Abstract
Background
High blood pressure (BP) is a risk factor for cerebrovascular disease, including stroke. Little is known about the importance of BP on the progression of microvascular disease of the brain that has been associated with functional decline in mobility and cognition in older people.
Methods and Results
This was a prospective cohort of subjects 75-89 years to determine relations among vascular risk factors, white matter hyperintensity volume (WMHs), and functional status. 99 subjects were enrolled using a balanced 3×3 matrix stratified by age and mobility performance and 72 subjects completed all sets of baseline and follow-up studies at 2 years. Subjects were excluded if there were medications, systemic or neurologic diseases that could compromise mobility. Ambulatory and clinic BP monitoring, magnetic resonance imaging (MRI), gait studies and neuropsychological testing were performed at baseline and after 24 months. Brain classification into normal white matter and T2-hyperintense WMH was performed using semi-automated segmentation. Quantitative measures of mobility and cognitive function were obtained longitudinally. Increased ambulatory systolic BP, but not clinic systolic BP, from baseline to 24 month follow-up was associated with increased WMH over that same period, as as well as measures of executive function/processing speed. Similar associations were observed for 24 hour BP, awake BP, and sleep BP, but not the surge between the sleep and awake time at the 24 month time point.
Conclusions
These data demonstrate for the first time the importance of 24-hour systolic BP in the progression of brain WMH burden associated with impairment of cognitive function in older people. The 24-hour systolic BP may be a potential target for intervention in the elderly to reduce vascular disease of the brain and impairment of function.
Keywords: systolic hypertension in the elderly, ambulatory blood pressure, cerebrovascular disease, cognitive function, mobility
Introduction
Small vessel disease of the brain may present as white matter hyperintensities (WMHs), and are commonly present in the magnetic resonance images (MRIs) of older persons with hypertension and other vascular disease risk factors (1). These WMHs are clinically relevant in older people as they are associated with functional deterioration of mobility (2), cognition (2,3) and stroke (4) and have been proposed as an intermediate marker in the research setting (5). We have previously demonstrated significant relationships between total and regional WMH and measures of mobility, indicating that lesion burden was a key predictor of low mobility performance (6) and frontal WMH predicted speed of cognitive functioning (7).
An important advantage of ambulatory BP measurement over clinic values is its enhanced reproducibility, including in older people (8). Numerous studies demonstrate that ambulatory BP is a better predictor of cardiac, renal, and cerebral disease in middle-aged and older people with hypertension (9). However, there are minimal data regarding the impact of 24-hour BP over time on cerebrovascular disease and its functional consequences, particularly in the elderly population. We report our findings of a longitudinal prospective cohort study that examined the relationships among ambulatory and clinic BP, progression of WMH, and functional capabilities in older people.
Methods
Patients
Men and women, 75-89 years, were recruited for this longitudinal study defining the relationships among BP, WMH accrual and mobility impairment. From 312 individuals screened, 164 were eligible, consenting individuals from whom 117 were assessed for possible enrollment. Eighteen patients were excluded due to disorders known to interfere with mobility such as arthritis and Parkinson’s disease or claustrophobia that disallowed MRI testing; one patient was excluded following discovery of a tentorial meningioma. This subject was replaced at baseline. Subjects were enrolled from the community and geriatric practices using a balanced 3×3 matrix which stratified age (75-79; 80-84 and ≥ 85) and mobility performance in terms of Short Physical Performance Battery (SPPB) scores (11-12; 9-10 and < 9) as described previously (10). Patients were excluded if they had underlying neurologic diagnoses that would impair mobility or cognitive function. Patients with severe or unstable cardiovascular disorders (e.g. myocardial infarction in last 6 months, decompensated heart failure, recent stroke) were excluded. There were 72 patients who had all sets of studies at baseline and following 24 months of observation.
Following informed consent, subjects underwent neurological and cognitive assessment, as well as BP monitoring, gait laboratory assessment, and MRI of the brain. Assessors were blinded to clinical, mobility and imaging outcomes. The study was approved by the institutional review boards at the University of Connecticut Health Center, Farmington CT; and at Hartford Hospital, Hartford, CT.
Assessment of Blood Pressure
Resting clinic BPs were measured in triplicate after sitting for 5 minutes by a semi-automated digital device (Omron HealthCare 705CP, Vernon Hills, Illinois) with an appropriate sized cuff and bladder and averaged. The 24-hour ambulatory BP monitoring (ABPM) was conducted using the Oscar II BP device (Suntech Medical Instruments, Morrisville, NC) which has been validated independently for precision and reliability (11). Blood pressures were obtained every 15 minutes from 6 am to 10 pm, and every 30 min from 10 pm to 6 am as previously described (8).Data were transferred to the Accuwin software program (Suntech Medical Instruments, Morrisville, NC) for analysis. Components of the ABPM for analysis included the 24-hour mean systolic and diastolic BP, the awake and sleep BPs, and the early morning surge BP (2 hour post-awakening BP – the 2 hour pre-awakening BP = early morning surge) as previously described (8).
Assessment of mobility
Mobility was assessed using the Tinetti Gait score (12) and laboratory testing of mobility performance that included timed stair descent, walk time and self-paced maximum velocity. Gait speed was measured as participants walk at a comfortable pace over an 8-meter course with 1 meter for acceleration and deceleration. In addition to gait speed, time to descend 3 stairs at a comfortable pace was used as a measure of walking-moving.
Assessment of cognitive function
Measures of executive functioning and process speed included the Trail Making Test (Trails Part B), the Stroop Color and Word Test, and the California Computerized Assessment Package (CalCAP) simple (SRT) and sequential reaction time (SQ1). Trail Making tests the speed of visual search, attention, mental flexibility and motor function (13). The Stroop Color and Word tests how well an individual suppresses a habitual response in favor of an unusual one thus assessing complex processing speed. Slower Stroop performance has been associated with greater WMH (14). The Cal CAP consists of 3 reaction time tests, a simple, choice, and serial reaction times. Simple reaction time measures general motor slowing. Choice reaction time is a go-no go paradigm, which measures response inhibition and processing speed. Serial reaction time measures divided attention and processing speed (13).
