Abstract
Consisting of N-terminated diphenylalanine, a new type of supramolecular hydrogelators forms hydrogels within a narrow pH window (pH 5.0 to 6.0) and selectively inhibits growth of HeLa cells, which provides important and useful insights for designing molecular nanofibers as potential nanomedicines.
This communication describes supramolecular hydrogelators consisting of N-terminated dipeptides that exhibit selective inhibitory effects against cancer cells. Over the last decade, peptides, as one class of essential biological molecules, are emerging as a favourite and useful choice of building blocks for making nanostructured biomaterials, especially in the form of nanofibers that serve as the network of hydrogels.1 For example, amphiphilic oligopeptides have become a versatile platform for developing functional, self-assembled nanofibers that promise applications in tissue engineering, regenerative medicine, drug delivery, and anticancer therapeutics.2 A simple dipeptide of phenylalanine also self-assembles to form stiff nanotubes that allow the casting of metal nanowires.3 These seminal works have largely stimulated the use of other small peptides as the basic motifs for designing small molecule gelators that not only self-assemble into nanofibers, but also result in molecular hydrogels, which promise applications ranging from biomedicine to energy.4 Among the reported molecular hydrogelators containing dipeptides motifs, some have already shown useful properties and promising applications, including separation of protein,5 response to the changes of pH or temperatures6 or ligand-receptor interactions,7 function as the matrices for three-dimensional cell-cultures,8 and the formation of vesicles for the delivery of oligonucleotide.9 These promising results suggest that it is worthwhile to further explore the potentials of molecular hydrogelators containing dipeptides motifs because of their versatility, low cost, and well-established chemistry.
Most of the dipeptidic molecular hydrogelators, explored so far, are C-terminated hydrogelators.10 Though there is one report of a cationic dipeptide that forms vesicles for the possible delivery of oligonucleotide,9 the properties of the N-terminated dipeptides are largely unknown, including their ability to serve as hydrogelators and their cytotoxicity. To address this less explored direction of the development of small peptide hydrogelators, we conjugate naphthalene to the carboxylate end of a diphenylalanine to generate three N-terminated dipeptides (1–3, Fig. 1), which successfully self-assemble into supramolecular nanofibers in water to afford stable hydrogels with the concentration of less than 0.8 wt% and within a relatively narrow pH range (pH = 5.0–6.0). The formation of the molecular nanofibers and hydrogels of 1, 2 and 3 likely arise from both aromatic-aromatic interaction and the proper protonation of the N-terminal amine group. Besides that, we find that these hydrogelators exhibit significant higher cytotoxicity to HeLa cells than to Ect1/E6E7 cells, a result that represents the first example of molecular hydrogelators selectively inhibit cancer cells. These results are significant because they may provide important insights for understanding cell specific cytotoxicity that are critical for the applications of hydrogelators and hydrogels.
Fig. 1.
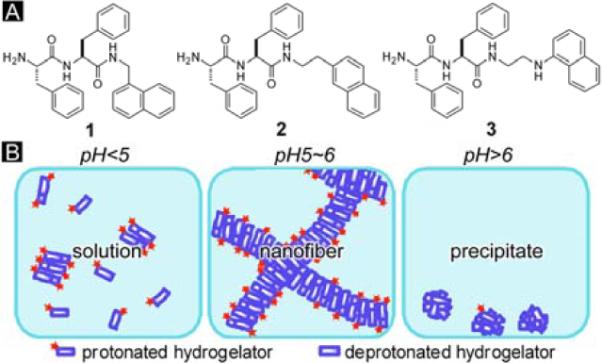
(A) Molecular structures of the N-terminated dipeptide hydrogelators 1 to 3. (B) Illustrated pH response of the hydrogelators.
Fig. 1 shows the structures of the N-terminated hydrogelators. 1, 2 and 3 all consist of di-phenylalanine and naphthalene motifs. Their structures differ at the linker between the two motifs and the position of substitution on the naphthalene group. 2 has a 2-substitute naphthalene; 1 and 3 both have 1-substitute naphthalene, but the linker of 3 has an extra secondary amine. These slight structural variations result in difference in the properties of the hydrogelation of these three compounds (Table 1). Each of three compounds is able to immobilize a large quantity of water and form a stable supramolecular hydrogel (with more than 99 wt% of water) at a proper pH. However, under same pH (Fig. 2A to C), hydrogelator 1, 2 and 3, at their optimal gelation concentrations, all give hydrogels of similar optical appearances despite the different concentrations. Due to the presence of 1-aminonaphthalene in its structure,11 the hydrogel of 3 is fluorescent.
