Abstract
FGFR3 mutations are common in low grade urothelial carcinoma and represent a potential therapeutic target in this disease. Their incidence and functional role in high grade urothelial carcinoma (HGUC), which displays an increased propensity for recurrence and muscularis propria invasion, is less well defined. We developed a mass spectrometry based genotyping assay to define the incidence of FGFR3 mutations in a large clinically annotated set of urothelial carcinomas. FGFR3 mutations were found in 17% of HGUC versus 84% of low-grade lesions. Retrospective pathologic review of the class of FGFR3 mutant HGUC revealed unique histologic features characterized by a bulky, exophytic component with branching papillary architecture as well as irregular nuclei with a koilocytoid appearance. The predictive value of this histologic appearance was confirmed using a prospective set of 49 additional HGUC. Prospective histologic review was able to correctly predict for the presence of an FGFR3 mutation in 13 of 24 HGUC specimens that exhibited the distinct morphology (54%). All 25 specimens lacking the defined histologic features were FGFR3 wild-type for a negative predictive value of 100%. Macrodissection of individual tumors confirmed the presence of the FGFR3 mutant allele in non-invasive and invasive, low and high-grade regions of individual tumors and in the lymph node metastases of patients whose tumors possessed the characteristic morphologic signature, suggesting that FGFR3 mutations are not restricted to the more clinically indolent regions of HGUCs. These data suggest that histologic screening of HGUC followed by confirmatory genotyping can be used to enrich for the population of HGUC most likely to harbor activating mutations in the FGFR3 receptor tyrosine kinase. Histologic review could thus aid in the development of targeted inhibitors of FGFR3 by facilitating the identification of the subset of patients most likely to harbor activating mutations in the FGFR3 gene.
Keywords: fibroblast growth factor receptor-3, FGFR3, urothelial carcinoma, morphology
Introduction
Bladder cancer (BC) is the ninth most common cancer worldwide and the fourth most common cancer in men in the United States, accounting for more than 70,000 cases a year and over 14,000 disease-related deaths in 2009 alone.[1] Of newly diagnosed cases, approximately 70% either lack demonstrable invasion or exhibit invasion restricted to the lamina propria.[2, 3] Following surgical resection or intravesical treatment, a majority of tumors will recur with 10%–30% ultimately developing invasion into the muscularis propria, which is strongly associated with disease-specific morbidity and mortality despite multimodality treatment.[4, 5]
Higher grade lesions are associated with a greater risk for tumor recurrence and invasion.[6–9] The advanced median age at diagnosis of 70 years renders a significant proportion of patients unsuitable candidates for treatments secondary to age-related comorbidities. Furthermore, bladder cancer requires prolonged monitoring and treatment of recurrent tumors. As a result, the cost of medical care for this disease was almost $3.7 billion in 2001, making it the fifth most expensive cancer to treat in the United States, and the cost per patient from the time of diagnosis to death is the highest among all cancers.[10, 11] These factors underscore the need for novel, less toxic, and more efficacious treatment approaches for patients with advanced urothelial carcinoma (UC) as well as the need to identify a priori those tumors at high risk for progression.
Recent successes in the targeting of activated oncogenic kinases have led to the hope that this paradigm can be similarly applied to patients with advanced UC as well as tumors with greater risk of recurrence or progression.[12–16] Such efforts have been impeded, however, by our limited understanding of the role of such kinases, if any, in mediating tumor progression in high-grade UC (HGUC). Fibroblast growth factor receptor-3 (FGFR3) is a receptor tyrosine kinase that is somatically altered in a number of malignancies including UC, multiple myeloma, and less commonly in cervical carcinoma.[17, 18] In UC, FGFR3 is activated through point mutations that are largely found within exons 7, 10 and 15.[19] These mutations promote constitutive receptor dimerization and kinase activation. Prior studies have reported that the majority of low-grade UC harbor activating mutations in FGFR3 [20–22] whereas the incidence and prognostic relevance of FGFR3 mutations in HGUC lesions remains poorly defined.
We examined a large cohort of clinically annotated bladder tumors with the goal of defining the frequency and morphologic features of FGFR3 mutations in patients with high-grade disease. Our results indicate that although FGFR3 mutations occur with lower frequency in high-grade tumors as compared to those with low-grade disease, FGFR3 mutation status defines a distinct morphologic subtype of HGUC. Furthermore, in contrast to the high prevalence of FGFR3 mutations in patients with favorable outcome, low-grade tumors, patients with HGUC whose tumors harbor a mutant FGFR3 allele have a similar risk of post-surgical recurrence and disease-specific mortality as those whose tumors are FGFR3 wild-type. These data support the testing of selective FGFR3 inhibitors in patients with HGUC. Furthermore, the association of FGFR3 mutations with a distinct papillary morphology suggests that histological examination may aid efforts to enrich clinical trials of agents targeting oncogenic FGFR3.
Materials and methods
Sample Selection and Characteristics
Frozen sections were collected from 153 high-grade bladder tumors, 150 of which were derived from cystectomies performed between 2004 and 2008 at Memorial Sloan-Kettering Cancer Center and archived within the Institutional Tumor Bank. Three samples containing high grade disease were obtained from a transurethral resection of bladder tumor (TURBT). All tissue samples had been snap-frozen in −80° C in liquid nitrogen following resection. All tumors were reviewed for tumor content and, if possible, macro-dissected to minimize stromal contamination. Twenty samples with minimal tumor content were excluded from analysis.
In those samples harboring FGFR3 mutations, invasive and non-invasive, high and low-grade components were macro-dissected from corresponding formalin-fixed paraffin-embedded (FFPE) sections when available and sequenced for hotspot mutations in FGFR3. Additionally, matched normal tissue from each tumor, taken from the bladder wall in an area without evidence of gross morphologic abnormalities, was sequenced for all cases with FGFR3 mutations.
An additional 63 FFPE low-grade tumors obtained by TURBT were analyzed for FGFR3 mutation status.
