Introduction
Recent advances in immunosuppression, operative techniques and patient management have improved short term one year survival rates after kidney, liver and heart transplantation to more than 80% at most experienced centers. In fact, organ allograft recipients without major post-operative complications are being discharged from the hospital within several weeks of the operation, a scenario almost unheard of even as little as a decade ago. These developments have shifted considerable attention in research away from short term problems such as acute rejection, to the major obstacles to long-term, morbidity-free survival: chronic rejection (CR), the subject of this review, and the adverse effects of maintenance immunosuppression, which is needed to prevent CR.
The nature and magnitude of the problem
CR can be broadly defined as a largely indolent, but progressive form of allograft injury characterized primarily by fibrointimal hyperplasia of arteries, or obliterative arteriopathy (OA), interstitial fibrosis and atrophy of parenchymal elements. For the most part, it is irreversible and eventually results in allograft failure. Currently, CR is the most significant medical obstacle to long term morbidity-free allograft survival. The incidence is thought to progressively increase with time after transplantation, but after a period of five years it affects about 10% of liver to around 60% of lung allograft recipients (table 1) [1–11].
Table 1.
Estimates of the incidence of chronic rejection in various solid organ allografts
| Allograft | Incidence of OA at 5 Years | References |
|---|---|---|
| Heart | 25% – 60% | [1], [2], [3] |
| Heart-Lung | 8% – 20% (OA) | [1], [4] |
| 50% – 60% (OB) | [1], [5], [7], [8] | |
| Lung | 28/45% (single/double lung) | [7] |
| Liver | 3–17%; 10% and decreasing | [9], [10] |
| Pancreas alone | 30–70%*(late graft loss) | [11] |
| Pancreas and kidney | 20–40%* | [11] |
Estimates based on graft survival rates after one year, with the assumption that most late graft failures are due to CR.
The term “chronic” implies a temporally prolonged course and in general, CR more indolently compromises organ function, in contrast to acute rejection that can precipitously cause allograft failure because of vascular necrosis/thrombosis and infarction. However, many cases of CR clearly evolve from severe or inadequately controlled acute rejection episodes and in patients not compliant with chronic maintenance immunosuppression. In such patients, persistent immunologic injury leads to a progressive decline in organ function over a period of weeks to months. There is however, a significant number of patients who do not fit these profiles, and slowly develop graft failure over a period of years. This more indolent presentation may be attributable to “clinically silent” rejection episodes that go undetected or to other factors.
Alloimmunity was the suspected etiology of CR when it was first reported over 3–4 decades ago [12, 13]. Indeed, from an immunological perspective, CR could be thought as an iatrogenically-induced autoimmune disease that shares many features with chronic graft versus host disease (CGVHD) However, CR appears to more selectively damage the arterial tree in comparison to autoimmune diseases and GVHD. In fact, obliteration of the arterial lumen because of fibrointimal hyperplasia, or obliterative arteriopathy (OA), is the pathognomonic lesion of CR.
The premise that CR is immunologically mediated, is best supported by the fact that isografts rarely suffer this complication and if they do, it is much delayed and less severe in comparison to allografts [14–17]. The importance of immunologic injury is also supported by many clinical studies, in which severe or persistent acute rejection [10, 18–26], inadequate immunosuppression [8, 27], donor/recipient racial or ethnic differences[28–32], sex mismatching[24, 33], viral infections [34–41] and the use of immune activating drugs are cited as risk factors [42].
The effect of MHC matching on long term allograft survival is also used as evidence of an immune etiology [28, 29,31,32,43–46]. However, the actual impact of imperfect matching on long term allograft survival has been less than expected [28,31,43,44,47–51] With complete six antigen matches, immunological causes of allograft failure are minimized [52,53] Any degree of mismatching usually results in a progressive attrition of graft function over time [28,29,31,32,43–46], which is in large part, related to CR. Liver allografts however, are an exception. They are relatively resistant to CR (table 1) [51] and HLA matching appears to play a dualistic role [54,55]: Mismatching increases the incidence of acute rejection, but MHC matching might contribute to a greater susceptibility to recurrent inflammatory liver disease [54].
Despite a convincing body of data, a level of uncertainty still exists about OA pathogenesis, largely because it is difficult to study in clinical material. Prior to graft failure or death when the entire organ can be histopathologically examined, invasive diagnostic procedures are often needed to make the diagnosis of CR. These tests are often subjectively interpreted. The issue is further confused by atherosclerotic donor disease [56–58]; the development of atherosclerosis after transplantation, and the observation in some studies that OA is associated with conventional risk factors for atherosclerosis [59–62]. Lastly, the various organs are affected differently by CR, so there is no generic definition that can equally applied across all organs. For example, in heart allografts, the primary manifestation of CR is OA and progressive ischemic damage [1,63–65]. In lung allografts, destruction of the small bronchioles (obliterative bronchiolitis) is the major insult limiting long-term survival [1,7,22,66–68], whereas the vascular disease is generally considered to be of secondary importance [1,7,22,66,67]. In the liver, both bile duct loss and OA (see below) together contribute to allograft failure [69–73]. Nevertheless, there are many similarities in the various manifestations of CR between solid organ allografts [63,74,75], which enable us to initially cover the topic from a common pathophysiological perspective. The organ-specific peculiarities that exist, often arise as a result of physioanatomic variations, some of which in turn, can influence the immunological mechanisms responsible for the injury.
Common features of chronic rejection
Introduction
In this section, we will review the common findings of CR in various solid organ allografts, which include: 1) graft vascular disease or obliterative arteriopathy(OA); 2) lymphohistiocytic interstitial inflammation; 3) patchy interstitial fibrosis, 4) destruction and atrophy of parenchymal cells; 5) destruction and atrophy of organ-associated lymphoid tissues and lymphatic vessels.
Graft vascular disease or obliterative arteriopathy (OA)
Distribution of the Lesions
The characterization of OA as a diffuse concentric intimal thickening involving the entire intra-organ arterial tree [63,64,74–76] is generally helpful in distinguishing it from atherosclerosis, which is endemic in the general population. However, in an individual lesion, it is not always possible to distinguish between the two disorders [60,77,78]. This is particularly true for organs such as the heart that are normally prone to atherosclerosis, but less of a problem in athero-resistant organs, like the liver. In an effort to avoid confusion, “obliterative arteriopathy” (OA) will be used throughout this review to refer to allograft arterial disease associated with rejection, whereas the term “atherosclerosis” (AS) will be used for the common endemic disease, realizing that the distinction is less than perfect.
The distribution of OA lesions in allografts is one of the key features used to distinguish it from AS. Whereas AS preferentially affects the large extracorporeal arteries [1,63,64,74], it has been repeatedly shown that OA more often involves both the extra-organ (e.g., epicardial, hepatic hilar, etc.) and first, second and third-order medium-sized intra-organ muscular arteries [1,63,64,74–76]. However, it has been our experience that OA lesions are not as diffuse as one might expect from a review of the pathology literature [1,63,64,74–76]. Even though smaller arteries are involved, the lesions in both large and smaller arteries are often patchy in distribution, and it has also been our impression that they often begin and evolve more quickly near branch points [63].
Small arteries (40–50 µm in external diameter) and arterioles commonly sampled in liver, heart and intestinal allograft biopsies can also be involved with OA; but it is much less common than large and medium-sized vessel disease [63,79] and the changes seen are less specific for the diagnosis of CR [63] Medial hypertrophy and/or mural hyalinization and eventually, frank destruction of the microvasculature, have been described in renal and liver allografts with CR [69,80,81]. Misorientation and compartmentalization of medial smooth muscle cells and adventitial fibrosis have been described in small subendocardial arteries from cardiac allografts [79], but these lesions do not necessarily correlate with the presence or absence of OA.
Arterial inflammation
Because OA is so strongly correlated with an alloimmune response, it is tempting to conclude that arterial inflammation causes the damage by recognizing normally expressed or upregulated class I and II donor MHC antigens on the endothelium [63,82–86]. However, arterial inflammation is not always observed [87,88] and fibrointimal hyperplasia can progress without it [88–92]. When inflammation is not seen, it is thought that antibody alone initiates the damage, or that the stereotypic vascular repair response can proceed without continued immunologic injury [89,92].
Even though OA can apparently occur without inflammation, most investigators would concede that cellular immunity importantly contributes to its development in many cases. The preferential localization of leukocytes in the adventitia and intima suggests that these are the most important antigenic targets or sites of damage [63,75,82–85,93]. The adventitia is rich in lymphatics and donor dendritic cells making it a site of both peripheral sensitization and a conduit for emigrating leukocytes in acute rejection [63,82,85,87,93,94]. When rejection is mild, the inflammation is usually limited to the adventitia [87]. When it is unusually severe, or when the recipient harbors anti-donor antibodies, mononuclear and/or neutrophilic endothelitis, respectively, are usually also present [74,77,82,95–101]. In many cases the intimal inflammation is quickly followed by fibrointimal hyperplasia, which marks the beginning of OA.
The inflammation often persists in OA lesions showing fibrointimal hyperplasia. At this stage, the lymphocytes present might be recognizing non-MHC antigen(s), like heat shock protein [102]. In any event, it is likely that toxic effector molecules and cytokines released by the inflammatory cells contribute to disruption of arterial wall homeostasis and arterial remodeling, since the two often co-exist [63,82–84,86]. However, the inflammatory cells often become separated from the endothelial layer by a concentric ring of intimal myofibroblasts (fig. 1). Thus, the inflammation is no longer directly associated with the endothelium. Instead, it forms a ring in the deep intima, which is separated from a cuff of adventitial macrophages by the media. The two inflammatory cell populations are connected with each other via a stream of individual inflammatory cells that permeate between vacuolated medial myocytes. This suggests that dynamic trafficking occurs between the adventitial and the deep intima, while the endothelium remains intact.
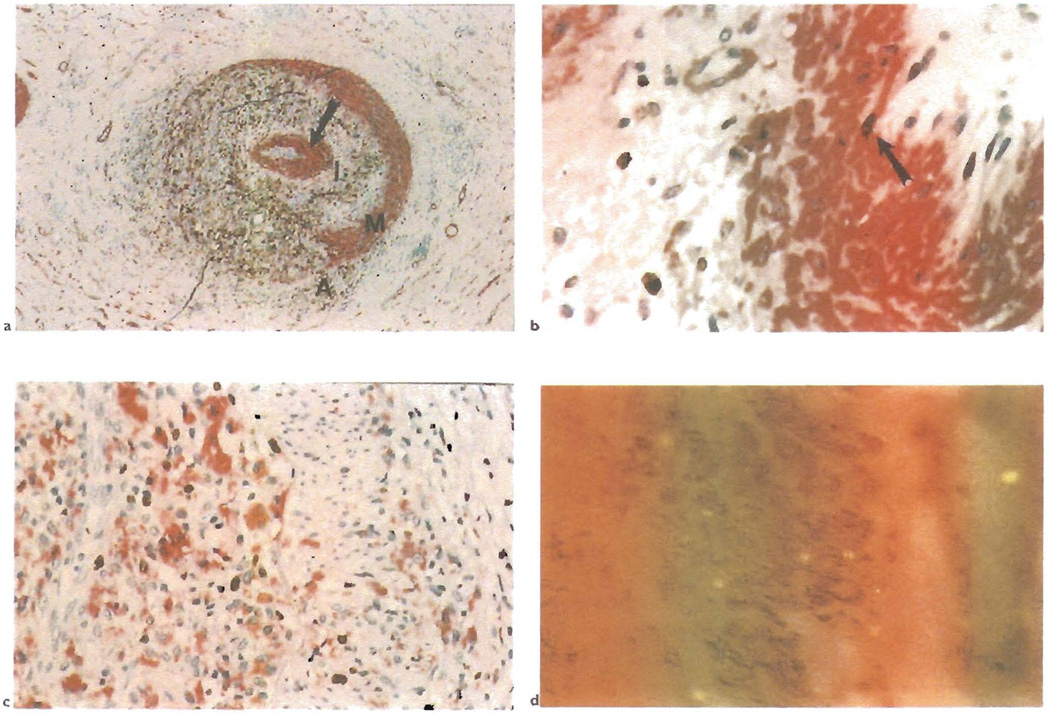
Fig. 1.
a) This section shows a hepatic artery branch in the hilum of a liver allograft. It is stained with a double immunolabeling procedure for smooth muscle actin(red) and macrophages(CD68: brown). Note the severe lumenal narrowing because of obliterative arteriopathy and destruction of the media along one half of its circumference(A = adventitia: M = media: I = intima). Note also the concentric ring of myofibroblasts(arrow) that separates the deep intimal macrophages from the endothelium, which is intact and uninflammed. Extension of the macrophages from the adventitia through to the deep intima suggests that some of the arterial inflammation may begin in the adventitia and may contribute to the development of OA without directly involving the intima. b) A higher magnification of the subendothelial ring of myofibroblasts shows them to be micotically active{arrow: double staining for smooth muscle actin (red) and proliferating cells. Ki-67 (brown)}. c) The ring of deep intimal inflammatory cells is also mitotically active{double staining for macrophages (CD68: red) and proliferating cells, Ki-67 (brown)}. d) Double labeling for smooth muscle cells(actin; brown) and male cells (Y chromosome in situ hybridization, yellow dot) shows that the intimal myofibroblasts in the female recipient of a male liver are of donor origin.
Immunophenotypic analyses have shown that the arterial inflammation consists primarily of an admixture of T-lymphocytes and macrophages [60,63,82,83,86,103]. In some studies, CD8+ T cells are the most common [83,103], a subset of which show perform positivity, identifying a cytolytic effector pathway [83,86] CD4/CD8 double negative and γδ T cells have been cultured in vitro from affected vessels [104]. The presence of occasional dendritic cells, signals ongoing antigenic presentation [82]. Macrophages however, often become the predominant inflammatory cell population, which may be related to the increased deposition of ground substance and lipid trapping, both of which stimulate phagocytosis(see below) [60,63,82,86,103,105–107]. The macrophages permeate the adventitia, media and intima, and proliferate within the artery [63]. Foam cell transformation is more common in early lesions and most often seen in liver allografts [60,63,72,74,75,105–108]. An adventitial-to-intimal macrophage size gradient is also often observed: those nearest to the lumen have abundant foamy cytoplasm, ones in the adventitia are smaller. Other inflammatory cells, such as dendritic cells [82], B cells [63,82], eosinophils [109] and plasma cells are much less commonly observed [63,82].
Mural histopathology
In practical terms, graft vascular disease can be equated with obliterative arteriopathy (OA), because vein involvement is both significantly less common and less severe [66,74,108]. The reason for preferential arterial targeting is uncertain, but hemodynamic factors, the prototypic arterial response to injury [110–112], endothelial antigenic differences and unique mechanical factors related to lymphatic disruption [87], are possible explanations.
It seems clear that OA evolves from a repair response to vessel wall injury, and all three layers of the artery wall are involved: intima, media and adventitia [63,74,111,113]. Documentation of the sequential changes have largely relied on a reconstruction of events from failed human allografts and autopsy specimens, and from detailed sequential analyses of experimental animal models. Common early findings include endothelial activation, which histologically manifests as hypertrophy or a “hobnail” appearance with eosinophilic transformation of the cytoplasm [63,85,87,114]. This impression is supported by the appearance of functional endothelial activation [114,115], including upregulation of class II MHC and adhesion molecules [63,87,116–120], and damage that can be objectively documented via a Fas-based apoptotic pathway [86]. In turn, endothelial damage results in loss of barrier function and the influx of clotting proteins(including fibrin), platelets, blood cells and lipids, all of which disrupt intimal homeostasis and contribute to the repair response and vascular narrowing(see below). Edema and an increased deposition of intercellular matrix and lipid [105], and a local turnover of cells [86,87] are seen in all three layers, but are especially common in the intima, which results in recruitment of medial myocytes and infiltration of foamy macrophages [74,77,82,95–100]. This is followed by intimal myofibroblast proliferation, that eventually replaces the entire intima. Breaks in the internal elastic lamina are variably present [63].
In the media, early changes include edema, and degeneration or frank necrosis of individual myocytes [87, 93]. Later, the media can become thinned [63, 81], presumably as a result of intimal migration of medial myocytes in response to injury. In fact, the media can be completely replaced by foam cells and/or fibroblasts so that the artery is no longer a flexible muscular conduit, but a rigid or loosely-wall tube formed by fibroblasts or foam cells, respectively.
Changes in the adventitia of arteries have received far less attention than they deserve. Early after transplantation, the adventitia is a primary site of sensitization, which results in inflammation and edema [87,94]. The adventitial inflammation often persists [63,82,87] and is followed by adventitial fibrosis [79,87] in the fully developed lesions. Ultimately, disruption of arterial wall homeostasis and lumenal narrowing predisposes to thrombosis and organ ischemia. Prototypic events are reconstructed in fig. 2.
