Abstract
Generation of reactive oxygen species (ROS) is a ubiquitous phenomenon in eukaryotic cells' life. Up to the 1990s of the past century, ROS have been solely considered as toxic species resulting in oxidative stress, pathogenesis and aging. However, there is now clear evidence that ROS are not merely toxic species but also—within certain concentrations—useful signaling molecules regulating physiological processes. During intense skeletal muscle contractile activity myotubes' mitochondria generate high ROS flows: this renders skeletal muscle a tissue where ROS hold a particular relevance. According to their hormetic nature, in muscles ROS may trigger different signaling pathways leading to diverging responses, from adaptation to cell death. Whether a “positive” or “negative” response will prevail depends on many variables such as, among others, the site of ROS production, the persistence of ROS flow or target cells' antioxidant status. In this light, a specific threshold of physiological ROS concentrations above which ROS exert negative, toxic effects is hard to determine, and the concept of “physiologically compatible” levels of ROS would better fit with such a dynamic scenario. In this review these concepts will be discussed along with the most relevant signaling pathways triggered and/or affected by ROS in skeletal muscle.
1. Introduction
Oxidative stressors, such as reactive oxygen species (ROS), have been initially and long considered as merely deleterious species to skeletal muscle tissue. Indeed, since the 1980s abundant evidence clearly indicated that ROS play a pathogenic role in inherited muscular dystrophies [1] and have then been identified as concausal factors in various muscular diseases [2–5]. However, and thereafter, accumulating evidence indicated that ROS, at least within concentrations emerging from physiological conditions, could also play a positive role in physiologically relevant processes in muscle cells. As an example, inflammation-derived ROS play a contradictory role in muscle repair [2]: in combination with other actors such as growth factors and chemokines, ROS participate in a cascade of events leading to muscle regeneration and repair; on the contrary, the local persistence of ROS sustained by infiltrated neutrophils may cause further injury by oxidatively damaging differentiating myoblasts and myotubes thus delaying the restitutio ad integrum. Similarly, ROS generated during exercise promote mitochondriogenesis (a key factor in muscle differentiation) via peroxisome proliferator-activated-receptor-gamma-coactivator-1α-(PGC-1α) activated signal transduction pathway [3] but, at higher and persistent levels, they might target mitochondria and mitochondrial DNA (mtDNA) turning into blockers of myogenic differentiation [4, 5]. Other examples of such diverging capacities—which strengthen the generally accepted notion that ROS act in a hormetic fashion—will be discussed thereafter. The prevalence of each of the two actions, that is, beneficial or detrimental, depends on the coincidence of various intrinsic and extrinsic factors among which the most prominent is the level and the duration of ROS targeting muscle cells; other variables are the source or the site of ROS generation, the antioxidant status of target cells, and their DNA repair capacity. The differentiation stage of muscle cells (satellite cell, differentiating myoblast or mature myotube) is also capable of redirecting the cell through different signaling pathways and of further modulating the ensuing cell response. Today ROS are known to trigger and/or affect many signaling pathways relevant to skeletal muscle cells' homeostasis and adaptation: here we will illustrate some of the signaling pathways triggered/affected by ROS in muscle tissue and their physiopathological implications (see Figure 1 for a visual summary).
Figure 1.
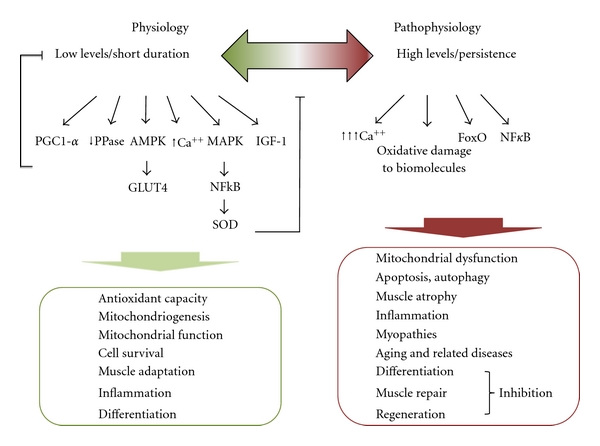
Major signaling pathways triggered and/or affected by ROS in skeletal muscle. Low levels of ROS activate specific key signaling molecules such as PGC-1α, AMPK, and MAPK, which control cellular mechanisms for muscle adaptation (oxidative metabolism, mitochondrial biogenesis, and mitochondrial functionality) as well as antioxidant enzymes that function as backregulators of intracellular ROS levels. Slight ROS accumulation also inhibits PPases and promotes the phosphorylation state of many proteins involved in the muscle signaling responses. Moreover, low levels of ROS play an important role in inducing upregulation of growth factors such as IGF-1, which has beneficial effects in muscle protein balance, supports oxidative metabolism, and contributes to the development of an oxidant-resistant phenotype, therefore preventing oxidative damage and chronic diseases. Thus, low levels of ROS elicit positive effects on physiological muscle responses. By contrast high levels of ROS cause functional oxidative damages of proteins, lipids, nucleic acids and cell components, induce a significant rise of intracellular [Ca2+], and promote signaling cascades for apoptosis or autophagy via NF-κB or FoxO paths. For these reasons high ROS levels are reputed to act as etiological, or at least exacerbating factors in muscle atrophy, sarcopenia, wasting, and chronic-/aging-related muscle diseases and myopathies. Depending on their level/persistence, ROS may also turn the same process from “physiologic” into “pathologic”, as in the case of inflammation.
