Abstract
This study was undertaken to examine the influence of seeding density, extracellular matrix and days in culture on bile acid transport proteins and hepatobiliary disposition of the model bile acid taurocholate. Mouse hepatocytes were cultured in a sandwich configuration on six-well Biocoat™ plates with an overlay of Matigel™ (BC/MG) or gelled-collagen (BC/GC) for 3 or 4 days at seeding densities of 1.0, 1.25 or 1.5 × 106 cells/well. The lower seeding densities of 1.0 and 1.25 × 106 cells/well resulted in good hepatocyte morphology and bile canalicular network formation, as visualized by 5- (and 6)-carboxy-2′,7′dichlorofluorescein accumulation. In general, taurocholate cellular accumulation tended to increase as a function of seeding density in BC/GC; cellular accumulation was significantly increased in hepatocytes cultured in BC/MG compared to BC/GC at the same seeding density on both days 3 and 4 of culture. In general, in vitro intrinsic biliary clearance of taurocholate was increased at higher seeding densities. Levels of bile acid transport proteins on days 3 and 4 were not markedly influenced by seeding density or extracellular matrix except for multidrug resistance protein 4 (Mrp4), which was inversely related to seeding density. Mrp4 levels decreased ~2- to 3-fold between seeding densities of 1.0 × 106 and 1.25 × 106 cells/well regardless of extracellular matrix; an additional ~3- to 5-fold decrease in Mrp4 protein was noted in BC/GC between seeding densities of 1.25 × 106 and 1.5 × 106 cells/well. Results suggest that seeding density, extracellular matrix and days in culture profoundly influence Mrp4 expression in sandwich-cultured mouse hepatocytes. Primary mouse hepatocytes seeded in a BC/MG configuration at densities of 1.25 × 106 cells/well or below, and cultured for 3 days, yielded optimal transport based on the probes studied. This work demonstrates the applicability of the sandwich-cultured model to mouse hepatocytes.
Keywords: Sandwich-cultured mouse hepatocytes, Mrp4, taurocholate, BEI, in vitro Clbiliary, carboxydichlorofluorescein diacetate
Introduction
Primary hepatocytes are a widely accepted in vitro tool to evaluate hepatic drug uptake, metabolism and cytochrome P450 induction. Maintaining hepatocytes between two layers of gelled collagen (sandwich-culture configuration) facilitates the development of intact canalicular networks, reestablishment of polarized excretory function and maintenance of hepatic transport protein expression and function 1–5. Dunn et al. first demonstrated that the sandwich configuration enhanced the morphology and viability of hepatocytes, and helped maintain normal secretion of many liver-specific proteins and organic compounds 6–8. Subsequent studies have demonstrated that the sandwich configuration facilitates the formation of gap junctions and functional bile canalicular networks during culture 2, 3. Several studies also have shown that sandwich-cultured hepatocytes are capable of performing a wide variety of cellular functions normally attributed to hepatocytes in vivo such as albumin secretion, bile acid synthesis and excretion and P450 enzyme induction, especially when compared to hepatocytes cultured under conventional conditions 2, 3, 8–11.
Sandwich-cultured hepatocytes have been used to investigate hepatic accumulation and excretion of a wide variety of substrates 12–14. In fact, the in vitro intrinsic biliary clearance generated from the sandwich-cultured hepatocyte model has been shown to correlate with in vivo biliary clearance data in rats 15, 16 and humans 17, 18. Thus, the sandwich-cultured hepatocyte model is very promising for characterizing biliary excretion and the hepatic disposition of drug candidates. With all relevant hepatic transport proteins and xenobiotic metabolizing enzymes expressed, substrate uptake, metabolism, and parent/metabolite efflux can be assessed. Recent work has demonstrated that sandwich-cultured primary mouse hepatocytes maintain metabolic competence longer than rat hepatocytes based on gene expression profiles and cytochrome P450 activity 19. Furthermore, the application of this method to hepatocytes from gene-disrupted mice in which specific transport proteins have been knocked out may provide new insight into the many roles of hepatic transport proteins. Numerous investigators have examined the influence of culture conditions on hepatic transport protein expression and function in sandwich-cultured rat and human hepatocytes 9, 20–23. However, culture conditions have not been optimized for transport protein expression and function in sandwich-cultured mouse hepatoctyes. As interest grows in utilizing the phenotypic diversity of various mouse strains (e.g., the Collaborative Cross 24) to predict drug-induced hepatotoxicity, higher throughput screening systems, such as sandwich-cultured mouse hepatocytes, may prove to be particularly useful.
Hepatic transport proteins play an important role in the vectorial transport of bile acids from sinusoidal blood into the bile canaliculi. Sodium taurocholate cotransporting polypeptide (Ntcp) is expressed exclusively in the liver and mediates sodium-dependent uptake of bile acids 25, 26. The organic anion-transporting polypeptides (Oatps), also localized on the basolateral membrane, translocate bile acids in a sodium-independent manner 26. The multidrug resistance proteins 3 and 4 (Mrp3 and Mrp4) are involved in the basolateral excretion of organic anions and bile acids from the hepatocyte across the sinusoidal membrane into blood 27–29. In addition, data generated in mice deficient in the organic solute transporter (Ost)-α subunit have suggested that Ostα-Ostβ, a heteromeric organic solute and steroid transporter, also is involved in the basolateral efflux of bile acids 30. The apical (canalicular) transport proteins involved in biliary excretion of bile acids include the bile salt export pump (Bsep) 31, the multidrug resistance protein 2 (Mrp2) 32, 33 and more recently, multidrug resistance P-glycoprotein (Mdr1a/1b) 34. Interestingly, triple knockout mice deficient in Mdr1a/1b and Bsep exhibited a significantly more severe phenotype than Bsep−/− and wild-type mice, including impaired bile formation, jaundice, flaccid gallbladder and hepatic inflammation. 34
The purpose of the present studies was to examine the influence of seeding density, extracellular matrix and days in culture on hepatic bile acid transport protein expression and taurocholate hepatobiliary disposition. The criteria used for the selection of optimal conditions were hepatocyte morphology, transport protein expression, substrate accumulation, as well as the biliary excretion index (BEI) and biliary clearance (in vitro Clbiliary) of taurocholate.
