6 months of age (Fig. 5A). When normalized by lean mass, food intake was significantly increased at 6 months (Fig. 5B). We further examined the effects of anorexigenic hormones in these females. While leptin (5 mg/kg, i.p.) significantly reduced food intake in controls, these anorexigenic effects were blunted in ERαlox/lox/POMC-Cre females (Fig. 5C). In contrast, anorexia induced by MTII, a melanocortin agonist, was indistinguishable in ERαlox/lox/POMC-Cre and controls (Fig. 5D). Collectively, these findings demonstrated that ERα in POMC neurons is required to maintain normal feeding behavior, at least partly through interacting with the anorexigenic leptin signals.
Figure 5.
Deletion of ERα in POMC neurons leads to hyperphagic and hypermetabolic phenotypes. (A) Daily food intake in 4-month old and 6-month old female mice fed with regular chow (n=12 or 13/genotype). (B) The same food intake data in (A) presented as daily food intake normalized by lean mass. (C) Effects of i.p. injections of leptin (5 mg/kg) on food intake in 12-week old chow-fed female mice (n=8/group). (D) Effects of i.p. injections of MTII (1 mg/kg) on food intake in 16-week old chow-fed female mice (n=8/group). (E–I) HFD-fed female mice (n=10 or 13/genotype) were acclimated to the TSE metabolic chambers. Average heat production (E), ambulatory movements (F) and rearing activities (G) during 24 hr, 12 hr dark cycle and 12 hr light cycle were measured at fed condition. (H–I) HFD was removed from the TSE metabolic chambers for 24 hrs, and average heat production (H) during 24 hr, 12 hr dark cycle and 12 hr light cycle was measured at fasted condition. (I) Body weight loss induced by 24 hr fasting was measured. Note: mice used in (E–I) were 20-week old littermates, and had comparable body weight (ERαlox/lox: 25.14±1.16 vs ERαlox/lox/POMC-Cre: 25.12±0.95, P>0.05), fat mass (ERαlox/lox: 8.84±0.13 vs ERαlox/lox/POMC-Cre: 8.80±0.11, P>0.05) and lean mass (ERαlox/lox: 14.28±0.27 vs ERαlox/lox/POMC-Cre: 14.72±0.22, P>0.05). (J) Plasma norepinephrine and epinephrine in chow-fed females (n=6/genotype). Data are presented as mean ± SEM, and * P<0.05 and **P<0.01 between ERαlox/lox/POMC-Cre mice and ERαlox/lox mice.
Increased energy expenditure, sympathetic outflow and plasma estradiol- 17β
Unexpectedly, at libitum ERαlox/lox/POMC-Cre females showed increased heat production (Fig. 5E). In addition, the ambulatory movements during the dark cycle were significantly elevated in the ERαlox/lox/POMC-Cre females (Fig. 5F), which may at least partly contribute to the increased energy expenditure. The rearing activities were comparable between the two genotypes (Fig. 5G). To further confirm the unexpected hypermetabolic phenotypes, we examined the energy expenditure during fasting. Consistent with results from at libitum mice, ERαlox/lox/POMC-Cre mice showed increased heat production during 24 hr fasting (Fig. 5H), which led to a significantly greater weight loss (Fig. 5I). Interestingly, ERαlox/lox/POMC-Cre females had significantly higher plasma norepinephrine than control mice, while plasma epinephrine levels were comparable (Fig. 5J). The elevated sympathetic tone may be the underlying mechanisms for elevated metabolism seen in these ERαlox/lox/POMC-Cre mice.
Although we cannot fully exclude the possibility that the increases in sympathetic outflow and energy expenditure in ERαlox/lox/POMC-Cre females are directly due to ERα deletion from POMC neurons, it is more likely that these phenotypes may result from compensatory actions of ERα in other CNS sites. Supporting this notion, we found that ERαlox/lox/POMC-Cre females have significantly higher plasma estradiol-17β levels (Fig. 6A). Presumably, the elevated estradiol- 17β would act on other CNS ERα sites (including SF1 neurons) to increase sympathetic outflow, energy expenditure and physical activity.