We followed the World Health Organization/International Society of Urological Pathology (WHO/ISUP) consensus classification of urothelial neoplasms of the urinary bladder for tumor grading.[23]
Clinical data were collected under an Institutional Review Board-approved waiver of authorization.
Morphologic Analysis
Detailed histopathologic examination of all hematoxylin & eosin (H&E) slides of the 133 samples was initially performed by a single genitourinary pathologist (HAA) and the morphologic characteristics of tumors with confirmed FGFR3 mutations, including tumor grade and stage, pattern of growth, the presence of a non-invasive component, and cellular and nuclear features, were confirmed with six pathologists, five of whom with expertise in genitourinary pathology (VER, SKT, OL, SWF, AG, YC). The tumors were also compared to FGFR3 wild type (wt) samples to identify any distinguishing features that correlated with mutation status. We later applied these morphologic characteristics to a separate, prospective cohort to determine whether their presence predicted for FGFR3 mutation status (described below).
Mutation Detection
Genomic DNA was extracted using the DNeasy Tissue kit (Qiagen Inc., Valencia, CA). In the cohort of 133 frozen tumors, bidirectional Sanger sequencing of all coding regions of FGFR3 was performed as previously reported [24, 25]. All primer sequences are available upon request. Tumors were screened for FGFR3 hotspot mutations using an iPLEX assay developed specifically for this purpose (Sequenom, Inc., San Diego, CA). Briefly, multiplexed PCR and extension primers were designed for a panel of known mutations. After PCR and single nucleotide extension reactions, the resulting extension products were analyzed using a matrix-assisted laser desorption/ionization–time-of-flight (MALDI-TOF) mass spectrometer as previously reported [24].
RESULTS
Detection of FGFR3 mutations by Sanger sequencing and MALDI-TOF mass spectrometry in high and low grade urothelial cancers
To determine the prevalence of FGFR3 mutations in HGUC, we sequenced all coding exons of FGFR3 for 133 frozen tumors, all of which exhibited high-grade morphology. Eighteen samples (13%) harbored FGFR3 mutations. Two samples contained non-hotspot mutations (D468N and S131L) and one of these samples (with the S131L mutation) also harbored a second S249C mutation. All mutations were confirmed as somatic by sequencing the corresponding normal tissue DNA.
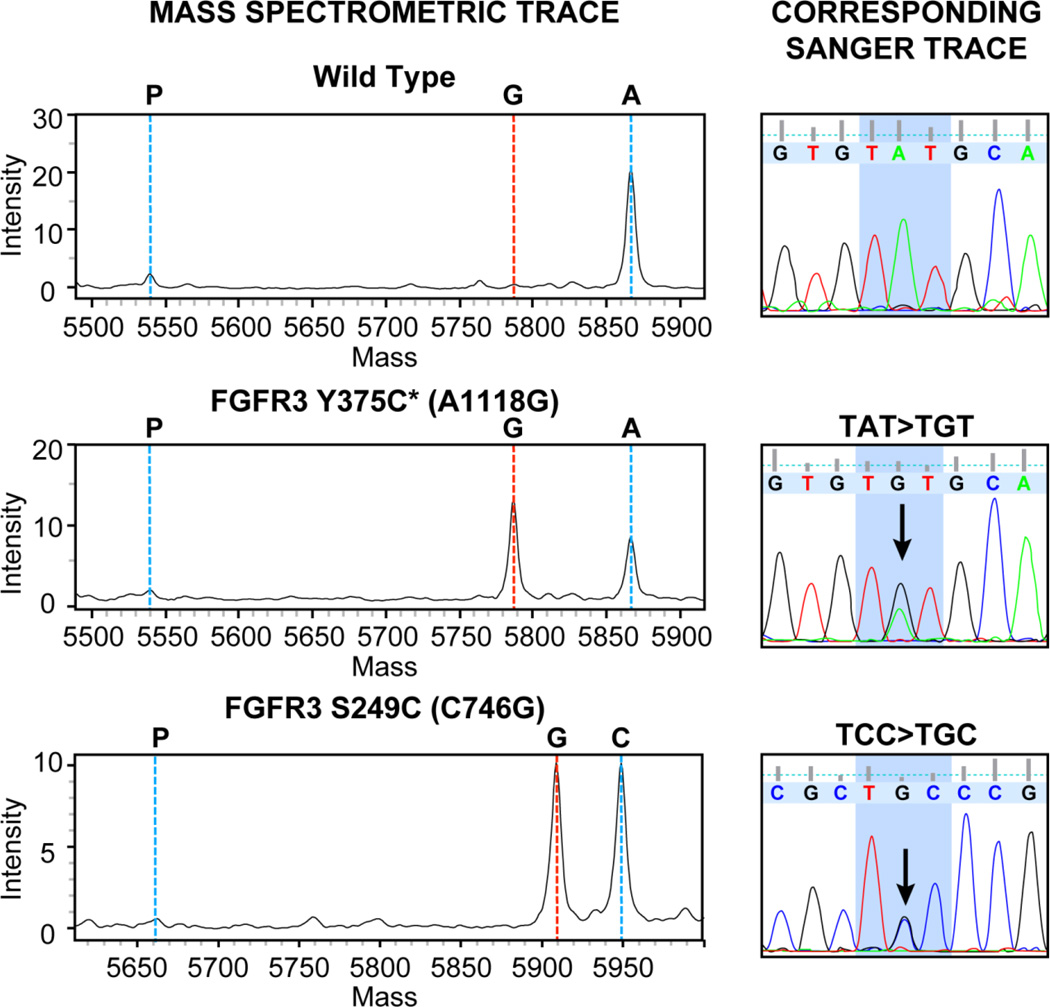
To facilitate rapid screening for FGFR3 mutations within our sample set, we developed a MALDI-TOF MS Sequenom assay (described above) to detect hotspot mutations within FGFR3, including both the more commonly reported R248C, S249C, and Y375C mutations as well as the less frequent G370C and S371C alterations. All hotspot mutations detected by Sanger were also detected by the Sequenom assay. Representative MS and corresponding Sanger traces for tumors with the S249C and Y375C FGFR3 mutations are shown in Figure 1.
