Abstract
Optimal intestinal calcium (Ca) absorption is necessary for the protection of bone and the prevention of osteoporosis. Ca absorption can be represented as the sum of a saturable pathway and a non-saturable pathway that is primarily dependent upon luminal Ca concentration. While models have been proposed to describe these transport components, significant gaps still exist in our understanding of these processes. Habitual low intake of Ca up-regulates the saturable transport pathway, a process mediated by increased renal production of 1,25 dihydroxyvitamin D (1,25(OH)2 D). Consistent with this, low vitamin D status as well as deletion/mutation of the vitamin D receptor (VDR) or 25 hydroxyvitamin D-1α hydroxylase (CYP27B1) genes limit Ca absorption by reducing the saturable pathway. There is some evidence that non-saturable Ca absorption in the ileum is also regulated by vitamin D status, but the mechanism is unclear. Treatment with a number of hormones can regulate Ca absorption in vivo [e.g. parathyroid hormone (PTH), thyroid hormone, growth hormone (GH)/insulin-like growth factor I (IGF-1), estrogen, testosterone]. However, some of these actions are indirect (i.e. mediated through the regulation of vitamin D metabolism or signaling), whereas only a few (e.g. estrogen, IGF-1) have been shown to persist in the absence of vitamin D signaling.
Keywords: vitamin D receptor, estrogen, IGF-1, pregnancy, kinetics
I. An Overview of Intestinal Calcium Absorption
Whole body calcium (Ca) metabolism is controlled by a three tissue axis of intestine, kidney, and bone in an effort to maintain serum Ca within a narrow range (1). In this light, intestinal Ca absorption from the diet is an essential process maintaining Ca balance and bone health.
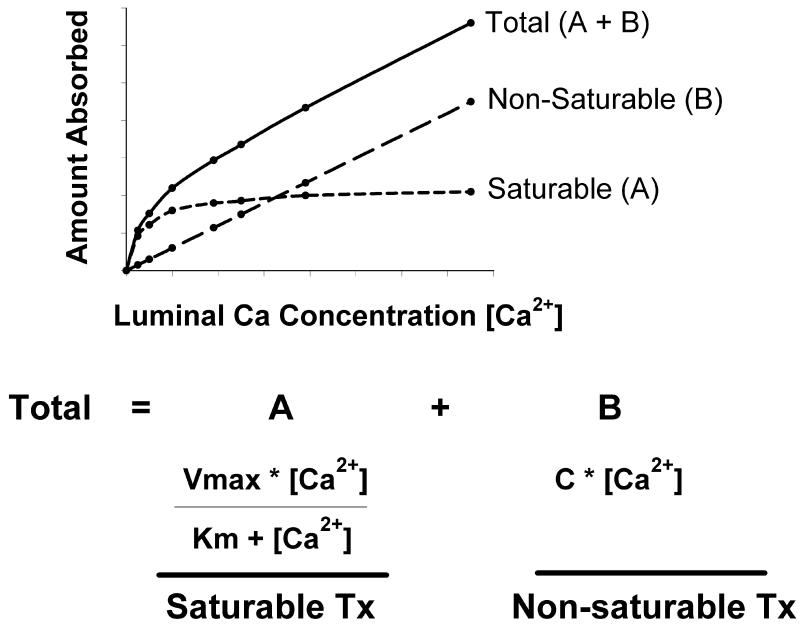
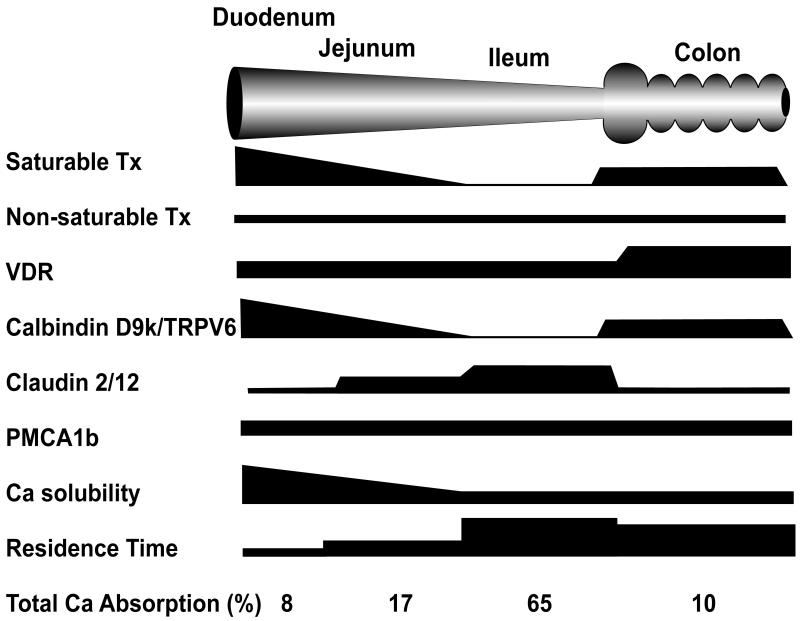
Ca absorption is moderately efficient in free living humans with 35% of a dietary load typically being absorbed. Kinetic modeling based on the efficiency of absorption across a wide range of luminal Ca concentrations shows that the transfer of Ca across the intestinal barrier occurs through both saturable (presumably transcellular) and non-saturable (presumably paracellular diffusion) pathways (2-5) (Figure 1). The existence of these two transport routes can be modeled mathematically using a Michaelis-Menten equation modified to include a linear component. The saturable component of Ca absorption is prevalent in the proximal small intestine (i.e. duodenum and jejunum) and is under nutritional and physiological regulation. This is an energy dependent pathway whereby Ca movement from the mucosal-to-serosal side of the intestinal barrier can occur against a concentration gradient (6). The saturable pathway is absent in the ileum (7), but some animal studies have reported that saturable Ca transport may be functional in the lower bowel (8-10). In contrast, passive transport occurs throughout the length of the intestine and is a non-saturable, linear function of luminal Ca concentration (e.g. 13% of luminal load per hour in humans (5)). The point at which the saturable component of Ca absorption from the small intestine of adults is at 50% maximum (Km) can be calculated from data published by Heaney et al. 4 and Sheik et al. (4,5). Assuming meal Ca levels are diluted by a factor of 2 in the stomach, the Km is equivalent to a meal level of approximately 265 mg Ca. However, even at 2 g of calcium in a single meal, the saturable component of Ca transport is still at only 90% capacity. For recommended levels of Ca intake (i.e 400-500 mg per meal), the saturable component of Ca absorption will account more than 60% of total Ca absorption in the small intestine demonstrating the importance of the saturable Ca absorption pathway under normal dietary loads. Under adequate-to-high Ca intakes, the proportion of Ca transported in any given segment is determined by the presence of the saturable and non-saturable pathways, the sojourn time through the intestinal tract, and the solubility of Ca within the intestinal segment (e.g. in the ileum and lower bowel, where pH is neutral or basic, Ca solubility may be < 20% that seen in the duodenum (11)). As a result, even though Ca solubility is low and the saturable pathway is absent or very low, the total amount of Ca absorption is greatest in the ileum since transit time through this segment is 10 or more times longer in comparison to the more proximal intestinal segments (12,13) (Figure 2).
Figure 1. Kinetic modeling of intestinal Ca absorption demonstrates the existence of saturable and non-saturable pathways.
Examination of Ca absorption under a range of luminal concentrations have demonstrated that total Ca transport is described by a curvilinear function. Total transport (A+B) is the sum of a saturable component (A) (defined by the Michaelis-Menton equation) and a concentration-dependent, non-saturable component (B) (defined by a linear equation). A = Saturable Ca Tx; B = Non-saturable Ca Tx; [Ca2+] = luminal concentration of Ca; C = the slope of the non-saturable linear component; Vmax = the maximum transport rate for the saturable transport component; Km = the luminal concentration of Ca at ½ Vmax. Tx = transport.
Figure 2. A schematic demonstrating the relationships between features proposed to be critical for intestinal Ca absorption.
Net Ca absorption is determined by many factors and different mechanisms for Ca absorption may be present in different intestinal segments. This schematic summarizes these features graphically in black. The height of the black bar signifies the relative importance or abundance of a feature for a given intestinal segment. Refer to the text for details about the various parameters listed in the figure. Tx = transport.
II. Regulation of Intestinal Ca Metabolism
The amount of Ca absorbed from the diet is a function of the amount of Ca consumed and the efficiency of the Ca absorption process. It is this latter process that is strongly regulated by physiological state (e.g. pregnancy and lactation, maturity/aging) and by habitual dietary Ca intake (1). In the next several sections we will review the evidence supporting the regulation of intestinal Ca absorption by vitamin D, describe the molecular mechanisms that have been proposed to account for intestinal Ca absorption, and finish by summarizing studies on the regulation of intestinal Ca absorption by other hormones.
A. Role of Vitamin D Status and Vitamin D Signaling in Intestinal Ca Absorption
The habitual consumption of low Ca diets is a major stimulus increasing the efficiency of intestinal Ca absorption. As discussed below, this effect is mediated through changes in the serum levels of specific hormones, particularly the active metabolite of vitamin D. While early studies suggested that intestinal Ca absorption is up-regulated by low dietary Ca (14,15), Pansu et al. (3) directly showed that feeding a low Ca diet to rats for 5 weeks (0.17% Ca vs. 0.44% in the reference group) increased the efficiency of duodenal Ca absorption by specifically increasing saturable Ca transport (Vmax increased 55%). Similarly, Dawson-Hughes et al. (16) found that in women, the efficiency of Ca absorption increased by 32% within 1 week of reducing dietary Ca intake from 2000 mg/d to 300 mg/d. It is now understood that the increased efficiency of Ca absorption resulting from habitual low Ca intake is due to an adaptation mediated by increased renal production of the active, hormonal form of vitamin D, 1,25 dihydroxyvitamin D [1,25(OH)2 D] (17).