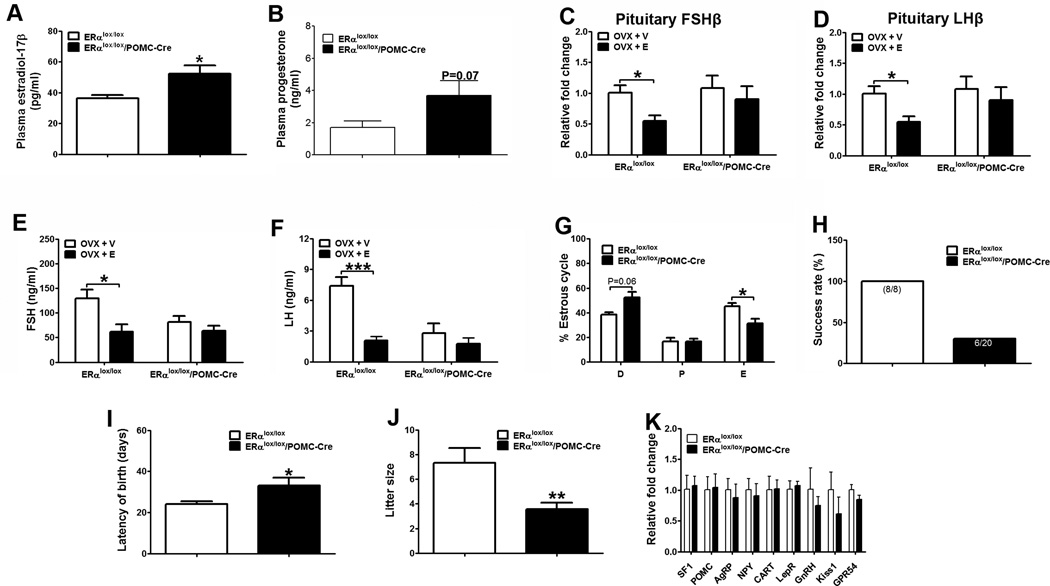
Figure 6.
Deletion of ERα in POMC neurons leads to fertility phenotypes. (A–B) Plasma estradiol-17β (A) and progesterone (B) at diestrus in 6-month old female mice fed with HFD (n=6 or 8/genotype). (C–D) Relative mRNA levels of FSHβ (C) or LHβ (D) in the pituitary measured 4 weeks after receiving ovriectomy plus estradiol-17β replacement (0.5 µg/day/mouse, OVX+E) or plus vehicle (OVX+V) (n=6/group). (E–F) Plasma FSH (E) or LH (F) measured in mice described in (C–D). (G) Length of diestrus, proestrus and estrus relative to the entire etrus cycles (n=8 or 17/genotype). (H) Percentage of mice that successfully delivered pups (n=8 or 20/genotype). (I) Averaged time period between mating day and birth day of pups (n=8 or 6/genotype). (J) Averaged litter size (n=8 or 6/genotype). Note: Only mice that successfully delivered pups in (H) were included in the analyses in (I and J). (K) Relative mRNA levels in the hypothalamus from 16-week old chow-fed females (n=6/genotype). Data are presented as mean ± SEM, and *P<0.05, **P<0.01, and ***P<0.001 between ERαlox/lox/POMC-Cre mice and ERαlox/lox mice.
Impaired negative feedback and fertility
Plasma progesterone in ERαlox/lox/POMC-Cre females trended to increase but failed to reach statistical significance (Fig. 6B). Although the basal FSH and LH levels in gonad intact females were not significantly altered (Supple Table 2), the estrogen-induced suppressions of FSH and LH were blunted in ERαlox/lox/POMC-Cre females. Specifically, estradiol-17β replacement in OVX control females significantly suppressed expression of FSH and LH subunits in the pituitary, whereas these negative feedback effects were blunted in ERαlox/lox/POMC-Cre females (Fig. 6C and 6D). Similar patterns were also observed in plasma FSH and LH (Fig. 6E and 6F). These results suggest that ERα in POMC neurons is required to mediate the negative feedback regulation. Of note, the plasma FSH/LH levels after OVX appeared to be lower in ERαlox/lox/POMC-Cre compared to control mice (Fig. 6E and 6F), while the pituitary mRNA levels of FSH/LH subunits in the same mice were comparable (Fig. 6C and 6D). This discrepancy suggests that the secretion of FSH/LH or the stability/degradation of plasma FSH/LH may be differently regulated in the OVX ERαlox/lox/POMC-Cre mice.