Table 1.
Gelation properties and 48h IC50 of the N-terminated hydrogelators.
| Compound | MGCa (mM / wt%) | pH | IC50 (μM / μg/ml) |
|
|---|---|---|---|---|
| HeLa | Ect1/E6E7 | |||
| 1 | 4.4 / 0.20 | 5.0–6.0 | 142.0 ± 3.6 / 64.1±1.7 | >200 / >90.2 |
| 2 | 6.5 / 0.30 | 5.0–6.0 | >200 / >93.1 | >200 / >93.1 |
| 3 | 16.7/ 0.80 | 5.0–6.0 | 150.2 ± 3.1 / 72.1±1.5 | >200 / >96.1 |
MGC: minimum gelation concentration.
Fig. 2.
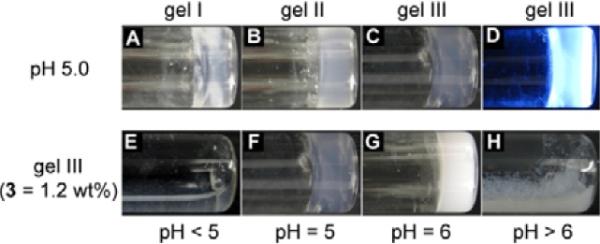
Optical images of (A) gel I ([1] = 0.4 wt%), (B) gel II ([2] = 0.4 wt%), (C) gel III ([3] = 1.2 wt%). (D) A fluorescent image of gel III (λex = 345 nm). Compound 3 (1.2 wt%) exists (E) as a clear solution at pH < 5.0, (F) a transparent gel at pH = 5.0, (G) an opaque gel at 5.0 < pH < 6.0, and (H) precipitates at pH > 6.0.
Fig. 2E to H demonstrate the phase transition of 3 induced by the change of pH. At the concentration of 1.2 wt%, 3 forms the hydrogel at pH 5 to 6, with the pellucidity gradually changing from transparent to opaque when the pH increases from 5 to 6. When the pH reaches above 6, 3 becomes insoluble in water and mainly exists as white precipitates; when the pH is below 5, 3 dissolves well in water and gives a clear solution. The formation of the hydrogel within a narrow pH range (Fig. 1B) likely arises from a balance of protonation/deprotonation of the N-terminal amine. The N-terminal primary amine, at relatively low pH (e.g., pH < 5.0 in this work), exists as ammonium groups due to the protonation of the amine group. The positive charges on the molecules cause the hydrogelators to repulse each other thus disfavor the formation of nanofibers (or the networks of the nanofibers), which result in solutions of the hydrogelators. At relatively high pH (e.g., pH > 6.0 in this work), the N-terminal amines exist as neutral amine groups due to deprotonation, significantly increases the hydrophobicity of the hydrogelators, thus promoting the aggregation and further precipitation of the hydrogelators. Only within a narrow pH window of weak acidic condition, the N-terminal amine group establishes the subtle balance of protonation/deprotonation that allows certain degree of aggregation (self-assembly and crosslinking of nanofibers) of the hydrogelators while preserves their strong interactions with water molecules, thus achieving hydrogelation. Despite the extra secondary amine in 3, which gives it higher solubility than the other two hydrogelators, the three hydrogelators share the same pH window for gelation (Table 1). The higher pKa (9.7) of secondary amine than primary amine12 makes it to be protonated within the pH window of hydrogelation, thus, like 1 and 2, partial protonation derived from the primary amine of 3 at pH 5.0–6.0 leads to hydrogelation.