For over 70 years we have known that Ca absorption in the gut is dependent upon adequate vitamin D (18,19). The efficiency of intestinal Ca absorption is reduced by >75% in vitamin D deficient animals (7) and in dialysis patients with compromised renal function and low circulating 1,25(OH)2 D levels (20). The deficit in Ca absorption caused by vitamin D deficiency can be restored by either repletion of vitamin D status or with injections of 1,25(OH)2 D. In rat duodenum (7) and in differentiated monolayers of the human intestinal cell line Caco-2 (21), the effect of 1,25(OH)2 D on Ca absorption is limited to the saturable component of transport, leading to an increase in Vmax (i.e. the maximal capacity) but not Km, suggesting that more transporters are produced following 1,25(OH)2 D treatment. The regulation of transcellular Ca movement by vitamin D status and 1,25(OH)2 D treatment has been clearly demonstrated in duodenum using ion microscopy to follow the movement of 44Ca across the absorptive epithelial cell. Chandra et al. (22) showed that while Ca normally flows from the apical side of the enterocyte through the epithelial cell over the course of 20 minutes; however, in vitamin D deficient chicks, Ca can enter the enterocyte, but is trapped in the region just below the microvilli. This group later showed that treating vitamin D deficient chicks with 1,25(OH)2 D caused a progressive redistribution of Ca from the brush border region into the cytoplasm to the basolateral membrane (23). The 1,25(OH)2 D effect was first seen 2-4 hours after the treatment, consistent with the induction of gene expression mediated through the vitamin D receptor (VDR). These data strongly supports the hypothesis that the 1,25(OH)2 D-regulated, saturable component of duodenal Ca absorption is a transcellular process. However, there is some evidence that the non-saturable portion of Ca absorption in the human ileum is also vitamin D sensitive; the slope of the non-saturable transport pathway is reduced in chronic renal disease patients and returns to normal after 1,25(OH)2 D injection (5).
a. Critical Role of VDR in Control of intestinal Ca Absorption
Since the 1970s, we’ve understood that many of the biological effects associated with 1,25(OH)2 D are dependent upon transcriptional events that require its binding to the nuclear VDR (24,25). The importance of the VDR for normal intestinal Ca absorption was shown experimentally using VDR knockout (KO) mice, where VDR deletion reduces Ca absorption efficiency by >70% in growing animals. The loss of Ca absorption causes the major phenotypes associated with VDR gene deletion, i.e. poor growth, low serum Ca, high serum parathyroid hormone (PTH), and severe osteomalacia (26,27). Two studies have used transgenic expression of VDR directed to the intestine to rescue the phenotype of the VDR KO mouse. Initial studies using the adenosine deaminase promoter/enhancer to drive transgenic VDR expression to the distal duodenum and jejunum showed that this is inadequate for recovery of the VDR KO mouse on a chow diet, but can improve the phenotype of VDR KO mice fed a high Ca rescue diet (28). This suggests that although the proximal duodenum and jejunum are highly responsive to 1,25(OH)2 D in normal mice, VDR function in additional segments are necessary to optimize intestinal Ca absorption and normalize Ca metabolism. Consistent with this hypothesis, villin promoter-mediated transgenic expression of VDR throughout the intestinal epithelium was sufficient to completely normalize duodenal Ca absorption, serum PTH and Ca, and bone mineral density in VDR KO mice (29). This work supports the hypothesis that the primary role for VDR signaling relevant to Ca metabolism is the control of intestinal Ca absorption efficiency.
It has been proposed that intestinal VDR level or function may be an important factor influencing intestinal Ca absorption under a variety of conditions. For example, loss of basal and vitamin D-responsive Ca absorption during aging (30,31) or after estrogen depletion (32,33) is associated with a reduction in intestinal VDR (34,35). Consistent with the importance of VDR level in these conditions, inducible over-expression of VDR in the intestinal cell line Caco-2 increases 1,25(OH)2 D-regulated transcellular Ca transport (36) while reduced intestinal VDR levels seen in mice heterozygous for the VDR KO allele blunts 1,25(OH)2 D-regulated intestinal Ca absorption efficiency (37).
Some have proposed that polymorphisms in the VDR gene can influence Ca absorption and the intestinal response to 1,25(OH)2 D. The F allele of the Fok I gene polymorphism disrupts the translation codon (ATG to ACG), shortens VDR protein by three amino acids, and makes it more transcriptionally active (38). Studies in children and in young Chinese women report that individuals homozygous for the shorter, more transcriptionally active F allele have greater Ca absorption efficiency compared to individuals with the f allele (39,40). However, Zmuda et al. (41) found no relationship between FokI genotype and Ca absorption in older African-American women. Polymorphisms within the distal and 3′ UTR regions of the VDR gene have been identified and are in strong linkage disequilibrium: BsmI (B/b), ApaI (A/a), and TaqI (T/t) (42,43). Unlike the FokI polymorphism, the impact of these polymorphisms on VDR function, transcription, or mRNA stability is unclear. None-the-less, several studies have shown that individuals homozygous for the “high bone density” BsmI b allele (42) have higher intestinal Ca absorption than those with the B allele (43-45), although other studies have not found an association between BsmI geneotype and calcium absorption (46-49). Another study using haplotypes of VDR polymorphisms found that Ca absorption was significantly increased in women with the haplotype associated with high bone mass (bbaaTT) compared to the other other VDR haplotypes even when corrected for dietary calcium intake, serum 1,25(OH)2D level and body weight (43). Collectively, these data support the hypothesis that variations in VDR level or function may influence intestinal Ca absorption.
b. Can High Vitamin D Status Increase Intestinal Ca Absorption?
There is some controversy regarding whether intestinal Ca absorption is responsive only to increased serum levels of 1,25(OH)2 D (7,50,51) or whether it can also increase in response to improved serum 25 hydroxyvitamin D [25(OH) D] levels, independent of renal conversion to 1,25(OH)2 D. Heaney et al. (52) reported the first suggestion of an independent effect of high serum 25(OH) D levels. They found that Ca absorption (measured by appearance of 45Ca in serum 5 hours after a meal containing 300 mg Ca) was increased by 25% after 4 weeks of treatment with 50 μg 25(OH) D/d even though serum 1,25(OH)2 D levels did not change. Heaney et al. (53) later showed that as serum 25(OH) D increased from 50 to 86.5 nmol/L, there was a 65% increase in Ca absorption efficiency in post-menopausal women (increased area under the curve of serum Ca 5 h after an oral load of 500 mg Ca). These studies suggest that the enzyme necessary for conversion of 25(OH) D to 1,25(OH)2 D in the kidney, 25 hydroxyvitamin D-1α hydroxylase (CYP27B1), is also present in the intestine. This hypothesis is supported by the fact that CYP27B1 promoter activity is seen in the jejunum and ileum of transgenic mice expressing a 1.5 kb CYP27B1 promoter-luciferase reporter gene (54) and by the detection of CYP27B1 mRNA (by RT-PCR) and protein (by immunohistochemistry) in the human duodenum (55). In addition, the mRNA level of a well-characterized vitamin D target gene, transient receptor potential cation channel, subfamily V, member 6 (TRPV6), correlated well with CYP27B1 mRNA in human duodenal biopsies, but not with serum 1,25 (OH)2 D (55), suggesting local production of the hormone accounts for the increase in TRPV6 expression.
Data from several reports do not strongly support a role for high vitamin D status as a regulator of intestinal Ca absorption. First, Abrams et al. (56) found that higher serum 25(OH) D levels were not consistently associated with higher Ca absorption efficiency in a cross-sectional study of 251 school age children. Also, studies by Need et al. (57,58) found that the efficiency of intestinal Ca absorption was normal across a wide range of vitamin D status levels [20-90 nmol/L 25(OH) D] in adults. Increasing vitamin D status was only beneficial when subjects were so deficient that their serum 25(OH) D levels were inadequate to support renal production of 1,25(OH)2 D. Hansen et al. (59) reported that raising serum 25(OH) D from 55 to 160 nmol/L in women with a 50,000 IU daily supplement of vitamin D2 for 15 days increased Ca absorption efficiency only modestly (13% above baseline as determined by a dual stable isotope technique). Thus, the hypothesis that improving vitamin D status, beyond that necessary to support adequate renal production of 1,25(OH)2 D, can regulate intestinal Ca absorption is not strongly supported by the literature. However, although a cross-sectional study by Aloia et al. (60) did not find an association between Ca absorption and serum 25(OH) D levels in 495 healthy women, they found that the positive relationship between serum 1,25(OH)2 D and Ca absorption was stronger for low serum 25(OH) D levels than for high levels. This interaction deserves further study.
B. Molecular Models of Ca absorption
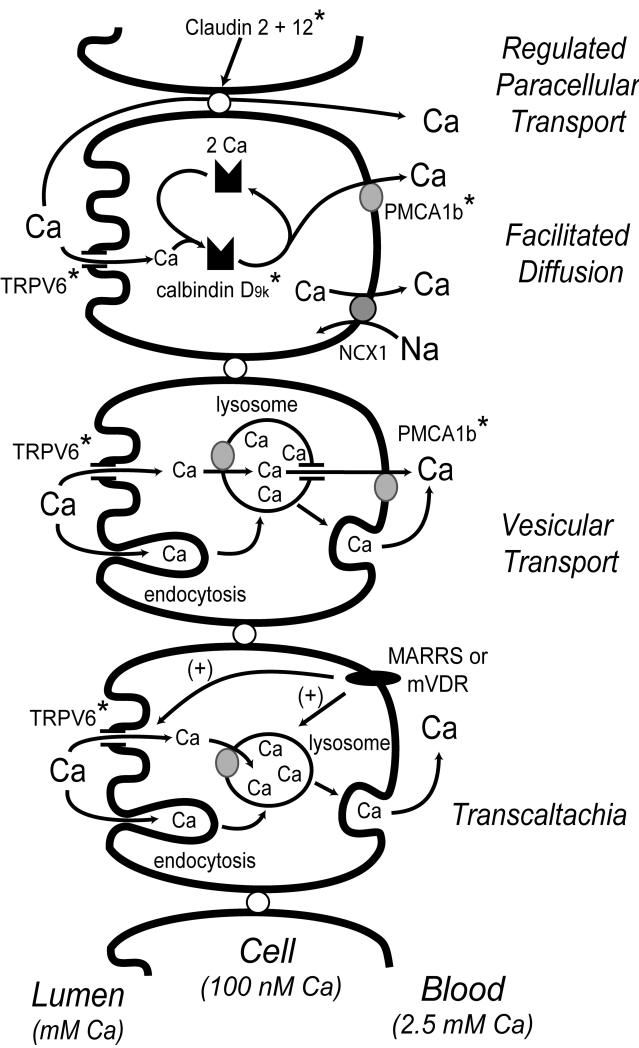
Four models have been proposed to explain the mechanism for intestinal Ca absorption: facilitated diffusion, vesicular trafficking, transcaltachia, and regulated paracellular transport. In the following section we will review each of these models with an emphasis towards relating them to the physiologic data defining Ca absorption and its regulation by vitamin D.
a. Facilitated Diffusion
The biochemical evidence supporting this model was summarized by Bronner et al. (61) in a critical review of transport data from isolated brush border membrane vesicles (BBMV), isolated basolateral membrane vesicles, and a variety of whole intestine transport methods conducted in rats and chicks (e.g. everted gut sacs, in situ transport, Ussing chambers). This review concluded that while both Ca uptake and Ca extrusion were vitamin D regulated processes, it was the intracellular diffusion of Ca that was the rate limiting step in the transcellular Ca transport process. From these data, Bronner et al. (61) and others have built the facilitated model shown in Figure 3.
Figure 3. Models for intestinal Ca absorption.