In addition, we found that the gonad intact ERαlox/lox/POMC-Cre females had abnormal estrous cycles. The length of estrous phase was significantly reduced, while the diestrus phase trended to be elongated in ERαlox/lox/POMC-Cre mice (Fig. 6G). No differences were observed in the length of proestrus phase (Fig. 6G). Further, female ERαlox/lox/POMC-Cre mice had impaired reproductive capacity. In particular, only 30% of ERαlox/lox/POMC-Cre females (6 out of 20) were able to conceive and deliver, while 100% of the age-matched control females gave birth (Fig. 6H). In addition, it took significantly longer for the 6 ERαlox/lox/POMC-Cre dams to conceive than the controls (Fig. 6I). The average litter size from these dams was significantly reduced (Fig. 6J). No major abnormalities were observed in the morphology of ovaries from ERαlox/lox/POMC-Cre females (Supple Fig. 5C). Collectively, these findings indicate that ERα in POMC neurons is required to maintain normal estrous cyclicity and female fertility.
Expression of hypothalamic genes
In an attempt to further characterize the effects of estrogen/ERα on the POMC neurons, we examined the gene expression profile in the hypothalamus of ERαlox/lox/POMC-Cre females. However, there was no significant difference in the expression of POMC, AgRP, NPY, CART, leptin receptors, SF1, GnRH, Kiss1 and GPR54 between ERαlox/lox/POMC-Cre females and controls (Fig. 6K).
Responses in pituitary functions
In addition to the POMC neurons in the brain, POMC (ACTH) cells also exist in the pituitary. We found that ERα mRNA was significantly reduced in the ERαlox/lox/POMC-Cre pituitary (Supple Fig. 5A), indicating that ERα is also deleted from these pituitary ACTH cells. However, we did not find any significant changes in POMC (ACTH) expression in the pituitary (Supple Fig. 5B). In addition, plasma levels of corticosterone at either basal or stressed conditions were comparable between ERαlox/lox/POMC-Cre females and controls (Supple Table 2). Morphologic analysis did not reveal any alterations in the pituitary (Supple Fig. 5B). In addition, we did not detect any significant changes in plasma T3/T4 levels (Supple Table 2). Therefore, it is unlikely that the metabolic and reproductive phenotypes observed in ERαlox/lox/POMC-Cre females are due to loss of ERα from the pituitary ACTH cells, although we cannot fully rule out this possibility.
Double deletion of ERα from SF1/POMC neurons affects feeding, energy expenditure and fat distribution
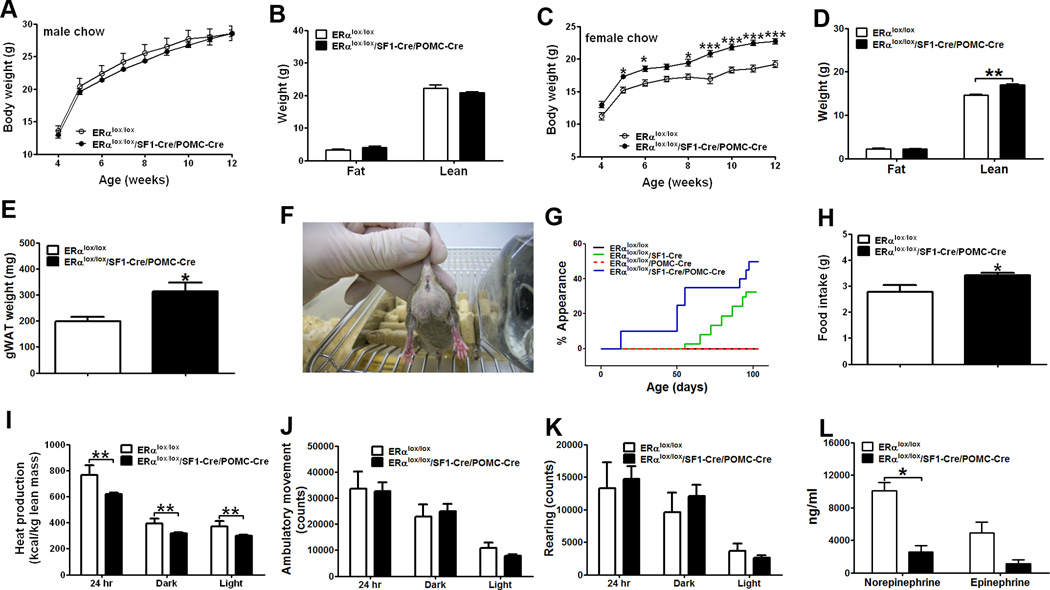
Our data indicated that ERα in SF1 neurons is required to regulate energy expenditure and fat distribution, while ERα in POMC neurons is required for the regulation of feeding. To further confirm this model, we generated mice lacking ERα in both SF1 and POMC neurons (ERαlox/lox/SF1-Cre/POMC-Cre) and the control (ERαlox/lox) littermates.