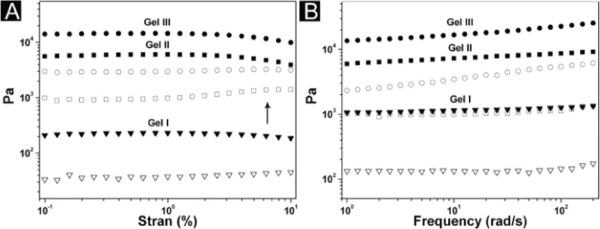
We use rheology to characterize the viscoelastic properties of the hydrogels formed by 1, 2 and 3. Fig. 3A shows the dependence of G′ (storage moduli) and G″ (loss moduli) of the hydrogels over a wide range of strain amplitude. Unlike gel I and gel III, whose moduli exhibit a trend of decrease at high strain amplitude (i.e., strain thinning13), gel II displays a weak strain overshoot in amplitude response: while G′ decreases with increasing strain amplitude, G″, with the increase of strain amplitude, rises first before drops rapidly. This weak strain overshoot indicates that the molecules of 2 align with each other and associate into superstructures (in this case, bundle of fibers) that can resist the deformation up a certain strain value (G″ increase) before a large deformation disrupts the structure (i.e., G″ starts to decrease). The strain resistant structure of gel II explains that G′ of gel II is larger than that of gel I while the concentration of hydrogelators are the same (Fig 3B). In frequency sweep, all three hydrogels have G′ and G″ essentially independent of frequency, in addition to G′ dominating G″ at all frequencies, thus indicating the elastic nature of the three hydrogels. The mechanical properties of hydrogels formed by N-terminal hydrogelators classify these hydrogels as extensively cross-linked networks.14
Fig. 3.
Rheological properties of hydrogels formed by 1, 2 and 3. (A) Strain sweep and (B) frequency sweep of gel I ([1] = 0.4 wt%: ▼), gel II 40 ([2] = 0.4 wt%: ■) and gel III ([3] = 1.2 wt%: ●). Storage moduli (G′: filled symbols); loss moduli (G″: open symbols). Arrow points at the weak strain overshoot of gel II.
Transmission electron micrographs (TEM) of uranyl acetate stained15 samples of the hydrogels reveal the nanostructures of the matrices of the hydrogels formed by these N-terminal hydrogelators. As the dominate structure of all the three hydrogels are nanofibers, each hydrogelator forms the nanofibers bearing a slightly different feature. Long and extended helical nanofibers with the pitch of about 40 nm (Fig. 4A, D) are the main morphology of the network of gel I; gel II consists of straight nanofibers that tend to align in parallel (Fig. 4B); the major morphology of the network of gel III is randomly tangled nanofibers. Unlike the nanofibers in gel I and gel II, nanofibers in gel III have irregular widths.
Fig. 4.
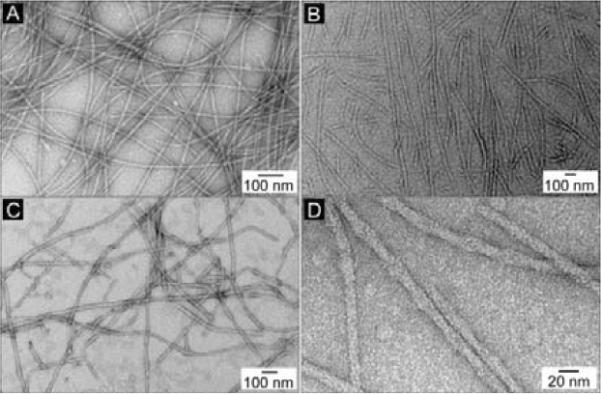
The TEM images of negative stained (A) gel I, (B) gel II, (C) gel III, and (D) the magnified image of the helical nanofibers in gel I.