Brain Magnetic Resonance Imaging and Quantitative White Matter Hyperintensity Volume
Magnetic resonance images (MRI) were acquired and white matter lesions quantified using a protocol described elsewhere (6). Briefly, MR high-resolution images were acquired using a 3-Tesla Siemens Allegra scanner (Erlangen, Germany). The brain was imaged with 3 sequences, namely a T1-weighted magnetization prepared rapid gradient echo (MPRAGE), a T2-weighted 3D-Fast Spin Echo (T2) and a fluid attenuated inversion recovery (FLAIR). Pre-processing included correction of magnetic field-related signal inhomogeneities and linear affine registration of FLAIR and T2 series to the MPRAGE series. A semi-automated segmentation method was used to assign each pixel in the intracranial cavity a value corresponding to either gray matter (GM), normal white matter (WM), white matter hyperintensities (WMH) or CSF based on both signal intensity and anatomic location. To account for brain size variability, the total volume of WMH was expressed as fraction of the intracranial cavity volume: WMH fraction = [total WMH (ml) / intracranial cavity volume (ml)] × 100%. All MR imaging analyses were performed blinded to clinical and gait laboratory data.
To validate the WMH segmentation method, we randomly selected 10 subjects in the study and compared WMH results to a ‘gold standard’ obtained on these subjects by an expert neuroradiologist in our group who manually outlines the WMH from FLAIR images. The WMH volumes obtained from the gold standard and from the study method outputs were 1.46 ± 1.14 and 1.62 ± 1.18 (as a percent of intracranial cavity volume or %ICV), respectively. The intraclass correlation coefficient for the 2 methods was 0.99 (95% CI: 0.98-0.99, p < 0.00001). The method’s reproducibility was assessed on 10 subjects whom participated in a test-retest experiment which consisted of 2 MR brain image acquisitions in the same day but more than 1 hour apart. For this experiment, the intraclass correlation coefficient was 0.99 (95% CI: 0.97-0.99, p < 0.00001). The proportion of pixels reproducibly classified as WMH were 77.5% ± 12.3% (95% CI: 68.7-86.3). Mean and standard error in WMH measurement was 0.02% ± 0.20 %ICV.
Statistical Analysis
SAS version 9.1 (SAS Institute, Inc., Cary, NC) was used for statistical analyses. Data were analyzed cross-sectionally (at 24 months) and longitudinally (change from baseline to 24 months). Change was calculated as (24 months – baseline) for all variables.
The primary analyses used the progression model of Diggle et al (15) to simultaneously model the both the cross-sectional associations of baseline BP with WMH, cognitive, and functional outcomes at baseline, and also the association of the change in BP between follow-up and baseline with the change in WMH, cognition, and function between follow-up and baseline. Chronological age, and LDL cholesterol levels, were included the models as possible confounders in all analyses. This method avoids the entangling cross-sectional and longitudinal effects. Random intercepts were included in the model in order to take into account the fact that individuals could contribute more than one observation to the analysis. Blood pressure measurements analyzed included clinic systolic, 24-hour mean systolic, awake systolic, sleep systolic and early morning surge systolic BPs. Outcomes analyzed included WMH, mobility and cognition. The WMH was corrected for the intracranial cavity volume, and expressed as a percent. Specific mobility measures included Tinetti Gait Score, time (seconds) to descend three stairs, and self-paced maximum velocity (m/sec)). Cognitive measures included trailmaking B, CalCAPSQ1, and Stroop-Color-Word. Of note, in this age population, the systolic BP has far more clinical importance than the diastolic BP (16), hence the focus for the analyses was on systolic BP.
To examine the association of WMH with functional status, we also conducted cross-sectional regression analyses for each of the dependent variables (mobility and cognition). These models were performed for the 24-month data. Final models for the mobility measures controlled for age and LDL cholesterol levels. A two-tailed level of α ≤0.05 was the threshold for statistical significance in all analyses.
Several different analyses were conducted to study whether there might exist threshold levels of clinic and 24-hour systolic BP that may be associated with the progression of WMH. Locally weighted scatterplot smoother (lowess) curves were constructed to visually depict the relationships. Secondly, the population was sub-divided into 2 groups of ambulatory systolic BP at 24 months (those with mean 24 hour BPs < 135 mmHg and ≥ 135 mmHg at 24 months and those who had a dipper versus non-dipper 24 hour profile (defined as a ≥ 10% decline in sleep vs awake BP for dippers and < 10% decline in sleep vs awake BP for nondippers). Total WMH, mobility and cognitive measures at 24 months were compared via the scatterplot smoother curves; relations among the 2 groups of 24-hour systolic BP with the various outcome measures, as well as age and LDL cholesterol, were evaluated with Kruskal-Wallis nonparametric tests..
Results
Patient characteristics
Ninety-nine participants, all white, were initially enrolled in the study with a mean age of 82 ± 3.8 years; 95 completed a baseline ambulatory BP recording. Two years later, a total of 23 patients had died, moved into a convalescent home, had a pacemaker implant, or declined participation in the 24-hour BP monitoring portion of the study, leaving 72 patients for the final analysis (Table 1). The characteristics of the 23 patients who dropped out of the study before the 24 month analysis time point were similar at baseline to the 72 patients who remained in the cohort analysis for age, gender, WMH and mobility but did have a significantly higher 24 hour systolic BP at baseline (approximately 7 mmHg higher, p = 0.021).
Table 1.