2. Generation of ROS in Skeletal Muscle Cells
Mitochondria are commonly considered as the predominant source of ROS in skeletal muscle cells [6, 7]. Increased mitochondrial ROS generation occurs during various and different situations, such as in the course of intense contractile activity [8] or in response to cytokines such as tumor necrosis factor-α (TNF-α) [9]. Early reports assumed that 2–5% of the total oxygen consumed by mitochondria may undergo one electron reduction with the generation of superoxide [10, 11]. More recent studies indicated that complexes I and III of the electron transport chain are the main sites of mitochondrial superoxide production [12, 13]. During exercise, it is assumed that the increased ROS generation in the course of contractile activity is due to the high oxygen consumption that takes place during increased mitochondrial activity. Indeed superoxide generation in skeletal muscle increases to about a 50- or 100-fold during aerobic contractions [14, 15].
However, recent evidence demonstrates that mitochondria may not be the prevalent source of ROS during exercise [8], and future studies are required to better elucidate the mitochondrial role in contraction-induced production of ROS in skeletal muscle. In 2002 St. Pierre and colleagues [16] reexamined the rate of mitochondrial ROS production and concluded that the total fraction of oxygen converted into superoxide was equal to 0.15%; this value is significantly lower than that (2–5%) estimated by other authors (see for example [17]). This lower rate of superoxide production takes account of the uncoupling proteins role (specifically UCP3 in skeletal muscle) as regulators of mitochondrial ROS production [18, 19] acting to prevent oxidative damage to mitochondria. In addition, growing evidence highlights that mitochondria produce more ROS during the basal state 4 of respiration as compared to state 3 (maximal ADP-stimulated respiration) [20–23]. Thus, since skeletal muscle mitochondria, during aerobic contractile activity, are predominantly in state 3, this limits their capacity of generating ROS during contractions [21–23].
Mitochondria are not the main and only source of ROS production in skeletal muscle during exercise. Indeed, other relevant sources of ROS production within muscle cells are nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) located within the sarcoplasmic reticulum, transverse tubules, and the sarcolemma [24, 25].
Phospholipase A2 (PLA2) is another known source of intracellular ROS production [17]. Arachidonic acid released from cell membranes by PLA2 is a substrate for ROS-generating enzyme systems such as the lipoxygenases [26]. Activation of PLA2 can stimulate NOXs [27] and increased PLA2 activity can promote ROS production in muscle mitochondria [28] and cytosol [29] and release ROS into the extracellular space [26]. Both calcium-dependent and -independent forms of PLA2 exist in skeletal muscle and both contribute to muscle ROS generation [17]. In particular, the calcium-independent isoforms are likely to be involved in cytosolic oxidant activity in skeletal muscle cells [29], whereas a 14-kDa calcium-dependent isoform located within mitochondria is reputed to stimulate mitochondrial ROS generation during contractile activity [30]. In this light it has been proposed [29] that the calcium-independent PLA2 is a major determinant of ROS activity under resting conditions, whereas during processes or stress elevating intracellular calcium concentration (contraction, inflammation, heat stress, etc.) the calcium-dependent PLA2 is activated to promote ROS production.
Finally, superoxide anion is known to be generated by xanthine oxidase (XO) in the cytosol of contracting rat skeletal muscles cells [31]. However, human skeletal muscles contain lower levels of XO than rat muscle cells, and the question whether XO plays an important role in superoxide production in human skeletal muscle is still open [31, 32]. More ROS-generating mechanisms may be operative at the same time, as in the case of prolonged muscle ischemia where mitochondrial and cytoplasmic (via XO) ROS production has been simultaneously scored [33].
ROS can be generated through the above mechanisms not only within muscle cells but also in their proximity. For instance, during inflammation (a pathophysiological state that substantially alters cellular oxidative/antioxidant homeostasis) infiltrated polymorphoneutrophils activate NOX producing ROS via the respiratory burst and many cytokines, which amplify in a feedforward cycle ROS production, are secreted within muscles [34]. For instance, during the early phase of muscle injury, inflammatory cytokines can bind to membrane receptors and activate specific ROS-generating enzymes, such as cyclooxygenase-2, NOX, and XO [35]. Endothelial cells from injured muscle are known to secrete TNF-α, interleukine (IL)-1, IL-6, and IL-8, providing a positive feedforward cycle [36]. Whereas a transient oxidative stress is necessary in inflamed muscle cells to exert an antiseptic function and to activate various signal transduction pathways relevant to the restitutio ad integrum, prolonged severe oxidative stress may imbalance intracellular antioxidant homeostasis and hence long-term muscle welfare.