Chow-fed male ERαlox/lox/SF1-Cre/POMC-Cre mice and controls showed comparable body weight and body composition (Fig. 7A and 7B). Female ERαlox/lox/SF1-Cre/POMC-Cre mice had significantly increased body weight (Fig. 7C), which was mainly reflected by increases in lean mass (Fig. 7D). Although the whole body fat mass was not different (Fig. 7D), the average weight of gonadal fat was significantly increased in ERαlox/lox/SF1-Cre/POMC-Cre females (Fig. 7E). Similar to ERαlox/lox/SF1-Cre females, subsets of ERαlox/lox/SF1-Cre/POMC-Cre females displayed the massive gonadal fat expansion (Fig. 7F). While 30% of ERαlox/lox/SF1-Cre females developed this phenotype during adulthood (2–3 months), 50% of ERαlox/lox/SF1-Cre/POMC-Cre females developed the same phenotype at earlier ages (as early as 2 weeks of age) (Fig. 7G). Further, ERαlox/lox/SF1-Cre/POMC-Cre females were not only hyperphagic (Fig. 7H), but also showed decreased heat production (Fig. 7I). No significant difference was observed in ambulatory movements and rearing activities in female mice (Fig. 7J and 7K). Finally, ERαlox/lox/SF1-Cre/POMC-Cre females had significantly lower plasma norepinephrine levels than controls; epinephrine levels were not significantly different (Fig. 7L). Collectively, these findings indicate that ERα expressed by SF1 and POMC neurons provides the coordinated control of food intake, energy expenditure and fat distribution.
Figure 7.
Deletion of ERα in both SF1 and POMC neurons produces hyperphagia and decreased energy expenditure. (A) Weekly body weight in male mice weaned on regular chow (n=6 or 15/genotype). (B) Body composition in 12-week old male mice fed with regular chow (n=6 or 15/genotype). (C) Weekly body weight in female mice weaned on regular chow (n=11 or 39/genotype). (D) Body composition in 12-week old female mice fed with regular chow (n=11 or 39/genotype). (E) Weight of gonadal WAT in 6-week old female mice fed with regular chow (n=7 or 9/genotype). (F) Photograph of an ERαlox/lox/SF1-Cre/POMC-Cre female mouse at the age of 6 weeks. (G) Appearance rate of abdominal obesity (defined by obvious abnormal expansion of the lower abdominal obesity as demonstrated in (E)). (H) Daily food intake was measured in 6-week old female mice fed with regular chow (n=6 or 17/genotype). (I–K) Chow-fed female mice (n=6 or 17/genotype) were acclimated to the TSE metabolic chambers and average heat production (I), ambulatory movement (J) and rearing activity (K) during 24 hr, 12 hr dark cycle and 12 hr light cycle was measured. Note: mice used in (H–K) were 6-week old littermates, and had comparable body weight (ERαlox/lox: 16.63±1.01 vs ERαlox/lox/SF1-Cre/POMC-Cre: 18.09±0.51, P>0.05) and fat mass (ERαlox/lox: 2.15±0.37 vs ERαlox/lox/SF1-Cre/POMC-Cre: 1.88±0.26, P<0.01), but different lean mass (ERαlox/lox: 12.33±0.35 vs ERαlox/lox/SF1-Cre/POMC-Cre: 13.69±0.22, P<0.01). (L) Plasma norepinephrine and epinephrine in chow-fed females (n=4/genotype, 6 weeks of age). Data are presented as mean ± SEM, and * P<0.05, **P<0.01, and ***P<0.001 between ERαlox/lox/SF1-Cre/POMC-Cre mice and ERαlox/lox mice.
Cre transgenes do not affect body weight
To rule out the possibility that the Cre transgenes used in the present study may have independently contributed to the metabolic phenotypes, we generated parallel cohorts of transgenic mice carrying only the Nestin-Cre, SF1-Cre or POMC-Cre transgene and their respective wildtype littermates. These mice were produced at the same genetic background as those conditional knock-out mice. No significant change in body weight was observed in these transgenic Cre mice (Supple Fig. 6).