Figure 1.
Representative MS and Sanger sequencing traces for two tumors harboring FGFR3 mutations Y375C and S249C. A wild type MS and Sanger trace are shown for comparison.
*The FGFR3b isoform found in epithelial tissue contains 2 additional amino acid residues compared to the FGFR3c isoform in mesenchymal cells. Therefore, the Y375C mutation in epithelial tumors is equivalent to the germline Y373C mutation detected in skeletal disorders.
High grade urothelial carcinomas expressing an FGFR3 mutation display a distinct exophytic, papillary morphology
The morphologic characteristics of the eighteen tumors harboring FGFR3 mutations were reviewed and compared to the cohort of tumors wild-type for FGFR3.[26] All eighteen FGFR3 mutant tumors were noted to be bulky and polypoid, with protrusion of the tumor mass into the lumen of the urinary bladder.
By histopathologic examination the following features were noted in the FGFR3 mutant tumors (Figure 2):
The presence of a prominent exophytic, papillary component of non-invasive tumor, characterized by long, slender, branching and sometimes complex papillary formations. This exophytic, papillary component was present in 17 of the 18 cases.
The papillae were lined by polygonal cells with distinct cell borders and clear to eosinophilic cytoplasm. The nuclei were variable in size with irregular and “wrinkled” nuclear membranes exhibiting a “koilocytoid” appearance. These morphologic features were most recognizable in the non-invasive papillary component, but were also seen at the base of the papillae at the pushing borders of the lesion. The extent of these features varied but they were identifiable in all eighteen cases.
In 11 of the 18 cases, tumors contained cells with darker nuclei and less abundant cytoplasm, giving the tumor a more basaloid appearance.
While all cases were high-grade carcinomas, in ten of eighteen FGFR3 mutant cases a component of low-grade cytomorphology was also detected. The extent of the low-grade component varied considerably from focal to abundant.
Similar morphologic features (polygonal cells with distinct cell borders and clear to eosinophilic cytoplasm, nuclear pleomorphism and irregular/“wrinkled” nuclear membrane) were seen in the invasive component in 8 cases. However, in the remaining 10 cases, the invasive tumor had a different appearance indistinguishable from conventional urothelial carcinoma (not otherwise specified).
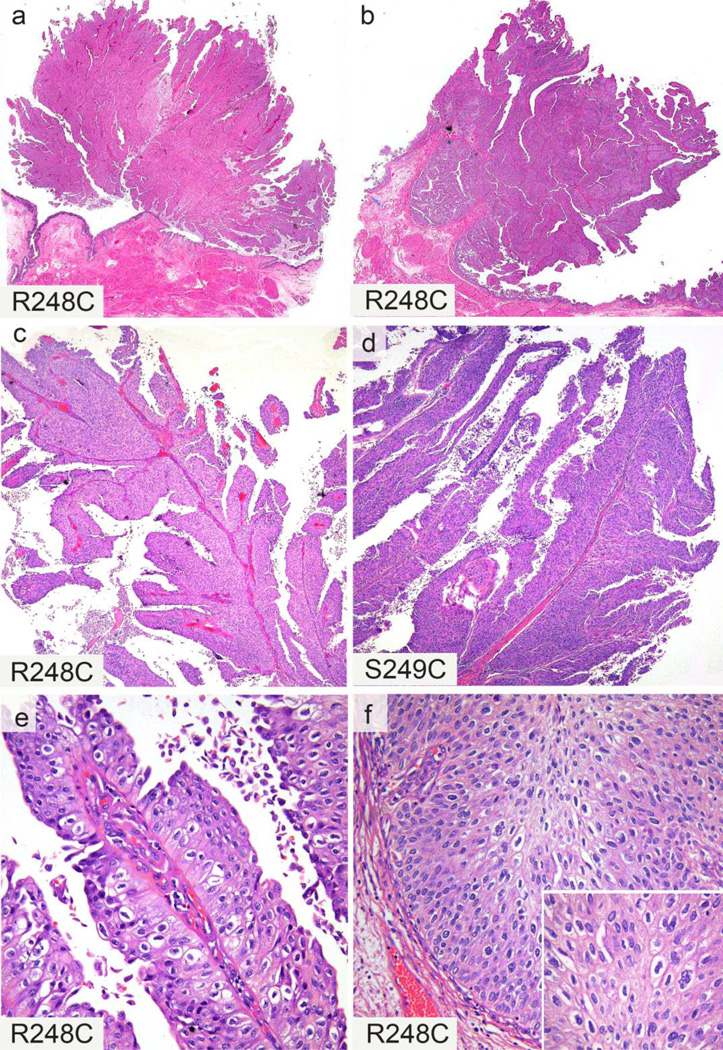
Figure 2.
Histopathologic features of high grade urothelial carcinoma with FGFR3 mutations. Two examples with prominent exophytic, papillary component of non-invasive tumor (a & b). Two examples with long, slender, branching papillary formations (c & d). Papillary structures lined by polygonal cells with distinct cell borders and clear to eosinophilic cytoplasm (e). Tumor nuclei with variable size and irregular “wrinkled” nuclear contour, resulting in a “koilocytoid” appearance (e, f, inset). These cellular features were most recognizable in the non-invasive papillary component (e), but were also seen at the base of the papillae at the pushing borders of the lesion (f). Corresponding FGFR3 mutations displayed on left lower corner of each image (a, c, e are from one case; b, f are from another case).
To determine whether the presence of these morphologic features were predictive of FGFR3 mutation status in a prospective cohort, we identified 24 additional tumors that possessed morphologic characteristics similar to the samples identified as FGFR3 mutant from 161 consecutive patients (15%). Specifically, all 24 samples exhibited a bulky exophytic tumor with papillary configuration and cell morphology characterized by distinct cell borders and irregular nuclei with “koilocytoid” appearance. These samples were selected from either cystectomies (n=13) or nephroureterectomies (n=9) and ureterectomies (n=2) for upper tract disease. Two separate tumors resected from the same cystectomy specimen were included in this set as were two pairs of matching primary tumor and lymph node metastasis. For comparison, we selected from the same consecutive cohort of 161 patients, 25 additional HGUC tumors, not otherwise specified, that specifically lacked the morphologic features identified in the FGFR3 mutated group (Figure 3).