Several models have been proposed to explain how Ca transverses the intestinal barrier. The facilitated diffusion, vesicular transport, and transcaltachia models are all transcellular absorption pathways. In contrast, claudin 12 and 2 have been proposed to provide selectivity for Ca to the tight junction complex. While most of the research suggests that Ca absorption is regulated by vitamin D at a transcriptional level (putative targets for VDR-mediated gene transcription are identified by an asterisk), the transcaltachia model describes rapid, 1,25(OH)2 D-induced, transepithelial Ca movement that requires a basolateral membrane receptor (i.e. either MARRS or a membrane localized version of VDR, mVDR). For details of how vitamin D regulates various aspects of these models, refer to the text.
In the facilitated diffusion model, vitamin D-dependent uptake of Ca across the brush border membrane into the enterocyte is mediated by TRPV6 (also known as CaT1 or ECAC2), an apical membrane Ca channel (62). TRPV6 mRNA level is reduced by more than 90% in the duodenum of VDR KO mice and the TRPV6 gene is strongly regulated by 1,25(OH)2 D in cultured intestinal cells (63-65) and in the duodenum of mice (51,64) and humans (66). In addition, induction of TRPV6 mRNA precedes the increase in duodenal Ca absorption that occurs following a single 1,25(OH)2 D injection (51). However, in contrast to the implication by some that TRPV6 induction is the rate limiting step in transcellular Ca transport, Ca can enter the enterocyte in vitamin D deficient chicks, but is trapped in the region just beneath the microvilli (22). Furthermore, in TRPV6 KO mice, intestinal Ca absorption across everted gut sacs was still responsive to 1,25(OH)2 D injection (67,68) and the improved absorption resulting from feeding a low Ca diet was reduced by only 40% (68). While there may be some compensation by other Ca channels for the loss of TRPV6 in the KO mouse, these data do not support the simple hypothesis that TRPV6 is the sole means by which Ca can enter the enterocyte during Ca absorption.
The central player in the facilitated diffusion model is the cytoplasmic Ca binding protein calbindin D. There are two forms of calbindin D, a 9 kd form found in mammalian intestine and mouse kidney (calbindin D9k) and a 28 kd form found in the avian intestine and kidney and in the mammalian kidney (calbindin D28k). Calbindins are small EF-hand proteins that can bind either 2 (D9k) or 4 (D28k) moles of Ca per mole of protein (69). Calbindin protein levels positively correlate to Ca absorption over a wide range of biological conditions (61). In vitamin D deficient animals and in VDR KO mice, calbindin levels in the intestine are significantly reduced (26,70). In addition, 1,25(OH)2 D injections significantly increase duodenal levels of calbindin, suggesting that calbindin D9k and D28k may be vitamin D target genes (71). Finally, disruption of Ca binding to calbindins with theophyline treatment adversely affects intestinal Ca absorption in rats (72). Collectively, these data suggest that calbindins are proteins that participate in Ca transport by either acting as an intracellular buffer (i.e. limiting second messenger signaling by Ca during the transport process) or as a ferry that permits Ca to move away from the apical membrane to the basolateral membrane (22,73).
However, several studies refute the hypothesis that calbindins are necessary for intestinal Ca absorption. First, several studies have shown that intestinal Ca absorption can be low even when calbindin protein levels are elevated (27,51,74), suggesting that elevated calbindin levels are not sufficient to maintain elevated intestinal Ca absorption under all conditions. In addition, although the facilitated diffusion model predicts that Ca absorption cannot occur in the absence of calbindin D, two different lines of calbindin D9k null mice are phenotypically normal and neither basal nor 1,25(OH)2 D-induced Ca absorption (measured using the everted sac technique) are affected (68,75-77). However, although both single calbindin D9k null mice and single TRPV6 null mice have normal duodenal Ca absorption after 1,25(OH)2 D treatment, the ability of double calbindin D9k/TRPV6 KO mice to increase Ca absorption in response to 1,25(OH)2 D is reduced by 60% compared to wild-type mice (68). This suggests that TRPV6 and calbindin D9k proteins together may have a special role in Ca absorption, and that their interaction is more complex than the current iteration of the facilitated diffusion model predicts.
The final step in the facilitated diffusion model is the extrusion of Ca from the cell. Favus et al. (6) used Ussing chambers to show that the ATPase inhibitor trifluoroperizine could reduce transcellular Ca transport in duodenal segments and block the increase in Ca transport induced by prior treatment with 1,25(OH)2 D. This ATP-dependent process is localized to the basolateral membrane and is necessary to move Ca up the concentration gradient that exists between the enterocyte cytoplasm and the serum. Wasserman et al., (78,79) later identified the plasma membrane Ca ATPase 1b (PMCA1b) as a basolateral protein whose protein and mRNA level was expressed throughout the chick intestine, reduced by vitamin D deficiency, and increased by vitamin D repletion (2-3 fold) or by consumption of low Ca diets. While some have suggested that the basolateral extrusion of Ca may also be mediated by a sodium-Ca exchanger (80), Favus et al. (6) reported that sodium-potassium pump inhibitors that disrupt the sodium gradient necessary for sodium-Ca exchange had no impact on Ca transport across duodenal segments mounted in Ussing chambers.
b. Vesicular Transport
Several observations support sequestration of Ca into vesicles within the cell as an alternative mechanism to the role proposed for calbindin D as a Ca ferry/buffer during transcellular intestinal Ca absorption (Figure 3). 1,25(OH)2 D treatment can increase the number of lysosomes in chick intestine (81) and the release of lysosomal enzymes from isolated rat enterocytes (82), suggesting there is a vitamin D-dependent increase in the activity and cycling of lysosomes. In addition, during Ca absorption: Ca accumulates in lysosome-like structures (83), the level of lysosomal Ca is increased 3.1-fold within 10 hours of 1,25(OH)2 D treatment (84), and Ca is associated with endosomes in the brush border membrane of intestinal epithelial cells prior to its appearance in lysosomes (85). Consistent with an essential role for lysosomes in intestinal Ca absorption, disrupting lysosomal pH with agents like quinacrine and chloroquine do not stop Ca entry into enterocytes (84) or ATP-mediated Ca extrusion (86), but it prevents lysosomal Ca accumulation and blocks Ca absorption (84). Collectively, these data suggest that vesicular movement is a legitimate pathway for uptake and movement of Ca through intestinal epithelial cells. It is not clear, however, what makes the vesicular transport pathway specific for Ca. In chick intestine, Nemere et al. (84) identified calbindin D28k in an endosome-like compartment and in lysosomes containing Ca after 1,25(OH)2 D treatment. However, similar observations have not been made in mammalian intestinal epithelial cells and, given the fact that Ca absorption is normal in calbindin D9k KO mice, a role for calbindin D as the factor defining Ca specificity to the vesicular transport system seems unlikely.
c. Transcaltachia
In contrast to the mechanisms described above, transcaltachia is a mode of Ca transport that occurs within minutes of exposing enterocytes to 1,25(OH)2 D. This is consistent with a body of literature on the existence of rapid 1,25(OH)2 D actions that are initiated at the cell membrane and that are independent of new transcriptional events, such as activating kinase signaling pathways and the opening of chloride channels (87). Transcaltachia has been directly demonstrated in the perfused chick duodenum where exposure to a physiologic dose of 1,25(OH)2 D for 14 minutes increased Ca appearance in the serosal perfusate by 40% (88). This effect occurs only in response to serosal exposure to 1,25(OH)2 D and it is lost in the intestine of vitamin D deficient chicks. This suggests that in absorptive epithelial cells in the intestine there is a membrane receptor on the basolateral surface of whose level, or whose downstream components, are dependent upon vitamin D regulated synthesis of new proteins. The exact mechanism that Ca follows through the cell during transcaltachia has not been determined with certainty, but these data are consistent with the vesicular transport model (89), as well as a need for the apical membrane Ca channel TRPV6 (90).
The existence of a specific basolateral membrane vitamin D binding protein was first shown in 1,25(OH)2 D-binding studies using chick duodenum (91). Some reports suggest that this represents a novel, non-nuclear role for the VDR (92) while other data indicates that transcaltachia is mediated through a new vitamin D binding protein called the membrane associated rapid response steroid binding protein (MARRS) (93). In support of the VDR hypothesis, mouse intestinal VDR was found associated with a caveolae-rich membrane fraction (92). Activation of the rapid, non-transcriptional roles mediated through the VDR is proposed to occur through a unique alternative ligand binding pocket (94); specific vitamin D analogs that are proposed to fit into this alternative binding pocket can stimulate rapid effects of 1,25(OH)2 D, including transcaltachia (95).
MARRS is a protein with multiple functions and names (e.g. ERp57, PLCα, PDIA3). Ribozyme mediated knockdown of MARRS was shown to reduce phosphate uptake into chick enterocytes (93) and studies recently reported at a national meeting found that intestine-specific deletion of MARRS in mice reduces 1,25(OH)2 D binding and disrupts 1,25(OH)2 D regulated Ca uptake into enterocytes (96). However, it is not yet clear whether MARRS deletion has an impact on intestinal Ca absorption, whole body Ca metabolism, or bone. In addition, there are other aspects to the transcaltachia model that limit its acceptance as a physiologically important pathway. For example, the rapid fluxes in serum 1,25(OH)2 D needed for transcaltachia have not been reported, particularly during the consumption of Ca-rich meals when transcaltachia would have to occur for the physiologic benefit of the process to be realized. Thus, for transcaltachia to gain full acceptance as an important mode for regulating intestinal Ca absorption, additional research is needed to place regulation through this mechanism into a more physiologic context.
d. Regulated Paracellular Movement through Tight Junctions
Although much of the research on intestinal Ca absorption has focused on explaining the vitamin D-regulated changes in saturable Ca transport that is prominent in the proximal small intestine, several studies have shown that vitamin D signaling increases diffusional, presumably paracellular fluxes across the intestine, particularly in the jejunum and ileum (5,97). Tudpor et al. (98) used Ussing chambers to find that 1,25(OH)2 D induced Ca absorption in the rat duodenum through a solvent drag mechanism sensitive to PI3K, PKC, and MEK inhibitors. Their finding that 1,25(OH)2 D induced ion movement and transepithelial electrical resistance without affecting manitol flux suggests that the effect was due to a change in the charge selectivity of the tight junction. The paradigm that tight junction selectivity may be relevant for transepithelial mineral transport was previously demonstrated in the kidney when mutations in the tight junction protein paracellin 1 (also known as claudin 16) were found to account for magnesium and Ca wasting associated with the genetic disease Familial hypomagnesmia with hypercalciuria and nephrocalcinosis (99). Recently, Fujita et al. (100) found that 1,25(OH)2 D treatment significantly increased claudin 2 and 12 mRNA levels in Caco-2 cells, that mRNA and protein levels for these proteins were significantly lower at 12 wk in the jejunum of VDR KO compared to wild type mice, and that siRNA against these claudins reduce Ca permeability in Caco-2 cell monolayers. While this is an intriguing new way to view Ca absorption, this model must be further tested prior to its acceptance. For example, it should be determined whether the loss of claudin 2 and 12 influence the saturable or non-saturable component of Ca absorption using kinetic modeling. If the impact of deletion has a significant impact on the linear diffusional component of Ca transport, this may explain the findings from Sheikh et al. (5) who found that the non-saturable component of ileal Ca absorption was reduced in chronic renal disease patients with low serum 1,25(OH)2 D levels. This would also be consistent with the observation that claudin 2 and 12 expression is highest in the ileum (101) rather than in the duodenum where 1,25(OH)2 D regulates the saturable component of Ca absorption. It is also interesting to reflect on the fact that claudin 2 expression is highest in the undifferentiated crypt cells of the small intestine (102) whereas, similar to what is seen for the absorption of other minerals (e.g. iron (103)), the expression of proteins proposed to be important for transcellular Ca movement (PMCA1b, calbindin D9k) increase as cells differentiate and migrate up the villus (80,104).