Characteristics of the Patient Population at Baseline and 24 Months
| Parameter | Baseline Mean (SD) |
24 Months Mean (SD) |
p-value |
|---|---|---|---|
| Age (years) | 82.1 (3.9) | 84.2 (3.9) | n/a |
| Gender (M:F) | 31:46 | 31:46 | n/a |
| Body mass index (kg/m2) | 26.7 (4.7) | 26.3 (4.5) | 0.02 |
| Blood Pressure (mmHg) (mean ± SD) | |||
| Clinic BP | 136/71 (16/9) | 136/68 (15/10) | 0.90/0.01 |
| 24-hour BP | 129/66 (12/6) | 131/67 (14/7) | 0.77/0.77 |
| Awake BP | 132/68 (12/7) | 132/68 (14/7) | 0.89/0.69 |
| Sleep BP | 122/60 (15/8) | 125/60 (17/10) | 0.11/0.37 |
| Early morning surge* BP | 9/10 (16/11) | 9/8 (16/10) | 0.91/0.37 |
| Biochemical studies | |||
| Total cholesterol (mg/dl) | 198 (39) | 180 (41) | <0.0001 |
| Low density cholesterol (mg/dl) | 126 (36) | 102 (33) | <0.0001 |
| High density cholesterol (mg/dl) | 56 (15) | 55 (16) | 0.06 |
| Triglycerides (mg/dl) | 100 (41) | 95 (41) | 0.25 |
| Serum glucose (mg/dl) | 99 (14) | 101 (17) | 0.09 |
| Insulin (uIU/ml) | 7.64 (7.92) | 8.02 (10.63) | 0.47 |
| hsCRP (mg/l) | 3.32 (3.99) | 3.49 (5.48) | 0.79 |
| Plasminogen activator inhibitor (mg/mL) | 21.9 (13.5) | 24.6 (19.3) | 0.08 |
| Magnetic Resonance Imaging | |||
| Total Brain Volume (ml) | 1402.2 (143.1) | 1401.6 (144.1) | 0.43 |
| White matter hyperintensity volume (ml) | 13.9 (12.8) | 20.5 (16.2) | <0.0001 |
| White matter hyperintensity/Total Brain Volume (%) | 1.00 (0.90) | 1.47 (1.20) | <0.0001 |
| Functional Assessments | |||
| Mobility parameters | |||
| Tinetti Gait | 11.24 (1.19) | 11.24 (1.31) | 0.92 |
| 8 foot walk time (sec) | 3.1 (0.7) | 3.2 (0.8) | 0.40 |
| Maximum velocity (m/sec) | 0.70 (0.15) | 0.70 (0.18) | 0.84 |
| Time to descend 3 stairs (msec) | 5019 (1121) | 6400 (2521) | <0.0001 |
| Cognitive studies | |||
| Trails B (sec) | 114 (68) | 130 (76.) | 0.05 |
| Stroop Color Word Reaction time (msec) | 27 (9.0) | 26 (9) | 0.23 |
| Sequential process time (msec) | 589 (149) | 545 (194) | 0.07 |
| Simple reaction time (msec) | 414 (132) | 417 (164) | 0.72 |
Of subjects enrolled, 70% had a history of hypertension, 13% coronary artery disease, 6% diabetes, and 48% dyslipidemia (LDL cholesterol > 130 mg/dl). Over the following 2 years, for the 72 participants there were no major changes in body weight, clinic or ambulatory BP and only 3 patients had major interval medical problems: development of stroke, heart failure or valvular disease. At baseline, 64% of subjects were taking antihypertensive drug therapy and 69% at 24 months (2 patients stopped antihypertensive therapy and 6 patients started or had changes in antihypertensive therapy). Clinical decisions related to drug therapies were made by the patients’ primary care physicians without input from study staff. Total and LDL cholesterol fell significantly in the cohort; at baseline, 50% of subjects were taking lipid-lowering drugs - 9 patients started cholesterol lowering medications while 8 discontinued them over 24 months. The mean volume of WMH increased significantly over the 2-year period. One of the 4 mobility measures increased significantly (time to descend stairs) as did one of the 4 cognitive measures (Trails B).
Impact of therapy and outcomes
Regression analyses which included use of anti-platelet therapy, anti-hypertensive drugs, and lipid lowering therapy to predict ambulatory BP and functional outcomes were evaluated. No therapy was a significant predictor in any of the models.
Blood pressure, white matter hyperintensity percent and function
The relationship of both change in clinic and ambulatory BP over the 24 month follow-up period with change WMH, mobility and cognitive measures at 24 months are shown by the results of the regression analyses in Table 2. Analyses were adjusted for age and LDL cholesterol at 24 months since the values of these 2 measures changed significantly over the 2 years and were correlated with total WMH at 24 months. The accrual of WMH from baseline were significantly associated with change from baseline in the 24-hour systolic BP, but not for changes in clinic systolic BP (Table 2). For mobility measures, walk time had a non-significant association with 24-hour systolic BP (p=0.076). Changes in Trailmaking B and Simple Reaction Time were associated with change in 24 hour systolic BP and sleep systolic BP; change in Trailmaking B was also associated with change in awake systolic BP. Stroop Color Word was the only functional parameter associated with change in surge systolic BP. (Table 2). There were no significant associations of any of the cognitive or physical measures, or of WMH, with any of the BP measures at baseline.
Table 2.