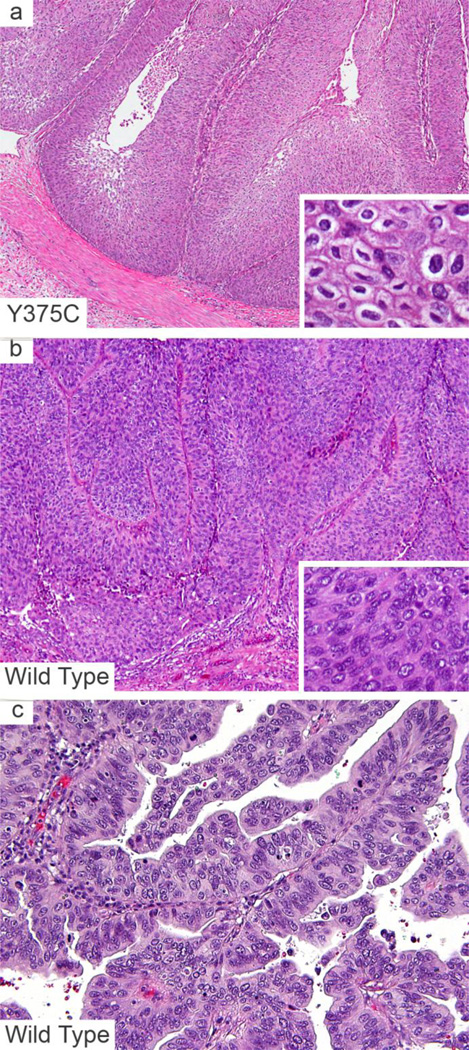
Figure 3.
Morphologic features of urothelial carcinoma with and without FGFR3 mutations. Non-invasive urothelial carcinoma with a Y375C mutation, depicting the papillary morphology, irregular nuclei, perinuclear clearing and distinct cell borders (a, inset). FGFR3 wild type tumors showing an inverted (b) and exophytic (c) growth pattern. Wild type tumors lack the morphologic features suggestive of FGFR3 mutations including the absence of distinct cell borders, raisinoid/wrinkled nuclei and perinuclear clearing (inset b, c).
The 49 samples were then profiled for FGFR3 hotspot mutations utilizing the Sequenom assay and putative mutations were subsequently confirmed by Sanger sequencing. All samples were run through the Sequenom platform twice, and results in all cases were concordant between the two runs. In three samples, PCR amplification prior to Sanger sequencing was unsuccessful and in one tumor, a mutation found by Sequenom could not be detected by Sanger. In summary, thirteen of the 24 tumors that exhibited the distinctive morphology identified previously harbored FGFR3 mutations, resulting in a positive predictive value of 54%, whereas all 25 tumors lacking the distinctive morphology were FGFR3 wild-type (negative predictive value of 100%). Notably, the identical FGFR3 mutations found in the primary tumor were also present in the metastatic component of the corresponding lymph node pairs. (Figure 3)
Because a prominent feature of the above-described FGFR3 mutation-associated morphology was the presence of a large exophytic, noninvasive component, and as FGFR3 mutations are commonly found in low grade, superficial bladder tumors, we isolated the invasive and non-invasive components of eight FGFR3 mutant tumors from FFPE sections by macro-dissection and genotyped DNA extracted from each component. In six of eight samples (75%), the FGFR3 mutation was detected in both the invasive and non-invasive components. Of the two remaining samples, one contained an R248C mutation in only the invasive section while the other possessed an S249C mutation only in a high-grade noninvasive region (Table 1). In an additional three samples, low and high-grade non-invasive components were isolated and sequenced. In all three cases, FGFR3 mutations were detected in both the low and high-grade components.
Table 1.
Distribution of FGFR3 Mutations in High and Low Grade, Primary and Metastatic Tumors
| ID | Mutation | High-grade non-invasive |
High- grade invasive |
|---|---|---|---|
| B012 | G370C | G370C | G370C |
| B060 | S249C | S249C | S249C |
| B072 | R248C | R248C | R248C |
| B124 | S249C | S249C | S249C |
| B126 | S249C | S249C | S249C |
| B138 | S371C | S371C | S371C |
| B148 | S249C | S249C | WT |
| B085 | R248C | WT | R248C |
| ID | Mutation | Low-grade | High- grade |
|---|---|---|---|
| B049 | S249C | S249C | S249C |
| B091 | Y375C | Y375C | Y375C |
| B107 | S249C | S249C | S249C |
| ID | Mutation | Primary | Metastasis |
|---|---|---|---|
| B164 | S249C | S249C | S249C |
| B166 | Y375C | Y375C | Y375C |
FGFR3 mutations were detected in macro-dissected FFPE sections containing predominantly high grade non-invasive or high grade invasive components from eight tumors. Six tumors possessed concordant mutations while mutations were detected in only the non-invasive or the invasive components of two tumors (marked in red). Concordant FGFR3 mutations were also found in both the low and high grade components of three tumors for which adequate paraffin material was available for sequencing. In two specimens, identical FGFR3 mutations were detected in the primary tumor and corresponding lymph node metastasis.