Experimental Details
Materials
Dulbecco’s modified Eagle’s medium (DMEM), MEM non-essential amino acids solution (100×), L-glutamine, insulin, penicillin G-streptomycin solution, and 5- (and 6)-carboxy-2′,7′dichlorofluorescein (CDF) diacetate (CDFDA) were purchased from Invitrogen (Carlsbad, CA). Collagenase (type 4), fetal bovine serum (FBS), sodium taurocholate, Triton X-100, dexamethasone, and Hanks’ balanced salt solution (HBSS) modified with (H-1387) or without (H-4891) calcium chloride were obtained from Sigma-Aldrich (St. Louis, MO). BioCoat™ collagen I plates, Matrigel™basement membrane matrix, rat tail collagen (type I), and ITS+™(insulin/transferrin/selenium) culture supplement were purchased from BD Biosciences Discovery Labware (Bedford, MA). [3H]Taurocholate (5 Ci/mmol, >97% purity) was obtained from PerkinElmer Life and Analytical Sciences (Boston, MA). Bio-Safe II™ liquid scintillation cocktail was obtained from Research Products International (Mt. Prospect, IL). Bicinchoninic acid (BCA) protein assay reagents and BSA for the protein assay standard were purchased from Pierce Chemical Co. (Rockford, IL). All other chemicals and reagents were of analytical grade and available from commercial sources.
Isolation and In Vitro Culture of Primary Mouse Hepatocytes
Male C57BL/6 mice (27.7–30.3 g) were purchased from Charles River Laboratories, Inc. (Raleigh, NC). Mice had free access to water and food prior to surgery. All animal procedures complied with the guidelines of the Institutional Animal Care and Use Committee (University of North Carolina, Chapel Hill, NC). Hepatocytes were isolated by a two-step collagenase perfusion, as described previously 35, with modifications. The inferior vena cava was cannulated and buffers were perfused retrograde to blood flow. Cell viability, determined by trypan blue exclusion, was 89–92.7%. In pilot studies, hepatocytes were seeded on six-well plates with gelled collagen solution, as described previously 21, or six-well Biocoat™ plates. In subsequent studies, hepatocytes were seeded at 1.0, 1.25 or 1.5 × 106 cells/well in 6-well BioCoat™ plates in DMEM without phenol red supplemented with 2 mM L-glutamine, 1% (v/v) MEM non-essential amino acids, 100 units penicillin G sodium, 100 μg streptomycin sulfate, 1 μM dexamethasone, 5% (v/v) FBS, and 10 μM insulin (day 0 of culture), and allowed to attach for 2–6 h in a humidified incubator (95% O2, 5% CO2) at 37°C. After cell attachment, culture plates were swirled gently and the culture medium was replaced with the same medium. Cells were overlaid 16–24 h (day 1 of culture) after seeding with ice-cold Matrigel™ basement membrane matrix (BC/MG) or gelled collagen (BC/GC). Matrigel was overlaid at a concentration of 0.25 mg/mL in 2 mL/well cold serum-free DMEM containing 2 mM L-glutamine, 1% (v/v) MEM non-essential amino acids, 100 units penicillin G sodium, 100 μg streptomycin sulfate, 0.1 μM dexamethasone, and 1% (v/v) ITS+™. Gelled collagen solution (~1.5 mg/mL) was prepared by adding 4 mL of rat tail type I collagen, 4 mL sterile deionized water, and 1 mL of 10X DMEM, and the pH was adjusted to 7.4 with 2N NaOH. Hepatocyte cultures were aspirated and the monolayer was overlaid with 0.1 mL/well of ice-cold neutralized type I gelled collagen and placed at 37°C in a humidified incubator for ~1 hour to allow the matrix to gel, followed by the addition of 1.5 mL/well of warm DMEM with the same supplements as the cells overlaid with matrigel. The culture medium was changed every 24 h until experiments were performed on day 3–4 of culture.
Accumulation Experiments
The method to determine substrate accumulation in sandwich-cultured hepatocytes has been described previously 16. Briefly, hepatocytes were rinsed twice with 2 mL warm HBSS containing Ca2+ (standard buffer) or Ca2+-free HBSS, and incubated with 2 mL of the same buffer for 10 min at 37°C to maintain tight junction integrity and bile canalicular networks (cells+bile), or disrupt tight junctions and open bile canalicular networks (cells), respectively. The buffer was removed, and the cells were incubated for 10 min at 37°C with 1.5 mL of [3H]taurocholate (1 μM) or CDFDA (2 μM) in standard buffer. Hepatocytes were rinsed vigorously three times with 2 mL ice-cold standard buffer following the incubation. CDFDA-treated hepatocytes were immediately viewed and digital images captured with a Zeiss Axiovert 200TV inverted phase contrast microscope. Taurocholate-treated hepatocytes were lysed with 1 mL 0.5% (v/v) Triton X-100 in phosphate-buffered saline by placing plates on an orbital shaker for a minimum of 20 min at room temperature. Taurocholate uptake was corrected for nonspecific binding by subtracting uptake on blank six-well Biocoat™ plates overlaid with Matrigel™ or gelled collagen. Data were normalized to protein concentration in each well, determined in duplicate aliquots using BCA protein assay reagent kit (Pierce) as instructed by the manufacturer. BSA, as supplied by the manufacturer, was used as a standard (0.2–2 mg/mL). The [3H]taurocholate samples were analyzed by liquid scintillation spectroscopy in a Packard Tri-Carb scintillation counter (PerkinElmer Life and Analytical Sciences).
Cytotoxicity Assay
The activity of lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis, into the medium of sandwich-cultured hepatocytes was measured using a cytotoxicity kit according to the manufacturer’s instructions (Roche, Indianapolis, IN). The degree of LDH release was expressed as a percentage of the maximum cellular LDH release, measured by adding 0.5% Triton X-100 to sandwich-cultured mouse hepatocytes at each seeding density.
Immunoblots
Cells were washed once with HBSS, and resuspended in lysis buffer consisting of 1% SDS, 1 mM EDTA and Complete protease inhibitor cocktail tablets (Roche Diagnostics, Mannheim, Germany). Protein concentrations were determined by the BCA assay. Whole-cell lysates (30–40 μg) were resolved on NuPAGE 4 to 20% Bis-Tris gel (Invitrogen Corp, Carlsbad, CA) and the proteins were transferred to polyvinylidene difluoride (PVDF) membranes. After blocking in 5% milk-Tris-buffered saline with Tween 20 (TBST) for 30 min, blots were incubated overnight at 4°C with the following antibodies: Mrp2 (Abcc2; M2III-6), Mrp3 (Abcc3; M3II-21), Mrp4 (Abcc4; M4I-10), Mdr1a/1b (Abcb1; C-219), breast cancer resistance protein [Bcrp (Abcg2); BXP-53], which were supplied by Alexis Biochemicals, San Diego, CA]; Bsep (Abcb11; K44) and Ntcp (Slc10a1; K4), which were a kind gift from Drs. Bruno Stieger and Peter Meier); and β-actin (C4, Chemicon, San Francisco, CA). After incubation with HRP-conjugated secondary antibody, signals were detected by chemiluminescent substrate Supersignal West Dura (Pierce, Rockford, IL) with a Bio-Rad VersaDoc imaging system; densitometry analysis was performed using Quantity One v4.1 software (Bio-Rad Laboratories, Hercules, CA).