Relations Among Clinic and Ambulatory Blood Pressure Components with White Matter Hyperintensity and Functional Parameters at 24 months
| 24-hour Systolic BP | Clinic systolic BP | Awake Systolic BP | Sleep systolic BP | Morning Surge in systolic BP |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 MONTHS | Estimate (95% CI) |
P- Value |
Estimate (95% CI) |
P- Value |
Estimate (95% CI) |
P- Value |
Estimate (95% CI) |
P- Value |
Estimate (95% CI) |
P- Value |
| Total WMH (%) | 0.026 (0.004, 0.048) |
0.02 | 0.006 (−0.013, 0.025) |
0.5 | 0.021 (−0.002, 0.043) |
0.07 | 0.02 (0.002, 0.038) |
0.03 | −0.001 (−0.02, 0.017) |
0.88 |
| Change in WMH (%) |
0.011 (0.001, 0.020) |
0.02 | 0.004 (−0.004, 0.012) |
0.33 | 0.01 (0.001, 0.02) |
0.03 | 0.007 (0, 0.015) |
0.05 | 0.001 (−0.007, 0.008) |
0.88 |
| Tinetti Gait | −0.013 (−0.038, 0.012) |
0.32 | −0.008 (−0.029, 0.013) |
0.44 | −0.013 (−0.037, 0.012) |
0.32 | −0.004 (−0.024, 0.016) |
0.69 | −0.01 (−0.03, 0.011) |
0.35 |
| Stair descent time (msec) |
17.016 (−30.04, 64.07) |
0.47 | −5.145 (−46.71, 36.42) |
0.81 | 0.006 (−0.041, 0.052) |
0.80 | 0.019 (−0.019, 0.056) |
0.32 | 0.004 (−0.033, 0.041) |
0.82 |
| Maximal Gait Velocity (m/sec) |
−0.003 (−0.014, 0.008) |
0.57 | −0.001 (−0.01, 0.008) |
0.82 | 0 (−0.003, 0.003) |
0.97 | −0.002 (−0.004, 0.001) |
0.25 | 0.001 (−0.002, 0.003) |
0.58 |
| Walk time (sec) | 0.011 (−0.005, 0.027) |
0.17 | −0.005 (−0.018, 0.008) |
0.42 | 0.006 (−0.009, 0.022) |
0.41 | 0.012 (−0.001, 0.024) |
0.07 | −0.001 (−0.014, 0.012) |
0.91 |
| Trail Making Test Part B (sec) |
0.596 (−0.60, 1.791) |
0.32 | 1.246 (0.071, 2.421) |
0.04 | 0.44 (−0.741, 1.622) |
0.46 | 0.916 (−0.031, 1.863) |
0.06 | −0.887 (−1.833, 0.058) |
0.07 |
| Stroop Color Word (msec) |
−0.105 (−0.247, 0.038) |
0.15 | −0.114 (−0.24, 0.011) |
0.07 | −0.082 (−0.224, 0.06) |
0.25 | −0.099 (−0.213, 0.015) |
0.09 | 0.085 (−0.028, 0.198) |
0.14 |
| Sequential Process time (msec) |
2.600 (−1.124, 6.323) |
0.17 | 0.47 (−2.615, 3.554) |
0.76 | 1.636 (−2.097, 5.37) |
0.39 | 2.611 (−0.375, 5.596) |
0.09 | −0.822 (−3.882, 2.239) |
0.59 |
| Simple Reaction time (msec) |
1.887 (−1.287, 5.061) |
0.24 | 0.47 (−2.152, 3.093) |
0.72 | 2.106 (−1.024, 5.236) |
0.18 | 0.917 (−1.644, 3.479) |
0.48 | −1.297 (−3.845, 1.251) |
0.31 |
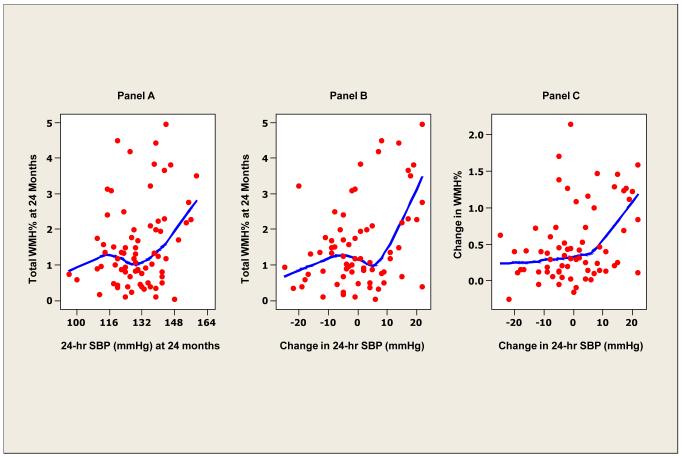
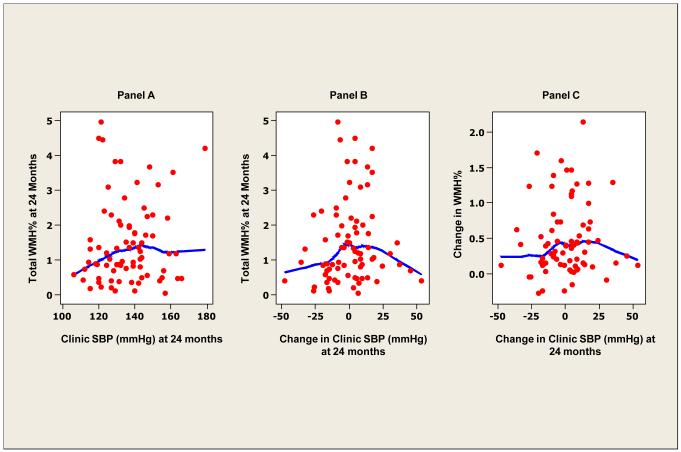
To study the possibility of thresholds in 24-hour systolic BP defining WMH and mobility parameters, smoothed (lowess) scatter plots of BP with WMH (Figure 1) were constructed. At higher levels of 24-hour systolic BP and larger changes in 24-hour systolic BP over time, WMH at 24 months was also higher (Figure 1, panels A and B). Larger increases in WMH were also observed with large changes in 24 hour systolic BP (Figure 1, panel C). In contrast, no relationship between clinic systolic and WMH (Figure 2, panel A) nor change in clinic systolic BP and WMH at 24 months was evident (Figure 2, panel B).
Figure 1. White matter hyperintensity and ambulatory blood pressure.