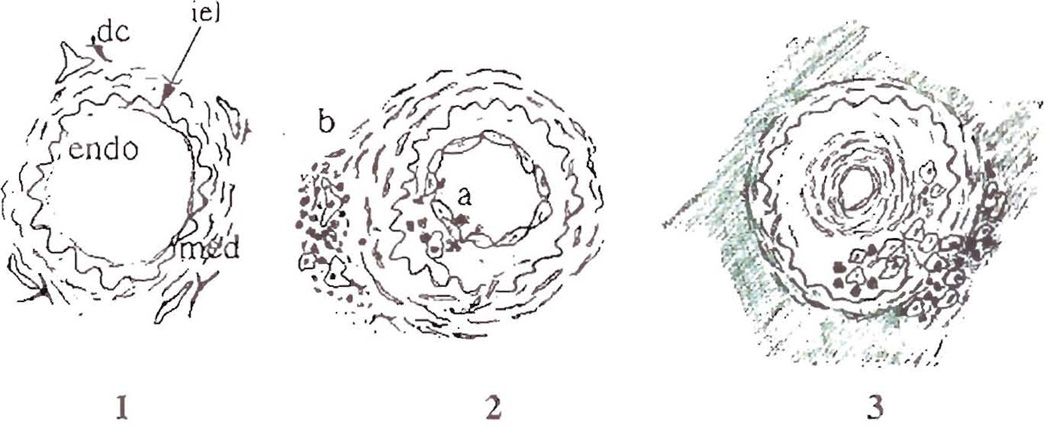
Fig. 2.
Schematic of the sequential steps in the development of obliterative arteriopathy, which is the common feature of chronic rejection in all solid organ allografts. A normal artery is shown in frame 1, illustrating the important anatomic features including the endothelial lining (endo), which is flattened and inactive-appearing, the internal elastic lamina (iel), the media (med) and the dendritic cells(dc) located near draining lymphatics in the adventitia of arteries. Frame 2 shows early arterial changes during acute rejection and two distinct, but not mutually exclusive pathways of arterial injury. In severe acute rejection(a) the endothelium and intima can be directly injured by antibodies/complement and/or inflammatory cells, which leads to an intimal repair response and myofibroblast proliferation. However, arterial damage can also occur in less severe rejection episodes, illustrated by pathway (b). Inflammatory cells accumulate near adventitial donor dendritic cells and draining lymphatics. This causes edema of the media and intima with damage of individual medial myocytes, and indirect injury of the endothelium. Endothelial hypertrophy and activation occur with both insults and the arterial repair response is triggered. Frame 3 illustrates some of the latter charges in the development of OA. A concentric ring of myofibroblasts develops immediately subjacent to the endothelium, which returns to a more quiescent appearance. The media also becomes thinner, presumably as a result or migration of myocytes to the intima and/or compensatory arterial dilatation. The macrophages and lymphocytes now accumulate in the deep intima and focally stream through the disrupted media. This results in a connection between the intimal inflammatory cells and the cuff of macrophages and lymphocytes in the adventitia. The arrangement suggests that adventitial to intimal trafficking of immune cells is an important entry route. It occurs at a time when fibrosis is developing in the adventitia around affected anteries and in the interstitium.
Lipid and glycosaminoglycan deposition
OA is not commonly thought of as a lipid-dependent disorder, but there is evidence to suggest that hyperlipidemia can hasten its development [59,61,62,97] and measurement of the lipid content of arteries affected by OA show increased mean total cholesterol, esterified cholesterol, free cholesterol, and phospholipid content and concentration [121]. However, grummous atheroma, calcification and cholesterol clefts are less frequently observed in OA than in AS. McManus and colleagues [105,121] compared the patterns of lipid and glycosaminoglycan deposition in arteries affected by OA to that seen in AS in the general population. The lipoproteins seen in OA consist primarily of Apo(a) and apoE, which is in contrast to AS, where apoB is more prominent. Also, the apoE deposits can be seen in endothelia and in the extracellular space of the superficial intima with mild disease early after transplantation, and in the deeper intima with severe OA [107]. Co-localization of glycosaminoglycans shows that biglycan is particularly prominent in the intima of evolving OA lesions, but not in AS. Decorin is present mainly in adventitia of all vessels and in the intima of AS. Prominent versican accumulation is seen in the intima and media of arteries with OA, associated with smooth muscle and foam cells and the intimal biglycan and versican deposits positively correlate with the extent of luminal narrowing in OA [106]. Interestingly, the areas of Apo(a) and apoE deposition are also the sites of prominent proteoglycan sediments, especially versican. McManus, et al hypothesize that arterial injury, either mechanical and/or immunologic, leads to dramatic increases in intimal lipoprotein permeability and increased lipid retention within the arterial wall. This presumably occurs because the lipid interacts with chondroitin sulfate proteoglycans, including versican, which in turn, results in accelerated lipid endocytosis by macrophages and smooth muscle cells [107], and foam cell transformation.
Interstitial inflammation
Chronically rejecting liver, heart, intestinal and pancreatic allografts often show very mild and patchy inflammation, whereas in renal allografts, it can be more pronounced because of withdrawal of immunosuppression prior to allograft nephrectomy. The inflammation consists mostly of lymphocytes, macrophages and plasma cells. Formation of nodular lymphoid aggregates, some of which also contain germinal centers, suggests ongoing antigen presentation and continual stimulation [87,122]. The aggregates are often located in the adventitia of arteries, and near the serosa or capsule of organs [87,122,123], which are also the site of draining lymphatics. Inflammatory cells are also individually scattered throughout the interstitium, where they are seen in close association with parenchymal cells undergoing damage and atrophy.
Immunophenotypic studies have shown primarily CD4+ and CD8+ T cells, occasional eosinophils [109] and macrophages [87,122,124–127], the latter which can be quite prominent. Compared to acute rejection, there are usually more B cells [116,127,128], and formation of B cell follicles is not uncommon. Increased transcripts for granzyme B; the presence of Th1-like cytokines IL-2 and IFN-γ [126] and the prominence of macrophages, suggests that CR is mediated by an immune response tilted toward the TH1 pathway.
Damage and atrophy of parenchymal cells and interstitial fibrosis
Epithelial cells which rest on a conventional basement membrane, particularly those that line conduits used for exchange of substances with the environment (e.g., bronchioles, bile ducts, pancreatic ducts, renal tubular epithelium), are particularly prone to damage during CR. There are at least several possible explanations for this observation, which are covered in greater detail in a recent review of the immunopathology of liver allograft rejection [129]. Basement membranes contain matrix components important to the migration, positioning and co-stimulation of T-cells [129]. In addition, epithelial cell “immunogenicity” is enhanced by unique antigenic profiles [129] and their ability to upregulate immunologically active adhesion and co-stimulatory molecules and to produce cytokines and growth factors [129,130]. Since the epithelial-lined conduits also transport antigens to and from the environment, they are often intermixed with antigen presenting cells, and surrounded by a rich lymphatic plexus (see below), which drains to the regional lymph nodes. This is normally a site of local immune activation to environmental and microbial antigens, which in an allograft, could trigger rejection. Lastly, the bile ducts and bronchioles are almost exclusively supplied by an arterial circulation, obliteration of which can cause ischemic injury. Thus, both direct immunologic injury and ischemia likely contribute to the damage and loss of small bile ducts and bronchioles during CR [69.131].
Patchy interstitial fibrosis is another hallmark feature of CR, but alone, it cannot be used as a reliable predictor of CR in peripheral biopsy samples [132]. Small scars are frequently seen as a result of previous biopsies or other insults. Nevertheless, fibrosis is a constant feature of CR, and it often manifests as expansion and collagenization of the connective tissue normally surrounding the arteries affected by OA. This is combined with more localized interstitial widening [63], especially in areas of ongoing immunologic damage, which are often associated with the deposition of tenascin and other matrix components [133,134], endothelin [135] and activation of interstitial myofibroblasts [134]. Larger scars representing healed infarcts and fibrosis in watershed regions supplied by the terminal circulation, suggest that ischemic injury also contributes to fibrogenesis [63].
Disruption of lymphatics and organ associated lymphoid tissues
Complete revascularization of an allograft results in the circulation of recipient immune cells through donor organ-associated lymphoid tissues(GALT, BALT, portal lymphoid tissue) [136–140] and regional donor lymph nodes [141]. Early after transplantation, this results in a “in vivo mixed lymphocyte response”, which typically manifests as acute rejection [136–139,141]. Transplantation also disrupts the efferent lymphatics, which results in organ edema and contributes to the re-implantation response. The lymphatic channels reconnect within two to three weeks [142, 143] in the absence of additional insults. Acute rejection however, results in the increased production of lymph fluid and at the same time, disrupts the lymphatic microvasculature, both of which contribute to the reappearance of graft edema and swelling during rejection reactions [142,144–147]. In CR, the organ-associated lymphoid tissue, such as the BALT [147,148] or GALT [139], is destroyed and replaced by fibrosis. Patchy interstitial fibrosis also focally disrupts intra-organ lymphatics [87, 149]. Both of these changes undoubtedly contribute to an inability of chronically rejecting allografts to adequately process infectious agents and antigens that are normally cleared via these pathways [8,37,40,148,150–152]. In addition, disruption of lymphatic drainage alone has been shown to produce arterial injury, which resembles changes seen with developing OA [153]. Thus, we [87] and others before us [149], have suggested that this potential mechanism of arterial injury might importantly contribute to the development of OA.
The direct injury model of pathogenesis
Cause(s) of the arterial injury
Animal models are an excellent way of controlling experimental conditions to isolate the various arterial insults. Many models that study immunological injury ameliorate acute rejection, so the allograft survives long enough to develop CR and OA. One approach exploits a weak immunological barrier, where only minor histocompatibility barriers are crossed(e.g. LEW to F-344 rats) [95–99,154,155]. Another approach uses transient treatment with various forms of immunosuppression with the most popular being a short course of anti-CD4 monoclonal antibodies [85,115]. The percentage of animals surviving long term and developing OA can be adjusted by titrating or adding immunosuppression, depending on the model.
In these models, there is relentless direct immunological injury to the allogeneic arterial endothelium from prolonged, non-lethal acute rejection, that appears to be mediated primarily by T lymphocytes, macrophages and anti-donor antibodies [85,95,98,99,114,115,154,156]. This quickly evolves into CR and OA, much like the clinical cases that directly evolve from an inadequately controlled acute rejection. Indeed, on the basis of these models and clinical studies [63, 75, 77, 157], many investigators would support the following pathogenesis of OA: direct lymphohistiocytic inflammation of allogeneic endothelium or alloantibody binding → endothelial cell damage → release of cytokines and growth factors by endothelial cells, myofibroblasts and inflammatory cells → disruption of intimal homeostasis → intimal myofibroblast proliferation → lumenal narrowing. For the purposes of discussion, this will be called the “DIRECT INJURY HYPOTHESIS”.
Substitution of immune deficient and/or knockout mice as recipients into these schemes, has further clarified the nature of the immunological injury by isolating the contribution of humoral and cellular components [88,91]. The development of OA in combined immune deficient mice treated with repeated injections of exogenous anti-donor antibodies [88] nicely illustrates the importance of antibodies. Other models also clearly show a role for antibody-mediated damage [158,159]. However, OA also develops in B cell knockout mice that presumably are incapable of an antibody response [88,91], showing an important contribution by cellular immunity. Other insults that could contribute to the arterial injury or repair response include preservation/peri-operative ischemic injury [160,161]; cytomegalovirus infection [34,64]; altered mechanical or hemodynamic forces because of either decreased renal nephron mass [6], or immunologically-mediated lymphatic disruption [87]; and other well-known atherogenic insults such as hyperlipidemia, hypertension, diabetes and smoking.
Mechanisms of myofibroblast proliferation
Experimental animal models have also nicely shown that the initial arterial insult may be immunologic(antigen dependent), but the stereotypic repair response that follows does not appear to require the persistent influence of alloimmunity at some stage in its development(antigen independent) [89, 90, 92]. Thus, regardless of the source or mechanism of injury, the response of the vessel wall is the final common pathway for the development of disease and ultimately, organ dysfunction. This has led to a natural pre-occupation with understanding the molecular mechanisms of myofibroblast proliferation [162]. To study this response, a number of investigators use the isolated aortic allograft model, introduced by Häyry [19]. Although it lacks the organ parenchyma [92, 94, 163–166], this model offers a quick way of screening agents that might be of potential therapeutic benefit(Häyry, personal communication).
Research to date has implicated multiple and redundant signaling molecules that initiate and/or promote smooth muscle cell proliferation and fibrogenesis [19,97,162,167–171], similar to the situation observed with AS in the general population [172–175]. This includes growth factors, cytokines and chemokines [17,19,97,118,156,162,167–171,176–180]. For example, it has been shown that platelet-derived growth factor A and B [167,168,181], fibroblast growth factor [167,170,182], insulin-like growth factor-1 [162] and interleukin-1[162] can be released by myofibroblasts, endothelial and inflammatory cells into the arterial intima and all of them can stimulate smooth muscle cells to migrate to the site of injury and proliferate. Macrophages appear to be an important source of molecules causing damage, and initiating the repair response and fibrogenesis [17,97,118,126,154–156,176,177,183]. This helps explain the inhibition of OA by an essential fatty acid deficient diet, which interferes with leukocyte chemotaxis [97]. Transforming growth factor-beta 1 might importantly contribute to fibrosis [134,167,169,171,179]. Interleukin-6 might has been linked to the glomerular proliferative lesions in chronic renal allograft rejection [118]. The reader is referred to several excellent reviews for a more detailed accounting of this complex area [19,21,161,162,167,172–174,183,184].
Manifestations of chronic rejection in specific organs
Liver allografts
Chronic liver allograft rejection usually is not seen before 2 months [185] after transplantation and most frequently develops after: 1) an unresolved episode of acute rejection; 2) multiple episodes of acute rejection; or 3) indolently over a period of months to years, with few or no clinically apparent acute cellular rejection episodes [27]. Risk factors have already been discussed. Often the only reliable early indicator of CR is persistent and preferential elevation of γ-glutamyl transpeptidase and alkaline phosphatase [27, 186], which is related to bile duct damage and loss [26,27,69,71,73,187–191]. If clinical symptoms are present, they usually resemble those of acute rejection, until allograft dysfunction becomes severe enough to cause jaundice [26,27,69,71,73,187–191]. Biliary sludging or appearance of biliary strictures, hepatic infarcts, and finally loss of hepatic synthetic function, which can manifest as coagulopathy, malnutrition, and hepatosplenomegaly [27]are late findings presaging allograft failure.
Chronic liver allograft rejection is histopathologically defined by two main features: severe damage and loss of small (< 60 µm) bile ducts and, as in all other solid organs, OA [26,27,69,71,73,187–191]. Cases with either isolated bile duct loss or foam cell arteriopathy alone may occur, but usually both features are found together [72, 73]. Unfortunately, arteries with pathognomonic changes are rarely present in needle biopsy specimens, and therefore what has been observed about OA in the liver has come from examination of failed allografts removed at the time of re-transplantation. Therefore, in biopsy specimens, significant weight is usually placed on damage and loss of small bile ducts, but ductopenia can be non-specific finding that occasionally occurs as a result of non-rejection-related complications [192].
As with CR in other organs, the inflammation is often inconspicuous, consisting of lymphocytes, plasma cells and macrophages [116, 126, 193]. Despite a paucity of portal inflammation, intra-epithelial lymphocytes are located adjacent to degenerating biliary epithelial cells [26,27,69,71,73,187–191], which show uneven spacing of individual epithelial cells, eosinophilic transformation of the cytoplasm and ducts only partially lined by bile duct cells (fig. 3) [194]. The portal infiltrate is T cell predominant in CR and contains both CD4 and CD8 subsets [116,126,193], with the latter predominating in and around the biliary epithelium [193]. Increased γ-interferon, IL-2, granzyme B transcripts [126] have also been detected, the last of which strongly suggests that a cytolytic effector pathway of injury is involved. The damaged ducts can also show "inappropriate" expression of the major ABO blood group antigens [195], the significance of which is uncertain. The diagnosis of CR at these “earlier” stages [192, 194, 196–198] can be difficult. Review of previous biopsies and clinical correlation typically show a patient with documented acute rejection, that has progressed to chronic injury and prolonged liver dysfunction, which has not responded to appropriate anti-rejection therapy [27]. The diagnosis of CR can also be supported by selective hepatic angiography that shows pruning of the intrahepatic arteries with poor peripheral filling and segmental narrowing [26,191,199,200].