Data Analysis
For accumulation studies, the biliary excretion index (BEI, %) and in vitro intrinsic biliary clearance (Clbiliary, mL/min/kg) were calculated using B-CLEAR® technology (Qualyst, Inc., Raleigh, NC):16
| (1) |
where substrate accumulation in the cells+bile compartments was determined in hepatocytes preincubated in standard buffer; cellular accumulation of substrate was determined in hepatocytes preincubated with Ca2+-free HBSS.
| (2) |
where AUC0-T represents the product of the incubation time (T) and the initial taurocholate concentration in the medium. In vitro Clbiliary values were scaled per kilogram body weight using 0.63, 0.72 and 0.78 mg protein per 1.0, 1.25, and 1.5 × 106 cells, respectively (the average value obtained in all preparations), 135 × 106 hepatocytes/g of mouse liver tissue 36, and 87.5 g of liver tissue per kg of body weight (1.75 g/0.02 kg mouse) 37.
Statistical analysis of the [3H]taurocholate cellular accumulation and in vitro intrinsic biliary clearance data was performed using a three-way analysis of variance for the factors of day in culture, seeding density and extracellular matrix, with Bonferroni’s post hoc test (SAS version 9.1, Cary, NC).
Results
Sandwich-Cultured Mouse Hepatocyte Viability and Bile Canalicular Network Formation
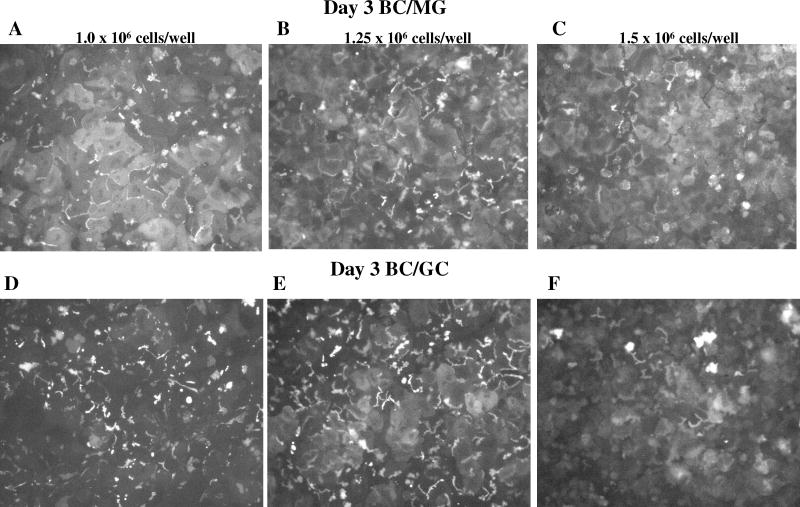
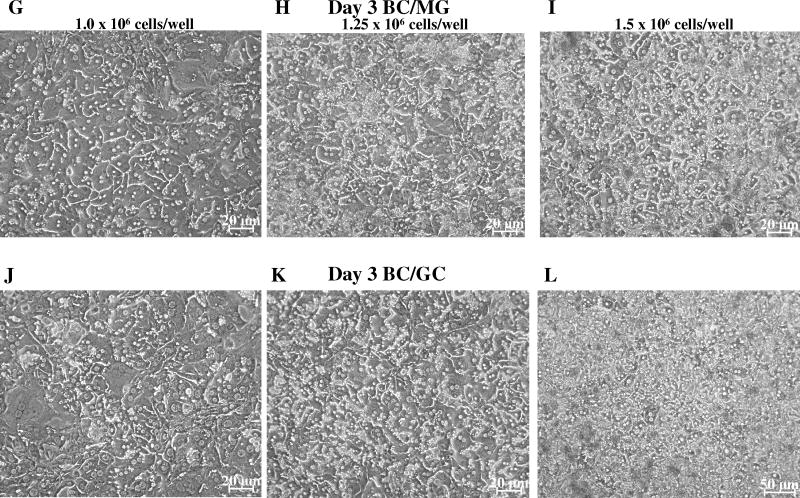
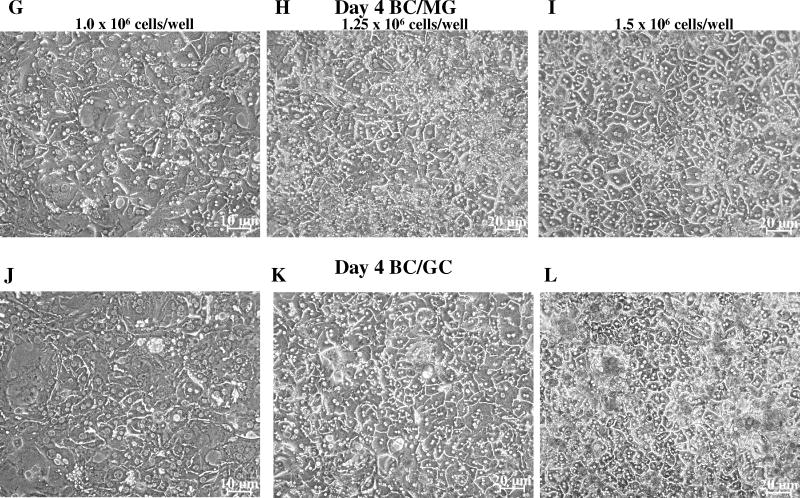
Initial experiments plating mouse hepatocytes on gelled collagen plates resulted in decreased attachment and adherence after overlay compared to Biocoat™ plates. Therefore, subsequent experiments utilized Biocoat™ plates only, on which hepatocytes flattened to form confluent monolayers. Hepatocyte morphology was evaluated by light microscopy, and canalicular network formation was determined by CDF imaging. Mouse hepatocytes cultured in sandwich-configuration and maintained in DMEM for up to 4 days were cuboidal in shape and formed extensive canalicular networks (Figures 1 and 2). The intensity of CDF accumulation in the bile canaliculi was more extensive in mouse hepatocytes cultured in the BC/GC configuration compared to the BC/MG configuration. The BC/MG matrix had greater intracellular accumulation of CDF compared to BC/GC. Based on the light microscopy images, the higher seeding density of 1.5 × 106 cells/well resulted in greater accumulation of dead cells and debris compared to the lower seeding densities (Figures 1 and 2). Based on LDH leakage, cell death primarily occurred in the first 24 hours before overlay; medium LDH levels during the first 24 hr after plating reached ~25% of Triton X-100 control. Cumulative LDH leakage after overlay through day 4 in culture was negligible (< 12% of Triton X-100 control), regardless of seeding density or extracellular matrix (data not shown).