Locally weighted scatterplot smoother plots of 24-hour average systolic BP and white matter hyperintensity (WMH) lesions (as percent of total intracranial volume) (panel A), change in 24-hour systolic BP and WMH (%) (panel B) and change in 24-hour systolic BP and change in WMH (%) at 24 months (panel C).
Figure 2. White matter hyperintensity and clinic blood pressure.
Locally weighted scatterplot smoother plots of clinic systolic BP and white matter hyperintensity (WMH) lesions (as percent of total intracranial volume) (panel A), change in clinic systolic BP and WMH (%) (panel B) and change in clinic systolic BP and change in WMH (%) at 24 months (panel C).
Using a threshold cut-point of 24-hour systolic BP of 135 mmHg (Clinical cut-point as defined by the American Society of Hypertension (17)) we also observed a significant difference in total WMH at 24 months but not for change in WMH (Table 3). In addition, several of the functional parameters showed more substantial decline in patients with 24-hour systolic BPs ≥ 135 mmHg versus those who had a 24-hour systolic BP < 135 mmHg.
Table 3.
White Matter Lesion Volume and Functional Parameters at 24 months According to 24-hour Blood Pressure Cut-Points Above and Below 135 mmHg
| 24-HOUR AMBULATORY BLOOD PRESSURE | |||
|---|---|---|---|
| <135 mmHg (n = 28) | ≥ 135 mmHg (n = 45) | P-value | |
| 24-hour Systolic BP (mmHg) | 122.0 ± 1.3 | 142.9 ± 1.2 | < 0.001 |
| Total WMH (%) | 1.26 ± 0.15 | 1.96 ± 0.26 | 0.03 |
| Change in WMH (%) * | 0.43 ± 0.07 | 0.62 ± 0.10 | 0.100 |
| Mobility assessments | |||
| Tinetti Gait | 11.5 ± 0.2 | 11.0 ± 0.3 | 0.184 |
| Stair descent time (sec) | 5.8 ± 0.4 | 6.8 ± 0.4 | 0.023 |
| Maximal gait velocity (m/s) | 2.5 ± 0.1 | 2.2 ± 0.1 | 0.051 |
| Walk time (sec) | 2.9 ± 0.1 | 3.5 ± 0.2 | 0.003 |
| Cognitive assessments | |||
| Trails B (sec) | 117.1 ± 8.2 | 136.5 ± 15.4 | 0.489 |
| Stroop Color Word (msec) | 27.5 ± 1.3 | 24.7 ± 1.9 | 0.122 |
| Sequential process time (msec) | 501.6 ± 30.8 | 613.8 ± 33.4 | 0.033 |
| Simple reaction time (msec) | 389.0 ± 21.1 | 461.8 ± 37.2 | 0.025 |
Data are mean ± standard error of the mean; WMH – white matter hyperintensity; m/s – meters/second;
- change in WML over 24 months; p-values were derived from Kruskal-Wallis testing.
White matter hyperintensity (WMH) volume and functional parameters were evaluated according to ‘dipping status’ at 24 months (Table 4). For the purposes of this analysis, subjects who had a decline in sleep systolic BP relative to awake systolic BP of > 10% were considered dippers or extreme dipper if > 20% (n = 26) and those who had a lack of decline in sleep systolic BP relative to awake systolic BP were considered non-dippers (<10% for a non-dipper and < 0% for a riser) (n = 46). Mobility function was better in dippers compared to non-dippers (Table 4) while there were no differences in WMH or cognitive function according to dipper status. Of note, various systolic BP variability measures (the standard deviation of the 24-hour mean systolic BP, the awake systolic BP , or the sleep systolic BP) were not associated with WMH or functional parameters.
Table 4.
White Matter Lesion Volume and Functional Parameters at 24 months according to Dipping Status
| Parameter | |||
|---|---|---|---|
| Risers/Non-dippers | Dippers/Extreme Dippers | p-value | |
| (n = 46) | (n = 26) | ||
| Clinic Systolic BP (mmHg) | 135.8 ± 2.2 | 135.6 ± 2.8 | 0.82 |
| 24-hr Systolic BP (mmHg) | 130.7 ± 2.0 | 128.7 ± 2.3 | 0.62 |
| Total WMH (%) | 1.6 ± 0.2 | 1.3 ± 0.2 | 0.57 |
| Change in WMH (%) | 0.53 ± 0.08 | 0.46 ± 0.08 | 0.78 |
| Mobility assessments | |||
| Tinetti Gait | 11.1 ± 0.2 | 11.5 ± 0.2 | 0.22 |
| Stair descent time (sec) | 6.5 ± 0.3 | 5.7 ± 0.6 | 0.02 |
| Maximal gait velocity (m/s) | 0.7 ± 0.02 | 0.8 ± 0.04 | 0.04 |
| Walk time (sec) | 3.3 ± 0.1 | 2.9 ± 0.1 | 0.02 |
| Cognitive assessments | |||
| Trails B (sec) | 135.6 ± 10.9 | 104.5 ± 8.3 | 0.12 |
| Stroop Color Word (msec) | 26.1 ± 1.4 | 26.9 ± 1.5 | 0.39 |
| Sequential process time (msec) | 551.7 ± 31.1 | 523.1 ± 35.8 | 0.78 |
| Simple reaction time (msec) | 434.3 ± 27.7 | 385.5 ± 22.3 | 0.3 |
Total white matter hyperintensity volume and function at 24 months
Regression analyses were also conducted to examine the relationships between the mobility and cognitive measures with WMH at 24 months. Following adjustment for age and LDL cholesterol levels, 3 of the 4 mobility measures and all of the cognitive measures were significantly related to WMH volume at 24 months.