Clinical profile of FGFR3 mutant high-grade urothelial cancers
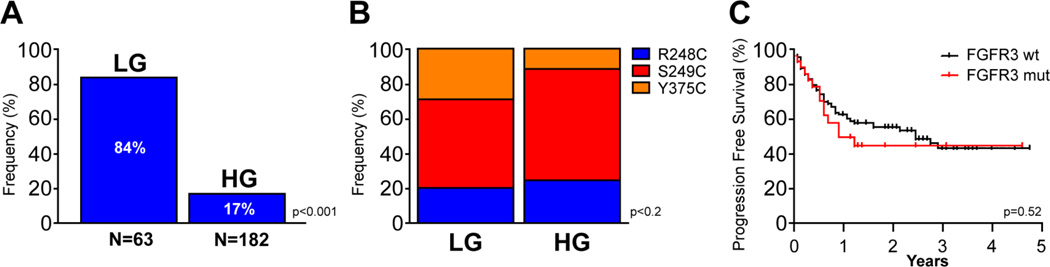
We compared the frequency and allele specificity of FGFR3 mutations in our 133 HGUC samples with sixty-three paraffin-embedded low-grade samples. As depicted in Figure 4A, 84% of the low-grade samples harbored an FGFR3 mutation, a rate consistent with that previously reported.[20, 27, 28] The distribution of mutant alleles was similar in the high and low-grade cohorts (Figure 4B). Notably, despite the association of FGFR3 mutations with a favorable prognosis in low grade UC, no statistically significant difference in outcome was observed in the high-grade cohort as a function of FGFR3 mutant status (Figure 4C).
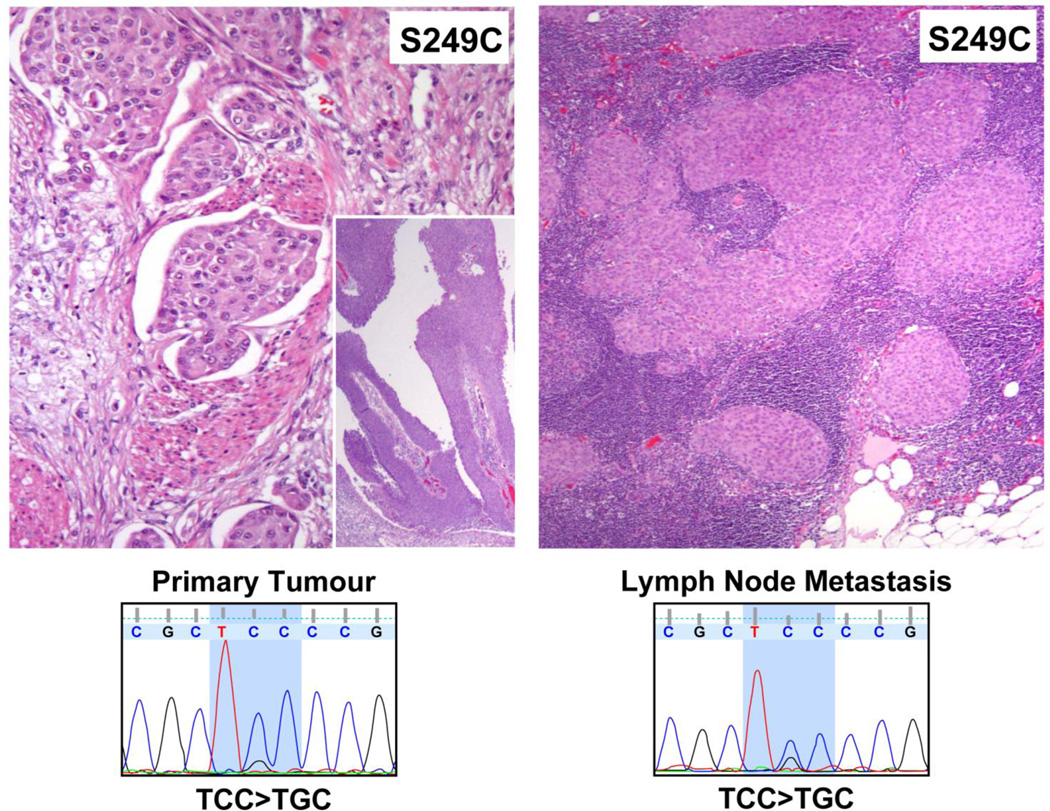
Figure 4.
Representative H&E stained section from a primary tumor invasive into muscularis propria; including non-invasive papillary component (inset) and matched lymph node metastasis are shown with corresponding Sanger traces demonstrating an FGFR3 S249C mutation in both samples.
The clinical characteristics and disease outcomes of all 30 unique FGFR3 mutant high-grade cases identified in both the retrospective and prospective cohorts are depicted in Table 2. Within this cohort, 14 of 30 patients (47%) developed tumor recurrence following cystectomy with 10 of 14 (71%) of these patients dead of disease with a median followup of 15.6 months. A comparison of the clinical characteristics between the FGFR3 mutant and wild type cohorts is provided in Table 3. Other than for an increased number of patients with muscularis propria-invasive disease or higher in the wild type cohort, the two populations were not significantly different with regards to gender, age, or clinical status.
Table 2.