Figure 1.
Effect of seeding density and extracellular matrix on cell morphology and bile canalicular network formation in day 3 sandwich-cultured mouse hepatocytes. Representative (n =3) CDF fluorescence (A – F) and light microscopy (G – L) images of mouse hepatocytes cultured in a Biocoat™/Matrigel™ (BC/MG; A–C & G–I) or Biocoat™/gelled-collagen (BC/GC; D–F & J–L) sandwich configuration in six-well plates (maintained with DMEM for 3 days); seeding densities (1.0, 1.25 or 1.5 × 106 cells/well) are noted at the top of each column. Sandwich-cultured mouse hepatocytes were incubated with 2 μM CDFDA for 10 min.
Figure 2.
Effect of seeding density and extracellular matrix on cell morphology and bile canalicular network formation in day 4 sandwich-cultured mouse hepatocytes. Representative (n = 3) CDF fluorescence (A – F) and light microscopy (G – L) images of mouse hepatocytes cultured in a Biocoat™/Matrigel™ (BC/MG; A–C & G–I) or Biocoat™/gelled-collagen (BC/GC; D–F & J–L) sandwich configuration in six-well plates (maintained with DMEM for 4 days); seeding densities (1.0, 1.25 or 1.5 × 106 cells/well) are noted at the top of each column. Sandwich-cultured mouse hepatocytes were incubated with 2 μM CDFDA for 10 min.
Transport Protein Expression
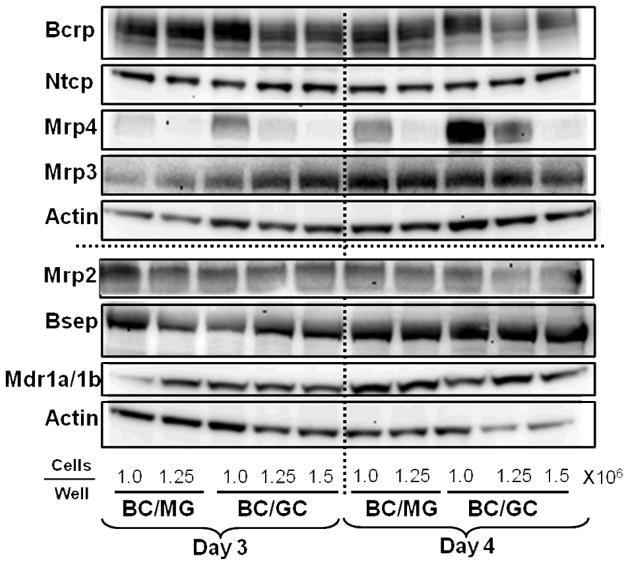
Representative immunoblots of Ntcp, Mrp4, Mrp3, Mrp2, Bsep, Bcrp, and Mdr1a/1b in sandwich-cultured mouse hepatocytes seeded at different densities on BC/MG and BC/GC are shown in Figure 3. Relevant bile acid transport proteins were expressed in sandwich-cultured mouse hepatocytes by day 3; culture configuration and seeding density had modest effects on Mrp3, Mrp2, Bcrp, Ntcp, Mdr1a/1b and Bsep protein. Interestingly, Mrp4 protein increased (~2-fold or greater) as seeding density decreased, regardless of extracellular matrix on both day 3 and 4 (Figures 3 and 4).
Figure 3.
Influence of seeding density, extracellular matrix and day in culture on transport protein levels in sandwich-cultured mouse hepatocytes. Representative immunoblots of Bcrp, Ntcp, Mrp4, Mrp3, Mrp2, Bsep, and Mdr1a/1b in mouse hepatocytes cultured in Biocoat™/Matrigel™ (BC/MG) or Biocoat™/gelled-collagen (BC/GC) sandwich configuration in six-well plates and maintained with DMEM for 3 or 4 days (n = 2). B-actin was used as a loading control.
Figure 4.
Relative expression of Mrp4 protein compared with β-actin in sandwich-cultured mouse hepatocytes cultured in Biocoat™/Matrigel™ (BC/MG) or Biocoat™/gelled-collagen (BC/GC) sandwich configuration in six-well plates and maintained with DMEM for 3–4 days. Densitometry was performed with Quantity One software (version 4.1; Bio-Rad, Hercules, CA).
Hepatobiliary Disposition of the Model Bile Acid Taurocholate
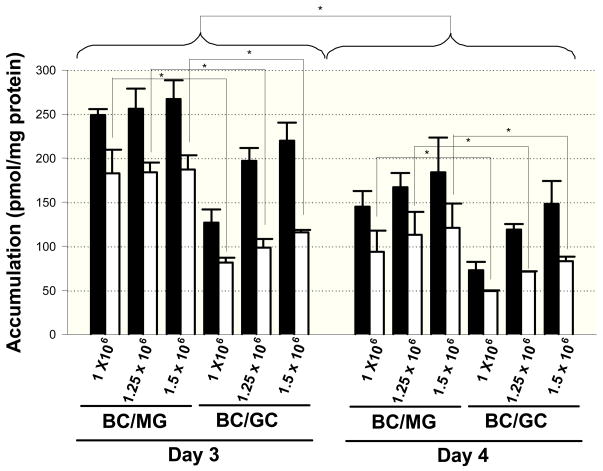
The influence of seeding density, overlay matrix composition and days in culture on the accumulation and biliary excretion of [3H]taurocholate was assessed in sandwich-cultured mouse hepatocytes. Cellular accumulation of [3H]taurocholate was significantly lower on day 4 compared to day 3 (Figure 5). [3H]Taurocholate cellular accumulation tended to increase as a function of seeding density for BC/GC; [3H]taurocholate cellular accumulation was significantly greater in mouse hepatocytes cultured in the BC/MG configuration compared to the BC/GC configuration at each seeding density on both days 3 and 4 of culture (Figure 5). In general, in vitro intrinsic biliary clearance of taurocholate was increased at higher seeding densities; taurocholate BEI values were not significantly different across seeding density (Table 1).
Figure 5.