Discussion
Genetic segregation of ERα functions in the brain
Estrogen/ERα system is known to regulate food intake, energy expenditure, physical activity (Gao et al., 2007) and fat distribution (Heine et al., 2000). ERα-expressing cells mediating these estrogenic effects are not identified prior to our study. The phenotypic comparison in four mouse models we generated provides evidence to support a segregation model that ERα expressed by distinct hypothalamic neurons mediates different functions of estrogens in the context of energy homeostasis.
Mice lacking ERα in the CNS have hyperphagia and decreased energy expenditure. These results indicate that CNS ERα is required to suppress food intake and increase energy expenditure. The anorexigenic effects of estrogens are further pinpointed to be mediated by ERα-positive POMC neurons, as mice lacking ERα in POMC neurons or in both POMC and SF1 neurons develop hyperphagia, while deletion of ERα only in SF1 neurons does not affect feeding.
We identify ERα-positive SF1 neurons as the key site where estrogens act to stimulate energy expenditure, since deletion of ERα in SF1 neurons produces hypometabolic phenotypes. The increased energy expenditure seen in ERαlox/lox/POMC-Cre mice is unexpected, as estrogens are known to activate POMC neurons (Malyala et al., 2008), and activation of POMC neurons would increase energy expenditure (Morton et al., 2006). The fact that ERαlox/lox/POMC-Cre mice have elevated estradiol-17β levels suggests that the increased energy expenditure may result from compensatory estrogenic actions in other ER sites. Since mice lacking ERα in the CNS show decreased energy expenditure despite the elevated estradiol-17β levels, these “compensatory” estrogen signals must be neuronal in origin. In addition, decreased energy expenditure in ERαlox/lox/SF1-Cre/POMC-Cre mice further pinpoints that ERα-positive SF1 neurons are at least one site where elevated estradiol- 17β acts to stimulate energy expenditure in ERαlox/lox/POMC-Cre mice.
Estrogen/ERα signals also suppress fat accumulation in the visceral adipose depot (Heine et al., 2000; Rogers et al., 2009). We show that CNS deletion of ERα leads to increased visceral fat distribution. Further, mice lacking ERα in SF1 neurons or in both SF1 and POMC neurons show increased visceral fat distribution, whereas deletion of ERα in POMC neurons alone does not influence fat distribution. Therefore, these findings indicate that ERα in SF1 neurons is required to mediate estrogenic effects on fat distribution.
Collectively, our results indicate that estrogenic effects on food intake, energy expenditure and fat distribution are mediated by segregated hypothalamic ERα populations. Thus, ERα in SF1 neurons is required to maintain normal energy expenditure and fat distribution, while ERα in POMC neurons regulates feeding.
Notably, CNS deletion of ERα leads to hypoactivity, indicating that CNS ERα is required to stimulate physical activity. However, this phenotype is not observed in mice lacking ERα in SF1 neurons, in POMC neurons or in both, which implies that other CNS neurons expressing ERα may be responsible for these effects. Of note, animals with ERα knocked down in the VMH display decreased physical activity (Musatov et al., 2007). Together with our observations, these findings suggest that non-SF1 neurons in the VMH may mediate estrogenic actions to regulate physical activity.
ERα mutations in male mice and men cause obesity (Heine et al., 2000; Smith et al., 1994). Consistently, we show that CNS deletion of ERα also promotes body weight gain in males. These findings highlight the physiological relevance of ERα in male brains in the regulation of energy balance. Notably, deletion of ERα in SF1 neurons, POMC neurons or both, does not affect body weight in male mice. Collectively, these findings indicate that other ERα sites in male brains contribute to the regulation of energy balance. Further efforts are warranted to unravel these unknown ERα sites in male brains.
Estrogenic actions in VMH SF1 neurons
The major metabolic phenotypes in mice lacking ERα in SF1 neurons include decreased energy expenditure (e.g. BAT thermogenesis) and increased lipid accumulation in gonadal WAT. Both these deficits could be attributed to decreased sympathetic tone (lower norepinephrine). Consistently, electric stimulation of the VMH increases sympathetic inputs to BAT and enhances thermogenesis (Saito et al., 1987). Lipid metabolism in gonadal WAT is also regulated by the sympathetic nervous system. For example, increased lipolysis in gonadal WAT is associated with elevated sympathetic inputs (Plum et al., 2007), while expended gonadal WAT is found to have decreased sympathetic inputs (Ramadori et al., 2010). Collectively, these findings support a model that estrogens act on ERα in VMH SF1 neurons to increase central sympathetic outflow, which leads to increased BAT thermogenesis and inhibits lipid storage in gonadal WAT.