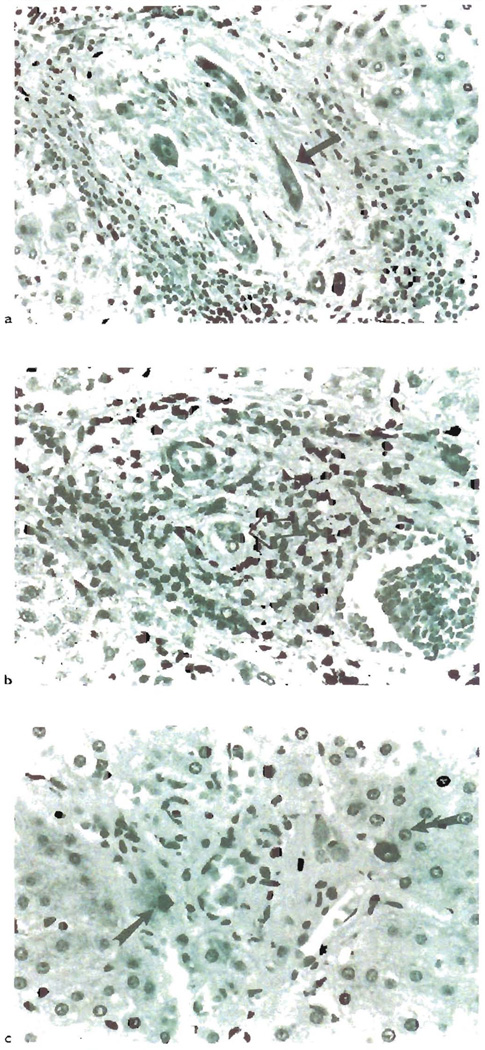
Fig. 3.
a) This section some of the most characteristic changes associated with the earlier phases of chronic liver allograft rejection. Note the “atrophic” appearance of the biliary epithelium(arrow). There is uneven spacing of the epithelial cells, eosinophilic transformation of the cytoplasm and local pyknosis. b) Interstingly, even though these duccal cells are “atrophic” appearing, they can show an increased proliferation rate, as determined by Ki-67 staining(arrow): c) Eventually, the small bile ducts are completely destroyed in chronic liver allograft rejection. Cytokeratin staining can be used to help identify the ducts, but it is usually not needed [288]. Whether these few periportal cytokeratin-19+ cells in this chronically rejected liver(arrows), represent progenitor cells capable of regenerating the ducts, is worthy of further study(see text).
Interestingly, despite the destruction of small ducts, a ductular reaction, or compensatory ductal proliferation in the marginal zone is not generally seen, unless the patient recovers. In such cases a “ductular reaction” has been described before clinical improvement [71,201,202]. Whether the recovery of ducts is related to proliferation of residual ductal epithelium, maturation of liver progenitor cells, or metaplasia of periportal hepatocytes, is a intriguing question about liver growth and development [129].
The portal tract connective tissue assumes a “scarred” appearance by routine light microscopy, which is related to a destruction of the peribiliary plexus [80]. An increase of extracellular matrix proteins fibronectin, tenascin, undulin, and collagen VI [134] have been described in the perivenular regions and this is associated with an accumulation of alpha-actin-positive cells and TGF-beta1-expressing macrophages in the areas [134].
Since the presence of foam cell arteriopathy can rarely be confirmed by a core needle biopsy, other "surrogate" features are often used to suggest the presence of OA. Features that suggest but do not prove the presence of foam cell arteriopathy include loss of arterioles and small (<20 µm) portal tract arteries, centrilobular hepatocellular swelling, perivenular sclerosis, and centrilobular hepatocyte dropout [26,27,69,71,73,187–191].
Lobular changes commonly seen in CR include centrilobular hepatocanalicular cholestasis, intra-sinusoidal foam cell clusters, mild spotty acidophilic necrosis of hepatocytes, centrilobular hepatocyte atrophy and/or ballooning and perivenular sclerosis [26,27,69,71,73,187–191]. The centrilobular degeneration and perivenular sclerosis may be related to either ischemia or damage from repeated bouts of “central venulitis” during acute rejection.
As with other organs, the diagnosis of CR is easier to establish in an explanted failed allograft. The presence of significant foam cell OA, should be seen in at least some of the muscular arteries in the hilum [26,27,69,71,73, 187–191]. Major hilar bile ducts may show sloughing of the epithelium, focal necrosis, intraluminal papillary hyperplasia of the epithelium, mural fibrosis and acute and chronic inflammation [203].
There are two fascinating aspects of chronic liver allograft rejection: its potential reversibility [71,202], which has already been discussed; and an appreciably lower incidence than other allografts (table 1, [51]). Experimental animal studies have also shown that a liver allograft can also protect other organs from the same donor from CR [87].
Resistance of liver allografts to CR can best be explained by the immunological theories of so called “hepatic tolerogenecity”. They can be broadly separated into two general categories, based on whether emphasis is placed on the parenchymal or non-parenchymal portion of the liver, which in turn, results in: 1) deletion or 2) functional anergy in the responding T cell repertoire. Release of soluble donor MHC class I antigen from the allograft, supporting the importance of the parenchyma, does not seem to be a tenable hypothesis [204]. Murine liver allografts are routinely accepted between strains of mice that show no difference between the class I loci, but are mismatched for class II [205]. In addition, fully allogeneic liver allografts from class I or II MHC deficient mice, which do not shed soluble MHC antigens [205,206], are also accepted. Moreover, other organs also secrete soluble MHC antigens [207] but they are routinely rejected, and infusion of exogenous soluble MHC usually leads to only slight graft prolongation.
One potential explanation for the importance of the parenchyma relies on the concept that allogeneic hepatocytes provide only one of two signals needed for allogeneic lymphocyte activation [208]. Lack of important co-stimulatory molecules is thought to result in the induction of anergy in the responding lymphocyte populations [208]. However, when transgenic mice that aberrantly express the allogeneic MHC class I molecule H-2Kb (Kb) in the liver, using a metallothionein promoter [209], are crossed with a strain that develops CD8+ T cells specific for the Kb molecule, lymphocyte-mediated “autoimmune” liver damage can be induced under certain conditions. Interestingly, most of the CD8+ cells responsive to Kb were eliminated by the intense intrahepatic activation, but some of the liver continued to show chronic low grade inflammation [209]. However, great care had to be taken to assure that alloantigen expression was limited to the liver [209]. Interpretation of these complicated experiments is difficult, but it appears that the liver may be able to effect an activation-induced partial clonal deletion of allogeneic lymphocytes [209], as originally proposed by Kamada [210]. This may represent a special circumstance, because alloantigen expression on other non-hematolymphoid cells such as pancreatic islets, does not lead to anergy [211], but to immunological “ignorance” [212], which is an important difference. Induction of true anergy may in fact, require presentation of antigen by professional antigen presenting cells.
It has been our contention that so called “hepatic tolerogenecity” is in large part, a result of hematolymphoid microchimerism [48,213,214] sustained by donor hematopoietic stem contained within the liver [215–217]. The immunologic mechanisms involved in graft acceptance is initially, a non-deletional, active process that appears to require the presence of donor APC [87,218]. In microchimeric recipients, the donor cells cause low grade proliferation and activation of the recipient immune system [51,87,218–220], including activation of B cells [219,221] and alterations in IL-4 production [222]. This in turn, leads to hyporesponsiveness to donor antigens and graft acceptance.
A recent series of investigations by Bishop et al [223–226] add credence to the viewpoint that hematolymphoid cell-induced activation of the recipient is important in liver graft tolerance. Comparing spontaneously accepting and rejecting rat strain combinations, they have shown that acceptance of the original liver allograft and subsequent grafts from the same donor, resides with a population of radio-sensitive hematolymphoid cells within the liver [226]. Destruction of the hematolymphoid cells by irradiation or blockage of the response by a short course of methylprednisolone reduces survival of subsequent donor strain skin grafts [226].
Heart
Obliterative arteriopathy is the major manifestation of CR in cardiac allografts. Unfortunately, cardiac allografts are also susceptible to AS that is endemic in the general population. This results in the frequent transmission of coronary artery disease from the donor to the recipient [56–58]. Thus, AS and OA both contribute to the arterial disease seen in allografts before and after cardiac transplantation [58,227–230].
Cardiac dysfunction associated with CR is largely because of ischemic damage to the heart. However, the classical symptoms of myocardial ischemia like angina pectoris, are thought not to be reliable indicators in the heart transplant recipient, since the allograft is denervated [3]. Nevertheless, in a series of 25 myocardial infarcts in heart transplant recipients [231], symptoms included chest pain (n=2), arm pain (n=3), weakness (n=16), dyspnea (n=11) and palpitations (n=8). Three episodes were clinically silent, detected only as new electrocardiographic changes during routine follow-up and two patients had old Q waves. Of the 8 patients hospitalized with symptoms, only 7 had typical Q-wave changes on electrocardiography; 5 had nonspecific ST-segment changes and 2 had no documented changes. Serial creatine phosphokinase levels were obtained in 13 patients, and values were elevated in 8. Other manifestations of CR and myocardial ischemia include cardiac enlargement with ventricular dilatation; loss of papillary muscle function and mitral regurgitation; ventricular wall dysfunction [3], constrictive pericarditis [232] and the development of congestive heart failure and/or cardiogenic shock, which can be misdiagnosed as infection [231].
One might expect that pre-existing coronary artery AS would accelerate the development of OA. Indeed, the use of older donors has been associated with a higher incidence of vascular abnormalities early after transplantation [57,229,233]. However, pre-existing donor artery disease does not necessarily impart an increased risk for the development of OA [57,234], which developed at different sites in the vessels. This suggests that at least some of the pathogenic mechanisms responsible for OA are different from those involved in AS. Clearly, more detailed investigation into the association between OA and AS is needed.
Some of the cardiac pathology can be detected by echocardiography or serial radionucleotide angiography before classic symptoms and signs of heart failure arise [3]. Although these methods of observation are less sensitive than histopathological examination of the entire allograft [235], they are more reliable than evaluation of endomyocardial biopsies, which can rarely detect OA (see below).
Gao et al. [236] originally classified the angiographic abnormalities into three categories: type A, discrete or tubular stenoses; type B, diffuse concentric narrowing; and type C, narrowed irregular vessels with occluded branches. Use of this classification or something similar, has proved to be a useful approach when applied to allograft coronary artery pathology. Type A lesions of the primary epicardial coronary vessels. with less frequent secondary branch involvement are most commonly encountered in non-allograft hearts. Not unexpectedly, the same type of lesions are detected in the allograft population shortly after transplantation, consistent with transmission of donor disease. However, type A lesions involving secondary and tertiary branches develop more frequently in allografts than in native hearts. In addition, type B and C lesions (diffuse narrowing, tapering and obliteration), are much more common in allograft coronary arteries than in native hearts and involve primary, secondary and tertiary branches [236]. Total vessel occlusion is found predominantly in the proximal or middle vessel segments of atherosclerotic disease, whereas distal involvement can be seen in up to one half of patients with OA. Failure to develop collateral vessels is also more common in allograft recipients with OA. Based on these observations, Gao et al. [236] concluded that coronary artery disease in transplant patients represents a mixture of typical AS and unique transplant-related progressive distal obliterative disease that occurs without collateral vessel development [236].
Several investigators have drawn the same conclusion using the more recently developed intravascular ultrasound [58,227–230]. This technique offers some advantages over angiography in terms of sensitivity [233,237,238] and the ability to more precisely characterize changes in coronary vessel shape and wall thickness [239], which enables one to follow the changes over time. In general, lesions frequently detected early after transplantation reflect donor AS in the general population: segmental and eccentric intimal thickening and lumenal narrowing, primarily involving the epicardial coronary arteries and their primary branches. Over time, more distal, diffuse, non-calcific and concentric intimal hyperplasia develops [58,228,230,238,240], which likely represents OA.
Serial intracoronary acetylcholine infusions and quantitative angiography have concluded that endothelial cell dysfunction is frequently observed in allograft coronary arteries and it often occurs early after transplantation [241,242]. A close association between endothelial dysfunction and intima abnormalities can be documented in some [241] but not all studies, presumably because of the episodic nature of the immune injury [227]. Other abnormalities include an impaired responsiveness of epicardial arteries to increased flow [242].
Except for rare cases [243], endomyocardial biopsies do not show changes that can be reliably used to make the diagnosis of CR [64,65,244–248]. Intimal thickening does not usually affect the small penetrating intra-cardiac arterioles present in endomyocardial biopsies [79], and collagen density in biopsy specimens does not appear to predictably increase with time in patients who develop CR [132]. There is however, some evidence that nodular sub-endocardial lymphocytic aggregates or Quilty lesions [244] may be associated with OA.
Quilty lesions are present in 4–25% of human endomyocardial allograft biopsies and are detected on at least one occasion in 58–78% of patients [246,247,249, 250]. Although they are not the equivalent of therapy-requiring acute rejection, some clinical studies show an association with acute rejection in the underlying myocardium [247, 251–254] or with OA [248]. Patients in the Stanford series with Quilty lesions who underwent transplantation in the early 1980’s had the highest incidence of graft vasculopathy [123], but more recently the incidence of OA has significantly decreased in these patients [123]. This perplexing observation might be explained by the fact that these patients had the most potential for improvement, particularly with the addition of new immunosuppressive regimens. For example, Tacrolimus has significantly lessened the incidence of Quilty lesions in Our heart transplant recipients [255] and significantly increased the half-life of kidney allografts [256], presumably by lessening CR.
Even though a causal association of Quilty lesions with OA is unlikely, these observations suggest that Quailty lesions might not be innocuous histopathological curiosities. Their presence in animal models with persistent alloactivation and developing OA [87] suggest that they serve as a signal of indolent ongoing immunological damage, which has the potential to contribute to the development of OA. Kemnitz et al. [253] offered an explanation for the possible association between Quilty lesions and OA by speculating that they may represent form of vascular rejection, since the heart develops embryologically from primitive vessels.
Consistent with an immune etiology [1,59,65,83,235, 245,257], it has been our experience in humans, that pure OA frequently involves both smaller intramyocardial arteries and epicardial coronaries and infrequently shows eccentrically situated and grummous atheromas and calcifications (fig. 4). In addition, the fibrosis in CR often extends along the connective tissue septal containing the affected arteries. This is in contrast to AS, that tends to show proximal epicardial coronary involvement by eccentric lesions with grummous atheromata and sparing of intramyocardial branches. OA can also clearly be shown to develop directly from Inflammatory and/or necrotizing arteritis is some cases [77,83,235], much like experimental animal models [85,87,95]. However, as already pointed out, even though OA involves both proximal and more distal portion of the arteries, OA is often not as diffuse or concentric as one might expect from a review of the literature [1,59,65,83,235,245,257]. In our experience, OA is often patchy in distribution (preferring branch points as epicardial vessels penetrate the myocardium), shows a significant adventitial components, can develop eccentrically, and frequently does not extend to the smallest arteries and arterioles present in endomyocardial biopsies [63, 79]. This is consistent with experimental animal models where the OA lesions are definitely a result of immune injury, but notoriously heterogeneous in distribution and severity [85,87,95]. In fact, the distribution is so heterogeneous that elaborate scoring systems are required to quantify the findings [85,87,95].
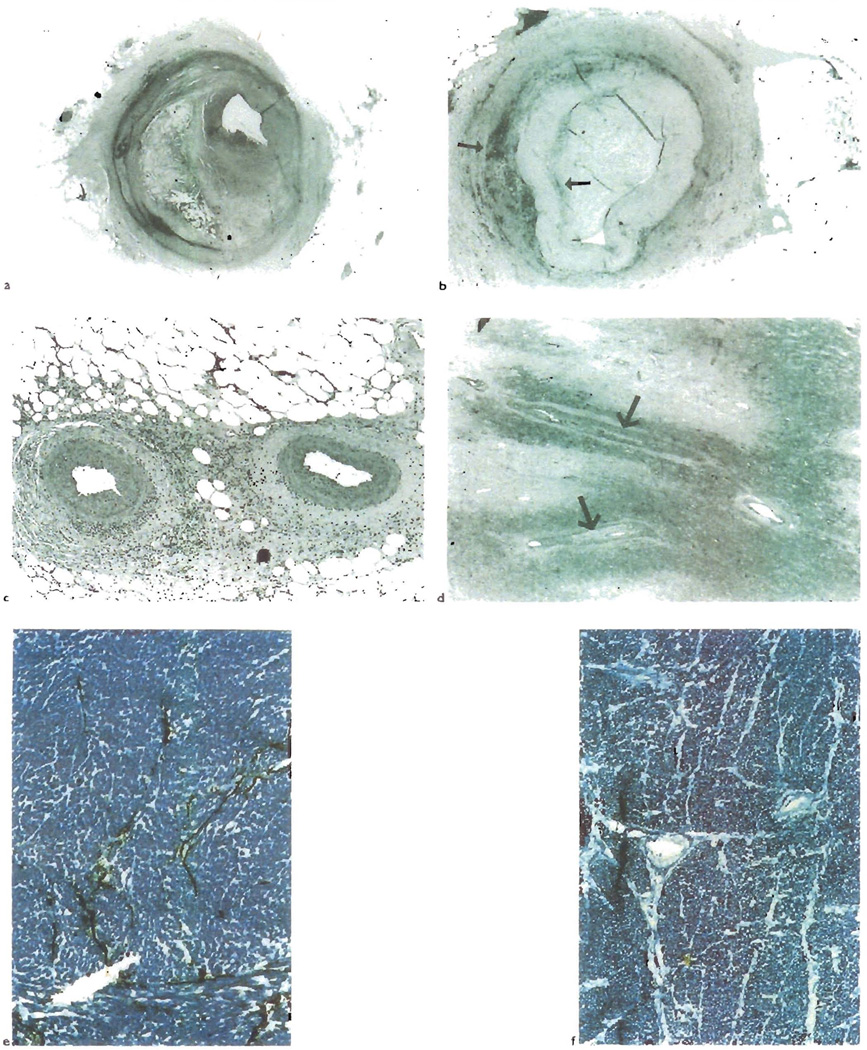
Fig. 4.
a) Atherosclerotic lesions involving the epicardial coronary arteries are frequently eccentric and show cholesterol clefts, as is seen in this lesion from a cardiac allograft. b) However, OA lesion can also have an eccentric distribution as shown here. Note the cuff of adventitial and deep intimal macrophages (arrows, CD68), similar to that seen in Figure 1 from a liver allograft. c) In contrast to acherosclerosis, OA often involves the distal branches of the epicardial coronary arteries and d) even those smaller intra-myocardial branches (arrows). There is also destruction of the lymphatic microvasculature of the heart in CR (e and f) Compare the lymphatic channels (black, histochemical stain for 5 nucleortidase) traversing the fibrous septae with arteries in a normal heart(e), with the lack of staining seen in cardiac allograft with CR(f)
However, in an individual lesion, the relative contribution of OA or AS may be difficult to determine. This is not surprising since atheromas are frequently inflamed with donor hematolymphoid cells and therefore would be more immunogenic than other constituents of the allograft. Conversely, McManus et al. [105,107,121] have shown the tendency for OA lesions to imbibe lipids. Thus, some histopathological studies of failed allografts and autopsy specimens have encountered some of the same difficulties as the imaging studies: Atherosclerosis and OA both contribute to the coronary artery disease seen in allografts. This might explain some of the more recent apparent inconsistencies in the distribution of OA noted by investigators using intravascular ultrasound [258].