Clinical Characteristics of FGFR3 Mutant Tumors
| Specimen ID |
Mutation | Age at Surgery |
Sex | Low Grade Component in Primary Tumor |
Primary Tumor Location |
Initial Stage | Stage at Surgery |
Clinical Status |
|---|---|---|---|---|---|---|---|---|
| B011 | G370C | 65 | M | N | Bladder | pT1 | pT1 | NED |
| B012 | G370C | 65 | M | Y | Bladder | pT2 | pT3N3 | DOD |
| B049 | S249C | 66 | M | Y | Bladder | pT1 | pT3N1 | DOD |
| B060 | S249C | 85 | M | N | Bladder | pT1 | pT1 | NED |
| B062 | D468N | 62 | M | N | Bladder | pT2 | pT3 | NED |
| B065 | S249C | 74 | M | Y | Bladder | pTa | pT1 | DOD |
| B072 | R248C | 75 | F | Y | Bladder | pTa | pT1 | NED |
| B078 | R248C | 62 | M | Y | Bladder | pT1 | pTa | DOD |
| B079 | S131L/S249C | 55 | M | Y | Bladder | pTa | pT1 | NED |
| B085 | R248C | 72 | M | Y | Bladder | pT1 | pT1 | DUK |
| B091 | Y375C | 61 | M | Y | Bladder | pT1 | pT2b | NED |
| B107 | S249C | 51 | F | Y | Bladder | pT1 | pT2b | NED |
| B118 | S249C | 75 | F | Y | Bladder | pTa | pTa | NED |
| B124 | S249C | 74 | M | Y | Bladder | pTa | pT3 | DOD |
| B126 | S249C | 86 | M | Y | Bladder | pT1 | pT3 | DOD |
| B128 | S249C | 52 | M | N | Bladder | pT2 | pT3 | AWD |
| B138 | S371C | 83 | F | N | Bladder | pT2 | pT2b | DOD |
| B148 | S249C | 81 | M | N | Bladder | pT1 | pT2aN1 | DOD |
| B154 | S249C | 85 | F | Y | Renal Pelvis | pTa | pT3a | NED |
| B156 | S249C | 68 | M | N | Bladder | pTa | pT2b | AWD |
| B157 | Y375C | 58 | M | Y | Ureter | pT2 | pT2 | NED |
| B158 | R248C | 54 | F | Y | Renal Pelvis | pTa | pTa | NED |
| B161 | S249C | 51 | F | N | Bladder | pTa | pT3 | AWD |
| B162 | R248C | 63 | M | N | Bladder | pT1 | pTa | NED |
| B163 | R248C | This sample is a separate tumor from the same cystectomy specimen as B162 | ||||||
| B164 | S249C | 57 | M | N | Bladder | pT1 | pT2bN3 | DOD |
| B165 | S249C | 76 | M | Y | Bladder | pTa | pTa | NED |
| B168 | Y375C | 51 | M | Y | Bladder/LN | pT2 | pT3N2 | NED |
| B171 | S249C | 70 | M | Y | Renal Pelvis | pTa | pT3bN2 | AWD |
| B175 | R248C | 73 | M | Y | Ureter | pTa | LG | NED |
| B177 | S249C | 80 | M | N | Bladder/LN | pTa | pT3N3 | DOD |
Table 3.
Comparison of Clinical Characteristics between FGFR3 mutant (mut) and wild type (wt) tumor samples
| Clinical Characteristics |
FGFR3 mut (%) |
FGFR3 wt (%) |
p-value (Fisher's exact test unless specified) |
|---|---|---|---|
| Gender | |||
| Male | 23 (77) | 112 (74) | =1.0000 |
| Female | 7 (23) | 39 (26) | |
| Cystectomy Stage | |||
| <pT2 | 13 (42) | 24 (16) | =0.0025 |
| Mean age (years) | 67.7 | 68.8 | |
| Clinical Status | |||
| DOD | 10 (33) | 40 (26) | =0.5519 (χ2) |
| AWD | 4 (13) | 9 (6) | |
| NED | 15 (50) | 69 (46) | |
| DUK | 1 (3) | 33 (22) | |
DISCUSSION
The recent success of selective kinases inhibitors in a subset of cancer types has generated hope that targeted inhibitors can be developed for a broader range of human cancer types. Thus far, this paradigm has been most effective with inhibitors of kinases that are mutationally activated. Examples include imatinib in patients with chronic myelogenous leukemia (abl) and gastro-intestinal stromal tumor (c-kit), erlotinib in non-small cell lung cancer (EGFR) and most recently PLX4032 in melanoma (BRAF).[12, 29–33] In the case of imatinib, abl translocations are pathognomonic for chronic myelogenous leukemia and thus pre-treatment patient selection beyond classical cytological characterization is not required in order to identify the population of patients most likely to respond. In contrast, it is now apparent that solid tumors as traditionally classified by tissue of origin harbor a diversity of mutations that confer overlapping selective advantage.
For example, EGFR, KRAS and BRAF mutations are all non-overlapping in non-small cell lung cancer.[34] While the mutual exclusivity of these alterations suggests that they all regulate overlapping downstream effectors of transformation, the specific oncogene mutated is highly predictive of response to targeted inhibitors of the pathway.[35, 36] Such observations suggest that the response of patients to targeted kinase inhibitors will depend strongly upon the complement of mutations within an individual patient’s tumor and that such predictors (both positive and negative) can be identified. The experience with EGFR targeted inhibitors in lung cancer further suggests that it is preferable to know the tumor genotype of patients prospectively in order to use this information to select the appropriate patients for trial.[12] This is particularly important if the frequency of a given mutation is low.
FGFR-3 is one of four members of the fibroblast growth factor receptor family of receptor tyrosine kinases that promote cell growth and proliferation.[37] Activation of this receptor family is mediated by receptor dimerization promoted by bivalent ligand binding facilitated by the co-association of an accessory molecule such as heparin.[37] Several prior studies have reported that activating mutations of FGFR-3 are particularly common in low-grade urothelial carcinoma.[20, 21, 38] Notably, the specific alleles found somatically mutated in bladder cancers when identified in the germline are associated with a variety of autosomal dominant skeletal disorders including severe achondroplasia with developmental delay and acanthosis nigricans as well as thanatophoric dysplasia.[17, 38, 39] Most of these FGFR3 mutations occur in the extracellular domain of FGFR-3 and promote constitutive receptor dimerization and activation. These findings along with additional preclinical studies using human cancer cell lines expressing mutant FGFR3 suggest that tumors expressing an activating FGFR3 mutant allele may be selectively sensitive to inhibitors of this kinase.[40]
As the bulk of the morbidity and mortality of bladder cancer is attributable to the development of muscularis propria-invasive high-grade disease, we sought to determine the frequency of FGFR3 mutations in high-grade bladder cancer and their prognostic relevance in this population. Although the frequency of FGFR3 mutation was lower in high grade (17%) versus low-grade (84%) urothelial cancers, in patients with HGUC, FGFR3 mutation was associated with an equally aggressive disease course as that of wild type tumors, with a high rate of recurrence and progression to muscularis propria-invasive disease. The poor outcome overall of patients with FGFR3 mutant HGUC justifies the testing of selective inhibitors of this kinase in patients with this disease. The high prevalence of FGFR3 mutations in low grade UC may, likewise, represent and attractive target for inhibition in this patient group.