C. Evidence for the Regulation of Ca Absorption by Other Hormones
a. Parathyroid Hormone
Classical studies using dietary Ca deprivation have identified PTH as a major hormonal regulator of Ca homeostasis. Once released, PTH has several important functions related to Ca metabolism: it promotes bone resorption by stimulating osteoclastic activity; it stimulates renal Ca reabsorption in the proximal renal tubule, and it increases in the efficiency of Ca absorption. However, the effect on Ca absorption is thought to be indirect and mediated through the ability of PTH to increase serum 1,25(OH)2 D levels; PTH stimulates the transcription of the gene for the renal enzyme, CYP27B1, and suppresses renal 24-hydroxylase (CYP24) mRNA levels (105,106).
Because of the effect that PTH has on systemic vitamin D metabolism, it has been difficult to directly test whether PTH has direct effects on intestinal Ca absorption in vivo. Nevertheless, there is some evidence suggesting that PTH may directly influence the intestine. The PTH receptor 1 has been detected in basolateral membrane fractions of rat intestinal epithelial cells (107), although it is not expressed at high levels relative to tissues like the kidney (from tissue expression data available at the BioGPS website, biogps.gnf.org/#goto=welcome). Nevertheless, PTH treatment of isolated rat enterocytes increases cell cyclic AMP levels and this activates Ca uptake through Ca channels (108,109). Direct in vitro evidence for PTH-induced transcaltachia comes from Nemere and Norman (110) who found that bovine PTH 1-34 stimulates Ca transport in isolated duodenal loops from chicks. However, while serum PTH levels vary significantly throughout the course of a day (i.e. high in the morning, lower after consuming Ca-rich meals), Ca absorption efficiency has not been shown to have a similar daily rhythm. In order to sufficiently test if PTH directly influences Ca absorption (and if this if physiologically relevant), an in vivo model would need to be generated with targeted deletion of intestinal PTH receptor 1.
b. Hormones regulating Growth and Metabolism
i. Thyroid Hormone
The thyroid hormones, thyroxine (T4) and triiodothyronine (T3), function primarily as regulators of metabolism. However, hyperthyroidism (i.e. overproduction of T4 or T3) has been associated with hypercalcemia and high bone turnover rates leading to osteopenia (111). Kumar et al. (112) found that Ca uptake was higher in BBMV isolated from the duodenum of hyperthyroid rats and lower in BBMV from hypothyroid rats. This suggests that thyroid hormones modulate intestinal Ca absorption. However, thyroid treatment increases serum PTH and 1,25(OH)2D levels, and so the effects of thyroid hormones on intestinal Ca absorption may be acting indirectly through the PTH-vitamin D axis. In contrast, T3 may have direct effects on the developing intestine. Using cultured embryonic chick intestinal segments, Cross and Peterlik found that while T3 treatment alone had no effect upon jejunal Ca uptake, it amplified the effect of 1,25(OH)2D on Ca transport by making the tissue more sensitive to the hormone (113). While this suggests T3 may regulate VDR levels in the intestine, this information isn’t available.
ii. Growth hormone and IGF-1
During childhood and adolescence, growth hormone (GH) and its physiologic mediator insulin-like growth factor I (IGF-1) have a major role in linear bone growth and accrual of bone mass (114-116). In addition, GH can also promote intestinal Ca absorption. Some studies suggest that this is indirectly mediated through activation of renal CYP27B1 and the elevation of serum 1,25(OH)2 D levels (117). However, we have shown that GH treatment significantly increases intestinal Ca absorption and duodenal calbindin D9k levels in aged rats without significantly increasing serum 1,25(OH)2 D levels (118). GH treatment can prevent the loss of total VDR that occurs with ovariectomized rats (35) suggesting that GH increases the cell sensitivity to 1,25(OH)2 D by regulating tissue VDR levels; however, this has not been shown directly. The effect of GH on Ca absorption is most likely mediated through IGF-1 and there is evidence that this effect is independent of vitamin D signaling. Fatayerji et al. (119) showed that in adult men, Ca absorption is positively correlated with IGF-1 and that age-related declines in IGF-1 have a significant negative impact on Ca absorption that could not be explained simply by a reduction in serum 1,25(OH)2 D levels. Similarly, while Ca absorption is reduced by 70% in mice lacking the VDR, there is evidence for vitamin D independent, growth-related regulation of intestinal Ca absorption in these mice (27). The vitamin D-independent mechanism by which the GH/IGF-1 axis may regulate intestinal Ca absorption is not clear at this time.
c. Sex Steroids
i. Estrogen
In addition to the well established effects that estrogen has on bone and bone cells (120-122), estrogen can influence Ca metabolism in several ways (123) In a comparison of pre and post-menopausal women, Heaney et al. (120) found that calcium balance fell significantly in post-menopausal women and that this was due to both a reduction in calcium absorption and an increase in urinary calcium loss. Estrogen loss can reduce the serum level of 1,25(OH)2 D but this can be reversed by estrogen repletion and is accompanied by an increase in fractional calcium absorption (124). This suggests that a primary effect of estrogen loss is disruption of the vitamin D endocrine system. However, a longitudinal study of women passing through menopause showed that while menopause was associated with decreased Ca absorption and increased serum Ca, there was no change in serum PTH or 1,25(OH)2 D level (125). While this suggests that estrogens may have a direct effect on calcium absorption, others have shown that estrogen may work by indirectly altering the intestinal responsiveness to 1,25(OH)2 D. For example, Gennari et al. (32) found that oophorectomy reduced basal and 1,25(OH)2 D induced intestinal Ca absorption in young women, and that this effect could be reversed by estrogen repletion. Several groups have suggested that the loss of intestinal vitamin D responsiveness in the absence of estrogen is due to the reduction in VDR levels that results from estrogen deficiency (33,35,126), although this hypothesis is not universally accepted (127).
Functional estrogen receptors (ERs) have been detected in cells isolated from rat small intestinal crypts (128). As a result, it is plausible that estrogen may directly affect intestinal biology (e.g. intestinal Ca absorption). In support of this hypothesis, Ten Bolscher et al. (129) found that treatment with a pharmacological dose of estradiol-benzoate for two days significantly increased intestinal Ca absorption in rats, and that this could be blocked by the pure ER antagonist, ICI 182780, suggesting estrogen can stimulate intestinal Ca absorption by activating an ER. Estradiol treatment has also been found to increase duodenal expression of TRPV6, calbindin D9k, and PMCA1b in the intestine of ovariectomized rats (130). The increase in TRPV6 mRNA occurred even in CYP27B1 null mice – indicating that the intestinal effects of estrogen are vitamin D independent. Similarly, van Cromphaut et al. (131) found that intestine TRPV6 mRNA levels fell 55% in ER alpha null mice and that pharmacologic treatment with estradiol increased duodenal TRPV6 mRNA levels 4-8 fold in both normal and VDR null mice. However, there is currently no evidence that ER alpha binds directly to the TRPV6 promoter.
ii. Testosterone
Several studies have shown that hypogonadism in men reduces bone mineral density (132,133), in part by the loss of signaling to osteoblasts through the androgen receptor (134). However, very little is known regarding the effects of testosterone on Ca absorption or vitamin D metabolism. In prepubertal boys, a 4-6 week course of testosterone increased intestinal Ca absorption by 61% (135). This was accompanied by a 67% increase in serum IGF-1 levels, so it is not clear if the intestinal effects of testosterone are direct. With aging, Ca absorption efficiency falls significantly in men, and this decline is related to changes in serum dehydroepiandrosterone sulphate (DHEAS), the sulfated form of the testosterone prohormone DHEA (136). The change in Ca absorption observed with aged men was not related to changes in serum 1,25(OH)2 D, suggesting changes in androgen signaling do not alter vitamin D metabolism. This is consistent with the observation that serum vitamin D metabolite levels are not altered by the changes in testosterone that accompany puberty (137).
d. Hormones of Pregnancy and Lactation
Fetal skeletal development during the third trimester of pregnancy and increased Ca loss during lactation are significant drains on maternal Ca metabolism. As such, maternal Ca requirements increase significantly during these periods.
Serum 1,25(OH)2 D levels and intestinal Ca absorption are both elevated during late pregnancy (138) and this is due in part to extrarenal 1,25(OH)2 D production by the placenta (139). Still, the impact of pregnancy on Ca absorption appears to have a vitamin D-independent component. In rats, the pregnancy-induced elevation in intestinal Ca absorption appears before fetal skeletal mineralization, changes in serum ionized Ca, or changes in serum 1,25(OH)2 D levels (140). In addition, intestinal Ca absorption is up-regulated during pregnancy in vitamin D deficient rats (141,142) and in VDR KO mice (143). The increase in Ca absorption during pregnancy is accompanied by a > 10-fold increase in TRPV6 mRNA levels in both pregnant wild-type and VDR null mice (131,143). However, the pregnancy-related factor regulating this vitamin D-independent increase in Ca absorption and TRPV6 mRNA is not yet known.
Ca absorption is up-regulated during lactation in rodents but not humans. As during pregnancy, vitamin D deficient rats are capable of up-regulating intestinal absorption during lactation (141,142,144). There is evidence that prolactin is the factor regulating Ca absorption during lactation – prolactin injections to vitamin D deficient male rats increased Ca absorption 30%, leading to a significant increase in serum Ca (145). The mechanism for this regulation is unclear but may include up-regulation of duodenal calbindin D9k and TRPV6 mRNA levels (131). In addition, prolactin can stimulate 1,25(OH)2 D production (146). Another intriguing hypothesis for the effect of prolactin is that it may stimulate Ca absorption through an L-type calcium channel in the distal jejunum and ileum. Morgan et al. (147) first observed that glucose increases intestinal Ca absorption in rat jejunum by depolarizing the enterocyte. This mechanism was inconsistent for a role for TRPV6, a protein that works best under the hyperpolarizating conditions of the duodenum, but was consistent with a role for the L-type calcium channel Cav 1.3. Later others showed that prolactin-regulated Ca transport across Caco-2 monolayers was inhibited by L-type Ca channel inhibitors and by siRNA against Cav 1.3 (148,149). Collectively, these data suggest that prolactin is capable of having both direct and indirect effects to increase intestinal Ca absorption.