Small intestine
Transplantation of the immunologically difficult small bowel, has only recently been clinically achieved to any significant extent [259–261]. This was largely because of ineffective control of acute rejection, which was made routinely possible by the use of FK506 (Tacrolimus) immunosuppression [262]. Thus, until recently, when the entire spectrum of human pathology was delineated [25], most of the CR changes small bowel allografts have relied on experimental animal models [139,140,263–265].
In experimental animals, CR of the small intestines often evolves from suboptimally controlled acute rejection [140]. It is characterized by OA, hyperplasia of the muscularis propria [266], apoptotic death of epithelial cells in the crypts of Lieberkuhn, villous blunting, focal fibrosis of the lamina propria, and destruction of donor mesenteric lymph nodes and the mucosal-associated lymphoid tissue(Peyer’s Patches) [140]. The last complication results in focal mucosal ulcers [139,263–267]. Although experience in humans is limited, detailed evaluation of the few chronically rejected human allografts [25] have shown the same set of histopathological changes as seen in the experimental animals (fig. 5).
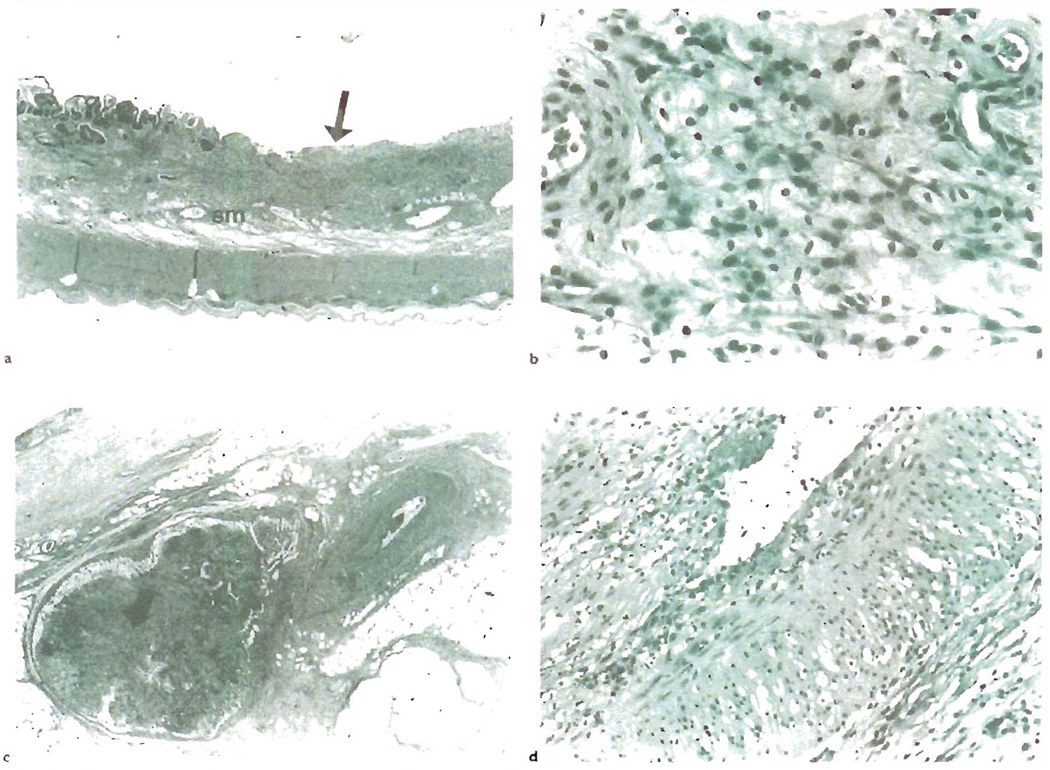
Fig. 5.
a) CR of human small bowel allograft shows focal, non-healing ulcers(arrow), as shown here, and thickening and fibrosis of the submucosa(sm). b) A higher magnification of the submucosa reveals abundant foam cell deposition, similar to that seen in the arterial intima of blood vessels with OA. c) Like other allografts, the arteries affected by OA are not commonly sampled in biopsies. This section shows a mesenteric artery with OA near a mesenteric lymph node with lymphold depletion and fibrosis(arrow). d) A higher magnification shows mild lymphocytic intimal inflammation, intimal foam cell deposition and endothelial cell hypertrophy. Note also the marked medial vacuolization, which is due to foam cell infiltration between myocytes and intercellular edema.
As with other allografts, the biopsy diagnosis of CR in humans is difficult. The vessels affected by OA, primarily the distal branches of the mesenteric arteries and the larger arteries of the serosa and muscularis propria, are not routinely sampled. Therefore, one is forced to rely on “surrogate” findings. These include focal, non-healing ulcers; a distinctive combination of regeneration and evident crypt epithelial apoprosis; and a disordered mucosal and villous architecture with fibrosis [25]. Typical clinical findings that support the diagnosis of CR include, persistent diarrhea with non-healing ulcers, repeated bouts of suboptimally controlled acute rejection; and either non-compliance with immunosuppressive medications or deliberate withdrawal of immunosuppression because of life-threatening infections [25]. Endoscopic and radiographic studies used to support the diagnosis of CR include focal loss of mucosal folds, focal ulcers and mural thickening, and pruning of the mesenteric arterial arcades, respectively.
Incompleteness of the direct injury model
In addition to immunological arterial injury, a number of clinical and experimental observations suggest that other factors contribute to the development of OA [6,16,51,63,70,82](table 2). The Direct Injury Model also does not address the immunological mechanisms responsible for persistent allograft injury. This is probably related to an incomplete understanding of the mechanisms of graft acceptance and a natural pre-occupation with the molecular mechanisms of the control of smooth muscle cell proliferation and fibrogenesis. In our opinion, one mechanical factor that should receive more attention is the destruction of organ-associated lymphoid tissues and lymphatic channels, which alone can cause arterial pathology. Similarly, destruction of the vasovasorum, could indirectly injure the wall of larger vessels and trigger a repair response [1]. Regardless, any hypothesis of OA pathogenesis will have to accommodate the inadequately explained findings listed in Table 2. In addition, it may be helpful to view CR as incomplete tolerance, by comparing recipients with CR to genetically-identical controls who are tolerant to the graft, instead of to isografts controls, as is commonly done in many experimental systems.
Table 2.
Observations not adequately explained by the Direct Injury Hypothesis (see text)
| Observation | Possible explanation(s) |
|---|---|
| Markers of severe acute rejection (alloantibodies or inflammatory arteritis), not always detected before development of OA. | -Severe acute rejection and inflammatory arteritis not required |
| Coronary arteries of composite heart-lung allograft recipients are less frequently and less severely affected than coronary arteries in isolated heart allografts (table 1). | -Mechanical factors involved -Antigen quality or quantity is of importance |
| Pulmonary arteries are less severely affected than coronary arteries in heart-lung transplant recipients [64] | -Mechanical factors involved -Antigenic or microenvironmental differences important |
| Inadequate explanation for progression of OA in absence of immunologic insult [17,89]. | -Initial injury triggers self-perpetuating repair response, perhaps driven by normal physiology |
| OA is not a feature of graft versus host disease | -Mechanical factors involved in organ removal and implantation important |
CR viewed as a “Incomplete Tolerance”
Implantation of any solid organ allograft results in a characteristic cycle of heightened immune activation, brought about the initial engagement of the donor and recipient lymphoid systems [48,214,218,268], followed by evolution toward a more stable relationship when immunosuppression can be considerably lowered or even withdrawn [269, 270]. In the past, this prototypic series of events was attributed to the presence of passenger leukocytes or donor hematolymphoid cells [271–273]. It was thought that they were essentially deleterious to allograft survival, and served only to directly sensitize the recipient [271–273]. When the donor cells were destroyed or died our, the donor/recipient immunological interface became less contentious. However, in some clinical [48,274–277] and experimental systems [87,278,279], the exact opposite was actually found: donor hematolymphoid cells persisted in successful long term allograft recipients, but were destroyed in recipients with CR. This phenomenon called “microchimerism” uncovered a paradox [218], resolution of which required a paradigm shift in transplantation immunobiology [214,218]. The new, Two-Way paradigm line of reasoning hypothesized that donor hematolymphoid cells are necessary, but alone, not sufficient for tolerance induction and thus, freedom from CR [48,87,213,214,218,278,279].
While attempting to study microchimerism, we inadvertently created a clinically relevant experimental animal model of CR [87,280]. In this model, genetically identical recipients were made CR-resistant or CR-prone by pretreatment with a donor liver allograft or bone marrow infusion, respectively [87], and then challenged with a cardiac allograft without immunosuppression. Both groups underwent a transient acute rejection within days after placement of the cardiac grates, but were operationally tolerant. The cardiac grafts were accepted and remain functional for more than 100 days. However, those pretreated with a bone marrow infusion were incompletely tolerant, because eventually they developed CR and OA, whereas those pretreated with a liver were resistant to CR.
A more in-depth analysis showed that the acute rejection crisis in the two groups could be separated on the basis of macrophage migration, cytokine production and alterations in MHC antigen expression. Acute rejection in the CR-prone animals was characterized by an influx and accumulation of recipient macrophages; persistent and strong upregulation of class II MHC antigens on the vascular endothelia; loss of intra- and extra-graft and donor hematolymphoid cells [87] and a cytokine mRNA response enriched in γ-interferon, IL-12 and macrophage activating chemokines(Murase, manuscript in preparation). In contrast, the strong upregulation of γ-interferon and IL-12 was not seen during acute rejection in the CR-resistant animals, even though there was evidence of graft inflammation by histology and immune activation by cytokine/chemokine mRNA analysis [87]. In addition, endothelial class II MHC expression in the CR-resistant recipients was weak and transient and donor hematolymphoid cells persisted in the graft and recipient lymphoid tissues.
Like other CR models, this one shows a clear link between CR and macrophage infiltration, which in turn, is likely related to the production of γ-interferon and other macrophage activating cytokines [97,177,180,267,281]. There is also evidence of persistent endothelial and smooth muscle cell activation, such as expression of MHC class II [87] antigens and adhesion molecules [17,85,97,114,115,118,155,156,163,176,241,282]. Unlike other models however, it shows that arterial remodeling starts to occurs during acute rejection before any intimal inflammation is seen. In addition, this model enables the investigator to compare and contrast completely tolerant recipients to those who are incompletely tolerant and develop CR. Major differences between these two groups are the persistence of donor hematolymphoid cells in the former, and heightened recipient macrophage influx in the latter [87]. Thus, our model more closely resembles the clinical cases that indolently develop CR.
The slower evolution of CR in some cases may be related to the mode of alloantigen presentation. As originally predicted by Batchelor [283], acute rejection is likely dominated by direct presentation of alloantigen by donor APCs, whereas CR may be primarily mediated via indirect alloantigen presentation by recipient APCs, consistent with the prominence of recipient APCs within the graft. Conversely, a bold and controversial hypothesis is that freedom from CR requires the persistence of donor hematolymphoid cells [48,87]. The idea is that to achieve tolerance, the donor immune system is required, since one of its normal physiological functions is to establish immunological boundaries, or the “self” [48,218]. Hopefully, the freedom from CR that is seen with augmentation of chimerism using harsh Conditioning regimens in experimental animals [284,285], will be realized in unconditioned human recipients as a lower incidence of CR [38,286,287]. Whether this line of reasoning is correct or not, only time will tell.