Accumulation in cells+bile (black bars) and cells (white bars) after a 10-min incubation with 1 μM [3H]taurocholate in mouse hepatocytes cultured in Biocoat™/Matrigel™ (BC/MG) or Biocoat™/gelled-collagen (BC/GC) sandwich configuration in six-well plates and maintained with DMEM for 3 or 4 days (Mean ± S.E.M., n=3). Cellular accumulation (white bars) of [3H]taurocholate was significantly increased on day 3 compared to day 4, and significantly greater when cultured in BC/MG configuration compared to the BC/GC configuration on both days 3 and 4 of culture. * p < 0.05
Table 1.
Mean biliary excretion index (BEI) and in vitro biliary clearance (in vitro Clbiliary) of [3H]taurocholate after a 10-min incubation with 1 μM [3H]taurocholate in sandwich-cultured mouse hepatocytes. (Mean ± SEM; n = 3 per group). Primary mouse hepatocytes were seeded at designated densities and cultured in Biocoat™/Matrigel™ (BC/MG) or Biocoat™/gelled-collagen (BC/GC) sandwich configurations in six-well plates and maintained with DMEM for 3 or 4 days. In vitro intrinsic biliary clearance of [3H]taurocholate was significantly increased as a function of seeding density
| Day in Culture | BC/MG | BC/GC | |||||
|---|---|---|---|---|---|---|---|
| 1.0 × 106 cells/well | 1.25 × 106 cells/well | 1.5 × 106 cells/well | 1.0 × 106 cells/well | 1.25 × 106 cells/well | 1.5 × 106 cells/well | ||
| 3 | BEI (%) | 26.3 ± 10.8 | 27.6 ± 5.5 | 29.6 ± 8.2 | 35.4 ± 3.9 | 49.9 ± 6.3 | 46.9 ± 6.4 |
| In Vitro Clbiliary (mL/min/kg) | 48.7 ± 20.5 | 60.4 ± 15.8 | 73.7 ± 24.6 | 33.6 ± 7.6† | 84.0 ± 15.6† | 96.0 ± 21.1† | |
|
| |||||||
| 4 | BEI (%) | 36.2 ± 9.0 | 32.5 ± 10.3 | 34.2 ± 5.0 | 32.4 ± 7.7 | 39.9 ± 3.5 | 43.5 ± 6.4 |
| In Vitro Clbiliary (mL/min/kg) | 38.3 ± 5.0** | 45.4 ± 10.6 | 57.8 ± 14.7** | 18.0 ± 6.4* | 40.4 ± 5.7* | 60.3 ± 19.3* | |
p < 0.05, 1.0 vs. 1.25 and 1.0 vs. 1.5 × 106 cells/well on day 3;
p<0.05, 1.0 vs. 1.25 and 1.0 vs. 1.5 × 106 cells/well on day 3;
p<0.05, 1.0 vs. 1.5 × 106 cells/well on day 4.
Discussion
Sandwich-cultured primary hepatocytes have been used extensively to study hepatic uptake, metabolism and efflux. To date, limited data have been published utilizing sandwich-cultured mouse hepatocytes to study hepatic transport mechanisms and biliary excretion. Previous reports have demonstrated the influence of culture conditions including the type of culture media, media supplements, extracellular matrix and confluency on cell morphology, bile canalicular network formation, substrate accumulation and BEI in both rat and cryopreserved human hepatoctyes 20–22. In the present study, the influence of seeding density, extracellular matrix and days in culture on protein levels of bile acid transporters, and the hepatobiliary disposition of taurocholate in sandwich-cultured mouse hepatocytes was assessed.
Both extracellular matrix overlay and seeding density had profound effects on the health, morphology, and bile canalicular network formation of sandwich-cultured mouse hepatocytes. The cell morphology of mouse hepatocytes cultured on Biocoat™ plates was similar to prior observations with rat hepatocytes; cells spread out to form a confluent monolayer resulting in a flattened appearance on this rigid collagen when compared to hepatocytes cultured on gelled collagen 2, 22. Mouse hepatocytes overlaid with Matrigel (BC/MG) exhibited a similar attachment efficiency and cell morphology compared to those overlaid with gelled collagen (BC/GC). However, Matrigel provided a more uniform, reproducible overlay. CDFDA, which is hydrolyzed to fluorescent CDF inside hepatocytes, is a good Mrp2 probe substrate 38 that has been used extensively to visualize the bile canaliculi 2, 20, 21. CDF was excreted into bile canaliculi in all culture conditions. However, more CDF accumulation in bile canalicular networks was observed by fluorescent microscopy in BC/GC compared to BC/MG at lower seeding densities, which may be attributed to more extensive formation of bile canalicular networks (Figures 1 and 2). Although, levels of Mrp2 were similar across extracellular matrix and days in culture (Figure 3), differences in the amount of functional Mrp2 protein on the apical membrane also may contribute to the increased accumulation of CDG in bile canalicular networks observed at the lower seeding densities. Furthermore, CDF intracellular accumulation was greater in mouse hepatocytes cultured on BC/MG compared to BC/GC based on visual inspection of the fluorescent microscope images (Figures 1 and 2); similar findings were noted for taurocholate cellular accumulation (Figure 5).
Mouse hepatocytes seeded at 1.5 × 106 cells/well resulted in greater accumulation of dead cells and decreased network formation, as visualized by CDF. Cell death occurred primarily during the first day of culture, shortly after hepatocytes were seeded, based on LDH measurements. Most of the unattached and dead cells were washed away when the medium was changed at 24 hr, prior to overlay, but some cell debris presumably was stuck to the collagen, as visualized in Fig. 1 and 2. The cell death observed in this study may be attributed primarily to collagenase digestion used during hepatocyte isolation, which has been shown by many investigators to influence cell viability 39–42, and cannot be explained by complete consumption of nutrients or growth factors in the cell medium. Greater cell death at higher seeding densities was similar to findings observed in mouse hepatocytes cultured at an overconfluent stage, which resulted in cell death by apoptosis 43. The observation that mouse hepatocytes perform better at a lower seeding density compared to other species (rat and human hepatocytes typically are seeded at ~1.75 × 106 cells/well on 6-well plates 44, 45) may be attributed to the increased oxygen demand in vivo in mice (liver blood flow in mice is ~90 ml/min/kg compared to ~55.2 and ~20.7 ml/min/kg in rats and humans, respectively 37.
Overlay matrix composition (BC/MG or BC/GC) and days in culture (day 3 or 4) appeared to have little effect on protein levels of Bsep, Bcrp, Ntcp, Mrp3, Mrp2 and Mdr1a/1b in sandwich-cultured mouse hepatocytes. Surprisingly, protein levels of Ntcp were well-maintained compared to sandwich-cultured rat hepatocytes, where significant downregulation of Ntcp protein3 (~80% decrease46) and a ~75–80% decrease in mRNA47 have been reported over days in culture.