The intracellular mechanisms by which estrogen/ERα regulate SF1 neurons are not fully understood. Park et al. reported that estrogens activate the rapid PI3K–Akt pathway in the VMH via an ERα-dependent mechanism (Park et al., 2011). Remarkably, restoration of ERα-associated rapid signaling pathways (including the PI3K–Akt pathway) at the global ERα null background is sufficient to rescue obesity (Park et al., 2011). In addition, we have previously shown that selective inhibition of PI3K in SF1 neurons leads to obesity (Xu et al., 2010). Collectively, these findings suggest that the PI3K pathway (and/or other rapid signaling pathways) in SF1 neurons may mediate anti-obesity effects of estrogens.
Alternatively, ERα may regulate SF1 neurons as a transcription factor. We show that mice lacking ERα in SF1 neurons have decreased expression of leptin receptors in the VMH. Consistent with a possible role of estrogen/ERα on expression of leptin receptors, these two receptors are found to be co-expressed by VMH neurons (Diano et al., 1998), and an estrogen response element (ERE) was found in the promoter region of leptin receptor gene (Lindell et al., 2001). In addition, selective deletion of leptin receptors in SF1 neurons produces similar obese phenotypes (Dhillon et al., 2006). Collectively, these data suggest that ERα in SF1 neurons may regulate energy homeostasis, at least partly, by modulating leptin sensitivity in the VMH.
Estrogenic actions in POMC neurons
Mice lacking ERα in POMC neurons develop hyperphagia, indicating that ERα signals in POMC neurons are physiologically relevant in the regulation of feeding. This regulation may be mediated by estrogenic actions on POMC neural activity, as estrogens have been shown to activate POMC neurons (Gao et al., 2007; Malyala et al., 2008). Alternatively, estrogens may regulate feeding partly via sensitizing responses to other anorexigenic hormones, such leptin. We previously demonstrated that estrogens potentiate the anorexigenic effects of leptin (Clegg et al., 2006). We now extend those findings to demonstrate that the effects of leptin on food intake are blunted in mice lacking ERα in POMC neurons. Importantly, the efficacy of MTII’s anorexia is not affected in these mice, suggesting that the impairments of estrogenic actions are constrained to the POMC neurons. Recent evidence indicates that estrogens, similar to leptin, induce STAT3 phosphorylation in the hypothalamus, and that the anti-obesity effects of estrogens are abolished in mice lacking STAT3 in the brain (Gao et al., 2007). Therefore, it is possible that estrogens may initiate signals in POMC neurons which ultimately converge on the leptin-induced pSTAT pathway to regulate food intake.
While the majority of POMC neurons reside in the ARH of the hypothalamus, a small subset of POMC neurons are also found in the NTS of the brainstem, which are implicated in the regulation of satiety (Fan et al., 2004). In addition, estrogens increase c-fos immunoreactivity in the NTS via ERα-mediated mechanisms (Asarian and Geary, 2007). Notably, our POMC-Cre induced efficient ERα deletion in the NTS, as well as the ARH. Therefore, these findings support an alternative model that estrogens may regulate food intake at least partly by acting on ERα expressed by NTS POMC neurons.
Hypothalamic ERα and reproduction
ERαlox/lox/SF1-Cre females develop infertility likely due to anovulation (the lack of ovarian corpus luteum). An important question remains as to whether this ovarian phenotype results from loss of ERα in VMH SF1 neurons or from loss of ERα in ovarian SF1 cells. Although we did not find significant reduction of ERα mRNA in the ovary of ERαlox/lox/SF1-Cre mice, we could not fully exclude the possibility that ERα is deleted in ovarian SF1 cells. On the other hand, it is interesting to note that our ERαlox/lox/SF1-Cre female mice recapitulate the ovarian phenotypes (numerous antral follicles and lack of corpus luteum) seen in ERαlox/lox/CAMKII-Cre mice, a brain-specific ERα knock-out model (Wintermantel et al., 2006). Therefore, it is possible that the ovarian phenotypes are caused by loss of ERα in SF1 neurons in the brain. Supporting this notion, genetic ablation of SF1 neurons in the brain leads to decreased or lacked corpora luteum and subfertility/infertility in female mice (Kim et al., 2010).