Acknowledgments
Supported by NIH I ROIDK49615-01 and the American Heart Association, Pennsylvania Affiliate
References
- 1.Billingham ME. Pathology of graft vascular disease after heart and heart-lung transplantation and its relationship to obliterative bronchiolitis. [Review][17 refs] Transplant Proc. 1995;27(3):2013–2016. [PubMed] [Google Scholar]
- 2.Cramer DV. Cardiac Graft Atherosclerosis. In: Paul LC, Solez K, editors. Organ Transplantation: long-term results. New York, NY: Marcel Dekker; 1992. pp. 173–195. [Google Scholar]
- 3.Balk AHMM, Weintar W. Chronic Heart Graft Rejection in the Clinical Setting. In: Paul LC, Solez K, editors. Organ Transplantation: long-term results. New York, NY: Marcel Dekker; 1992. pp. 187–195. [Google Scholar]
- 4.Berry GJ, Rizeq MN, Weiss LM, Billingham ME. Graft coronary disease in pediatric heart and combined heart-lung transplant recipients: a study of fifteen cases. Journal of Heart & Lung Transplantation. 1993;12(6 Pt 2):S309–S319. [PubMed] [Google Scholar]
- 5.Radley-Smith RC, Burke M, Pomerance A, Yacoub MH. Graft vessell disease and obliterative bronchiolitis after heart/lung transplantation in children. Transplant Proceed. 1995;27:2017–2018. [PubMed] [Google Scholar]
- 6.Mackenzie HS, Tullios SG, Heemann UW, et al. Nephron supply is a major determinant of long-term renal allograft outcome in rats. J Clin Invest. 1994;94(5):2148–2152. doi: 10.1172/JCI117571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahlers T, Haverich A, Schafers HJ, et al. Chronic rejection following lung transplantation. Incidence, time pattern and consequences. European Journal of Cardio Thoracic Surgery. 1993;7(6):319–323. doi: 10.1016/1010-7940(93)90174-a. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead B, Rees P, Sorensen K, et al. Incidence of obliterative bronchiolitis after heart-lung transplantation in children. Journal of Heart & Lung Transplantation. 1993;12(6 Pt 1):903–908. [PubMed] [Google Scholar]
- 9.Wiesner RH, Ludwig J, Van Hoek B, Krom RAF. Chronic Hepatic Allograft Rejection: a review of duclopenic rejection and the vanishing bile duct syndrome. In: Paul LC, Solez K, editors. Organ Transplantation: long-term results. New York, N.Y.: Marcel Dekker; 1992. pp. 197–216. [Google Scholar]
- 10.Wiesner RH, Ludwig J, van Hoek B, Krom RA. Current concepts in cell-mediated hepatic allograft rejection leading to ductopenia and liver failure. [Review] Hepatology. 1991;14(4 pt 1):721–729. doi: 10.1016/0270-9139(91)90064-3. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland DER, Moudry-Munn K, Gillingham K. Long-Term Outcome of Pancreas Transplants Functioning at One Year. In: Paul LC, Solez K, editors. Organ Transplantation: long-term results. New York, N.Y.: Marcel Dekker; 1992. pp. 217–246. [Google Scholar]
- 12.Hume DM, Merrill JP, Miller BF, Thom GW. Experience with renal homo-transplantation in the human: Report of nine cases. J Clin Invest. 1955;34:327–383. doi: 10.1172/JCI103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter KA, Thomson WB, Owen K, Kenyon JR, Mobray JF, Peart WS. Obliterative vascular changes in four human kidney homotransplants. British Medical Journal. 1963;2:639. doi: 10.1136/bmj.2.5358.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veith FJ, Montefusco CM, Blumcke S, Hagstrom JW. Long-term fate of lung autografts charged with providing total pulmonary function. I. Light and electron microscopic studies. Ann Surg. 1979;190(5):648–653. doi: 10.1097/00000658-197911000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norin AJ, Goodell EM, Kamholz SL, Veith FJ, Blumenstock DA. Immunologic, morphologic, and functional evaluation of long-term-surviving beagle lung allograft recipients treated with lethal total-body irradiation, autologous bone marrow, and metholrexate. Transplantation. 1987;44(2):179–184. doi: 10.1097/00007890-198708000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Tullius SG, Heemann U, Hancock WW, Azuma H, Tilney NL. Long-term kidney isografts develop functional and morphologic changes that mimic those of chronic allograft rejection. Ann Surg. 1994;220(4):425–432. doi: 10.1097/00000658-199410000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadeau KC, Azuma H, Tilney NL. Sequential cytokine dynamics in chronic rejection of rat renal allografts: roles for cytokines RANTES and MCP-I. PNAS. 1995;92(19):8729–8733. doi: 10.1073/pnas.92.19.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anonymous. Randomised trial comparing racrolimus (FK506) and cyclosprin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group. [see comments] Lancet. 1994;344(8920):423–428. [PubMed] [Google Scholar]
- 19.Harry P, Mennander A, Yilmaz S, et al. Towards understanding the pathophysiology of chronic rejection. [Review] Clinical Investigator. 1992;70(9):780–790. doi: 10.1007/BF00180748. [DOI] [PubMed] [Google Scholar]
- 20.Matas A. Chronic rejection in renal transplant recipients--risk factors and correlates. Clinical Transplantation. 1994;8(3 Pt 2):332–335. [PubMed] [Google Scholar]
- 21.Matas AJ, Burke JF, Jr, Devault GA, Jr, Monnco A, Pirsch JD. Chronic rejection. [Review] Journal of the American Society of Nephrology. 1994;4(8 Suppl):S23–S29. doi: 10.1681/ASN.V48s23. [DOI] [PubMed] [Google Scholar]
- 22.Griffith BP, Hardesty RL, Armitage JM, et al. A decade of lung transplantation. Ann Surg. 1993;218(3):310–318. doi: 10.1097/00000658-199309000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tejani A, Corles L, Stablein D. Clinical correlates of chronic rejection in pediatric renal transplantation. A report of the North American Pediatric Renal Transplant Cooperative study. Transplantation. 1996;61(7):1054–1058. doi: 10.1097/00007890-199604150-00011. [DOI] [PubMed] [Google Scholar]
- 24.Candinas D, Gunson BK, Nightingale P, Hubscher S, McMaster P, Neuberger JM. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet. 1995;346(8983):1117–1121. doi: 10.1016/s0140-6736(95)91797-7. [DOI] [PubMed] [Google Scholar]
- 25.Lee RG, Nakamura K, Tsamandas AC, et al. Pathology of human intestinal transplantation. [see comments] Gastroenterology. 1996;110(6):1820–1834. doi: 10.1053/gast.1996.v110.pm8964408. [DOI] [PubMed] [Google Scholar]
- 26.Lowes J, Hubscher S, Neuberger J. Chronic rejection of the liver allograft. Gastroenterology Clinics of North America. 1993;22:401–420. [PubMed] [Google Scholar]
- 27.Panel IW. Terminology for hepatic allograft rejection. Hepatology. 1995;22:648–654. [PubMed] [Google Scholar]
- 28.Jarcho J, Naftel DC, Shroyer TW, et al. Influence of HLA mismatch on rejection after heart transplantation: a multiinstitutional study. The Cardiac Transplant Research Database Group. Journal of Heart & Lung Transplantation. 1994;13(4):583–595. [PubMed] [Google Scholar]
- 29.Katznelson S, Gjectson DW, Cecka JM. The effect of race and ethnicity on kidney allograft outcome. Clinical Transplants. 1995:379–394. [PubMed] [Google Scholar]
- 30.Devlin JJ, JG OG, Tan KC, Calne RY, Williams R. Ethnic variations in patient and graft survival after liver transplantation. Identification of new risk factor for chronic allograft rejection. Transplantation. 1993;56(6):1381–1384. doi: 10.1097/00007890-199312000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Held PJ, Kahan BD, Hunsicker LG, et al. The impact of HLA mismatches on the survival of first cadaveric kidney transplants. [see comments] New England Journal of Medicine. 1994;331(12):765–770. doi: 10.1056/NEJM199409223311203. [DOI] [PubMed] [Google Scholar]
- 32.Koyama H, Cecka JM, Terasaki PI. Kidney transplants in black recipients. HLA matching and other factors affecting long-term graft survival. Transplantation. 1994;57(7):1064–1068. [PubMed] [Google Scholar]
- 33.Mehra MR, Stapleton DD, Ventura HO, et al. Influence of donor and recipient gender on cardiac allograft vasculopathy. An intravascular ultrasound study. Circulation. 1994;90(5 Pt 2):1178–1182. [PubMed] [Google Scholar]
- 34.Gratian MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261(24):3561–3566. [PubMed] [Google Scholar]
- 35.Keenan RJ, Lega ME, Dummer JS, et al. Cytomegalovirus serologic status and postoperative infection correlated with risk of developing chronic rejection after pulmonary transplantation. Transplantation. 1991;51(2):433–438. doi: 10.1097/00007890-199102000-00032. [DOI] [PubMed] [Google Scholar]
- 36.Jakel KT, Loning T, Arndt R, Rodiger W. Rejection, herpesvirus infection, and Ki-67 expression in endomyocardial biopsy specimens from heart transplant recipients. Pathology, Research & Practice. 1992;188((1–2)):27–36. doi: 10.1016/s0344-0338(11)81152-0. [DOI] [PubMed] [Google Scholar]
- 37.Manez R, White LT, Linden P, et al. The influence of HLA matching on cytomegalovirus hepatinis and chromic rejection after liver transplantation. Transplantation. 1993;55(5):1067–1071. doi: 10.1097/00007890-199305000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeevi A, Pavlick M, Lombardozzi S, et al. Immune status of recipients following bone marrow-augmented solid organ transplantation. Transplantation. 1995;59(4):616–620. doi: 10.1097/00007890-199502270-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajbat B, Missov E, Serre I, Balder P, Chaptal PA. Short-term development of transplant-related coronary artery disease in orthotopic cardiac allograft recipients. Minerva Cardioangiologica. 1995;43(10):435–438. [PubMed] [Google Scholar]
- 40.Siddiqui MT, Garrity ER, Husain AN. Bronchiolitis obliterans organizing pneumonia-like reactions: a nonspecific response or an atypical form of rejection or infection in lung allograft recipients? Hum Pathol. 1996;27(7):714–719. doi: 10.1016/s0046-8177(96)90403-7. [DOI] [PubMed] [Google Scholar]
- 41.Lautenschlager I, Hockerstedt K, Jalanko H, et al. Persistent Cytomega-lovirus In Liver Allografts With Chronic Rejection. Hepatology. 1997;25(1):190–194. doi: 10.1053/jhep.1997.v25.pm0008985289. [DOI] [PubMed] [Google Scholar]
- 42.Feray C, Samuel D, Gigou M, et al. An open trial of interferon alfa recombinant for hepatitis C after liver transplantation: antiviral effects and risk of rejection. Hepatology. 1995;22(4 Pt 1):1084–1089. [PubMed] [Google Scholar]
- 43.Doxiadis II, Smits JM, Schrender GM, et al. Association between specific HLA combinations and probability of kidney allograft loss: the taboo concept. Lancet. 1996;348(9031):850–853. doi: 10.1016/s0140-6736(96)02296-9. [DOI] [PubMed] [Google Scholar]
- 44.Kerman RH, Kimball PM, Lindholm A, et al. Influence of HLA matching on rejections and short- and long-term primary cadaveric allograft survival. Transplantation. 1993;56(5):1242–1247. doi: 10.1097/00007890-199311000-00037. [DOI] [PubMed] [Google Scholar]
- 45.Matas AJ, Burke JF, Jr, DeVault GA, Jr, Monaco A, Pirsch JD. Chronic rejection. [Review][51 ref] Journal of the American Society of Nephrology. 1994;4(8 Suppl):S23–S29. doi: 10.1681/ASN.V48s23. [DOI] [PubMed] [Google Scholar]
- 46.Zhou YC, Cecka JM. Effect of HLA matching on renal transplant survival. Clinical Transplants. 1993:499–510. [PubMed] [Google Scholar]
- 47.Nikaein A, Backman L, Jennings L, et al. HLA compatibility and liver transplant outcome. Improved patient survival by HLA and cross-matching. [see comments] Transplantation. 1994;58(7):786–792. [PubMed] [Google Scholar]
- 48.Starzl TE, Demetris AJ, Murase N, IIdstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. [see comments] [Review] Lancet. 1992;339(8809):1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starzl TF, Rao AS, Trucco M, Fontes P, Fung JJ, Demetris AJ. Explanation for loss of the HLA matching effect. [Review][16 refs] Transplant proc. 1995;27(1):57–60. [PMC free article] [PubMed] [Google Scholar]
- 50.Starzl TE, Trucco M, Rao A, Demetris A. The role of HLA typing in clinical kidney transplants: 30 years later. The blind-folding of HLA matching. [editorial] Clinical Transplants. 1993;423 [PMC free article] [PubMed] [Google Scholar]
- 51.Demetris AJ, Murase N, Delancy CP, Woan M, Fung JJ, Starzl TE. The liver allograft, chronic (ductopenic) rejection, and microchimerism: What can they teach us? Transplant Proc. 1995;27:67–70. [PMC free article] [PubMed] [Google Scholar]
- 52.Takemoto S, Cecka JM, Gjerison DW, Terasaki PI. Six-antigen-matched transplants. Causes of failure. Transplantation. 1993;55(5):1005–1008. doi: 10.1097/00007890-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Baltzan MA, Shoker AS, Baltzan RB, George D. HLA-identify--long-term renal graft survival, acute vascular, chronic vascular, and acute interstilial rejection. Transplantation. 1996;61(6):881–885. doi: 10.1097/00007890-199603270-00006. [DOI] [PubMed] [Google Scholar]
- 54.Markus BH, Duquesnoy RJ, Gordon RD, et al. Histocompatibility and liver transplant outcome. Does HLA exert a dualistic effect? Transplantation. 1988;46(3):372–377. [PMC free article] [PubMed] [Google Scholar]
- 55.Donaldson P, Underhill J, Doherty D, et al. Influence of Human leukocyte antigen matching on liver allograft survival and rejection: “the dualistic effect”. Hepatology. 1993;17(6):1008–1015. [PubMed] [Google Scholar]
- 56.Tuzcu EM, Hobbs RE, Rincon G, et al. Occult and frequent transmission of atherosclerotic coronary disease with cardiac transplantation. Insights front intravascular ultrasound. Circulation. 1995;91(6):1706–1713. doi: 10.1161/01.cir.91.6.1706. [DOI] [PubMed] [Google Scholar]
- 57.Botas J, Pinto FJ, Chenzbraun A, et al. Influence of preexistent donor coronary artery disease on the progression of transplant vasculopathy. An intravascular ultrasound study. Circulation. 1995;92(5):1126–1132. doi: 10.1161/01.cir.92.5.1126. [DOI] [PubMed] [Google Scholar]
- 58.Klauss V, Ackermann K, Spes CH, et al. Coronary Plaque Morphologic Characteristics Early and Late After Heart Transplantation – In Vivo Analysis With Intravascular Ultrasonography. American Heart Journal. 1997;133(1):29–35. doi: 10.1016/s0002-8703(97)70244-8. [DOI] [PubMed] [Google Scholar]
- 59.Winters GL, Kendall TJ, Radio SJ, et al. Posttransplant obesity and hyperlipidemia: major predictors of severity of coronary arteriopathy in failed human heart allografts. Journal of Heart Transplantation. 1990;9(4):364–371. [PubMed] [Google Scholar]
- 60.Vollmer E, Bosse A, Bogeholz J, et al. Apolipoproteins and immunohistological differentiation of cells in the arterial wall of kidneys in transplant arteriopathy. Morphological parallels with atherosclerosis. Pathology, Research & Practice. 1991;187(8):957–962. doi: 10.1016/S0344-0338(11)81067-8. [DOI] [PubMed] [Google Scholar]
- 61.Raisanen-Sokolowski A, Tilly-Kiesi M, Ustinov J, et al. Hyperlipidemia accelerates allograft arteriosclerosis (chronic rejection) in the rat. Arteriosclerosis & Thrombosis. 1994;14(12):2032–2042. doi: 10.1161/01.atv.14.12.2032. [DOI] [PubMed] [Google Scholar]
- 62.Gao SZ, Schroeder JS, Alderman EL, et al. Clinical and laboratory correlates of accelerated coronary artery disease in the cardiac transplant patient. Circulation. 1987;76(5 Pt 2):V56–V61. [PubMed] [Google Scholar]
- 63.Demetris AJ, Zerbe T, Banner B. Morphology of solid organ allograft arteriopathy: identification of proliferating intimal cell populations. Transplant Proc. 1989;21(4):3667–3669. [PubMed] [Google Scholar]
- 64.Billingham ME. Pathology and etiology of chronic rejection of the heart. Clinical Transplantation. 1994;8(3 Pt 2):289–292. [PubMed] [Google Scholar]
- 65.Tazelaar HD, Edwards WD. Pathology of cardiac transplantation: recipient hearts (chronic heart failure) and donor hearts (acute and chronic rejection). [Review] Mayo Clinic Proceedings. 1992;67(7):685–696. doi: 10.1016/s0025-6196(12)60726-5. [DOI] [PubMed] [Google Scholar]
- 66.Yousem SA, Paradis IL, Dauber JH, et al. Pulmonary arteriosclerosis in long-term human heart-lung transplant recipients. Transplantation. 1989;47:564–569. [PubMed] [Google Scholar]
- 67.Sarris GE, Smith JA, Shomway NE, et al. Long-term results of combined heart-lung transplantation: the Stanford experience. Journal of Heart & Lung Transplantation. 1994;13(6):940–949. [PubMed] [Google Scholar]
- 68.Cagle PT, Brown RW, Frost A, Kellar C, Yousem SA. Diagnosis of chronic lung transplant rejection by transbronchial biopsy. Mod Pathol. 1995;8(2):137–142. [PubMed] [Google Scholar]
- 69.Oguma S, Belle S, Starzl TE, Demetris AJ. A histometric analysis of chronically rejected human liver allografts: insights into the mechanisms of bile duct loss: direct immunologic and ischemic factors. Hepatology. 1989;9(2):204–209. doi: 10.1002/hep.1840090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oguma S, Zerbe T, Banner B, Belle S, Starzl TE, Demetris AJ. Chronic liver allograft rejection and obliterative arteriopathy: possible pathogenic mechanisms. Transplant Proc. 1989;21(1 Pt 2):2203–2207. [PMC free article] [PubMed] [Google Scholar]