Another profound observation noted in the present studies was that Mrp4 protein levels were higher at lower seeding densities (Figures 3 and 4). Mrp4 is localized on the basolateral membrane of hepatocytes and has been shown to efflux bile acids in a glutathione-dependent manner.28 Mrp4 plays an important role in detoxifying the liver; bile-duct ligated Mrp4−/− mice exhibited increased liver toxicity based on histological analysis, with a 4-fold decrease in serum bile acid concentrations and increased serum liver enzymes, compared to bile duct-ligated wild-type mice.48 Although levels of the basolateral efflux transport protein Mrp3 were increased modestly in common bile duct-ligated mice compared to sham-operated controls, 49 in bile duct-ligated mice lacking Mrp3, serum and liver bile acid concentrations were similar compared to sham-operated wild-type controls.50,51 Furthermore, Mrp4 protein was increased more than 2-fold in bile duct-ligated wild-type mice while Mrp3 protein was unchanged compared to sham-operated wild-type controls.48 As an adaptive response to cholestasis, MRP3 and MRP4 are upregulated to efflux bile acids across the basolateral membrane, thereby reducing intracellular accumulation of bile acids in hepatocytes and subsequent toxicity due to the detergent effects on the cell membrane and resulting mitochondrial dysfunction.53, 54 At lower, seeding densitites in sandwich-cultured mouse hepatocytes, Mrp4 may be unregulated as a compensatory mechanism to increase efflux of toxic bile acids; no change in Mrp3 protein was observed in sandwich-cultured mouse hepatocytes across extracellular matrix and days in cultured (Figure 3).
Taurocholate accumulation in mouse hepatocytes cultured on both BC/MG and BC/GC supported the observation that Mrp4 protein decreased as a function of increased seeding density (Figures 4 and 5). Clearly, on day 4 of culture, cellular accumulation (white bars) of [3H]taurocholate increased from 93.6 to 121 pmol/mg protein in BC/MG, and from 49.2 to 83.0 pmol/mg protein in BC/GC in mouse hepatocytes seeded at 1.0 × 106 and 1.5 × 106 cells/well, respectively (Figure 5). This could not be attributed to differences in the primary bile acid transport proteins because total protein levels of Ntcp, Mdr1a/1b and Bsep were similar across seeding density and collagen matrix (Figure 4). However, Mrp4 was decreased ~3-fold when mouse hepatocytes were seeded at a density of 1.25 × 106 cells/well compared to 1.0 × 106 cells/well on BC/MG, and decreased ~8-fold when mouse hepatocytes were seeded at a density of 1.5 × 106 cells/well compared to 1.0 × 106 cells/well on BC/GC (Figure 4). Perhaps the increased Mrp4 expression at lower seeding densities resulted in redirection of some taurocholate excretion across the basolateral rather than the canalicular membrane, thus reducing the cellular accumulation of taurocholate compared to the higher seeding density in which Mrp4 expression was ~3- to 8-fold lower and cellular accumulation was higher (Table 1, Figures 3–5). Furthermore, the cellular accumulation of [3H]taurocholate in BC/MG was higher than BC/GC for all seeding densities, whereas Mrp4 protein was lower in BC/MG compared to BC/GC at a given seeding density.
The hepatocyte accumulation of [3H]taurocholate in sandwich-cultured mouse hepatocytes was increased when compared to sandwich-cultured rat and human hepatocytes. The cellular accumulation of taurocholate on day 3 following a 10-min incubation with 1 μM [3H]taurocholate in sandwich-cultured mouse hepatocytes seeded at 1.5 × 106 cells/well was 187 ± 16 and 116 ± 3 pmol/mg protein on BC/MG and BC/GC, respectively (Figure 5). This was much greater than the reported cellular accumulation of taurocholate on day 4 following a 10-min incubation with 1 μM [3H]taurocholate in sandwich-cultured rat hepatocytes on BC/MG and BC/GC (<1044, 55 and ~5016 pmol/mg protein, respectively), or day 7 sandwich-cultured human hepatocytes (67.0 ± 25.018 and 65.8 ± 11.356 pmol/mg protein, both cultured on BC/MG). The in vitro intrinsic biliary clearance also was greater in mouse sandwich-cultured hepatocytes relative to rat and human sandwich-cultured hepatocytes. On day 3, the in vitro Clbiliary in mouse sandwich-cultured hepatocytes seeded at 1.5 × 106 cells/well on BC/MG and BC/GC, was 73.7 ± 24.6 and 96 ± 21.1 mL/min/kg, respectively, compared to 34.9 ± 12.044 and ~5516 mL/min/kg, respectively, in day 4 rat sandwich-cultured hepatocytes, and 15.8 ± 4.518 and 15.2 ± 5.056 mL/min/kg (both cultured on BC/MG only) in day 7 human sandwich-cultured hepatocytes. The differences in taurocholate accumulation and in vitro intrinsic biliary clearance are controlled by the transport proteins responsible for uptake, biliary excretion and basolateral efflux of bile acids. The observed differences are not due to the affinity of these hepatic transport proteins for taurocholate in mouse compared to rat and human hepatocytes because the Km values for NTCP/Ntcp, OATPs/Oatps and BSEP/Bsep are similar between species.48, 57–61 Km values have been reported for human MRP4 (25.8 μM).27 Therefore, the reason for greater taurocholate accumulation and in vitro intrinsic biliary clearance in sandwich-cultured mouse hepatocytes relative to sandwich-cultured rat and human hepatocytes may be attributed, in part, to maintenance of Ntcp and decreased Mrp4 (Figure 4) protein when seeded at 1.5 × 106 cells/well. Alternatively, species-dependent differences in the taurocholate Km for Mrp4 may exist.
Conclusion
In summary, extracellular matrix, seeding density and days in culture impact Mrp4 protein expression, bile canalicular network formation and taurocholate hepatobiliary disposition in sandwich-cultured primary mouse hepatocytes. Mouse hepatocytes seeded at densities of 1.25 × 106 cells/well or 1.0 × 106 cells/well, resulted in improved attachment, more extensive bile canalicular network formation and overall healthier hepatocytes. Total protein levels of Mrp3, Mrp2, Ntcp, Bcrp, Mdr1a/1b and Bsep were similar across seeding density and collagen matrix. Taurocholate accumulation was greater in BC/MG compared to BC/GC, and tended to increase as a function of seeding density for BC/GC. In vitro intrinsic biliary clearance was greatest at 1.5 × 106 cells/well. Hepatocellular accumulation and in vitro intrinsic biliary clearance of taurocholate were greater on day 3 compared to day 4. Primary mouse hepatocytes seeded in a BC/MG configuration at densities of 1.25 × 106 cells/well or 1.0 × 106 cells/well, and cultured for 3 days, yielded optimal transport based on the probes utilized in this study. This higher-throughput in vitro system could maximize the use of gene-disrupted mice from various strains to study hepatic function and to investigate the influence of gene disruption on hepatobiliary drug/metabolite disposition and hepatotoxicity.