Discussion
Principal findings
Prior studies have shown that cumulative clinic BP and out-of-office BP measurements are linked to WMH and its progression (18, 19) and that progression of white-matter grade is associated with cognitive decline (20). In the present study, we demonstrate that ambulatory systolic BP but not clinic systolic BP is associated with volumetric WMH progression and its effect on the function of older persons evaluated in a longitudinal fashion. In our cohort of subjects 75 to 90 years of age, we evaluated progression of WMH over 2 years for those who had office and ambulatory BP as well as volumetric MRI. Regression analyses were performed to assess the relations among changes in BP, WMH, mobility and cognitive function. Our data demonstrate that the 24-hour average systolic BP is associated with microvascular brain disease, characterized by volumetric MRI-derived WM lesion volume – and is also significantly associated with impairment of several measures of mobility and cognition. Most notably, worsening ambulatory BP was associated with accrual of WMH and impaired executive function, even after controlling for baseline BP in a longitudinal progression model (15). In contrast, the clinic systolic BP was not related to progression of WMH or functional measures.
Clinical threshold analyses sub-dividing the data into groups of normal (< 135 mmHg) versus abnormal (≥ 135 mmHg) ambulatory systolic BP at 2 years, demonstrated significant differences for WMH and the functional indexes. In the higher 24-hour systolic BP group, WMH was increased and stair descent times and walk times were slower compared to the normal 24-hour BP group while gait velocity was less. Gait speed in the higher ambulatory systolic BP group decreased 0.3 m/sec more than that in the normal ambulatory systolic BP group and stair descent time was 1 second slower in this group as well. While these differences may appear small, they represent changes at 2 years. Mobility limitations linked to WMH occur gradually so that this decrement is part of a long-term process that may compromise gait velocity over 10 or more years (21). Finally, there were also significant relationships among the 24-hour systolic BP values and WMH values at 2 years with some measures of executive functioning-processing speed. Thus, our results are novel and demonstrate for the first time that 24-hour ambulatory BP, not clinic BP, predicts progression of WMH volume within 2 years and is linked to significant and clinically important functional decline in older people.
Blood pressure reproducibility
One likely reason for our findings that 24-hour BP as well as changes in sleep systolic BP predict cerebrovascular disease and functional outcomes in the elderly but clinic BP does not, is the markedly enhanced reproducibility of ambulatory and sleep BP over that of static clinic BP measurements (8, 22). In fact, in the population studied herein, while there were only small changes in office, 24-hour, awake and sleep mean BP values between baseline and 2 years later, the variability (standard deviation of the differences between visits were much lower for 24-hour BP compared to the office BP (11.7/5.9 mmHg versus 17.8/9.0 mmHg, p < 0.01) (8). However, the reproducibility of the 24-hour BP was also substantially better than that of the early morning BP. These findings may also explain in part why 24-hour and awake systolic BP have stronger relationships with WMH and mobility than does to the morning surge in BP.
Prior investigations
Prior cross-sectional studies have demonstrated a relationship between ambulatory BP and WMH in middle-aged or older people (23-27). In contrast to the present study, however, the majority of these cross-sectional studies used more qualitative assessments of white matter lesions (present or absent) (26,27), total brain volume measurements (25), or a semi-quantitative scoring system of WMH (24). The present study obtained segmentation maps and total WMH volume in milliliters (quantified by multiplying voxel volume (mm3) by the number of WMH pixels (divided by 1000)) (6). This methodology allows for quantification of white matter lesion burden in brain regions with pathways supporting mobility (2, 6). Hence, it is possible that a combination of improved reproducibility of BP assessment over time using ambulatory monitoring (8) along with more precise measures of WMH played a role in our ability to demonstrate meaningful impact on function despite the relatively small number of patients in our cohort.
The results of the present study add to a large body of data demonstrating the superiority of 24-hour BP over that of clinic (or doctor’s office) BP in predicting hypertensive target organ disease, particularly cardiac size and structure (28,29) and renal impairment with proteinuria (30). In addition, ambulatory BP monitoring has been shown to be predictive of renal abnormalities in individuals with no history of hypertension (31).
Study limitations
The proportion of subjects from this cohort of subjects with a mean age of 82 years at baseline who were available for assessment at year 2 declined substantially due to death and disability. Of the 23 subjects unavailable, in only 5 cases was this due to unwillingness to wear the ABP recorder. Of the 72 patients who wore the monitor twice, 71 had complete data (nocturnal BP was missing in 1 subject). However, despite the reduction in available study patients at 24 months, there were highly significant findings relating changes in 24-hour systolic BP with changes in WMH and significant findings relating 24-hour systolic BP at 24 months with mobility assessments.
The methodology used in our study was not specifically designed to identify and separate leukoariosis and lacunae. Leukoariosis is a combination of white matter pallor, demyelination and microvascular ischemia and appears as hyperintense (WMH) signal in T2 weighted MRI scans. Lacunes are discrete microvascular infarcts due to occlusion (usually atherosclerosis) of a small penetrating vessel and appear as small cavities 3 to 15 mm in diameter with signal intensity comparable to that of cerebrospinal fluid. In our study it is not likely that large lacunes were included in the final mapping of WMH. However, we cannot exclude the presence of very small lacunae – hence areas identified as WMH may be a combination of both leukoaraiosis and possibly small silent lacunae. However, due to expert over-reads for the segmentation mapping to remove potential artifacts, we expect that the impact of potential inclusion of small lacunae in the final total WMH volumes to be minimal. Finally, many statistical tests are being reported so it is possible that some of the significant associations are due to chance; however, it should be noted that the association of 24 hour BP with WMH, mobility, and cognition were a priori hypotheses.