III. Conclusions
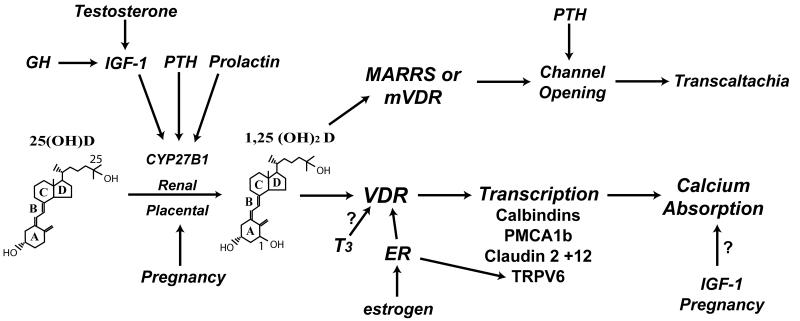
Intestinal Ca absorption is determined by the amount of Ca in a given meal, the habitual Ca intake, the physiological state of an individual, the solubility of Ca in the various intestinal segments, and gut transit time. The vitamin D metabolite, 1,25(OH)2 D, is the primary regulator of intestinal Ca absorption efficiency. Although it is clear that signaling through the VDR is necessary for vitamin D-mediated intestinal Ca absorption, the exact mechanism that Ca travels to make it through the intestinal barrier is still in question. Each of the three models that we presented to explain transcellular Ca transport (i.e. facilitated diffusion, vesicular transport, transcaltachia) has weaknesses that must be resolved through additional research. A recently proposed model for regulated paracellular Ca movement through tight junctions is not consistent with other reports showing that Ca movement is transcellular in the duodenum, but may help explain data indicating that vitamin D signaling can control non-saturable, presumably paracellular movement across the ileum. Thus, vitamin D signaling may utilize different mechanisms to regulate intestinal Ca absorption depending upon the segment of intestine studied. In addition to vitamin D, other hormones are important for the maintenance of optimal Ca absorption (Figure 4). Several hormones influence Ca absorption indirectly through the regulation of renal 1,25(OH)2 D production. Nonetheless, several hormones have vitamin D independent actions on Ca absorption (e.g. IGF-1, estrogen, factors associated with pregnancy) that deserve further inquiry.
Figure 4. Hormonal regulation of intestinal Ca absorption.
A number of hormones are known to influence calcium absorption. The strongest regulator is the hormonal form of vitamin D, 1,25(OH)2 D which regulates gene transcription through VDR or transcaltachia through membrane bound VDR or MARRS. Some hormones work indirectly by increasing the renal conversion of 25(OH) D to 1,25(OH)2 D, e.g. GH, IGF-1, PTH. Estrogen may activate the ER to regulate cellular VDR levels or directly regulate genes controlling intestinal Ca absorption like TRPV6. Several hormones have effects that are either suggestive [e.g. thyroid hormone (T3) on VDR] or whose mechanism is not clear (e.g. vitamin D independent effects of pregnancy and IGF-1 on Ca absorption).
Acknowledgements
This work was supported by NIH award DK054111 to JCF.
Abbreviations
- 1,25(OH)2 D
1,25 dihydroxyvitamin D
- 25(OH) D
25 hydroxyvitamin D
- BBMV
brush border membrane vesicles
- Ca
Calcium
- CYP24
24-hydroxylase
- CYP27B1
25 hydroxyvitamin D-1α hydroxylase
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulphate
- ER
estrogen receptor
- GH
growth hormone
- IGF-1
insulin-like growth factor I
- KO
knockout
- MARRS
membrane associated rapid response steroid binding protein
- PMCA1b
plasma membrane Ca ATPase 1b
- PTH
parathyroid hormone
- T3
triiodothyronine
- T4
thyroxine
- TRPV6
transient receptor potential cation channel, subfamily V, member 6
- VDR
Vitamin D Receptor
Footnotes
Declaration of Interest None
Reference List
- 1.Fleet JC. Molecular Regulation of Calcium Metabolism. In: Weaver CM, Heaney RP, editors. Calcium in Human Health. Humana Press; Totowa, NJ: 2006. pp. 163–190. [Google Scholar]
- 2.Wasserman RH, Taylor AN. Some aspects of the intestinal absorption of calcium, with special reference to vitamin D. In: Comar CL, Bronner F, editors. Mineral Metabolism, An Advanced Treatise. Academic Press; New York: 1969. pp. 321–403. [Google Scholar]
- 3.Pansu D, Bellaton C, Bronner F. Effect of Ca intake on saturable and nonsaturable components of duodenal Ca transport. Am J Physiol. 1981;240:32–7. doi: 10.1152/ajpgi.1981.240.1.G32. [DOI] [PubMed] [Google Scholar]
- 4.Heaney RP, Saville PD, Recker RR. Calcium absorption as a function of calcium intake. Journal of Laboratory & Clinical Medicine. 1975;85:881–90. [PubMed] [Google Scholar]
- 5.Sheikh MS, Schiller LR, Fordtran JS. In vivo intestinal absorption of calcium in humans. Miner Electrolyte Metab. 1990;16:130–146. [PubMed] [Google Scholar]
- 6.Favus MJ, Angeid-Backman E, Breyer MD, Coe FL. Effects of trifluoperazine,ouabain, and ethacrynic acid on intestinal calcium. Am J Physiol. 1983;244:G111–G115. doi: 10.1152/ajpgi.1983.244.2.G111. [DOI] [PubMed] [Google Scholar]
- 7.Pansu D, Bellaton C, Roche C, Bronner F. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am J Physiol. 1983;244:G695–G700. doi: 10.1152/ajpgi.1983.244.6.G695. [DOI] [PubMed] [Google Scholar]
- 8.Favus MJ, Kathpalia SC, Coe FL. Kinetic characteristics of calcium absorption and secretion by rat colon. Am J Physiol. 1981;240:G350–G354. doi: 10.1152/ajpgi.1981.240.5.G350. [DOI] [PubMed] [Google Scholar]
- 9.Grinstead WC, Pak CYC, Krejs GJ. Effect of 1,25-Dihydroxyvitamin-D3 on Calcium-Absorption in the Colon of Healthy Humans. American Journal of Physiology. 1984;247:G189–G192. doi: 10.1152/ajpgi.1984.247.2.G189. [DOI] [PubMed] [Google Scholar]
- 10.Karbach U, Feldmeier H. The cecum is the site with the highest calcium absorption in rat intestine. Dig Dis Sci. 1993;38:1815–1824. doi: 10.1007/BF01296104. [DOI] [PubMed] [Google Scholar]
- 11.Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966;11:503–521. doi: 10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- 12.Marcus CS, Lengemann FW. Absorption of Ca45 and Sr85 from solid and liquid food at various levels of the alimentary tract of the rat. J Nut. 1962;77:155–160. doi: 10.1093/jn/77.2.155. [DOI] [PubMed] [Google Scholar]
- 13.Duflos C, Bellaton C, Pansu D, Bronner F. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J Nutr. 1995;125:2348–2355. doi: 10.1093/jn/125.9.2348. [DOI] [PubMed] [Google Scholar]
- 14.Rottensten KV. The effect of body stores on the efficiency of calcium utilization. Biochem J. 1938;32:1285–1292. doi: 10.1042/bj0321285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leichsenring JM, Norris LM, Lamison SA, WILSON ED, PATTON MB. The effect of level of intake on calcium and phosphorus metabolism in college women. J Nutr. 1951;45:407–418. doi: 10.1093/jn/45.3.407. [DOI] [PubMed] [Google Scholar]
- 16.Dawson-Hughes B, Harris S, Kramich C, Dallal G, Rasmussen HM. Calcium retention and hormone levels in black and white women on high- and low-calcium diets. J Bone Min Res. 1993;8:779–787. doi: 10.1002/jbmr.5650080702. [DOI] [PubMed] [Google Scholar]
- 17.Favus MJ, Walling MW, Kimberg DV. Effects of dietary calcium restriction and chronic thyroparathyroidectomy on the metabolism of (3H)25-hydroxyvitamin D3 and the active transport of calcium by rat intestine. J Clin Invest. 1974;53:1139–1148. doi: 10.1172/JCI107652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolaysen R. Studies upon the mode of action of vitamin D: The absorption of calcium chloride, xylose and sodium sulphate from isolated loops of the small intestine and of calcium chloride from the abdominal cavity in the rat. Biochem J. 1937;31:323–328. doi: 10.1042/bj0310323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GERSHOFF SN, HEGSTED DM. Effect of vitamin D and Ca:P ratios on chick gastrointestinal tract. Am J Physiol. 1956;187:203–206. doi: 10.1152/ajplegacy.1956.187.2.203. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh MS, Ramirez A, Emmett M, Santa AC, Schiller LR, Fordtran JS. Role of vitamin D-dependent and vitamin D-independent mechanisms in absorption of food calcium. J Clin Invest. 1988;81:126–132. doi: 10.1172/JCI113283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giuliano AR, Wood RJ. Vitamin D-regulated calcium transport in Caco-2 cells: unique in vitro model. Am J Physiol. 1991;260:G207–G212. doi: 10.1152/ajpgi.1991.260.2.G207. [DOI] [PubMed] [Google Scholar]
- 22.Chandra S, Fullmer CS, Smith CA, Wasserman RH, Morrison GH. Ion microscopic imaging of calcium transport in the intestinal tissue of vitamin D-deficient and vitamin D-replete chickens: a 44Ca stable isotope study. Proc Natl Acad Sci U S A. 1990;87:5715–5719. doi: 10.1073/pnas.87.15.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fullmer CS, Chandra S, Smith CA, Morrison GH, Wasserman RH. Ion microscopic imaging of calcium during 1,25-dihydroxyvitamin D-mediated intestinal absorption. Histochem Cell Biol. 1996;106:215–222. doi: 10.1007/BF02484403. [DOI] [PubMed] [Google Scholar]
- 24.Haussler MR, Norman AW. Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci U S A. 1969;62:155–162. doi: 10.1073/pnas.62.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 26.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A. 2001 Nov 6;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y, Kato S, Fleet JC. Vitamin D Receptor (VDR) Knockout Mice Reveal VDR-Independent Regulation of Intestinal Calcium Absorption and ECaC2 and Calbindin D9k mRNA. J Nutr. 2003;133:374–380. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- 28.Marks HD, Fleet JC, Peleg S. Transgenic expression of the human Vitamin D receptor (hVDR) in the duodenum of VDR-null mice attenuates the age-dependent decline in calcium absorption. J Steroid Biochem Mol Biol. 2007 Jan 4; doi: 10.1016/j.jsbmb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136:1317–2. doi: 10.1053/j.gastro.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullamore JR, Gallagher JC, Wilkinson R, Nordin BEC, Marshall DH. Effect of age on calcium absorption. Lancet. 1970;2:535–537. doi: 10.1016/s0140-6736(70)91344-9. [DOI] [PubMed] [Google Scholar]