The low frequency of FGFR3 mutation in the high-grade cohort, however, raises a number of logistical hurdles to the accrual of sufficient patients with advanced bladder cancer to trials of selective FGFR-3 inhibitors. With the goal of facilitating the identification of high-grade tumors harboring FGFR3 mutations, we compared in detail the morphologic appearance of FGFR3 mutant and wild-type tumors. Our retrospective review of 133 high-grade tumors found that FGFR3 mutation was associated with specific, easily recognizable histopathologic features that were characterized by the presence of a prominent exophytic, papillary component lined with polygonal cells possessing koilocytoid nuclei.
Our prospectively identified case control test set of 24 tumors exhibiting the predictive morphologic features and the 25 tumors lacking these features, further supports the importance of careful histopathologic review, as FGFR3 mutations were identified in 54% of the cases in the former group whereas all 25 control cases of the latter group were FGFR3 wild type. These results suggest that detailed histological evaluation of HGUC can aid in the identification of patients with FGFR3 mutations and thus facilitate the recruitment of such patients to trials of selective FGFR-3 inhibitors. Interestingly, the suggestive morphology of FGFR3 mutation accurately predicted the presence of such mutations in primary tumors of the upper tract, including renal pelvis and ureter, in addition to urinary bladder tumors. This observation may help to expand the applicability of FGFR3 targeting approaches to include tumors of the upper urinary tract as well.
Notably, 11 of 24 (46%) of the tumors in the prospective set that displayed some or all the features that predict for the presence of FGFR3 mutations were wild-type for the hotspot mutations included with our Sequenom assay. As the prospective set consisted entirely of FFPE samples, it is possible that in some of these cases, our failure to detect FGFR3 mutations may have been attributable to the lower sensitivity of this assay in FFPE as compared to frozen tissue. Alternatively, such cases may have harbored non-hotspot FGFR3 mutants not included within our Sequenom panel or genomic alterations that phenocopy FGFR3 mutation such as receptor amplification or FGF ligand overexpression. In support of this latter possibility, two FGFR3 mutant tumors from the 133 frozen samples in which all FGFR3 coding exons were sequenced contained non-hotspot mutations (D468N and S131L), which have not been reported in the Catalogue of Somatic Mutations in Human Cancer but similarly possessed an extensive exophytic papillary component indistinguishable from the tumors harboring the commonly recurrent hotspot mutations.
The presence of an FGFR3 mutation in HGUC does not ensure that these tumors remain dependent upon this kinase. Prior studies of urothelial cancer have shown that significant genetic heterogeneity is present in bladder tumors resected from individual patients as well as within regions of the same tumor. It is possible that FGFR3 mutations are key drivers of tumor growth and survival in low-grade tumors but that additional genetic events relegate FGFR3 mutations to passenger status in the high-grade tumors in which they are found. Twenty of the 31 FGFR3 mutant high-grade tumors in our series exhibited evidence of a low-grade component. Careful dissection and genetic analysis of the high- and low-grade components from three such tumors clearly demonstrated that the FGFR3 mutation was present in the high-grade component and confirmed that our finding of FGFR3 mutations in such tumors was not artifactual due to the presence and intermixing of a second low-grade component. Furthermore, given the concordance of the mutant alleles between high and low-grade components, our data suggest that in such tumors, the low-grade disease was in fact a precursor to the high-grade disease. These results also suggest that a subset of high and low grade tumors may possess overlap of activated signaling pathways. As for the FGFR3 patients for which no low-grade component could be detected, this result could be explained by either sampling error or the replacement of the low-grade tumor by overgrowth of the high-grade component. Alternatively, in some of these cases, no preceding low-grade component may have existed.
Finally, as FGFR3 mutations are predominantly found in superficial low grade UC, it was possible that the detected mutations within our high-grade set may have been present only in the exophytic, non-invasive regions of the tumors whereas the invasive component may have been FGFR3 wild-type. Using the Sequenom assay on FFPE material consisting of only the invasive component, however, we were able to confirm the presence of an FGFR3 mutation in the invasive component for all but one of the tumors for which sufficient tissue was available for analysis (Table 1). This is consistent with a previous report by Tomlinson et al.[27] Moreover, in both FGFR3 mutant cases for which metastatic material from pelvic lymph nodes was available, the identifiable FGFR3 mutant was detectable in both the primary and metastatic lesion. These data provide further support to the contention that FGFR3 mutations are present in a subset of high-grade metastatic urothelial cancers. Future studies will be needed, however, to confirm that mutant FGFR3 remains biologically relevant in this context and thus represents a rational therapeutic target.
Figure 5.
Incidence and Distribution of FGFR3 mutations in low and high grade UC. A, A significantly higher incidence of FGFR3 mutations were detected in 63 low grade samples as compared to 182 high grade samples (p value as indicated, log-rank test). B, Comparison of the distribution of FGFR3 hotspot mutations (R248C, S249C, and Y375C) between low and high grade tumors. C, Kaplan-Meier plot of progression-free survival of 182 patients with high grade bladder cancer as a function of FGFR3 mutation status.
Acknowledgements
This work was support in part by the American Association for Cancer Research-Henry Shepard Bladder Cancer Research Fund, the Wiener fund, the Abrams Foundation, and an American Society of Clinical Oncology Young Investigator Award (GI). We are grateful for the technical assistance and support of members of the Geoffrey Beene Translational Oncology Core including Kety Huberman, Igor Dolgalev, and Sabrena Thomas.