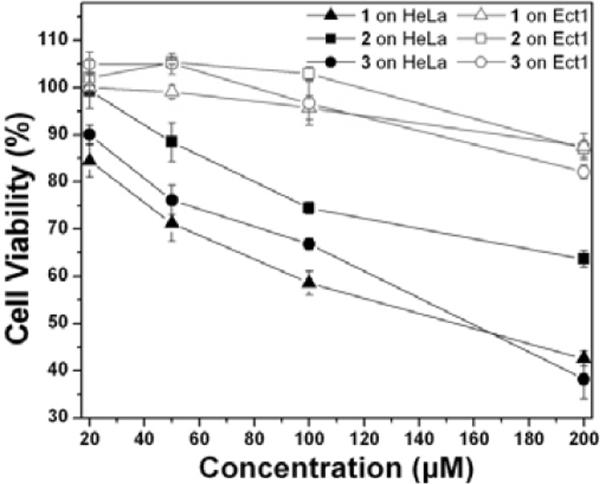
We use the MTT cell viability assay to examine the cytotoxicity of the N-terminal hydrogelators against HeLa cells and its counterpart cell line Ect1/E6E7 cells after treatment for 48 hours. HeLa, an epithelial cell line from cervical tumor, is the most widely used cell line in biology; Ect1/E6E7 is immortalized normal epithelial cell from cervix. There two cell lines serve as each other's reference, making a cancer-normal cell pair that is largely used as model cell pair in cancer study.16 Though hydrogelators 1, 2 and 3 exhibit similar cytotoxicity towards same cell lines, it is clear that 1, 2 and 3 all have lower cytotoxicity against Ect1/E6E7 cells than against HeLa cells (Fig. 5). Same cytotoxicity tests was performed on another cancer cell line T98G to verify if the cytotoxicity of the N-terminated hydrogelators only occurs on HeLa cells or is a general behavior on cancer cells. IC50 values of N-terminated hydrogelators on T98G (Fig S1) are in the same order of magnitude with HeLa cells and 2 is also being slightly less toxic than 1 and 3, which demonstrate that N-terminated hydrogelators are generally toxic towards cancer cells. 2 differs from the other two compounds as it has 2-substitued naphthyl instead of 1-substitued naphthyl group. The cytotoxicity of 2 on HeLa cells is also lower than that of 1 or 3, thus implying a possible correlation between substitution patterns with the cytotoxicity. The difference of the cytotoxicity against the two cell lines increases with the concentration, and the difference is as large as 40% (in the case of 3) at the highest concentration tested.
Fig. 5.
48h Cytotoxicity vs concentration curves of 1–3 on cancerous cells (HeLa) and counterpart normal cells (Ect1/E6E7).
The cause of difference in cytotoxicity of the N-terminated hydrogelators toward cancer and normal cell lines, we speculate, unlikely originates from the positive charges provided by the hydrogelators since the excess of positive charge should also lead to the death of Ect1/E6E7 cells, but might lie in one or the combination of the following reasons: first, normal cells might have a poor permeability to the N-terminated hydrogelators; second, as acidification is a significant feature of cancer cells,17 normal cells has a pH of 7.4–7.6, so the N-terminated hydrogelators are less likely to self-assemble in normal cells than in cancer cells; third, while the growth of Ect1/E6E7 cells is serum-free, the growth of HeLa cells is serum-required, so the N-terminated hydrogelators may interact with some essential proteins through ionic bond and consequently inhibit growth of HeLa cells. This ability to distinguish cancer and normal cell has not been discovered on any of the C-terminal hydrogelators (though it is possible for the precursors of hydrogelators18), which makes it a unique property of the N-terminated hydrogelators that warrants further investigation.
In conclusion, we design and synthesize a series of dipeptides based hydrogelators with exposed N-terminal amine that, unlike their C-terminal analogues, form hydrogels only within a narrow pH range. These N-terminated hydrogelators, as a new group in the family of dipeptide based hydrogelators, not only provide an alternative option for designing functional hydrogelators that allow bioactive groups to be conjugated through their C-terminals, but also have a unique property of selective inhibition of the growth of HeLa cells, which is of significance to the understanding of cellular response to hydrogel and hydrogelators and may eventually lead to a route for the applications of supramolecular hydrogel and hydrogelators in cancer therapies.
Supplementary Material
Acknowledgments
‡ This work was partially supported by NIH (R01CA142746). We thank the EM facility at Brandeis University for assistance.