- 71.Freese DK, Snover DC, Sharp HL, Gross CR, Savick SK, Payne WD. Chronic rejection after liver transplantation: a study of clinical, histological and immunological features. Hepatology. 1991;13:882–891. [PubMed] [Google Scholar]
- 72.Deligeorgi-Politi H, Wight DG, Calne RY, White DG. Chronic rejection of liver transplants revisited [published erratum appears in Transpl Int. 1995, 8(2): 163] Transplant Int. 1994;7(6):442–447. doi: 10.1007/BF00346040. [DOI] [PubMed] [Google Scholar]
- 73.Wight DA. Chronic liver transplant rejection: definition and diagnosis. Transplant Proc. 1996;28(1):465–467. [PubMed] [Google Scholar]
- 74.Radio S, Wood S, Wilson J, Lin H, Winters G, McManus B. Allograft vascular disease: comparison of heart and other grafted organs. [Review] Transplant Proc. 1996;28(1):496–499. [PubMed] [Google Scholar]
- 75.Sibley RK. Morphologic features or chronic rejection in kidney and less commonly transplanted organs. Clinical Transplantation. 1994;8(3 Pt 2):293–298. [PubMed] [Google Scholar]
- 76.Lin H, Wilson JE, Kendall TJ, et al. Comparable proximal and distal severity of intimal thickening and size of epicardial coronary arteries in transplant arteriopathy of human cardiac allografts. Journal of Heart & Lung Transplantation. 1994;13(5):824–833. [PubMed] [Google Scholar]
- 77.Davies H, al-Tikriti S. Coronary arterial pathology in the transplanted human heart. [see comments] International Journal of Cardiology. 1989;25(1):99–117. doi: 10.1016/0167-5273(89)90169-1. [DOI] [PubMed] [Google Scholar]
- 78.Loire R, Tabib A, Roux N. Late anatomical lesions after cardiac transplantation. Study of 44 autopsies beyond 6-months survival. [French] Annales de Cardiologie et d Angeiologie. 1993;42(9):452–459. [PubMed] [Google Scholar]
- 79.Boyle JJ, Lawrie G, McPhaden AR, Richens D, Lindop GB. Arterial lesions associated with medial disorganization and fibrosis in endomyocardial biopsies from human cardiac allografts. Histopathology. 1995;27(5):439–444. doi: 10.1111/j.1365-2559.1995.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto Y, McCaughan GW, Painter DM, Bishop GA. Evidence that portal tract microvascular destruction precedes bile duct loss in human liver allografts rejection. Transplantation. 1993;56(1):69–75. doi: 10.1097/00007890-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 81.Porter K. Renal Transplantation. In: Heptinstall RH, editor. Pathology of the Kidney. 4th ed. vol III. Boston: Little, Brown and Company; 1992. pp. 1799–l934. [Google Scholar]
- 82.Oguma S, Banner B, Zerbe T, Starzl T, Demetris AJ. Participation of dendritic cells in vascular lesions of chronic rejection of human allografts. Lancet. 1988;2(8617):933–936. doi: 10.1016/s0140-6736(88)92600-1. [DOI] [PubMed] [Google Scholar]
- 83.Hruban RH, Beschorner WE, Baumgartner WA, et al. Accelerated arteriosclerosis in heart transplant recipients is associated with a T-lymphocyte-mediated endothelialitis. Amer J Pathol. 1990;137(4):871–882. [PMC free article] [PubMed] [Google Scholar]
- 84.Gouldesbrough DR, Axelsen RA. Arterial endothelialitis in chronic renal allograft rejection: a histopathologicol and immonocytochemical study. [see comment] Nephrology, Dialysis, Transplantation. 1994;9(1):35–40. [PubMed] [Google Scholar]
- 85.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. I. Time course and immunogenetic and immunopathological considerations. Amer J Pathol. 1994;144(2):260–274. [PMC free article] [PubMed] [Google Scholar]
- 86.Dong C, Wilson JE, Winters GL, McManus BM. Human transplant coronary artery disease: pathological evidence for Fas-mediated apoptotic cytotoxicty in allograft arteriopathy. Laboratory Investigation. 1996;74(5):921–931. [PubMed] [Google Scholar]
- 87.Demetris AJ, Murase N, Ye Q, et al. An analysis of chronic rejection and obliterative arteriopathy: possible contributions of donor antigen presenting cells and lymphatic disruption. Amer J Pathol. 1997;150:563–578. [PMC free article] [PubMed] [Google Scholar]
- 88.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary artherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994;152(10):5135–5141. [PubMed] [Google Scholar]
- 89.Tullius SG, Haocock WW, Heemann U, Azuma H, Tilney NL. Reversibility of chronic renal allograft rejection. Critical effect of time after transplantation suggests both host immune dependent and independent phases of progressive injury. Transplantation. 1994;58(1):93–99. [PubMed] [Google Scholar]
- 90.Schmid C, Heemann U, Tilney NL. Retransplantation reverses monounclear infiltration but not myointimal proliferation in a rat model of chronic cardiac allograft rejection. Transplantation. 1996;61(12):1695–1699. doi: 10.1097/00007890-199606270-00005. [DOI] [PubMed] [Google Scholar]
- 91.Russell PS, Chase CM, Colvin RB. Contributions of cellular and humoral immunity to arteriopathic lesions in transplanted mouse hearts. In: Paul LC, Foegh M, editors. Sixth International Alexis Carrel Conference on Graft Atherosclerosis and Chronic Rejection; Banff, Canada. 1996. [Google Scholar]
- 92.Mennander A, Hayry P. Reversibility of allograft arteriosclerosis after re-transplantation to donor strain. Transplantation. 1996;62(4):526–529. doi: 10.1097/00007890-199608270-00016. [DOI] [PubMed] [Google Scholar]
- 93.Mennander A, Paavonen T, Hayry P. Intimal thickening and medial necrosis in allograft arteriosclerosis (chronic rejection) are independently regulated. Arteriosclerosis & Thrombosis. 1993;13(7):1019–1025. doi: 10.1161/01.atv.13.7.1019. [DOI] [PubMed] [Google Scholar]
- 94.Plissonnier D, Nochy D, Poncet P, et al. Sequential immunological targeting of chronic experimental arterial allograft. Transplantation. 1995;60(5):414–424. doi: 10.1097/00007890-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 95.Adams DH, Tilney NL, Collins JJ, Karnovsky MJ. Experimental Graft Atherosclerosis. I. The Lewis-to-F344 model. Transplantation. 1992;53:1115–1119. [PubMed] [Google Scholar]
- 96.Adams DH, Wyner LR, Karnovsky MJ. Experimental graft atherosclerosis. II Immunocytochemical analysis of lesion development. Transplantation. 1993;56:794–799. doi: 10.1097/00007890-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 97.Adams DH, Russell ME, Hancock WW, Sayegh MH, Wyner LR, Karnovsky MJ. Chronic rejection in experimental cardiac transplantation: studies in the Lewis-F344 model. [Review] Immunol Rev. 1993;134:5–19. doi: 10.1111/j.1600-065x.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 98.Cramer DV, Oian S, Harnaha J, Chapman FA, Starzl TE, Makowka L. Accelerated graft arteriosclerosis is enhanced by sensitization of the recipient to donor lymphocytes. Transplant Proc. 1989;21(4):3714–3715. [PMC free article] [PubMed] [Google Scholar]
- 99.Cramer DV, Qian SQ, Harnaha J, et al. Cardiac transplantation in the rat: I. The effect of histocompatibility differences on graft arteriosclerosis. Transplantation. 1989;47(3):414–419. doi: 10.1097/00007890-198903000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paul LC, Hayry P, Foegh M, et al. Diagnostic criteria for chronic rejection/accelerated graft atherosclerosis in heart and kidney transplants: joint proposal from the Fourth Alexis Carrel Conference on Chronic Rejection and Accelerated Arteriosclerosis in Transplanted Organs. [Review] Transplant Proc. 1993;25(2):2022–2023. [PubMed] [Google Scholar]
- 101.Foerster A. Vascular rejection in cardiac transplantation. A morphological study of 25 human cardiac allografts. Apnus. 1992;100(4):367–376. [PubMed] [Google Scholar]
- 102.Duquesnoy RJ, Mutase N, Suzow J, et al. Evidence for heat shock protein immunity in a rat cardiac allograft model of chronic rejection. Journal of Heart Lung Transplantation. 1996;15:S63. doi: 10.1097/00007890-199901150-00026. [DOI] [PubMed] [Google Scholar]
- 103.Muller-Hermelink HK, Dammrich JR. Obliterative transplant vasculopathy: pathogenesis and pathologie mechanisms. Verhandlungender Deutschen Gesellschaft fur Pathologie. 1989;73:193–206. [PubMed] [Google Scholar]
- 104.Duquesnoy RJ, Kaufman C, Zerbe TR, Woan MC, Zeevi A. Presence of CD4, CD8 double-negative and T cell receptor-gamma-delta-positive T cells in lymphocyte cultures propagated from coronary arteries from heart transplant patients with graft coronary disease. J Heart Lung Transplant. 1992;11(3 Pt 2):S83–S86. [PubMed] [Google Scholar]
- 105.McManus BM, Malcom G, Kendall TJ, et al. Lipid overload and proteoglycan expression in chronic rejection of the human transplanted heart. Clinical Transplantation. 1994;8(3 Pt 2):336–340. [PubMed] [Google Scholar]
- 106.Lin H, Wilson JE, Roberts CR, et al. Biglycan, Decorin, and Versican Protein Expression Patterns In Coronary Arteriopathy Of Human Cardiac Allografts – Distinctness As Compared to Native Atherosclerosis. Journal of Heart & Lung Transplantation. 1996;15(12):1233–1247. [PubMed] [Google Scholar]
- 107.Lin H, Ignatescu M, Wilson JE, et al. Prominence Of Apolipoproteins, B (a), and E In the Intimae Of Coronary Arteries In Transplanted Human Hearts – Geographic Relationship to Vessel Wall Proteoglycans. Journal of Heart & Lung Transplantation. 1996;15(12):1223–1232. [PubMed] [Google Scholar]
- 108.Liu G, Butany J, Wanless IR, Cameron R, Greig P, Levy G. The vascular pathology of human hepatic allografts. Hum Pathol. 1993;24(2):182–188. doi: 10.1016/0046-8177(93)90298-u. [DOI] [PubMed] [Google Scholar]
- 109.Nolan CR, Saenz KP, Thomas CAr, Murphy KD. Role of the eosinophil in chronic vascular rejection of renal allografts. Amer J Kid Dis. 1995;26(4):634–642. doi: 10.1016/0272-6386(95)90601-0. [DOI] [PubMed] [Google Scholar]
- 110.Ross R. Rous-Whipple Award Lecture. Atherosclerosis: a defense mechanism gone away. [Review] (139 refs) Amer J Pathol. 1993;143(4):987–1002. [PMC free article] [PubMed] [Google Scholar]
- 111.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. [Review] [127 refs] Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 112.Ross R. Cell biology of atherosclerosis. [Review][58 refs] Annual Review of Physiology. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 113.Dong C, Redenbach D, Wood S, Battistini B, Wilson JE, McManus BM. The pathogenesis of cardiac allograft vasculopathy. [Review][54 refs] Current Opinion in Cardiology. 1996;11(2):183–190. doi: 10.1097/00001573-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 114.Orosz CG. Endothelial activation and chronic allograft rejection. Clinical Transplantation. 1994;8(3 Pt 2):299–303. [PubMed] [Google Scholar]
- 115.Orosz CG. Local cellular immunology of experimental transplant vascular sclerosis. [Review] Clinical Transplantation. 1996;10(1 Pt 2):100–103. [PubMed] [Google Scholar]
- 116.Demetris AJ, Lasky S, Thiel DHV, Starzl TE, Whiteside T. Induction of DR/IA antigens in human liver allografts: An immunocytochemical and clinico-pathologic analysis of twenty failed grafts. Transplantation. 1985;40:504–509. doi: 10.1097/00007890-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qiao J, Ruan X, Tento A, Czer LSC, Blanche C, Fishbein MC. Expression of cell adhesion molecules in human cardiac allograft rejection. J Heart Lung Transplant. 1992;11:920–925. [PubMed] [Google Scholar]
- 118.Hancock WH, Whitley WD, Tullius SG, et al. Cytokines, adhesion molecules, and the pathogenesis of chronic rejection of rat renal allografts. Transplantation. 1993;56(3):643–650. doi: 10.1097/00007890-199309000-00028. [DOI] [PubMed] [Google Scholar]
- 119.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. III. Effects of recipient treatment with a monoclonal antibody to interferon-gamma. Transplantation. 1994;57(9):1367–1371. [PubMed] [Google Scholar]
- 120.Molossi S, Clausell N, Sett S, Rabinovitch M. ICAM-I and VCAM-I expression in accelerated cardiac allograft arteriopathy and myocardial rejection are influenced differently by cyclosporine A and tumour necrosis factor-alpha blockade. Journal of Pathology. 1995;176(2):175–182. doi: 10.1002/path.1711760211. [DOI] [PubMed] [Google Scholar]
- 121.McManus BM, Horley KJ, Wilson JE, et al. Prominence of coronary arterial wall lipids in human heart allografts. Implications for pathogenesis of allograft arteriopathy. Amer J Pathol. 1995;147(2):293–308. [PMC free article] [PubMed] [Google Scholar]
- 122.Luthringer DJ, Yamashita JT, Czer LS, Trento A, Fishbein MC. Nature and significance of epicardial lymphoid infiltrates in cardiac allografts. Journal of Heart & Lung Transplantation. 1995;14(3):S37–S43. [PubMed] [Google Scholar]
- 123.Joshi A, Masek MABW, Brown J, Weiss LM, Billingham ME. “Quilty” revisited, a 10-year perspective. Hum Pathol. 1995;26(5):547–557. doi: 10.1016/0046-8177(95)90252-x. [DOI] [PubMed] [Google Scholar]
- 124.Stein-Oakley AN, Jablonski P, Tzanidis A, et al. Development of chronic injury and nature of interstitial infiltrate in a model of chronic renal allograft rejection. Transplantation. 1993;56(6):1299–1305. doi: 10.1097/00007890-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 125.Azuma H, Nadeau KC, Ishibashi M, Tilney NL. Prevention of functional, structural, and molecular changes of chronic rejection of rat renal allografts by a specific macrophage inhibitor. Transplantation. 1995;60(12):1577–1582. doi: 10.1097/00007890-199560120-00034. [DOI] [PubMed] [Google Scholar]
- 126.Hayashi M, Martinez OM, Garcia-Kennedy R, So S, Esquivel CO, Krams SM. Expression of cytokines and immune mediators during chronic liver allograft rejection. Transplantation. 1995;60(12):1533–1538. doi: 10.1097/00007890-199560120-00027. [DOI] [PubMed] [Google Scholar]
- 127.Mattorri L, Ghidoni P, Palazzi P, Stasi P. Renal allograft rejection: immnuohistochemistry of inflammatory cellular subsets and Vascular lesions. Basic & Applied Histochemistry. 1986;30(2):267–277. [PubMed] [Google Scholar]
- 128.Winter JB, Clelland C, Gouw AS, Prop J. Distinct phenotypes of infiltrating cells during acute and chronic lung rejection in human heart-lung transplants. Transplantation. 1995;59(1):63–69. doi: 10.1097/00007890-199501150-00012. [DOI] [PubMed] [Google Scholar]
- 129.Demetris AJ, Sirica AE, editors. Pathobiology of the Biliary Tree. Boca Raton: CRC Press; 1996. Immunopathology of the Human Biliary Tree. (in press) [Google Scholar]
- 130.Mauck KA, Hosenpud JD. The bronchial epithelium: a potential allogeneic target for chronic rejection after lung transplantation. Journal of Heart & Lung Transplantation. 1996;15(7):709–714. [PubMed] [Google Scholar]
- 131.Baudel EM, Dromer C, Dubrez J, et al. Intermediate-term results after enbloc double-lung transplantation with bronchial arterial revascularization. Bordeaux Lung and Heart-Lung Transplant Group. J Thorac & Cardiovasc Surg. 1996;112(5):1292–1299. doi: 10.1016/s0022-5223(96)70143-5. [DOI] [PubMed] [Google Scholar]
- 132.Fornes P, Heudes D, Simon D, Guillemain R, Amrein C, Bruneval P. Influence of acute or chronic rejection on myocardial collagen density in serial endomyocardial biopsy specimens from cardiac allografts. Journal of Heart & Lung Transplantation. 1996;15(8):796–803. [PubMed] [Google Scholar]
- 133.Truong LD, Foster SV, Barrios R, et al. Tenascin is an ubiquitous extracellular matrix protein of human renal interstioum in normal and pathologic conditions. Nephron. 1996;72(4):579–586. doi: 10.1159/000188943. [DOI] [PubMed] [Google Scholar]
- 134.Demirci G, Nashan B, Pichlmayr R. Fibrosis in chronic rejection of human liver allografts: expression patterns of transforming growth factor-TGFbeta1 and TGF-beta3. Transplantation. 1996;62(12):1776–1783. doi: 10.1097/00007890-199612270-00016. [DOI] [PubMed] [Google Scholar]
- 135.Forbes RD, Cernacck P, Zheng S, Gomersall M, Guttmann RD. Increased endothelin expression in a rat cardiac allograft model of chronic vascular rejection. Transplantation. 1996;61(5):791–797. doi: 10.1097/00007890-199603150-00020. [DOI] [PubMed] [Google Scholar]
- 136.Prop J, Kuijpers K, Petersen AH, Bartels HL, Nieuwenhuis P, Wildevour CR. Why are lung allografts more vigorously rejected than hearts? Journal of Heart Transplantation. 1985;4(4):433–436. [PubMed] [Google Scholar]
- 137.Prop J, Wildevuur CR, Nieuwenhuis P. Lung allograft rejection in the rat II. Specific immunological properties of lung grafts. Transplantation. 1985;40(2):126–131. doi: 10.1097/00007890-198508000-00003. [DOI] [PubMed] [Google Scholar]
- 138.Prop J, Nieuwenhois P, Wildevuur CR. Lung allograft rejection in the rat. I. Accelerated rejection caused by graft lymphocytes. Transplantation. 1985;40(1):25–30. doi: 10.1097/00007890-198507000-00006. [DOI] [PubMed] [Google Scholar]
- 139.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of multivisceral allografts in rats: a sequential analysis with comparison to isolated orthotopic small-bowel and liver grafts. Surgery. 1990;108(5):880–889. [PMC free article] [PubMed] [Google Scholar]
- 140.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small-bowel allotransplantation under FK 506. Surgery. 1991;110(1):87–98. [PMC free article] [PubMed] [Google Scholar]