Deletion of ERα in POMC neurons impaired negative feedback regulation of estrogens on the HPG axis. The negative feedback is primarily mediated by ERα (Couse et al., 2003). ERα in pituitary LH cells partially mediate this effect, as deletion of ERα in LH cells blunts estrogen-induced inhibition on LH secretion (Singh et al., 2009). Alternatively, estrogens may act on gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus to inhibit the HPG axis. As GnRH neurons do not express ERα (Shivers et al., 1983), interneurons must exist to relay the negative feedback signals to GnRH neurons. Our observations that the negative feedback is impaired in ERαlox/lox/POMC-Cre females indicate that POMC neurons may be one of these interneurons. Supporting this notion, ARH POMC neurons project to the rostral preoptic area, where GnRH neurons are concentrated (Simonian et al., 1999). The possible signals that originate from POMC neurons and synapse on GnRH neurons are unclear. Recent studies from lean hypogonadotropic ewes suggested that α-MSH mediates leptin effects to restore pulsatile LH secretion (Backholer et al., 2010). Additionally, estrogens are implicated to increase the secretion of another POMC gene product, β-endorphin (Lagrange et al., 1994), which inhibits GnRH neurons (Lagrange et al., 1995). These findings support a model that estrogens act on ERα in POMC neurons to modulate secretion of POMC peptides (e.g. α-MSH and/or β-endorphin), which in turn act on GnRH neurons to mediate the negative feedback on HPG axis.
Other ERα populations in the brain may also contribute the negative feedback regulation. For example, Kiss1 neurons are required for the tonic stimulation of GnRH/LH secretion (Dungan et al., 2007). Interestingly, Kiss1 neurons in the ARH co-express ERα (Cravo et al., 2011) and estrogens reduce ARH Kiss1 expression (Smith et al., 2005). Of note, Kiss1 neurons and POMC neurons are exclusively segregated in the ARH (Cravo et al., 2011). Therefore, it is possible that ERα in ARH Kiss1 neurons may provide an alternative pathway, in addition to ERα in POMC neurons, to mediate the negative feedback.
Conclusions
Using mice lacking ERα in the CNS, in SF1 neurons, in POMC neurons, and in both SF1 and POMC neurons, we have substantially narrowed down the critical ERα sites that mediate the estrogenic effects on energy homeostasis and reproduction. These results provide genetic evidence to segregate physiological functions of ERα cells in the context of obesity and infertility, and may provide rational targets for the development of highly selective hormone replacement therapies which may be used to combat obesity and infertility in women.
Materials and Methods
Animals
Care of all animals and procedures were approved by the University of Cincinnati, UT Southwestern Medical Center and Baylor College of Medicine. Mice were housed in a temperature-controlled environment in groups of two to five at 22°C-24°C using a 12 hr light/12 hr dark cycle. Some cohorts were singly housed to measure food intake. The mice were fed either standard phytoestrogen-free chow (#2916, Harlan-Teklad, Madison, WI) or 42% high fat diet (#88137, Harlan Teklad) and water was provided ad libitum.
Body weight, food intake and body composition
Body weight was measured weekly. Food intake was measured daily, and average daily food intake was calculated using data from at least 4 continuous days (7-day data was used in 5-week old ERαlox/lox/SF1-Cre group). Body composition was determined using quantitative magnetic resonance (QMR).
Assessment of Negative Feedback
Twelve-week old chow-fed female ERαlox/lox/POMC-Cre mice and their ERαlox/lox controls were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and received bilateral ovriectomy, followed by subcutaneous implantations of pellets containing estradiol-17β (0.5 µg/day/mouse with 60-day releasing capacity, OVX+E) or containing vehicle (OVX+V). Four weeks later, these mice were sacrificed in the afternoon (between 1 pm to 4 pm) after deep anesthesia. The pituitary was quickly isolated; trunk blood was collected and processed to collect plasma. Expression of FSH and LH in the pituitary and plasma FSH/LH were analyzed as described in Supplemental Materials.