Footnotes
†Electronic Supplementary Information (ESI) available: [Figure S1 to S5]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Dawn A, Shiraki T, Haraguchi S, Tamaru S.-i., Shinkai S. Chem-Asian J. 2011;6:266. doi: 10.1002/asia.201000217. [DOI] [PubMed] [Google Scholar]; Ulijn RV, Smith AM. Chem. Soc. Rev. 2008;37:664. doi: 10.1039/b609047h. [DOI] [PubMed] [Google Scholar]; Ikeda M, Ochi R, Wada A, Hamachi I. Chem. Sci. 2010;1:491. [Google Scholar]
- 2.Zhang S. Nat. Biotech. 2004;22:151. [Google Scholar]; Cui H, Webber MJ, Stupp SI. Biopolymers (pept. Sci.) 2010;94:1. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reches M, Gazit E. Science. 2003;300:625. doi: 10.1126/science.1082387. [DOI] [PubMed] [Google Scholar]; Goerbitz CH. Chem. Commun. 2006:2332. doi: 10.1039/b603080g. [DOI] [PubMed] [Google Scholar]
- 4.Sangeetha NM, Maitra U. Chem. Soc. Rev. 2005;34:821. doi: 10.1039/b417081b. [DOI] [PubMed] [Google Scholar]; Gao Y, Kuang Y, Guo ZF, Guo ZH, Krauss IJ, Xu B. J. Am. Chem. Soc. 2009;131:13576. doi: 10.1021/ja904411z. [DOI] [PubMed] [Google Scholar]; Suzuki Y, Tanihara M, Nishimura Y, Suzuki K, Kakimaru Y, Shimizu Y. J. Biomed. Mater. Res. 1998;42:112. doi: 10.1002/(sici)1097-4636(199810)42:1<112::aid-jbm14>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]; Yang ZM, Xu KM, Wang L, Gu HW, Wei H, Zhang MJ, Xu B. Chem. Commun. 2005:4414. doi: 10.1039/b507314f. [DOI] [PubMed] [Google Scholar]; Hasırcı KLV, Gresser JD, Wise DL, Trantolo DJ. J. Biotechnol. 2001;86:135. doi: 10.1016/s0168-1656(00)00409-0. [DOI] [PubMed] [Google Scholar]
- 5.Yamamichi S, Jinno Y, Haraya N, Oyoshi T, Tomitori H, Kashiwagi K, Yamanaka M. Chem. Commun. 2011 doi: 10.1039/c1cc13826j. [DOI] [PubMed] [Google Scholar]
- 6.Pal A, Shrivastava S, Dey J. Chem. Commun. 2009:6997. doi: 10.1039/b914665b. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Gu HW, Yang ZM, Xu B. J. Am. Chem. Soc. 2003;125:13680. doi: 10.1021/ja036817k. [DOI] [PubMed] [Google Scholar]
- 8.Mahler A, Reches M, Rechter M, Cohen S, Gazit E. Adv. Mater. 2006;18:1365. [Google Scholar]; Jayawarna V, Ali M, Jowitt TA, Miller AF, Saiani A, Gough JE, Ulijn RV. Adv. Mater. 2006;18:611. [Google Scholar]
- 9.Yan X, He Q, Wang K, Duan L, Cui Y, Li J. Angew. Chem. Int. Ed. Engl. 2007;46:2431. doi: 10.1002/anie.200603387. [DOI] [PubMed] [Google Scholar]
- 10.Zhou M, Smith AM, Das AK, Hodson NW, Collins RF, Ulijn RV, Gough JE. Biomaterials. 2009;30:2523. doi: 10.1016/j.biomaterials.2009.01.010. [DOI] [PubMed] [Google Scholar]; Zhang Y, Kuang Y, Gao Y, Xu B. Langmuir. 2010;27:529. doi: 10.1021/la1020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruckert I, Demeter A, Morawski O, Kuhnle W, Tauer E, Zachariasse KA. J. Phys. Chem. A. 1999;103:1958. [Google Scholar]; Suzuki K, Tanabe H, Tobita S, Shizuka H. J. Phys. Chem. A. 1997;101:4496. [Google Scholar]
- 12.Hall HK., Jr. J. Am. Chem. Soc. 1957;79:5441. [Google Scholar]
- 13.Greenfield MA, Hoffman JR, Olvera de la Cruz M, Stupp SI. Langmuir. 2009;26:3641. doi: 10.1021/la9030969. [DOI] [PubMed] [Google Scholar]
- 14.Mezger TG. The rheology handbook: for users of rotational and oscillatory rheometers. Vincentz Network; 2006. [Google Scholar]
- 15.Frado LL, Craig R. J. Mol. Bio. 1992;223:391. doi: 10.1016/0022-2836(92)90659-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen CL, Hsieh FC, Lieblein JC, Brown J, Chan C, Wallace JA, Cheng G, Hall BM, Lin J. Br. J. Cancer. 2007;96:591. doi: 10.1038/sj.bjc.6603597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaupel P, Kallinowski F, Okunieff P. Cancer Res. 1989;49:6449. [PubMed] [Google Scholar]
- 18.Yang ZM, Xu KM, Guo ZF, Guo ZH, Xu B. Adv. Mater. 2007;19:3152. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.