- 141.Fung J, Zeevi A, Demetris AJ, et al. Origin of lymph node derived lymphocytes in human hepatic allografts. Clin Transplant. 1989;3:316–324. [PMC free article] [PubMed] [Google Scholar]
- 142.Malek P, Vrubel J, Kole J. Lymphatic aspects of experimental and clinical renal transplantation. Bulletin de la Societe Internationale de Chirurgic. 1969;28(1):110–114. [PubMed] [Google Scholar]
- 143.Kocandrle V, Houttuin E, Prohaska JV. Regeneration of the lymphatics after autotransplantation and homotransplantation of the entire small intestine Surgery. Gynecology & Obstetrics. 1966;122(3):587–592. [PubMed] [Google Scholar]
- 144.Malek P, Vrubel J. Lymphatic system and organ transplantation. [Review] Lymphology. 1968;1(1):4–22. [PubMed] [Google Scholar]
- 145.Cockett AT, Sakai A, Netto IC. Kidney lymphatics: an important network in transplantation. Transactions of the American Association of Genito Urinary Surgeons. 1973;65:73–76. [PubMed] [Google Scholar]
- 146.Eliska O, Eliskova M, Mirejovsky P. Lymph vessels of the transplanted kidney. Nephron. 1986;44(2):136–141. doi: 10.1159/000184218. [DOI] [PubMed] [Google Scholar]
- 147.Ruggiero R, Fietsam R, Jr, Thomas GA, et al. Detection of canine allograft lung rejection by pulmonary lymphoscintigraphy. J Thorac & Cardiovasc Surg. 1994;108(2):253–258. [PubMed] [Google Scholar]
- 148.Hruban RH, Beschorner WE, Baumgartner WA, et al. Depletion of bronchus-associated lymphoid tissue associated with lung allograft rejection. Amer J Pathol. 1988;132(1):6–11. [PMC free article] [PubMed] [Google Scholar]
- 149.Kline IK, Thomas PA. Canine lung allograft lymphatic alterations. Ann Thorac Surg. 1976;21(6):532–535. doi: 10.1016/s0003-4975(10)63924-0. [DOI] [PubMed] [Google Scholar]
- 150.Durham JR, Nakhleh RE, Levine A, Levine TB. Persistence of interstitial inflammation after episodes of cardiac rejection associated with systemic infection. Journal of Heart & Lung Transplantation. 1995;14(4):774–780. [PubMed] [Google Scholar]
- 151.Heemann OW, Tullius SG, Schmid C, Philipp T, Tilney NL. Infection-associated cellular activation accelerates chronic renal allograft rejection in rats. Transplant Int. 1996;9(2):137–140. doi: 10.1007/BF00336391. [DOI] [PubMed] [Google Scholar]
- 152.Wallwork J. Risk factors for chronic rejection in heart and lungs–why do hearts and lungs rot? Clinical Transplantation. 1994;8(3 Pt 2):341–344. [PubMed] [Google Scholar]
- 153.Solli F, Jellinck H, Schneider F, Lengyel E, Berczi V, Kekesi V. Lymphatic arteriopathy: damage to the wall of the canine femoral artery after lymphatic blockade. Lymphology. 1991;24(2):54–59. [PubMed] [Google Scholar]
- 154.Russell ME, Adams DH, Wyner LR, Yamashita Y, Halnon NJ, Karnovsky MJ. Early and persistent induction of monocyte chemoattractant protein 1 in rat cardiac allografts. PNAS. 1993;90(13):6086–6090. doi: 10.1073/pnas.90.13.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Karnovsky MJ, Russell ME, Hancock W, Sayegh MH, Adams DH. Chronic rejection in experimental cardiac transplantation in a rat model. Clinical Transplantation. 1994;8(3 Pt 2):308–312. [PubMed] [Google Scholar]
- 156.Russell ME, Wallace AF, Hancock WW, et al. Upregulation of cytokines associated with macrophage activation in the Lewis-to-F344 rat transplantation model of chronic cardiac rejection. Transplantation. 1995;59(4):572–578. [PubMed] [Google Scholar]
- 157.van Saase JL, van der Woude FJ, Thorogood J, et al. The relation between acute vascular and interstitial renal allograft rejection and subsequent chronic rejection. Transplantation. 1995;59(9):1280–1285. [PubMed] [Google Scholar]
- 158.Paul LC. Functional and histologic characteristics of chronic renal allograft rejection. Clinical Transplantation. 1994;8(3 Pt 2):319–323. [PubMed] [Google Scholar]
- 159.Rose ML. Role of antibody and indirect antigen presentation in transplant-associated coronary artery vasculopathy. [Review][75 refs] Journal of Heart & Lung Transplantation. 1996;15(4):342–349. [PubMed] [Google Scholar]
- 160.Gaudin PB, Rayburn BK, Hutchins GM, et al. Peritransplant injury to the myocardium associated with the development of accelerated arteriosclerosis in heart transplant recipients [see comments] Amer J Surg Pathol. 1994;18(4):338–346. doi: 10.1097/00000478-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 161.Day JD, Rayburn BK, Gaudin PB, et al. Cardiac allograft vasculopathy: the central pathogenetic role of ischemia-induced endothelial cell injury. [Review][84 refs] Journal of Heart & Lung Transplantation. 1995;14(6 Pt 2):S142–S149. [PubMed] [Google Scholar]
- 162.Hayry P, Isoniemi H, Yilmaz S, et al. Chronic allograft rejection. Immunol Rev. 1993;134:33–81. doi: 10.1111/j.1600-065x.1993.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 163.Lemström KB, Räisänen-Sokolowski AK, Häyry PJ, Koskinen PK. Triple drug immunosuppression significantly reduces aortic allograft arteriosclerosis in the rat. Arteriosclerosis, Thrombosis & Vascular Biology. 1996;16(4):553–564. doi: 10.1161/01.atv.16.4.553. [DOI] [PubMed] [Google Scholar]
- 164.Geraghty JG, Stoltenberg RL, Sollinger HW, Hullett DA. Vascular smooth muscle cells and neointimal hyperplasia in chronic transplant rejection. Transplantation. 1996;62(4):502–509. doi: 10.1097/00007890-199608270-00013. [DOI] [PubMed] [Google Scholar]
- 165.Graves LM, Bornfeldt KE, Raines EW, et al. Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. PNAS. 1993;90(21):10300–10304. doi: 10.1073/pnas.90.21.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koulack J, McAlister VC, Giacomantonio CA, Bitter-Suermann H, MacDonald AS, Lee TD. Development of a mouse aortic transplant model of chronic rejection. Microsurgery. 1995;16(2):110–113. doi: 10.1002/micr.1920160213. [DOI] [PubMed] [Google Scholar]
- 167.Paul LC, Saito K, Davidoff A, Benediktsson H. Growth factor transcripts in rat renal transplants. Amer J Kid Dis. 1996;28(3):44l–450. doi: 10.1016/s0272-6386(96)90504-1. [DOI] [PubMed] [Google Scholar]
- 168.Alpers CE, Davis CL, Barr D, Marsh CL, Hudkins KL. Identification of platelet-derived growth factor A and B chains in human renal vascular rejection. Amer J Pathol. 1996;148(2):439–451. [PMC free article] [PubMed] [Google Scholar]
- 169.Horvath LZ, Friess H, Schilling M, et al. Altered expression of transforming growth factor-beta S in chronic renal rejection. Kidney International. 1996;50(2):489–498. doi: 10.1038/ki.1996.340. [DOI] [PubMed] [Google Scholar]
- 170.Kerby JD, Verran DJ, Luo KL, et al. Immunolocalization of FGF-1 and receptors in human renal allograft vasculopathy associated with chronic rejection. Transplantation. 1996;62(4):467–475. doi: 10.1097/00007890-199608270-00008. [DOI] [PubMed] [Google Scholar]
- 171.Walgenbach KJ, Heeckt PF, Stanson JD, Whiteside TL, Bauer AJ. Increased expression of transforming growth factor-beta during chronic rejection. Transplant Proc. 1996;28(5):2450. [PubMed] [Google Scholar]
- 172.Raines EW, Ross R. Multiple growth factors are associated with lesions of atherosclerosis: specificity or redundancy? [Review][70 refs] Bioessays. 1996;18(4):271–282. doi: 10.1002/bies.950180405. [DOI] [PubMed] [Google Scholar]
- 173.Raines EW, Ross R. Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. [Review][32 refs] British Heart Journal. 1993;69(1 Suppl):S30–S37. doi: 10.1136/hrt.69.1_suppl.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bornfeldt KE, Raines EW, Graves LM, Skinner MP, Krebs EG, Ross R. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. [Review][67 refs] Annals of the New York Academy of Sciences. 1995;766:416–430. doi: 10.1111/j.1749-6632.1995.tb26691.x. [DOI] [PubMed] [Google Scholar]
- 175.Bornfeldt KE, Raines EW, Nakano T, Graves LM, Krebs EG, Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994;93(3):1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Russell ME, Wallace AF, Wyner LR, Newell JB, Karnovsky MJ. Upregulation and modulation of inducible nitric oxide synthase in rat cardiac allografts with chronic rejection and transplant arteriosclerosis. Circulation. 1995;92(3):457–464. doi: 10.1161/01.cir.92.3.457. [DOI] [PubMed] [Google Scholar]
- 177.Utans U, Arceci RJ, Yamashita Y, Russell ME. Cloning and characterization of allograft inflammatory factor-I: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. J Clin Invest. 1995;95(6):2954–2962. doi: 10.1172/JCI118003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Utans U, Liang P, Wyner LR, Karnovsky MJ, Russell ME. Chronic cardiac rejection: identification of five upregulated genes in transplanted hearts by differential mRNA display. PNAS. 1994;91(14):6463–6467. doi: 10.1073/pnas.91.14.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Waltenberger J, Wanders A, Fellström B, Miyazono K, Heldin CH, Funa K. Induction of transforming growth factor-beta during cardiac allograft rejection. J Immunol. 1993;151(2):1147–1157. [PubMed] [Google Scholar]
- 180.Wasowska B, Wieder KJ, Hancock WW, et al. Cytokine and alloantibody networks in long term cardiac allografts in rat recipients treated with rapamycin. J Immunol. 1996;156(1):395–404. [PubMed] [Google Scholar]
- 181.Katsuda S, Coltrera MD, Ross R, Gown AM. Human atherosclerosis. IV. Immunocytochemical analysis of cell activation and proliferation in lesions of young adults. Amer J Pathol. 1993;142(6):1787–1793. [PMC free article] [PubMed] [Google Scholar]
- 182.Zhao XM, Citrin BS, Miller GG, et al. Association of acidic fibroblast growth factor and untreated low grade rejection with cardiac allograft vasculopathy. Transplantation. 1995;59(7):1005–1010. doi: 10.1097/00007890-199504150-00015. [DOI] [PubMed] [Google Scholar]
- 183.Azuma H, Tilney NL. Chronic graft rejection. [Review] Current Opinion in Immunology. 1994;6(5):770–776. doi: 10.1016/0952-7915(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 184.Laine J, Holmberg C, Hayry P. Chronic rejection and late renal allograft dysfunction. [Review] Pediatric Nephrology. 1996;10(2):221–229. doi: 10.1007/BF00862088. [DOI] [PubMed] [Google Scholar]
- 185.Ludwig J, Wiesner RH, Batts KP, Perkins JD, Krom RAF. The acute vanishing bile duct syndrome (acute irreversible rejection) after orthotopic liver transplantation. Hepatology. 1987;7:476–483. doi: 10.1002/hep.1840070311. [DOI] [PubMed] [Google Scholar]
- 186.Pappo O, Ramos H, Starzl TE, Fung JJ, Demetris AJ. Structural integrity and identification of causes of liver allograft dysfunction occurring more than 5 years after transplantation. The American Journal of Surgical Pathology. 1995;19:192–206. doi: 10.1097/00000478-199502000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fennell RH. Ductular damage in liver transplant rejection: its similarity to that of primary biliary cirrhosis and graft-versus-host disease. Pathol Annu. 1981;1981:289–294. [PubMed] [Google Scholar]
- 188.Portmann B, Neuberger I, Williams R. Intrahepatic bile duct lesions. In: Calne RY, editor. Liver Transplantation, the Cambridge-Kings College Hospital Experience. London: Grune & Stratton; 1983. p. 279. [Google Scholar]
- 189.Grond J, Gouw AS, Poppema S, Sloof MJH, Gips CH. Chronic rejection in liver transplants: a histopathologic analysis of failed grafts and antecedent serial biopsics. Transplant Proc. 1986;18:128–135. [Google Scholar]
- 190.Vierling JM, R.H. Fennell J. Histopathology of early and late human hepatic allograft rejection. Evidence of progressive destruction of interlobular bile ducts. Hepatology. 1985;4:1076–1082. doi: 10.1002/hep.1840050603. [DOI] [PubMed] [Google Scholar]
- 191.Hoek BV, Wiesner R, Krom R, Ludwig J, Moore S. Severe ductopenic rejection following liver transplantation: incidence, time of onset, risk factors, treatment and outcome. Seminars in Liver Disease. 1992;12:41–50. doi: 10.1055/s-2007-1007375. [DOI] [PubMed] [Google Scholar]
- 192.Demetris AJ, Fung JJ, Todo S, et al. Conversion of liver allograft recipients from cyclosporine to FK506 immunosuppressive therapy--a clinicopathologic study of 96 patients. Transplantation. 1992;53(5):1056–1062. doi: 10.1097/00007890-199205000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McCaughan GW, Davies JS, Waugh JA, et al. A quantitative analysis of T lymphocyte populations in human liver allografts undergoing rejection: the use of monoclonal antibodies and double immunolabeling. Hepatology. 1990;12(6):1305–1313. doi: 10.1002/hep.1840120610. [DOI] [PubMed] [Google Scholar]
- 194.Demetris AJ, Seaberg EC, Batts K, et al. Chronic Liver Allograft Rejection: a NIDDK inter-institutional study analyzing the reliability of current criteria and proposal of an expanded definition. Amer J Surg Pathol. 1997 doi: 10.1097/00000478-199801000-00004. (submitted) [DOI] [PubMed] [Google Scholar]
- 195.Bloom S, Fleming K, Chapman R, Neuberger J, Hubscher S. Inappropriate expression of blood group antigens in hepatic allografts. Hepatology. 1994;19(4):876–881. [PubMed] [Google Scholar]
- 196.Demetris AJ, Fung JJ, Todo S, et al. Pathologic observations in human allograft recipients treated with FK 506. Transplant Proc. 1990;22(1):25–34. [PMC free article] [PubMed] [Google Scholar]
- 197.Demetris AJ, Banner B, Fung J, Shapiro R, Jordan M, Starzl TE. Histopathology of human renal allograft rejection under FK 506: a comparison with cyclosporine. Transplant Proc. 1991;23(1 Pt 2):944–946. [PMC free article] [PubMed] [Google Scholar]
- 198.Hubscher S, Neuberger JM. Chronic Rejection of the Liver Allograft. In: Neuberger J, Adams D, editors. Immunology of Liver Transplantation. London: Edward Arnold; 1993. pp. 216–229. [Google Scholar]
- 199.White RM, Zajko AB, Demetris AJ, Bron KM, Dekker A, Starzl TE. Liver transplant rejection: angiographic findings in 35 patients. American Journal of Roentgenology. 1987;148(6):1095–1098. doi: 10.2214/ajr.148.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Devlin J, Page AC, O'Grady J, Portmann B, Karani J, Williams R. Angiographically determined arteriopathy in liver graft dysfunction and surviva1. J Hepatol. 1993;18:68–73. doi: 10.1016/s0168-8278(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 201.Fan X, Sibalic V, Niederer E, Wuthrich RP. The proinflammatory cytokine interleukin-12 occurs as a cell membrane-bound form on macrophages. Biochemical & Biophysical Research Communications. 1996;225(3):1063–1067. doi: 10.1006/bbrc.1996.1295. [DOI] [PubMed] [Google Scholar]
- 202.Hubscher SG, Neuberger JM, Buckels JAC, Elias E, McMaster P. Vanishing bile-duct syndrome after liver transplantation – is it reversible? Transplantation. 1991;51:1004–1110. doi: 10.1097/00007890-199105000-00014. [DOI] [PubMed] [Google Scholar]
- 203.Demetris AJ, Markus BH, Burnharo J, et al. Antibody deposition in liver allografts with chronic rejection. Transplant Proc. 1987;19(4 Suppl 5):121–125. [PubMed] [Google Scholar]
- 204.Davies HS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–527. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 205.Qian S, Sun H, Demetris AJ, Fu F, Starzl TE, Fung JJ. Liver graft induced donor specific unresponsiveness without class I and/or class II antigen differences. Transplant Proc. 1993;25(1 Pt 1):362–363. [PMC free article] [PubMed] [Google Scholar]
- 206.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rhynes VK, McDonald JC, Gelder FE, et al. Soluble HLA class I in the serum of transplant recipients. Ann Surg. 1993;217(5):485–489. doi: 10.1097/00000658-199305010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Matzinger P. Tolerance, danger, and the extended family. Ann Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 209.Bertolino P, Heath WR, Hardy CL, Morahan G, Miller JF. Peripheral delenon of autoreactive CD8+ T cells in transgenic mice expressing H-2Kb in the liver. Eur J Immunol. 1995;25(7):1932–1942. doi: 10.1002/eji.1830250721. [DOI] [PubMed] [Google Scholar]
- 210.Kamada N. The immunology of experimental liver transplantation in the rat. Immunology. 1985;55:369–389. [PMC free article] [PubMed] [Google Scholar]
- 211.Slattery RM, Miller JF, Heath WR, Charlton B. Failure of a protective major histocompatibility complex class II molecule to delete autoreactive T cells in autoimmune diabetes. PNAS. 1993;90(22):10808–10810. doi: 10.1073/pnas.90.22.10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Miller JF, Heath WR. Self-ignorance in the peripheral T-cell pool. [Review] Immunol Rev. 1993;133:131–150. doi: 10.1111/j.1600-065x.1993.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 213.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance [see comments]. [Review] Hepatology. 1993;17(6):1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 214.Starzl TE, Demetris AJ. Transplantation Milestones: viewed with one- and two-way paradigms of tolerance. JAMA. 1995;273:876–879. doi: 10.1001/jama.273.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Murase N, Starzl TE, Ye Q, et al. Multilineage hematopoietic reconstitution of supralethally irradiated rats by syngeneic whole organ transplantation: with particular reference to the liver. Transplantation. 1996;61:1–4. doi: 10.1097/00007890-199601150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nature Medicine. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 217.Hays EF, Hays DM, Golde DW. Hematopoietic stem cells in mouse liver. Exp Hematol. 1978;6:18. [PubMed] [Google Scholar]