- 31.Nordin BE, Need AG, Morris HA, O’Loughlin PD, Horowitz M. Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr. 2004;80:998–1002. doi: 10.1093/ajcn/80.4.998. [DOI] [PubMed] [Google Scholar]
- 32.Gennari C, Agnusdei D, Nardi P, Civitelli R. Estrogen preserves a normal intestinal responsiveness to 1,25- dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab. 1990;71:1288–1293. doi: 10.1210/jcem-71-5-1288. [DOI] [PubMed] [Google Scholar]
- 33.Liel Y, Shany S, Smirnoff P, Schwartz B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999;140:280–285. doi: 10.1210/endo.140.1.6408. [DOI] [PubMed] [Google Scholar]
- 34.Ebeling PR, Sandgren ME, Dimagno EP, Lane AW, DeLuca HF, Riggs BL. Evidence of an age-related decrease in intestinal responsiveness to vitamin-D - relationship between serum 1,25-dihydroxyvitamin-D3 and intestinal vitamin-D receptor concentrations in normal women. J Clin Endocrinol Metab. 1992;75:176–182. doi: 10.1210/jcem.75.1.1320048. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Noland KA, Kalu DN. Modulation of intestinal vitamin D receptor by ovariectomy, estrogen and growth hormone. Mech Ageing Dev. 1997 Dec 15;99:109–122. doi: 10.1016/s0047-6374(97)00094-8. [DOI] [PubMed] [Google Scholar]
- 36.Shao A, Wood RJ, Fleet JC. Increased vitamin D receptor level enhances 1,25-dihydroxyvitamin D3- mediated gene expression and calcium transport in Caco-2 cells. J Bone Miner Res. 2001;16:615–624. doi: 10.1359/jbmr.2001.16.4.615. [DOI] [PubMed] [Google Scholar]
- 37.Song Y, Fleet JC. Intestinal Resistance to 1,25 Dihydroxyvitamin D in Mice Heterozygous for the Vitamin D Receptor Knockout Allele. Endocrinology. 2007 Mar 1;148:1396–1402. doi: 10.1210/en.2006-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jurutka P, Remus L, Whitfield K, Thompson P, Hsieh J, Zitzer H, Tavakkoli P, Galligan M, Dang H, Haussler C, Haussler M. The polymorphic N terminus in human vitamin D receptor isoforms influences trascriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol. 2000;14:401–420. doi: 10.1210/mend.14.3.0435. [DOI] [PubMed] [Google Scholar]
- 39.Ames SK, Ellis KJ, Gunn SK, Copeland KC, Abrams SA. Vitamin D receptor gene Fok1 polymorphisms predicts calcium absorption and bone mineral density in children. J Bone Miner Res. 1999;14:740–746. doi: 10.1359/jbmr.1999.14.5.740. [DOI] [PubMed] [Google Scholar]
- 40.Huang ZW, Dong J, Piao JH, Li WD, Tian Y, Xu J, Yang XG. [Relationship between the absorption of dietary calcium and the Fok I polymorphism of VDR gene in young women] Zhonghua Yu Fang Yi Xue Za Zhi. 2006;40:75–78. [PubMed] [Google Scholar]
- 41.Zmuda JM, Cauley JA, Danielson ME, Theobald TM, Ferrell RE. Vitamin D receptor translation initiation codon polymorphism and markers of osteoporotic risk in older African-American women. Osteoporosis International. 1999;9:214–219. doi: 10.1007/s001980050139. [DOI] [PubMed] [Google Scholar]
- 42.Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, Sambrook PN, Eisman JA. Prediction of bone density from vitamin D receptor alleles. Nature. 1994 Jan 20;367:284–287. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- 43.Wishart JM, Horowitz M, Need AG, Scopacasa F, Morris HA, Clifton PM, Nordin BE. Relations between calcium intake, calcitriol, polymorphisms of the vitamin D receptor gene, and calcium absorption in premenopausal women. Am J Clin Nutr. 1997;65:798–802. doi: 10.1093/ajcn/65.3.798. [DOI] [PubMed] [Google Scholar]
- 44.Dawson-Hughes B, Harris SS, Finneran S. Calcium absorption on high and low calcium intakes in relation to vitamin D receptor genotype. J Clin Endocrinol Metab. 1995;80:3657–3661. doi: 10.1210/jcem.80.12.8530616. [DOI] [PubMed] [Google Scholar]
- 45.Gennari L, Becherini L, Masi L, Gonnelli S, Cepollaro C, Martini S, Mansani R, Brandi ML. Vitamin D receptor genotypes and intestinal calcium absorption in postmenopausal women. Calcif Tissue Int. 1997;61:460–463. doi: 10.1007/s002239900368. [DOI] [PubMed] [Google Scholar]
- 46.Abrams SA, Griffin IJ, Hawthorne KM, Chen Z, Gunn SK, Wilde M, Darlington G, Shypailo RJ, Ellis KJ. Vitamin D receptor Fok1 polymorphisms affect calcium absorption, kinetics, and bone mineralization rates during puberty. J Bone Miner Res. 2005;20:945–953. doi: 10.1359/JBMR.050114. [DOI] [PubMed] [Google Scholar]
- 47.Francis RM, Harrington F, Turner E, Papiha SS, Datta HK. Vitamin D receptor gene polymorphism is men and its effect on bone density and calcium absorption. Clin Endocrinol. 1997;46:83–86. doi: 10.1046/j.1365-2265.1997.d01-1735.x. [DOI] [PubMed] [Google Scholar]
- 48.Kinyamu HK, Gallagher JC, Knezetic JA, DeLuca HF, Prahl JM, Lanspa SJ. Effect of vitamin D receptor genotypes on calcium absorption, duodenal vitamin D receptor concentration, and serum 1,25 dihydroxyvitamin D levels in normal women. Calcified Tissue International. 1997;60:491–495. doi: 10.1007/s002239900269. [DOI] [PubMed] [Google Scholar]
- 49.Laaksonen M, Karkkainen M, Outila T, Vanninen T, Ray C, Lamberg-Allardt C. Vitamin D receptor gene BsmI-polymorphism in Finnish premenopausal and postmenopausal women: its association with bone mineral density, markers of bone turnover, and intestinal calcium absorption, with adjustment for lifestyle factors. Journal of Bone and Mineral Metabolism. 2002;20:383–390. doi: 10.1007/s007740200055. [DOI] [PubMed] [Google Scholar]
- 50.Boyle IT, Gray RW, Ohmdahl JL, DeLuca HF, Schilling RF. The mechanism of adaptation of intestinal calcium absorption to low dietary calcium. J Lab Clin Med. 1971;78:813. [PubMed] [Google Scholar]
- 51.Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 52.Heaney RP, Barger-Lux MJ, Dowell MS, Chen TC, Holick MF. Calcium absorptive effects of vitamin D and its major metabolites. J Clin Endocrinol Metab. 1997;82:4111–4116. doi: 10.1210/jcem.82.12.4412. [DOI] [PubMed] [Google Scholar]
- 53.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium Absorption Varies within the Reference Range for Serum 25-Hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 54.Anderson PH, Hendrix I, Sawyer RK, Zarrinkalam R, Manavis J, Sarvestani GT, May BK, Morris HA. Co-expression of CYP27B1 enzyme with the 1.5kb CYP27B1 promoter-luciferase transgene in the mouse. Mol Cell Endocrinol. 2008 Mar 26;285:1–9. doi: 10.1016/j.mce.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Balesaria S, Sangha S, Walters JR. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1193–G1197. doi: 10.1152/ajpgi.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abrams SA, Hicks PD, Hawthorne KM. Higher serum 25-hydroxyvitamin D levels in school-age children are inconsistently associated with increased calcium absorption. J Clin Endocrinol Metab. 2009;94:2421–2427. doi: 10.1210/jc.2008-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Need AG, Nordin BE. Misconceptions - vitamin D insufficiency causes malabsorption of calcium. Bone. 2008;42:1021–1024. doi: 10.1016/j.bone.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Need AG, O’Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D Metabolites and Calcium Absorption in Severe Vitamin D Deficiency. J Bone Miner Res. 2008 Jul 2; doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 59.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Engelke JA, Shafer MM. Vitamin D insufficiency: disease or no disease? J Bone Miner Res. 2008;23:1052–1060. doi: 10.1359/JBMR.080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aloia JF, Chen DG, Yeh JK, Chen H. Serum vitamin D metabolites and intestinal calcium absorption efficiency in women. Am J Clin Nutr. 2010;92:835–840. doi: 10.3945/ajcn.2010.29553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol. 1986;250:G561–G569. doi: 10.1152/ajpgi.1986.250.5.G561. [DOI] [PubMed] [Google Scholar]
- 62.Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediated intestinal calcium absorption. J Biol Chem. 1999;274:22739–22746. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- 63.Fleet JC, Eksir F, Hance KW, Wood RJ. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;283:G618–G625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- 64.Meyer MB, Zella LA, Nerenz RD, Pike JW. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem. 2007 Jun 7; doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- 65.Cui M, Zhao Y, Hance KW, Shao A, Wood RJ, Fleet JC. Effects of MAPK signaling on 1,25-dihydroxyvitamin D-mediated CYP24 gene expression in the enterocyte-like cell line, Caco-2. J Cell Physiol. 2009;219:132–142. doi: 10.1002/jcp.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walters JR, Balesaria S, Chavele KM, Taylor V, Berry JL, Khair U, Barley NF, van Heel DA, Field J, Hayat JO, Bhattacharjee A, Jeffery R, Poulsom R. Calcium channel TRPV6 expression in human duodenum: different relationships to the vitamin D system and aging in men and women. J Bone Miner Res. 2006;21:1770–1777. doi: 10.1359/jbmr.060721. [DOI] [PubMed] [Google Scholar]
- 67.Kutuzova GD, Sundersingh F, Vaughan J, Tadi BP, Ansay SE, Christakos S, DeLuca HF. TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc Natl Acad Sci U S A. 2008 Dec 16;105:19655–19659. doi: 10.1073/pnas.0810761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149:3196–3205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christakos S, Gill R, Lee S, Li H. Molecular aspects of the calbindins. Journal of Nutrition. 1992;122:678–682. doi: 10.1093/jn/122.suppl_3.678. [DOI] [PubMed] [Google Scholar]