Acknowledgments
The authors would like to thank Yiwei Rong, for her technical expertise in the isolation of mouse hepatocytes. We sincerely thank Drs. Bruno Stieger and Peter Meier for providing Ntcp and Bsep antibodies. This research was supported by a grant from the National Institutes of Health (R01 GM41935).
Abbreviations used
- Ntcp
Sodium taurocholate cotransporting polypeptide
- Mrp4
multidrug resistance protein 4
- Mrp3
multidrug resistance protein 3
- Ost
organic solute transporter
- Bsep
bile salt export pump
- Mrp2
multidrug resistance protein 2
- Bcrp
breast cancer resistance protein
- Mdr1a/1b
multidrug resistance P-glycoprotein
- BEI
Biliary excretion index
- in vitro Clbiliary
biliary clearance
- DMEM
Dulbecco’s modified Eagle’s medium
- CDFDA
5- (and 6)-carboxy-2′,7′dichlorofluorescein diacetate
- FBS
fetal bovine serum
- HBSS
Hanks’ balanced salt solution
- BCA
Bicinchoninic acid
- MG
Matrigel™ basement membrane matrix
- GC
gelled rat tail collagen type I
- PVDF
polyvinylidene difluoride
- TBST
Tris-buffered saline with Tween 20
References
- 1.Hoffmaster KA, Turncliff RZ, LeCluyse EL, Kim RB, Meier PJ, Brouwer KLR. Pharm Res. 2004;21(7):1294–302. doi: 10.1023/b:pham.0000033018.97745.0d. [DOI] [PubMed] [Google Scholar]
- 2.LeCluyse EL, Audus KL, Hochman JH. Am J Physiol. 1994;266(6 Pt 1):C1764–74. doi: 10.1152/ajpcell.1994.266.6.C1764. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Brouwer KL, Gan LS, Brouwer KR, Stieger B, Meier PJ, Audus KL, LeCluyse EL. Pharm Res. 1998;15(10):1533–9. doi: 10.1023/a:1011994831139. [DOI] [PubMed] [Google Scholar]
- 4.Turncliff RZ, Meier PJ, Brouwer KL. Drug Metab Dispos. 2004;32(8):834–9. doi: 10.1124/dmd.32.8.834. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Tian X, Chandra P, Brouwer KLR. Mol Pharmacol. 2005;67(4):1334–41. doi: 10.1124/mol.104.004481. [DOI] [PubMed] [Google Scholar]
- 6.Dunn JC, Tompkins RG, Yarmush ML. J Cell Biol. 1992;116(4):1043–53. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Faseb J. 1989;3(2):174–7. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 8.Dunn JC, Tompkins RG, Yarmush ML. Biotechnol Prog. 1991;7(3):237–45. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 9.Turncliff RZ, Meier PJ, Brouwer KLR. Drug Metab Dispos. 2004;32(8):834–9. doi: 10.1124/dmd.32.8.834. [DOI] [PubMed] [Google Scholar]
- 10.Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, Wang H. J Pharmacol Exp Ther. 2007;320(1):72–80. doi: 10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahi J, Shord SS, Lindley C, Ferguson S, LeCluyse EL. J Biochem Mol Toxicol. 2009;23(1):43–58. doi: 10.1002/jbt.20264. [DOI] [PubMed] [Google Scholar]
- 12.Annaert PP, Brouwer KL. Drug Metab Dispos. 2005;33(3):388–94. doi: 10.1124/dmd.104.001669. [DOI] [PubMed] [Google Scholar]
- 13.Govindarajan R, Endres CJ, Whittington D, LeCluyse E, Pastor-Anglada M, Tse CM, Unadkat JD. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G570–80. doi: 10.1152/ajpgi.00542.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmaster KA, Zamek-Gliszczynski MJ, Pollack GM, Brouwer KL. Drug Metab Dispos. 2005;33(2):287–93. doi: 10.1124/dmd.104.001420. [DOI] [PubMed] [Google Scholar]
- 15.Abe K, Bridges AS, Yue W, Brouwer KL. J Pharmacol Exp Ther. 2008;326(3):983–90. doi: 10.1124/jpet.108.138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Chism JP, LeCluyse EL, Brouwer KR, Brouwer KL. Drug Metab Dispos. 1999;27(6):637–44. [PubMed] [Google Scholar]
- 17.Abe K, Bridges AS, Brouwer KL. Drug Metab Dispos. 2009;37(3):447–52. doi: 10.1124/dmd.108.023465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghibellini G, Vasist LS, Leslie EM, Heizer WD, Kowalsky RJ, Calvo BF, Brouwer KL. Clin Pharmacol Ther. 2007;81(3):406–13. doi: 10.1038/sj.clpt.6100059. [DOI] [PubMed] [Google Scholar]
- 19.Mathijs K, Kienhuis AS, Brauers KJ, Jennen DG, Lahoz A, Kleinjans JC, van Delft JH. Drug Metab Dispos. 2009;37(6):1305–11. doi: 10.1124/dmd.108.025775. [DOI] [PubMed] [Google Scholar]
- 20.Bi YA, Kazolias D, Duignan DB. Drug Metab Dispos. 2006;34(9):1658–65. doi: 10.1124/dmd.105.009118. [DOI] [PubMed] [Google Scholar]
- 21.Chandra P, Lecluyse EL, Brouwer KL. In Vitro Cell Dev Biol Anim. 2001;37(6):380–5. doi: 10.1007/BF02577575. [DOI] [PubMed] [Google Scholar]
- 22.Turncliff RZ, Tian X, Brouwer KLR. Biochem Pharmacol. 2006;71(10):1520–9. doi: 10.1016/j.bcp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Rose KA, Kostrubsky V, Sahi J. Mol Pharm. 2006;3(3):266–74. doi: 10.1021/mp0501022. [DOI] [PubMed] [Google Scholar]
- 24.Threadgill DW, Hunter KW, Williams RW. Mamm Genome. 2002;13(4):175–8. doi: 10.1007/s00335-001-4001-Y. [DOI] [PubMed] [Google Scholar]
- 25.Mita S, Suzuki H, Akita H, Hayashi H, Onuki R, Hofmann AF, Sugiyama Y. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G550–6. doi: 10.1152/ajpgi.00364.2005. [DOI] [PubMed] [Google Scholar]
- 26.Pauli-Magnus C, Meier PJ. Hepatology. 2006;44(4):778–87. doi: 10.1002/hep.21359. [DOI] [PubMed] [Google Scholar]