Implications of the findings
The data suggest that an intervention targeting mean 24-h systolic BP might reduce progression of microvascular disease and thus favorably impact function. Of interest is that the mean values in the highest tertile of clinic and 24-hour systolic BP are 153 and 144 mmHg, respectively. Only the Hypertension in the Very Elderly Trial (HYVET) (32) has studied the benefits of therapy in a similar age group to the present study. As clinic systolic BP was targeted and not ambulatory BP, it is not known what the goal of therapy for ambulatory BP should be in patients over 80 years of age. In HYVET, differences in clinic systolic BP of 15 mmHg translated into reductions in stroke mortality within 2 years. In our longitudinal study, a 14 mmHg difference in 24- hour systolic BP was associated with 40% less WMH and correspondingly improved function within a range that would be considered normal for this age group by many clinicians. Based on these findings, a clinical trial comparing WMH volume and functional measures of mobility and cognition in groups whose ambulatory BP is maintained at the higher versus lower ends of the normal systolic BP spectrum seems warranted.
Clinical Commentary of the Findings.
High blood pressure (BP) is a risk factor for cerebrovascular disease, including stroke. Little is known about the importance of BP on the progression of microvascular disease of the brain that has been associated with functional decline in mobility and cognition in older people. In this prospective cohort study of older people averaging 82 years old, relations among clinic and ambulatory BP, white matter hyperintensity volume (WMHs), and functional status were determined over 2 years. Changes in the 24-hour ambulatory systolic BP, but not clinic systolic BP, was associated with the amount of WMH accrued at the 24 month follow-up, and the progression of WMH from baseline, as well as measures of executive function/processing speed. Higher levels of 24-hour systolic BP were associated with WMH and mobility measures at 2 years; no such relation was seen with clinic systolic BP. Hence, these data demonstrate the importance of 24-hour systolic BP in the progression of brain WMH burden associated with impairment of function in older people. The 24-hour systolic BP may be a potential target for intervention in the elderly to reduce vascular disease of the brain and impairment of function.
Table 2.
continued. Relations Among Changes in Clinic and Ambulatory Blood Pressure Components with White Matter Hyperintensity and Functional Parameters at 24 months
| Change in 24-hour Systolic BP |
Change in Clinic Systolic BP |
Change in Awake Systolic BP |
Change in Sleep Systolic BP |
Change in Morning Surge in Systolic BP |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 MONTHS | Estimate (95% CI) |
P- Value |
Estimate (95% CI) |
P- Value |
Estimate (95% CI) |
P-Value | Estimate (95% CI) |
P- Value |
Estimate (95% CI) |
P- Value |
| Total WMH (%) | 0.02 (0.01, 0.029) |
0.000 | 0.003 (−0.004, 0.009) |
0.42 | 0.016 (0.007, 0.026) |
0.001 | 0.013 (0.005, 0.022) |
0.004 | −0.002 (−0.009, 0.005) |
0.641 |
| Tinetti Gait | −0.015 (−0.057, 0.028) |
0.503 | 0.004 (−0.022, 0.029) |
0.776 | −0.013 (−0.053, 0.027) |
0.521 | −0.021 (−0.057, 0.015) |
0.262 | 0.005 (−0.023, 0.033) |
0.73 |
| Stair descent time (msec) |
6.973 (−23.35, 37.29) |
0.654 | −4.693 (−24.53, 15.15) |
0.645 | −7.905 (−35.41, 19.60) |
0.576 | 16.281 (−9.135, 41.70) |
0.215 | −3.8 (−22.91, 15.31) |
0.698 |
| Maximal Gait Velocity (m/sec) |
−0.001 (−0.008, 0.005) |
0.694 | −0.002 (−0.006, 0.001) |
0.219 | 0.001 (−0.005, 0.008) |
0.649 | −0.002 (−0.008, 0.003) |
0.436 | 0.002 (−0.002, 0.007) |
0.303 |
| Walk time (sec) | 0.01 (−0.001, 0.022) |
0.076 | 0 (−0.007, 0.007) |
0.945 | 0.007 (−0.004, 0.017) |
0.23 | 0.007 (−0.003, 0.017) |
0.161 | 0.003 (−0.004, 0.01) |
0.423 |
| Trail Making Test Part B (sec) |
1.081 (0.128, 2.035) |
0.030 | 0.153 (−0.58, 0.887) |
0.684 | 0.937 (0.027, 1.847) |
0.048 | 0.898 (0.086, 1.709) |
0.034 | −0.121 (−0.804, 0.561) |
0.729 |
| Stroop Color Word (msec) |
−0.106 (−0.228, 0.016) |
0.093 | −0.016 (−0.094, 0.061) |
0.678 | −0.088 (−0.204, 0.028) |
0.142 | −0.092 (−0.195, 0.011) |
0.086 | 0.078 (0.007, 0.149) |
0.036 |
| Sequential Process time (msec) |
0.874 (−2.561, 4.31) |
0.620 | −1.876 (−4.07, 0.319) |
0.099 | −0.662 (−3.95, 2.626) |
0.695 | 1.618 (−1.305, 4.54) |
0.282 | −0.453 (−2.767, 1.862) |
0.703 |
| Simple Reaction time (msec) |
3.913 (1.13, 6.696) |
0.008 | 0.508 (−1.364, 2.38) |
0.596 | 2.223 (−0.378, 4.824) |
0.099 | 2.608 (0.233, 4.982) |
0.035 | −0.488 (−2.374, 1.398) |
0.614 |
Significant p-values shown in bold typeface
Acknowledgments
Funding Sources National Institutes of Health (R01 AG022092; R01 DA024667)
Footnotes
Conflict of Interest Disclosures. None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 2.Wakefield DB, Moscufo N, Guttmann CR, Kuchel GA, Kaplan RF, Pearlson G, Wolfson L. White matter hyperintensities predict functional decline in voiding, mobility and cognition in older adults. J Am Geriatr Soc. 2010;58:275–281. doi: 10.1111/j.1532-5415.2009.02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffens DC, Potter GG, McQuoid DR, MacFall JR, Payne ME, Burke JR, Plassman BL, Welsh-Bohmer KA. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2007;15:839–849. doi: 10.1097/JGP.0b013e318048a1a0. [DOI] [PubMed] [Google Scholar]