Statistics
The data are presented as mean ± SEM. Statistical analyses were performed using SigmaStat 2.03. After confirming normal distribution of data, comparisons between two genotypes were made by the unpaired two-tailed Student's t-test; repeated-measures ANOVA were used to compare changes over time between two genotypes. Two-way ANOVA analyses were used to assess the interactions between genotypes and treatments (e.g. saline versus drugs). P < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgment
We thank the Mouse Metabolic Phenotyping Core at UTSW Center (supported by NIH PL1 DK081182 and UL1 RR024923), Ms. Charlotte Lee, and Ms. Danielle Lauzon for the technical support. This work was supported by grants from American Heart Association (BGI) and from the NIH (R00DK085330, R01DK093587 and P30 DK079638-03 to YX, HD061539 to CFE, R01DK088423, RL1 DK081185, R37DK53301 and R01DK071320 to JKE, and DK073689 to DJC), from American Diabetes Association (YX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. doi: 10.1210/en.2007-0341. [DOI] [PubMed] [Google Scholar]
- Backholer K, Bowden M, Gamber K, Bjorbaek C, Iqbal J, Clarke IJ. Melanocortins mimic the effects of leptin to restore reproductive function in lean hypogonadotropic ewes. Neuroendocrinology. 2010;91:27–40. doi: 10.1159/000260060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeci AM, Paciaroni M, Caso V, Agnelli G. Hormone replacement therapy and stroke. Curr Vasc Pharmacol. 2008;6:112–123. doi: 10.2174/157016108783955338. [DOI] [PubMed] [Google Scholar]
- Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- Castaneda TR, Jurgens H, Wiedmer P, Pfluger P, Diano S, Horvath TL, Tang-Christensen M, Tschop MH. Obesity and the neuroendocrine control of energy homeostasis: the role of spontaneous locomotor activity. J Nutr. 2005;135:1314–1319. doi: 10.1093/jn/135.5.1314. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Diano S, Kalra SP, Sakamoto H, Horvath TL. Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain Res. 1998;812:256–259. doi: 10.1016/s0006-8993(98)00936-6. [DOI] [PubMed] [Google Scholar]
- Dulloo AG. Biomedicine. A sympathetic defense against obesity. Science. 2002;297:780–781. doi: 10.1126/science.1074923. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of Body Fat Mass as a Major Determinant of Metabolic Rate in Mice. Diabetes. 2010 doi: 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Li S, Zhao H, Peng B, Tobet SA, Elmquist JK, Parker KL, Zhao L. CNS-specific ablation of steroidogenic factor 1 results in impaired female reproductive function. Mol Endocrinol. 2010;24:1240–1250. doi: 10.1210/me.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008;34:317–327. doi: 10.1016/j.diabet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ. The potency of mu-opioid hyperpolarization of hypothalamic arcuate neurons is rapidly attenuated by 17 beta-estradiol. J Neurosci. 1994;14:6196–6204. doi: 10.1523/JNEUROSCI.14-10-06196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ. Estradiol-17 beta and mu-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- Lindell K, Bennett PA, Itoh Y, Robinson IC, Carlsson LM, Carlsson B. Leptin receptor 5'untranslated regions in the rat: relative abundance, genomic organization and relation to putative response elements. Mol Cell Endocrinol. 2001;172:37–45. doi: 10.1016/s0303-7207(00)00382-8. [DOI] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant DN, Kelly MJ, Ronnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27:1020–1027. doi: 10.1038/sj.ijo.0802378. [DOI] [PubMed] [Google Scholar]
- Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. Genetic rescue of nonclassical ERalpha signaling normalizes energy balance in obese Eralpha-null mutant mice. J Clin Invest. 2011;121:604–612. doi: 10.1172/JCI41702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Bruning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009 doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation accelerates norepinephrine turnover in brown adipose tissue of rats. Life Sci. 1987;41:193–197. doi: 10.1016/0024-3205(87)90493-0. [DOI] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304:345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Singh SP, Wolfe A, Ng Y, DiVall SA, Buggs C, Levine JE, Wondisford FE, Radovick S. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1) Biol Reprod. 2009;81:488–496. doi: 10.1095/biolreprod.108.075259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren BG. The benefits of oestrogen following menopause: why hormone replacement therapy should be offered to postmenopausal women. Med J Aust. 2009;190:321–325. doi: 10.5694/j.1326-5377.2009.tb02423.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, Lee CE, Choi MJ, Lauzon DA, Dhillon H, Lowell BB, Zigman JM, Zhao JJ, Elmquist JK. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 2010;12:88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development. 2001;128:147–154. doi: 10.1242/dev.128.2.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.