- 27.Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G640–9. doi: 10.1152/ajpgi.00354.2005. [DOI] [PubMed] [Google Scholar]
- 28.Rius M, Nies AT, Hummel-Eisenbeiss J, Jedlitschky G, Keppler D. Hepatology. 2003;38(2):374–84. doi: 10.1053/jhep.2003.50331. [DOI] [PubMed] [Google Scholar]
- 29.Zelcer N, Saeki T, Bot I, Kuil A, Borst P. Biochem J. 2003;369(Pt 1):23–30. doi: 10.1042/BJ20021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballatori N, Fang F, Christian WV, Li N, Hammond CL. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G179–G186. doi: 10.1152/ajpgi.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. J Biol Chem. 1998;273(16):10046–50. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 32.Gerk PM, Li W, Megaraj V, Vore M. J Pharmacol Exp Ther. 2007;320(2):893–9. doi: 10.1124/jpet.106.106922. [DOI] [PubMed] [Google Scholar]
- 33.Jedlitschky G, Hoffmann U, Kroemer HK. Expert Opin Drug Metab Toxicol. 2006;2(3):351–66. doi: 10.1517/17425255.2.3.351. [DOI] [PubMed] [Google Scholar]
- 34.Wang R, Chen HL, Liu L, Sheps JA, Phillips MJ, Ling V. Hepatology. 2009 doi: 10.1002/hep.23089. [DOI] [PubMed] [Google Scholar]
- 35.LeCluyse EL, Bullock PL, Parkinson A, Hochman JH. Pharm Biotechnol. 1996;8:121–59. doi: 10.1007/978-1-4899-1863-5_9. [DOI] [PubMed] [Google Scholar]
- 36.Sohlenius-Sternbeck AK. Toxicol In Vitro. 2006;20(8):1582–6. doi: 10.1016/j.tiv.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Davies B, Morris T. Pharm Res. 1993;10(7):1093–5. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 38.Kitamura T, Jansen P, Hardenbrook C, Kamimoto Y, Gatmaitan Z, Arias IM. Proc Natl Acad Sci U S A. 1990;87(9):3557–61. doi: 10.1073/pnas.87.9.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF. In Vitro. 1981;17(10):913–25. doi: 10.1007/BF02618288. [DOI] [PubMed] [Google Scholar]
- 40.Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Trump BF. In Vitro. 1981;17(10):926–34. doi: 10.1007/BF02618289. [DOI] [PubMed] [Google Scholar]
- 41.Seglen PO. Exp Cell Res. 1973;82(2):391–8. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- 42.Williams GM, Bermudez E, San RH, Goldblatt PJ, Laspia MF. In Vitro. 1978;14(10):824–37. doi: 10.1007/BF02616152. [DOI] [PubMed] [Google Scholar]
- 43.Shinzawa K, Watanabe Y, Akaike T. Cell Death Differ. 1995;2(2):133–40. [PubMed] [Google Scholar]
- 44.Wolf KK, Brouwer KR, Pollack GM, Brouwer KL. Drug Metab Dispos. 2008;36(10):2086–92. doi: 10.1124/dmd.108.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yue W, Abe K, Brouwer KL. Mol Pharm. 2009;6(1):134–43. doi: 10.1021/mp800100e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swift B, Pfeifer ND, Brouwer KLR. Drug Metabolism Reviews. doi: 10.3109/03602530903491881. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luttringer O, Theil FP, Lave T, Wernli-Kuratli K, Guentert TW, de Saizieu A. Biochem Pharmacol. 2002;64(11):1637–50. doi: 10.1016/s0006-2952(02)01382-5. [DOI] [PubMed] [Google Scholar]
- 48.Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. Hepatology. 2006;43(5):1013–21. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- 49.Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Hepatology. 2009 doi: 10.1002/hep.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zelcer N, van de Wetering K, de Waart R, Scheffer GL, Marschall HU, Wielinga PR, Kuil A, Kunne C, Smith A, van der Valk M, Wijnholds J, Elferink RO, Borst P. J Hepatol. 2006;44(4):768–75. doi: 10.1016/j.jhep.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, Lerro A, Kruh GD. Mol Pharmacol. 2005;68(1):160–8. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- 52.Delzenne NM, Calderon PB, Taper HS, Roberfroid MB. Toxicol Lett. 1992;61(2–3):291–304. doi: 10.1016/0378-4274(92)90156-e. [DOI] [PubMed] [Google Scholar]
- 53.Gores GJ, Miyoshi H, Botla R, Aguilar HI, Bronk SF. Biochim Biophys Acta. 1998;1366(1–2):167–75. doi: 10.1016/s0005-2728(98)00111-x. [DOI] [PubMed] [Google Scholar]
- 54.McRae MP, Lowe CM, Tian X, Bourdet DL, Ho RH, Leake BF, Kim RB, Brouwer KLR, Kashuba AD. J Pharmacol Exp Ther. 2006;318(3):1068–75. doi: 10.1124/jpet.106.102657. [DOI] [PubMed] [Google Scholar]
- 55.Swift B, Tian X, Brouwer KL. Pharm Res. 2009 doi: 10.1007/s11095-009-9909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. Gastroenterology. 2002;123(5):1649–58. doi: 10.1053/gast.2002.36591. [DOI] [PubMed] [Google Scholar]
- 57.Funk C, Pantze M, Jehle L, Ponelle C, Scheuermann G, Lazendic M, Gasser R. Toxicology. 2001;167(1):83–98. doi: 10.1016/s0300-483x(01)00460-7. [DOI] [PubMed] [Google Scholar]
- 58.Saeki T, Takahashi N, Kanamoto R, Iwami K. Biosci Biotechnol Biochem. 2002;66(5):1116–8. doi: 10.1271/bbb.66.1116. [DOI] [PubMed] [Google Scholar]
- 59.Hata S, Wang P, Eftychiou N, Ananthanarayanan M, Batta A, Salen G, Pang KS, Wolkoff AW. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G829–39. doi: 10.1152/ajpgi.00352.2002. [DOI] [PubMed] [Google Scholar]
- 60.Hagenbuch B, Meier PJ. J Clin Invest. 1994;93(3):1326–31. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]