- 70.Wasserman RH, Taylor AN. Vitamin D3-induced calcium-binding proteins in chick intestinal mucosa. Science. 1966;252:791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- 71.Bronner F, Buckley M. The molecular nature of 1,25-(OH)2-D3-induced calcium-binding protein biosynthesis in the rat. Adv Exp Med Biol. 1982;151:355–360. doi: 10.1007/978-1-4684-4259-5_41. [DOI] [PubMed] [Google Scholar]
- 72.Pansu D, Bellaton C, Roche C, Bronner F. Theophylline inhibits active Ca transport in rat intestine by inhibiting Ca binding by CaBP. Prog Clin Biol Res. 1988;252:115–120. [PubMed] [Google Scholar]
- 73.Feher JJ, Fullmer CS, Wasserman RH. Role of facilitated diffusion of calcium by calbindin in intestinal calcium absorption. Am J Physiol. 1992;262:C517–C526. doi: 10.1152/ajpcell.1992.262.2.C517. [DOI] [PubMed] [Google Scholar]
- 74.Spencer R, Charman M, Wilson PW, Lawson DEM. The relationship between vitamin D-stimulated calcium transport and intestinal calcium-binding protein in the chicken. Biochem J. 1978;170:93–101. doi: 10.1042/bj1700093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee GS, Lee KY, Choi KC, Ryu YH, Paik SG, Oh GT, Jeung EB. Phenotype of a calbindin-D9k gene knockout is compensated for by the induction of other calcium transporter genes in a mouse model. J Bone Miner Res. 2007;22:1968–1978. doi: 10.1359/jbmr.070801. [DOI] [PubMed] [Google Scholar]
- 76.Akhter S, Kutuzova GD, Christakos S, DeLuca HF. Calbindin D9k is not required for 1,25-dihydroxyvitamin D3-mediated Ca2+ absorption in small intestine. Arch Biochem Biophys. 2007 Apr 15;460:227–232. doi: 10.1016/j.abb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 77.Kutuzova GD, Akhter S, Christakos S, Vanhooke J, Kimmel-Jehan C, DeLuca HF. Calbindin D(9k) knockout mice are indistinguishable from wild-type mice in phenotype and serum calcium level. Proc Natl Acad Sci U S A. 2006 Aug 15;103:12377–12381. doi: 10.1073/pnas.0605252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wasserman RH, Smith CA, Brindak ME, Detalamoni N, Fullmer CS, Penniston JT, Kumar R. Vitamin-D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology. 1992;102:886–894. doi: 10.1016/0016-5085(92)90174-w. [DOI] [PubMed] [Google Scholar]
- 79.Cai Q, Chandler JS, Wasserman RH, Kumar R, Penniston JT. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc Natl Acad Sci U S A. 1993 Feb 15;90:1345–1349. doi: 10.1073/pnas.90.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Corven EJ, Roche C, Van Os CH. Distribution of Ca2+-ATPase, ATP-dependent Ca2+-transport, calmodulin and vitamin D-dependent Ca2+-binding protein along the villus-crypt axis in rat duodenum. Biochim Biophys Acta. 1985 Nov 7;820:274–282. doi: 10.1016/0005-2736(85)90121-x. [DOI] [PubMed] [Google Scholar]
- 81.Davis WL, Jones RG. Lysosomal proliferation in rachitic avian intestinal absorptive cells following 1,25-dihydroxycholecalciferol. Tissue and Cell. 1982;14:585–95. doi: 10.1016/0040-8166(82)90049-0. [DOI] [PubMed] [Google Scholar]
- 82.Nemere I, Szego CM. Early actions of parathyroid hormone and 1,25-dihydroxycholecalciferol on isolated epithelial cells from rat intestine: 1. Limited lysosomal enzyme release and calcium uptake. Endocrinology. 1981;108:1450–1462. doi: 10.1210/endo-108-4-1450. [DOI] [PubMed] [Google Scholar]
- 83.Warner RR, Coleman JR. Electron probe analysis of calcium transport by small intestine. Journal of Cell Biology. 1975;64:54–74. doi: 10.1083/jcb.64.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nemere I, Leathers V, Norman AW. 1, 25 dihydroxyvitamin D3-mediated intestinal calcium transport. Biochemical identification of lysozomes containing calcium and calcium-binding protein (calbindin-D28k) J Biol Chem. 1986;261:16106–16114. [PubMed] [Google Scholar]
- 85.Nemere I, Norman AW. 1,25-Dihydroxyvitamin D3-mediated vesicular transport of calcium in intestine: time-course studies. Endocrinology. 1988;122:2962–9. doi: 10.1210/endo-122-6-2962. [DOI] [PubMed] [Google Scholar]
- 86.Favus MJ, Tembe V, Tanklefsky MD, Ambrosic KA, Nellans HN. Effects of quinacrine on calcium active transport by rat intestinal epithelium. Am J Physiol. 1989;257:G818–G822. doi: 10.1152/ajpgi.1989.257.5.G818. [DOI] [PubMed] [Google Scholar]
- 87.Fleet JC. Rapid, membrane-initiated actions of 1,25 dihydroxyvitamin d: what are they and what do they mean? J Nutr. 2004;134:3215–3218. doi: 10.1093/jn/134.12.3215. [DOI] [PubMed] [Google Scholar]
- 88.Nemere I, Yoshimoto Y, Norman AW. Calcium transport in perfused duodena from normal chicks: Enhancement within fourteen minutes of exposure to 1,25 dihydroxyvitamin D3. Endocrinology. 1984;115:1476–1483. doi: 10.1210/endo-115-4-1476. [DOI] [PubMed] [Google Scholar]
- 89.Nemere I, Norman AW. Transcaltachia, vesicular calcium transport, and microtubule-associated calbindin-D28K: emerging views of 1,25-dihydroxyvitamin D3-mediated intestinal calcium absorption. Miner Electrolyte Metab. 1990;16:109–114. [PubMed] [Google Scholar]
- 90.Khanal RC, Peters TM, Smith NM, Nemere I. Membrane receptor-initiated signaling in 1,25(OH)2D3-stimulated calcium uptake in intestinal epithelial cells. J Cell Biochem. 2008 Nov 1;105:1109–1116. doi: 10.1002/jcb.21913. [DOI] [PubMed] [Google Scholar]
- 91.Nemere I, Dormanen MC, Hammond MW, Okamura WH, Norman AW. Identification of a specific binding protein for 1α , 25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J Biol Chem. 1994;269:23750–23756. [PubMed] [Google Scholar]
- 92.Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1{alpha},25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004 Jul 22;18:2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- 93.Nemere I, Farach-Carson MC, Rohe B, Sterling TM, Norman AW, Boyan BD, Safford SE. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc Natl Acad Sci U S A. 2004 May 11;101:7392–7397. doi: 10.1073/pnas.0402207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mizwicki MT, Keidel D, Bula CM, Bishop JE, Zanello LP, Wurtz JM, Moras D, Norman AW. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci U S A. 2004 Aug 31;101:12876–12881. doi: 10.1073/pnas.0403606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norman AW, Bishop JE, Bula CM, Olivera CJ, Mizwicki MT, Zanello LP, Ishida H, Okamura WH. Molecular tools for study of genomic and rapid signal transduction responses initiated by 1 alpha,25(OH)(2)-vitamin D(3) Steroids. 2002;67:457–466. doi: 10.1016/s0039-128x(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 96.Nemere I, Garbi N, Hammerling G. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS receptor/PDIA3/Erp57. FASEB J. 2010 doi: 10.1074/jbc.M110.116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karbach U. Paracellular Calcium Transport Across the Small Intestine. Journal of Nutrition. 1992;122:672–677. doi: 10.1093/jn/122.suppl_3.672. [DOI] [PubMed] [Google Scholar]
- 98.Tudpor K, Teerapornpuntakit J, Jantarajit W, Krishnamra N, Charoenphandhu N. 1,25-dihydroxyvitamin d(3) rapidly stimulates the solvent drag-induced paracellular calcium transport in the duodenum of female rats. J Physiol Sci. 2008;58:297–307. doi: 10.2170/physiolsci.RP002308. [DOI] [PubMed] [Google Scholar]
- 99.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriquez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 100.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912–1921. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem. 2006;54:933–944. doi: 10.1369/jhc.6A6944.2006. [DOI] [PubMed] [Google Scholar]
- 102.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 103.Trinder D, Oates PS, Thomas C, Sadleir J, Morgan EH. Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut. 2000;46:270–276. doi: 10.1136/gut.46.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L, Klopot A, Freund JN, Dowling LN, Krasinski SD, Fleet JC. Control of Differentiation-Induced Calbindin-D9k Gene Expression in Caco-2 Cells by Cdx-2 adn HNF-1α. Am J Physiol. 2004 Jun 24;287:G943–G953. doi: 10.1152/ajpgi.00121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zierold C, Mings JA, DeLuca HF. Regulation of 25-hydroxyvitamin D3-24-hydroxylase mRNA by 1,25-dihydroxyvitamin D3 and parathyroid hormone. J Cell Biochem. 2003;88:234–237. doi: 10.1002/jcb.10341. [DOI] [PubMed] [Google Scholar]
- 106.Armbrecht HJ, Boltz MA, Hodam TL. PTH increases renal 25(OH)D3-1alpha -hydroxylase (CYP1alpha) mRNA but not renal 1,25(OH)2D3 production in adult rats. Am J Physiol Renal Physiol. 2003;284:F1032–F1036. doi: 10.1152/ajprenal.00306.2002. [DOI] [PubMed] [Google Scholar]
- 107.Gentili C, Morelli S, de Boland AR. Characterization of PTH/PTHrP receptor in rat duodenum: Effects of ageing. Journal of Cellular Biochemistry. 2003 Apr 15;88:1157–1167. doi: 10.1002/jcb.10472. [DOI] [PubMed] [Google Scholar]
- 108.Nemere I, Szego CM. Early actions of parathyroid hormone and 1,25-dihydroxycholecalciferol on isolated epithelial cells from rat intestine. 2. Analyses of additivity, contribution of calcium, and modulatory influence of indomethacin. Endocrinology. 1981;109:2180–2187. doi: 10.1210/endo-109-6-2180. [DOI] [PubMed] [Google Scholar]
- 109.Picotto G, Massheimer V, Boland R. Parathyroid hormone stimulates calcium influx and the cAMP messenger system in rat enterocytes. American Journal of Physiology-Cell Physiology. 1997;42:C1349–C1353. doi: 10.1152/ajpcell.1997.273.4.C1349. [DOI] [PubMed] [Google Scholar]