Footnotes
Conflict of interest: None
Statement of author contributions
HA and GI conceived and carried out the experiments. DS supervised the design and execution of multiple aspects of this work. AH and MJ assisted with collection of sequencing data and analysis. VR, ST, OL, SF, AG and YC contributed to data validation. BB and GD assisted in acquisition of clinical material. DB, MM, AB and JR collected and analyzed clinical data. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997;158:62–67. doi: 10.1097/00005392-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Grossman HB, O'Donnell MA, Cookson MS, Greenberg RE, Keane TE. Bacillus calmette-guerin failures and beyond: contemporary management of non-muscle-invasive bladder cancer. Rev Urol. 2008;10:281–289. [PMC free article] [PubMed] [Google Scholar]
- 4.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 5.Milowsky MI, Stadler WM, Bajorin DF. Integration of neoadjuvant and adjuvant chemotherapy and cystectomy in the treatment of muscle-invasive bladder cancer. BJU Int. 2008;102:1339–1344. doi: 10.1111/j.1464-410X.2008.07980.x. [DOI] [PubMed] [Google Scholar]
- 6.Jordan AM, Weingarten J, Murphy WM. Transitional cell neoplasms of the urinary bladder. Can biologic potential be predicted from histologic grading? Cancer. 1987;60:2766–2774. doi: 10.1002/1097-0142(19871201)60:11<2766::aid-cncr2820601129>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Herr HW. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol. 2000;163:60–61. discussion 61-62. [PubMed] [Google Scholar]
- 8.Smits G, Schaafsma E, Kiemeney L, Caris C, Debruyne F, Witjes JA. Microstaging of pT1 transitional cell carcinoma of the bladder: identification of subgroups with distinct risks of progression. Urology. 1998;52:1009–1013. doi: 10.1016/s0090-4295(98)00374-4. discussion 1013-1004. [DOI] [PubMed] [Google Scholar]
- 9.Herr HW. Tumour progression and survival in patients with T1G3 bladder tumours: 15-year outcome. Br J Urol. 1997;80:762–765. doi: 10.1046/j.1464-410x.1997.00431.x. [DOI] [PubMed] [Google Scholar]
- 10.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 11.Sievert K, Amend B, Nagele U, Schilling D, Bedke J, Horstmann M, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009;27:295–300. doi: 10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 13.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 14.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Frias ML, de Frutos CA, Bermejo E, Nieto MA. Review of the recently defined molecular mechanisms underlying thanatophoric dysplasia and their potential therapeutic implications for achondroplasia. Am J Med Genet A. 2010;152A:245–255. doi: 10.1002/ajmg.a.33188. [DOI] [PubMed] [Google Scholar]
- 18.Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 19.di Martino E, L'Hote CG, Kennedy W, Tomlinson DC, Knowles MA. Mutant fibroblast growth factor receptor 3 induces intracellular signaling and cellular transformation in a cell type- and mutation-specific manner. Oncogene. 2009;28:4306–4316. doi: 10.1038/onc.2009.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billerey C, Chopin D, Aubriot-Lorton MH, Ricol D, Gil Diez de Medina S, Van Rhijn B, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955–1959. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez S, Lopez-Knowles E, Lloreta J, Kogevinas M, Amoros A, Tardon A, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol. 2006;24:3664–3671. doi: 10.1200/JCO.2005.05.1771. [DOI] [PubMed] [Google Scholar]
- 22.van Rhijn BW, Vis AN, van der Kwast TH, Kirkels WJ, Radvanyi F, Ooms EC, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol. 2003;21:1912–1921. doi: 10.1200/JCO.2003.05.073. [DOI] [PubMed] [Google Scholar]
- 23.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. The American journal of surgical pathology. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Pratilas CA, Hanrahan AJ, Halilovic E, Persaud Y, Soh J, Chitale D, et al. Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res. 2008;68:9375–9383. doi: 10.1158/0008-5472.CAN-08-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janakiraman M, Vakiani E, Zeng Z, Pratilas CA, Taylor BS, Chitale D, et al. Genomic and biological characterization of exon 4 KRAS mutations in human cancer. Cancer Res. 2010;70:5901–5911. doi: 10.1158/0008-5472.CAN-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Ahmadie HA, Liu O, Iyer GV, Heguy A, Gopalan A, Fine SW, et al. Unique Morphologic Characteristics of High Grade Urothelial Carcinoma with Fibroblast Growth Factor Receptor-3 (FGFR3) Gene Mutations. Mod Pathol. 2010;23:768. [Google Scholar]
- 27.Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol. 2007;213:91–98. doi: 10.1002/path.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura T, Suzuki H, Ohashi T, Asano K, Kiyota H, Eto Y. The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer. 2001;92:2555–2561. doi: 10.1002/1097-0142(20011115)92:10<2555::aid-cncr1607>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–4817. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- 30.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 31.Demetri GD. Targeting the molecular pathophysiology of gastrointestinal stromal tumors with imatinib. Mechanisms, successes, and challenges to rational drug development. Hematol Oncol Clin North Am. 2002;16:1115–1124. doi: 10.1016/s0889-8588(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 32.Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 33.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J Clin Oncol. 2010;28:4769–4777. doi: 10.1200/JCO.2009.27.4365. [DOI] [PubMed] [Google Scholar]
- 35.Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barton S, Starling N, Swanton C. Predictive Molecular Markers of Response to Epidermal Growth Factor Receptor(EGFR) Family-Targeted Therapies. Curr Cancer Drug Targets. 2010;10:799–812. doi: 10.2174/156800910793357925. [DOI] [PubMed] [Google Scholar]
- 37.Haugsten EM, Wiedlocha A, Olsnes S, Wesche J. Roles of Fibroblast Growth Factor Receptors in Carcinogenesis. Mol Cancer Res. 2010;8:1439–1452. doi: 10.1158/1541-7786.MCR-10-0168. [DOI] [PubMed] [Google Scholar]
- 38.van Rhijn BW, van Tilborg AA, Lurkin I, Bonaventure J, de Vries A, Thiery JP, et al. Novel fibroblast growth factor receptor 3 (FGFR3) mutations in bladder cancer previously identified in non-lethal skeletal disorders. Eur J Hum Genet. 2002;10:819–824. doi: 10.1038/sj.ejhg.5200883. [DOI] [PubMed] [Google Scholar]
- 39.Webster MK, Donoghue DJ. FGFR activation in skeletal disorders: too much of a good thing. Trends Genet. 1997;13:178–182. doi: 10.1016/s0168-9525(97)01131-1. [DOI] [PubMed] [Google Scholar]
- 40.Tomlinson DC, Hurst CD, Knowles MA. Knockdown by shRNA identifies S249C mutant FGFR3 as a potential therapeutic target in bladder cancer. Oncogene. 2007;26:5889–5899. doi: 10.1038/sj.onc.1210399. [DOI] [PMC free article] [PubMed] [Google Scholar]