- 4.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ., Jr White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 5.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. Epub ahead of print. Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moscufo N, Guttmann CR, Meier D, Csapo I, Hildenbrand PG, Healy BC, Schmidt JA, Wolfson L. Brain regional lesion burden and impaired mobility in the elderly. Neurobiol Aging. 2011;32:646–54. doi: 10.1016/j.neurobiolaging.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan RF, Cohen RA, Moscufo N, Guttmann C, Chasman J, Buttaro M, Hall CH, Wolfson L. Demographic and biological influences of cognitive reserve. J Clin Exp Neuropsychol. 2009;31:868–876. doi: 10.1080/13803390802635174. [DOI] [PubMed] [Google Scholar]
- 8.Campbell P, Ghuman N, Wakefield D, Wolfson L, White WB. Long-term reproducibility of ambulatory blood pressure is superior to office blood pressure in the very elderly. J Hum Hypertension. 2010;24:749–854. doi: 10.1038/jhh.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghuman N, Campbell P, White WB. Role of ambulatory and home blood pressure recording in clinical practice. Curr Cardiol Rep. 2009;11:414–421. doi: 10.1007/s11886-009-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guralnik JM, Simonsick EM, Ferrucci L, Glynn R, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 11.Cameron JD, Stevenson I, Reed E, McGrath BP, Dart AM, Kingwell BA. Accuracy of automated auscultatory blood pressure measurement during supine exercise and treadmill stress electrocardiogram-testing. Blood Press Monit. 2004;9:269–275. doi: 10.1097/00126097-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80:429–434. doi: 10.1016/0002-9343(86)90717-5. [DOI] [PubMed] [Google Scholar]
- 13.Strauss E, Sherman MS. A compendium of neuropsychological tests. 3rd ed Oxford; New York: 2006. Strauss. [Google Scholar]
- 14.Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol. 1993;50:818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- 15.Diggle PJ, Liang K-Y, Zeger SL, Heagerty P. Analysis of Longitudinal Data. 2nd Edition Oxford University Press; 2002. [Google Scholar]
- 16.White WB. Systolic versus diastolic blood pressure versus pulse pressure. Curr Cardiol Rep. 2002;4:463–467. doi: 10.1007/s11886-002-0107-4. [DOI] [PubMed] [Google Scholar]
- 17.Pickering TG, White WB. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2010;4:56–61. doi: 10.1016/j.jash.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, Shibata DK, Knopman DS, Jack CR, Mosley TH., Jr Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41:3–8. doi: 10.1161/STROKEAHA.109.566992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and the brain: A 5-year follow-up. Neurology. 2005;64:1846–1852. doi: 10.1212/01.WNL.0000164712.24389.BB. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth WT, Arnold AM, Beauchamp NJ, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O’Leary DH, Furberg CD. Incidence, Manifestations, and Predictors of Worsening White Matter on Serial Cranial Magnetic Resonance Imaging in the Elderly: The Cardiovascular Health Study. Stroke. 2005;35:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 21.Wolfson L. Gait and balance dysfunction: a model of the interaction of age and disease. Neuroscientist. 2001;7:178–183. doi: 10.1177/107385840100700212. [DOI] [PubMed] [Google Scholar]
- 22.Mansoor GA, McCabe EJ, White WB. Long-term reproducibility of ambulatory blood pressure. J Hypertens. 1994;12:703–708. [PubMed] [Google Scholar]
- 23.Schwartz GL, Bailey KR, Mosley T, Knopman DS, Jack CR, Jr, Canzanello VJ, Turner ST. Association of ambulatory blood pressure with ischemic brain injury. Hypertension. 2007;49:1228–1234. doi: 10.1161/HYPERTENSIONAHA.106.078691. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein IB, Bartzokis G, Hance DB, Shapiro D. Relationship between blood pressure and subcortical lesions in healthy elderly people. Stroke. 1998;29:765–772. doi: 10.1161/01.str.29.4.765. [DOI] [PubMed] [Google Scholar]
- 25.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens. 2008;26:1636–1641. doi: 10.1097/HJH.0b013e3283018333. [DOI] [PubMed] [Google Scholar]
- 26.Sierra C, de la Sierra A, Mercader J, Gomez-Angelats E, Urbano-Marquez A, Coca A. Silent cerebral white matter lesions in middle-aged essential hypertensive patients. J Hypertens. 2002;20:519–524. doi: 10.1097/00004872-200203000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Angelats E, de la Sierra A, Sierra C, Parati G, Mancia M, Coco A. Blood pressure variability and silent cerebral damage in essential hypertension. Am J Hypertens. 2004;17:696–700. doi: 10.1016/j.amjhyper.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 28.White WB, Schulman P, McCabe EJ, Dey HM. Average daily blood pressure, not office blood pressure, determines cardiac function in patients with hypertension. JAMA. 1989;261:873–877. [PubMed] [Google Scholar]
- 29.White WB. Relating cardiovascular risk to out-of-office blood pressure and the importance of controlling blood pressure 24 hours a day. Am J Med. 2008;121(8 suppl):S2–S7. doi: 10.1016/j.amjmed.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Palatini P, Mormino P, Santonastaso M, Mos L, Dal Follo M, Zanata G, Pessina AC. Target organ damage in stage 1 hypertensive subjects with white coat and sustained hypertension: results from the HARVEST study. Hypertension. 1998;21:57–63. doi: 10.1161/01.hyp.31.1.57. [DOI] [PubMed] [Google Scholar]
- 31.Clausen P, Jensen JS, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Ambulatory blood pressure and urinary albumin excretion in clinically healthy subjects. Hypertension. 1998;32:71–77. doi: 10.1161/01.hyp.32.1.71. [DOI] [PubMed] [Google Scholar]
- 32.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nitkin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]