- 110.Nemere I, Norman AW. Parathyroid hormone stimulates calcium transport in perfused duodena from normal chicks: comparison with the rapid (transcaltachic) effect of 1,25-dihydroxyvitamin D3. Endocrinology. 1986;119:1406–8. doi: 10.1210/endo-119-3-1406. [DOI] [PubMed] [Google Scholar]
- 111.Pantazi H, Papapetrou PD. Changes in parameters of bone and mineral metabolism during therapy for hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1099–1106. doi: 10.1210/jcem.85.3.6457. [DOI] [PubMed] [Google Scholar]
- 112.Kumar V, Prasad R. Thyroid hormones stimulate calcium transport systems in rat intestine. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2003 Nov 20;1639:185–194. doi: 10.1016/j.bbadis.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 113.Cross HS, Peterlik M. Calcium and Inorganic-Phosphate Transport in Embryonic Chick Intestine - Triiodothyronine Enhances the Genomic Action of 1,25-Dihydroxycholecalciferol. Journal of Nutrition. 1988;118:1529–1534. doi: 10.1093/jn/118.12.1529. [DOI] [PubMed] [Google Scholar]
- 114.Mora S, Pitukcheewanont P, Nelson JC, Gilsanz V. Serum levels of insulin-like growth factor I and the density, volume, and cross-sectional area of cortical bone in children. J Clin Endocrinol Metab. 1999;84:2780–2783. doi: 10.1210/jcem.84.8.5874. [DOI] [PubMed] [Google Scholar]
- 115.Boot AM, Engels MA, Boerma GJ, Krenning EP, De Muinck Keizer-Schrama SM. Changes in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiency. J Clin Endocrinol Metab. 1997;82:2423–2428. doi: 10.1210/jcem.82.8.4149. [DOI] [PubMed] [Google Scholar]
- 116.Rudman D, Feller AG, Nagrajm HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 117.Zoidis E, Gosteli-Peter M, Ghirlanda-Keller C, Meinel L, Zapf J, Schmid C. IGF-I and GH stimulate Phex mRNA expression in lungs and bones and 1,25-dihydroxyvitamin D(3) production in hypophysectomized rats. Eur J Endocrinol. 2002;146:97–105. doi: 10.1530/eje.0.1460097. [DOI] [PubMed] [Google Scholar]
- 118.Fleet JC, Bruns ME, Hock JM, Wood RJ. Growth hormone and parathyroid hormone stimulate intestinal calcium absorption in aged female rats. Endocrinology. 1994;134:1755–1760. doi: 10.1210/endo.134.4.8137740. [DOI] [PubMed] [Google Scholar]
- 119.Fatayerji D, Mawer EB, Eastell R. The role of insulin-like growth factor I in age-related changes in calcium homeostasis in men. J Clin Endocrinol Metab. 2000;85:4657–4662. doi: 10.1210/jcem.85.12.7031. [DOI] [PubMed] [Google Scholar]
- 120.Heaney RP, Recker RR, Saville PD. Menopausal changes in bone remodeling. J Lab Clin Med. 1978;92:964–970. [PubMed] [Google Scholar]
- 121.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 122.Sarma U, Edwards M, Motoyoshi K, Flanagan AM. Inhibition of bone resorption by 17beta-estradiol in human bone marrow cultures. J Cell Physiol. 1998;175:99–108. doi: 10.1002/(SICI)1097-4652(199804)175:1<99::AID-JCP11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 123.Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 124.Gallagher JC, Riggs BL, DeLuca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1980;51:1359–1364. doi: 10.1210/jcem-51-6-1359. [DOI] [PubMed] [Google Scholar]
- 125.Nordin BE, Wishart JM, Clifton PM, McArthur R, Scopacasa F, Need AG, Morris HA, O’Loughlin PD, Horowitz M. A longitudinal study of bone-related biochemical changes at the menopause. Clin Endocrinol (Oxf ) 2004;61:123–130. doi: 10.1111/j.1365-2265.2004.02066.x. [DOI] [PubMed] [Google Scholar]
- 126.Arjmandi BH, Holis BW, Kalu DN. In vivo effect of 17 β-estradiol on intestinal calcium absorption in rats. Bone and Mineral. 1994;26:181–189. doi: 10.1016/s0169-6009(08)80062-1. [DOI] [PubMed] [Google Scholar]
- 127.Colin EM, van den Bemd GJ, Van Aken M, Christakos S, De Jonge HR, DeLuca HF, Prahl JM, Birkenhager JC, Buurman CJ, Pols HA, van Leeuwen JP. Evidence for involvement of 17beta-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the Rat. J Bone Miner Res. 1999;14:57–64. doi: 10.1359/jbmr.1999.14.1.57. [DOI] [PubMed] [Google Scholar]
- 128.Thomas ML, Xu X, Norfleet AM, Watson CS. The presence of functional estrogen receptors in intestinal epithelial cells. Endocrinology. 1993;132:426–430. doi: 10.1210/endo.132.1.8419141. [DOI] [PubMed] [Google Scholar]
- 129.Ten Bolscher M, Netelenbos JC, Barto R, Van Buuren LM, Van der vijgh WJ. Estrogen regulation of intestinal calcium absorption in the intact and ovariectomized adult rat. J Bone Miner Res. 1999;14:1197–1202. doi: 10.1359/jbmr.1999.14.7.1197. [DOI] [PubMed] [Google Scholar]
- 130.Van Abel M, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ. Regulation of the Epithelial Ca2+ Channels in Small Intestine as Studied by Quantitative mRNA Detection. Am J Physiol Gastrointest Liver Physiol. 2003 Mar 5; doi: 10.1152/ajpgi.00036.2003. [DOI] [PubMed] [Google Scholar]
- 131.Bouillon R, Van Cromphaut S, Carmeliet G. Intestinal calcium absorption: Molecular vitamin D mediated mechanisms. J Cell Biochem. 2003;88:332–339. doi: 10.1002/jcb.10360. [DOI] [PubMed] [Google Scholar]
- 132.Orwoll ES, Klein RF. Osteoporosis in men. Endocr Rev. 1995;16:87–116. doi: 10.1210/edrv-16-1-87. [DOI] [PubMed] [Google Scholar]
- 133.Finkelstein JS, Klibanski A, Neer RM. A longitudinal evaluation of bone mineral density in adult men with histories of delayed puberty. J Clin Endocrinol Metab. 1996;81:1152–1155. doi: 10.1210/jcem.81.3.8772591. [DOI] [PubMed] [Google Scholar]
- 134.Colvard DS, Eriksen EF, Keeting PE, Wilson EM, Lubahn DB, French FS, Riggs BL, Spelsberg TC. Identification of androgen receptors in normal human osteoblast-like cells. Proc Natl Acad Sci U S A. 1989;86:854–857. doi: 10.1073/pnas.86.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mauras N, Haymond MW, Darmaun D, Vieira NE, Abrams SA, Yergey AL. Calcium and protein kinetics in prepubertal boys. Positive effects of testosterone. J Clin Invest. 1994;93:1014–1019. doi: 10.1172/JCI117049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen RY, Nordin BE, Need AG, Scopacasa F, Wishart J, Morris HA, Horowitz M. Relationship between calcium absorption and plasma dehydroepiandrosterone sulphate (DHEAS) in healthy males. Clin Endocrinol (Oxf ) 2008 Apr 14; doi: 10.1111/j.1365-2265.2008.03272.x. [DOI] [PubMed] [Google Scholar]
- 137.Krabbe S, Hummer L, Christiansen C. Serum levels of vitamin D metabolites and testosterone in male puberty. J Clin Endocrinol Metab. 1986;62:503–507. doi: 10.1210/jcem-62-3-503. [DOI] [PubMed] [Google Scholar]
- 138.Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67:693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- 139.Breslau NA, Zerwekh JE. Relationship of estrogen and pregnancy to calcium homeostasis in pseudohypoparathyroidism. J Clin Endocrinol Metab. 1986;62:45–51. doi: 10.1210/jcem-62-1-45. [DOI] [PubMed] [Google Scholar]
- 140.Quan-Sheng D, Miller SC. Calciotrophic hormone levels and calcium absorption during pregnancy in rats. Am J Physiol. 1989;257:E118–E123. doi: 10.1152/ajpendo.1989.257.1.E118. [DOI] [PubMed] [Google Scholar]
- 141.Halloran BP, DeLuca HF. Calcium-Transport in Small-Intestine During Pregnancy and Lactation. American Journal of Physiology. 1980;239:E64–E68. doi: 10.1152/ajpendo.1980.239.1.E64. [DOI] [PubMed] [Google Scholar]
- 142.Brommage R, Baxter DC, Gierke LW. Vitamin D-independent intestinal calcium and phosphorus absorption during reproduction. American Journal of Physiology. 1990;259:631–8. doi: 10.1152/ajpgi.1990.259.4.G631. [DOI] [PubMed] [Google Scholar]
- 143.Fudge NJ, Kovacs CS. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology. 2010;151:886–895. doi: 10.1210/en.2009-1010. [DOI] [PubMed] [Google Scholar]
- 144.Boass A, Toverud SU, Pike JW, Haussler MR. Calcium metabolism during lactation: enhanced intestinal calcium absorption in vitamin D-deprived, hypocalcemic rats. Endocrinology. 1981;109:900–907. doi: 10.1210/endo-109-3-900. [DOI] [PubMed] [Google Scholar]
- 145.Pahuja DN, DeLuca HF. Stimulation of Intestinal Calcium-Transport and Bone Calcium Mobilization by Prolactin in Vitamin-D-Deficient Rats. Science. 1981;214:1038–1039. doi: 10.1126/science.7302575. [DOI] [PubMed] [Google Scholar]
- 146.Robinson CJ, Spanos E, James MF, Pike JW, Haussler MR, Makeen AM, Hillyard CJ, MacIntyre I. Role of Prolactin in Vitamin-D Metabolism and Calcium-Absorption During Lactation in the Rat. Journal of Endocrinology. 1982;94:443–453. doi: 10.1677/joe.0.0940443. [DOI] [PubMed] [Google Scholar]
- 147.Morgan EL, Mace OJ, Affleck J, Kellett GL. Apical GLUT2 and Cav1.3: regulation of rat intestinal glucose and calcium absorption. J Physiol. 2007 Apr 15;580:593–604. doi: 10.1113/jphysiol.2006.124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thongon N, Nakkrasae LI, Thongbunchoo J, Krishnamra N, Charoenphandhu N. Enhancement of calcium transport in Caco-2 monolayer through PKCzeta-dependent Cav1.3-mediated transcellular and rectifying paracellular pathways by prolactin. Am J Physiol Cell Physiol. 2009;296:C1373–C1382. doi: 10.1152/ajpcell.00053.2009. [DOI] [PubMed] [Google Scholar]
- 149.Nakkrasae LI, Thongon N, Thongbunchoo J, Krishnamra N, Charoenphandhu N. Transepithelial calcium transport in prolactin-exposed intestine-like Caco-2 monolayer after combinatorial knockdown of TRPV5, TRPV6 and Ca(v)1.3. J Physiol Sci. 2010;60:9–17. doi: 10.1007/s12576-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]