- 218.Demetris AJ, Murase N, Rao AS, Starzl TE. The role of passenger leukocytes in rejection and “tolerance” after solid organ transplantation: a potential explanation of a paradox. In: Touraine JL, et al., editors. Rejection and Tolerance. Netherlands: Kluwer Academic Publishers; 1994. pp. 325–392. [Google Scholar]
- 219.Delaney CP, Murase N, Chen-Woan M, et al. Allogeneic hematolymphoid microchimerism and prevention of autoimmune disease in the rat: a relationship between allo- and autoimmunity. The Journal of Clinical Investigation. 1996;97:217–225. doi: 10.1172/JCI118393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liégeois A, Escourrou J, Ouvré E, Charriere J. Microchimerism: a stable state of low-ratio proliferation of allogeneic bone marrow. Transplant Proc. 1977;9:273–276. [PubMed] [Google Scholar]
- 221.Bandeira A, Coutinho A, Carnaud C, Jacquemart F, Forni L. Transplantation tolerance correlates with high levels of T- and B-lymphocyte activity. Proceedings of the National Academy of Science (USA) 1989;86:272–276. doi: 10.1073/pnas.86.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Orloff MS, DeMara EM, Coppage ML, et al. Alterations of the interleukin-4 pathway in production of tolerance by mixed hematopoietic chimerism. Surgery. 1995;118(2):212–219. doi: 10.1016/s0039-6060(05)80326-5. [DOI] [PubMed] [Google Scholar]
- 223.Sun J, McCaughan GW, Matsumoto Y, Sheil AG, Gallagher ND, Bishop GA. Tolerance to rat liver allografts. I. Differences between tolerance and rejection are more marked in the B cell compared with the T cell or cytokine response. Transplantation. 1994;57(9):1349–1357. [PubMed] [Google Scholar]
- 224.Sun J, Sheil R, McCaughan G, Jung S, Gallagher N, Bishop G. Strong tolerance mediated by allografting in the rat is due to donor liver leukocytes. Transplant Proc. 1995;27(6):3578. [PubMed] [Google Scholar]
- 225.Sun J, McCaughan GW, Gallagher ND, Sheil AG, Bishop GA. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation. 1995;60(3):233–236. doi: 10.1097/00007890-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 226.Bishop GA, Sun J, DeCruz DJ, et al. Tolerance to rat liver allografts. III. Donor cell migration and tolerance-associated cytokine production in peripheral lymphoid tissues. J Immunol. 1996;156(12):4925–4931. [PubMed] [Google Scholar]
- 227.Anderson TJ, Meredith IT, Uehala A, et al. Functional significance of intimal thickening as detected by intravascular ultrasound early and late after cardiac transplantation. Circulation. 1993;88(3):1093–1100. doi: 10.1161/01.cir.88.3.1093. [DOI] [PubMed] [Google Scholar]
- 228.Chenzbraun A, Pinto FJ, Alderman EL, et al. Distribution and morphologic features of coronary artery disease in cardiac allografts: an intracoronary ultrasound study. Journal of the American Society of Echocardiography. 1995;8(1):1–8. doi: 10.1016/s0894-7317(05)80351-7. [DOI] [PubMed] [Google Scholar]
- 229.Escobar A, Ventura HO, Stapleton DD, et al. Cardiac allograft vasculopathy assessed by intravascular ultrasonography and nonimmunologic risk factors. American Journal of Cardiology. 1994;74(10):1042–1046. doi: 10.1016/0002-9149(94)90856-7. [DOI] [PubMed] [Google Scholar]
- 230.Tuzcu EM, De Franco AC, Goormastic M, et al. Dichoromous pattern of coronary atherosclerosis 1 to 9 years after transplantation: insights from systematic intravascular ultrasound imaging. Journal of the American College of Cardiology. 1996;27(4):839–846. doi: 10.1016/0735-1097(95)00564-1. [DOI] [PubMed] [Google Scholar]
- 231.Gao SZ, Schroeder JS, Hunt SA, Billingham ME, Valantine HA, Stinson EB. Acute myocardial infarction in cardiac transplant recipients. American Journal of Cardiology. 1989;64(18):1093–1097. doi: 10.1016/0002-9149(89)90858-8. [DOI] [PubMed] [Google Scholar]
- 232.Hinkamp TJ, Sullivan HJ, Montoya A, Park S, Bartlett L, Pifarre R. Chronic cardiac rejection masking as constrictive pericarditis. Ann Thorac Surg. 1994;57(6):1579–1583. doi: 10.1016/0003-4975(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 233.Pflugfelder PW, Boughner DR, Rudas L, Kostuk WJ. Enhanced detection of cardiac allograft arterial disease with intracoronary ultrasonographic imaging. American Heart Journal. 1993;125(6):1583–1591. doi: 10.1016/0002-8703(93)90744-t. [DOI] [PubMed] [Google Scholar]
- 234.Drinkwater DC, Laks H, Blitz A, et al. Outcomes Of Patients Undergoing Transplantation With Older Donor Hearts. Journal of Heart & Lung Transplantation. 1996;15(7):684–691. [PubMed] [Google Scholar]
- 235.Johnson DE, Gao SZ, Schroeder JS, DeCampli WM, Billingham ME. The spectrum of coronary artery pathologic findings in human cardiac allografts. Journal of Heart Transplantation. 1989;8(5):349–359. [PubMed] [Google Scholar]
- 236.Gao SZ, Alderman EL, Schroeder JS, Silverman JF, Hunt SA. Accelerated coronary vascular disease in the heart transplants patient: coronary arteriographic findings. Journal of the American College of Cardiology. 1988;12(2):334–340. doi: 10.1016/0735-1097(88)90402-0. [DOI] [PubMed] [Google Scholar]
- 237.Mehra MR, Ventura HO, Stapleton DD, Smart FW, Collins TC, Ramee SR. Presence of severe intimal thickening by intravascular ultrasonography predicts cardiac events in cardiac allograft vasculopathy. Journal of Heart & Lung Transplantation. 1995;14(4):632–639. [PubMed] [Google Scholar]
- 238.Schroeder JS, Gao SZ, Hunt SA, Stinson EB. Accelerated graft coronary artery disease: diagnosis and prevention. [Review][55 refs] Journal of Heart & Lung Transplantation. 1992;11(4 Pt 2):S258–S265. [PubMed] [Google Scholar]
- 239.Lowry RW, Kleiman NS, Raizner AE, Young JB. Is intravascular ultrasound better than quantitative coronary arteriography to assess cardiac allograft arteriopathy? Catheterization & Cardiovascular Diagnosis. 1994;31(2):110–115. doi: 10.1002/ccd.1810310204. [DOI] [PubMed] [Google Scholar]
- 240.Rickenbacher PR, Pinto FJ, Chenzhraun A, et al. Incidence and severity of transplant coronary artery disease early and up to 15 years after transplantation as detected by intravascular ultrasound [see comments] Journal of the American College of Cardiology. 1995;25(1):171–177. doi: 10.1016/0735-1097(94)00323-i. [DOI] [PubMed] [Google Scholar]
- 241.Davis SF, Yeung AC, Meredith TT, et al. Early endothelial dysfunction predicts the development of transplant coronary artery disease at 1 year post-transplant. Circulation. 1996;93(3):457–462. doi: 10.1161/01.cir.93.3.457. [DOI] [PubMed] [Google Scholar]
- 242.Mugge A, Heublein B, Kuhn M, et al. Impaired coronary dilator responses to substance P and impaired flow-dependent dilator responses in heart transplant patients with graft vasculopathy. Journal of the American College of Cardiology. 1993;21(1):163–170. doi: 10.1016/0735-1097(93)90732-g. [DOI] [PubMed] [Google Scholar]
- 243.Palmer DC, Tsai CC, Roodman ST, et al. Heart graft arteriosclerosis. An ominous finding on endomyocardial biopsy. Transplantation. 1985;39(4):385–388. [PubMed] [Google Scholar]
- 244.Billingham ME. The postsurgical heart. Amer J Cardiovasc Pathol. 1988;1(3):319–334. [PubMed] [Google Scholar]
- 245.Billingham ME. Cardiac Transplantation. In: Sale GE, editor. The Pathology of Organ Transplantation. Boston: Butterworths; 1990. pp. l33–152. [Google Scholar]
- 246.Winters GL, Costanzo-Nordin MR. Pathological findings in 2300 consecutive endomyocardial biopsies. Mod Pathol. 1991;4(4):441–448. [PubMed] [Google Scholar]
- 247.Pardo-Mindan FJ, Lozano MD, Contreras-Mejuto F, de Alava E. Pathology of heart transplant through endomyocardial biopsy. Sem Diag Pathol. 1992;9(3):238–248. [PubMed] [Google Scholar]
- 248.Stovin PGI, Ai-Tikriti SA. The correlation of various features of rejection in myocardial biopsies after human heart transplantation. Path Res Pract. 1989;185:836–842. doi: 10.1016/s0344-0338(89)80283-3. [DOI] [PubMed] [Google Scholar]
- 249.Suit PF, Kottke-Marchant K, Ratliff NB, Pippenger CE, Easely K. Comparison of whole-blood cyclosporine levels and the frequency of endomyocardial lymphocytic infiltrates (the guilty lesion) in cardiac transplantation. Transplantation. 1989;48(4):618–621. [PubMed] [Google Scholar]
- 250.Kottke-Marchant K, Ratliff NB. Endomyocardial lymphocytic infiltrates in cardiac transplant recipients. Arch Pathol Lab Med. 1989;113:690–698. [PubMed] [Google Scholar]
- 251.Kemnitz J, Cohnert T, Schafters H, et al. A classification of cardiac allograft rejection. Amer J Surg Pathol. 1987;11:503–515. doi: 10.1097/00000478-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 252.Forbes RDC, Rowan RA, Billingham ME. Endocardial infiltrates in human heart transplants: a serial biopsy analysis comparing four immunosuppression protocols. Hum Pathol. 1990;21:850–855. doi: 10.1016/0046-8177(90)90055-a. [DOI] [PubMed] [Google Scholar]
- 253.Kemnitz J, Cremer J, Schaefers H, et al. Some aspects of changed histopathologic appearance of acute rejection in cardiac allografts after prophylactic application of OKT3. J Heart Lung Transplant. 1991;10:366–372. [PubMed] [Google Scholar]
- 254.Costanzo-Nordin MR, Winters GL, Fisher SG, et al. Endocardial infiltrates in the transplanted heart. J Heart & Lung Transplant. 1993;12(5):741–747. [PubMed] [Google Scholar]
- 255.Tsamandas AC, Pham S, Seaberg E, et al. Cardiac Transplantation under Tacrolimus (FK506) immunosuppression: histopathologic observations and comparison to a Cyclosporine-based regimen with ATG induction. J Heart and Lung Transplantation. 1997 (in press) [PMC free article] [PubMed] [Google Scholar]
- 256.Gjertson DW, Cecka JM, Terasaki PI. The relative effects of FK506 and cyclosporine on short and long-term kidney graft survival. Transplantation. 1995;60:1384–1388. doi: 10.1097/00007890-199560120-00002. [DOI] [PubMed] [Google Scholar]
- 257.Johnson DE, Alderman EL, Schroeder JS, et al. Transplant coronary artery disease: histopathologic correlations with angiographic morphology. Journal of the American College of Cardiology. 1991;17(2):449–457. doi: 10.1016/s0735-1097(10)80114-7. [DOI] [PubMed] [Google Scholar]
- 258.Miller LW. Allograft coronary disease: lessons from new diagnostic methods. In: Paul LC, Foegh M, editors. Sixth International Alexis Carrel Conference on Graft Atherosclerosis and Chronic Rejection; Banff, Canada. 1996. [Google Scholar]
- 259.Todo S, Tzakis A, Reyes J, et al. Clinical small bowel or small bowel plus liver transplantation under FK 506. Transplant Proc. 1991;23(6):3093–3095. [PMC free article] [PubMed] [Google Scholar]
- 260.Tzakis AG, Todo S, Reyes J, et al. Intestinal transplantation in children under FK 506 immunosuppression. Journal of Pediatric Surgery. 1993;28(8):1040–1043. doi: 10.1016/0022-3468(93)90514-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222(3):270–280. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Starzl TE, Fung JJ, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Langrehr JM, Hoffman RA, Demetris AJ, et al. Evidence that indefinite survival of small bowel allografts achieved by a brief course of cyclosporine or FK506 is not due to systemic hyporesponsiveness. Transplantation. 1992;54(3):505–510. doi: 10.1097/00007890-199209000-00022. [DOI] [PubMed] [Google Scholar]
- 264.Langrehr JM, Banner B, Lee KK, Schraut WH. Clinical course, morphology, and treatment of chronically rejecting small bowel allografts. Transplantation. 1993;55(2):242–250. doi: 10.1097/00007890-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 265.Langrehr JM, Demetris AJ, Banner B, et al. Mucosal recipient-type mononuclear repopulation and low-grade chronic rejection occur simultaneously in indefinitely surviving recipients of small bowel allografts. Transplantation. 1994;7(2):71–78. doi: 10.1007/BF00336465. [DOI] [PubMed] [Google Scholar]
- 266.Heeckt PF, Lee KK, Halfter WM, Schraut WH, Bauer AJ. Functional, impairment of enteric smooth muscle and nerves caused by chronic intestinal allograft rejection regresses after FK506 rescue [published erratum appears in Transplantation 1995 Mar 27;59(6);928] Transplantation. 1995;59(2):159–164. [PubMed] [Google Scholar]
- 267.Su GL, Walgenbach KJ, Heeckt PH, et al. Increased expression of interferon-gamma in a rat model of chronic intestinal allograft rejection. Transplantation. 1996;62(2):242–248. doi: 10.1097/00007890-199607270-00016. [DOI] [PubMed] [Google Scholar]
- 268.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 269.Mazariegos GV, Ramos H, Shapiro R, Zeevi A, Fung JJ, Starzl TE. Weaning of immunosuppression in long-term recipients of living related renal transplants: a preliminary study. Transplant Proc. 1995;27(1):207–209. [PMC free article] [PubMed] [Google Scholar]
- 270.Ramos HC, Reyes J, Abu-Elmagd K, et al. Weaning of immuosuppression in long-term liver transplant recipients. Transplantation. 1995;59(2):212–217. doi: 10.1097/00007890-199501270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Austyn JM, Steinman RM. The passenger leukocyte – a fresh look. Transpl Rev. 1988;2:139–176. [Google Scholar]
- 272.Austyn JM, Kupiec-Weglinski JW, Hankins DF, Morris PJ. Migration patterns of dendritic cells in the mouse: Homing to T-cell dependent areas of spleen, and binding with marginal zone. J Exp Med. 1988;167:646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Steinman RM. The dendritic cell system and its role in immunogenicity. [Review] Ann Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 274.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism and donor-specific non-reactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55(6):1272–1277. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.McSherry C, Hertz MJ, Jackson AM, et al. Allogeneic microchimerism and donor antigen-specific hyporeactivity in lung transplant recipients. Clinical Transplantation. 1995;9(6):442–449. [PubMed] [Google Scholar]
- 276.Burlingham J, Grailer AP, Fechner JH, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness(clonal energy) in a tolerant renal transplant recipient. Transplantation. 1995;59:1147–1155. [PubMed] [Google Scholar]
- 277.Reinsmoen NL, Jackson A, McSherry C, et al. Organ-specific patterns of donor antigen-specific hyporeactivity and peripheral blood allogeneic microchimerism in lung, kidney and liver recipients. Transplantation. 1995;60:1546–1554. doi: 10.1097/00007890-199560120-00029. [DOI] [PubMed] [Google Scholar]
- 278.Demetris AJ, Murase N, Starzl TE. Donor dendritic cells after liver and heart allotransplantation under short-term immunosuppression [letter] Lancet. 1992;339(8809):1610. doi: 10.1016/0140-6736(92)91875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, microchimerism, and GVH reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25(6):3337–3344. [PMC free article] [PubMed] [Google Scholar]
- 280.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation. 1995;60:158–171. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Utans U, Quist WC, McManus BM, et al. Allograft inflammatory factory-1. A cytokine-responsive macrophage molecule expressed in transplanted human hearts. Transplantation. 1996;61(9):1387–1392. doi: 10.1097/00007890-199605150-00018. [DOI] [PubMed] [Google Scholar]
- 282.Libby P, Tanaka H. The pathogenesis of coronary arteriosclerosis (“chronic rejection”) in transplanted hearts. Clinical Transplantation. 1994;8(3 Pt 2):313–318. [PubMed] [Google Scholar]
- 283.Batchelor JR, Braun MY. Distinct T cells mediating acute and chronic rejection. In: Touraine JJ, editor. Rejection and Tolerance. vol 25. Dordrecht: Kluwer Academic Publishers; 1994. pp. 103–110. [Google Scholar]
- 284.Blom D, Morrissey NJ, Mesonero C, et al. Induction of specific tolerance through mixed hematopoietic chimerism prevents chronic renal allograft rejection in a rat model. Surgery. 1996;120(2):213–219. doi: 10.1016/s0039-6060(96)80290-x. [DOI] [PubMed] [Google Scholar]
- 285.Orloff MS, DeMara EM, Coppage ML, et al. Prevention of chronic rejection and graft arteriosclerosis by tolerance induction. Transplantation. 1995;59(2):282–288. [PubMed] [Google Scholar]
- 286.McDaniel DO, Naftilan J, Hulvey K, et al. Peripheral blood chimerism in renal allograft recipients transfused with donor bone marrow. Transplantation. 1994;57:852–856. doi: 10.1097/00007890-199403270-00014. [DOI] [PubMed] [Google Scholar]
- 287.Fontes P, Rao A, Demetris AJ, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Harrison RF, Patsiaoura K, Hubscher SG. Cytokeratin immunostaining for detection of biliary epithelium: its use in counting bile ducts in cases of liver allograft rejection. Journal of Clinical Pathology. 1994;47(4):303–308. doi: 10.1136/jcp.47.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]