The oxygen-centered species that mostly arises from the processes described so far is superoxide anion, but its significance in ROS signaling pathways seems to be limited to a role as a precursor signaling molecule. Indeed superoxide undergoes enzymatic or spontaneous dismutation, a process generating H2O2. H2O2 is a nonradical, is a weak oxidant with a relatively long half-life allowing its diffusion within cells and across cell membranes [37], reacts with many different cellular molecules, and activates a wide number of signaling pathways. These properties render H2O2 the most relevant ROS signaling molecule in cells [38]. In contrast, H2O2 undergoing Fenton chemistry in the presence of redox active free iron ions or other transition metals can give rise to hydroxyl radicals, which react immediately with any surrounding biomolecules exerting most of the deleterious effects associated with oxidative stress. In this light, iron homeostasis can be considered as a comodulator of ROS signaling and effects. In particular, since skeletal muscle contains 10 to 15% of body iron—mainly in myoglobin and mitochondria—it could be particularly sensitive to alterations of iron homeostasis: accordingly it has been recently reported that levels of muscle nonheme iron and the iron transport protein, transferrin were elevated in senescence, suggesting that iron load is a significant component of sarcopenia [39].
3. Antioxidants and Modulation of Muscle Cells' Sensitivity to ROS
As it will be discussed throughout this review, the net effect of ROS on cells' signaling pathways and fate depends also on the cellular antioxidant capacity. The antioxidant network consists of enzymes, such as catalase, glutathione peroxidase (GPx), thioredoxin reductases (TRxs), superoxide dismutases (SODs), and soluble antioxidants such as glutathione (GSH) and vitamin E. Depending on its efficacy, the antioxidant cellular network plays a primary role in maintaining ROS below a physiologically compatible threshold level, thus allowing ROS to serve, theoretically, as signaling molecules and avoiding them to exert direct toxic effects. Many antioxidant enzymes are known to be induced in response to increased ROS generation. The increased ROS flux occurring in the course of strenuous exercise, through redox-sensitive mechanisms, induces the expression of γ-glutamylcysteinyl synthetase, the rate-limiting enzyme of GSH synthesis, of GPx, and of MnSOD [40]. Nuclear Factor KappaB (NF-κB), activator protein 1 (AP-1), and mitogen-activated protein kinases (MAPKs) have been identified as the major signaling pathways that can be activated by exercise-derived ROS and directly involved in the induction of the above antioxidant enzymes [40]. For instance, in the signaling path of MnSOD gene expression, NF-κB and AP-1 play an important role [41]: both NF-κB and AP-1 binding sites are present in the promoter of the mammalian MnSOD gene, and ROS have been shown to activate their binding.
The above responses, whose extent might be genetically predetermined, represent a fundamental adaptive countermeasure to conditions potentially resulting in frank oxidative stress. A specific modulator of ROS activity, working in an “antioxidant-like” fashion in ROS-mediated autophagy and apoptosis (see also “ROS mediate autophagy and apoptosis”), is the p53-inducible glycolysis and apoptosis regulator (TIGAR), a p53-target gene. Indeed, TIGAR can reduce ROS levels in response to nutrient starvation or metabolic stress, thus inhibiting autophagy and apoptosis independently of mammalian target of rapamycin (mTOR) or p53 modulation [42]. The TIGAR protein functions as a fructose-2,6-bisphosphatase and contributes to the regulation of intracellular ROS levels by modulation of the glycolytic pathway [43]. By decreasing glycolytic rate and redirecting glycolytic intermediates to the oxidative branch of the pentose phosphate pathway, TIGAR causes an increase in NADPH production, which favours ROS scavenging through GPx/glutathione reductase and GSH cycle. In this manner, TIGAR lowers the sensitivity of cells to ROS-induced p53-dependent apoptosis [43].
As to the influence of physical exercise, it caused an activation of MAP kinases in gastrocnemius muscle from rats; this in turn activated the NF-κB pathway and consequently the expression of SOD and adaptation to exercise through increased expression of endothelial and inducible nitric oxide synthase [40]. All these responses were silenced when ROS production was prevented by allopurinol [40]. Thus ROS act as signals in exercise because their scavenging prevents activation of important signaling pathways promoting useful adaptations in cells. Because these signals result in an upregulation of powerful antioxidant enzymes, exercise itself can be considered an antioxidant.
Besides the established soluble cellular antioxidants, creatine (Cr) is emerging as a pleiotropic molecule capable of influencing muscle cell's trophism, differentiation, and sensitivity to ROS [44, 45]. Cr has a high tropism for skeletal muscles, where most of body Cr is stored and has been shown to exert direct and indirect antioxidant activity in proliferating and differentiating C2C12 myoblasts [44]. A recent article by Young et al. [46] showed that two TRxs situated in the mitochondria and cytoplasm, respectively, were increased in Cr-treated C2C12 myoblasts: peroxiredoxin-4, a type 2 peroxiredoxin, and thioredoxin-dependent peroxide reductase.
As it will be discussed below, the 5′-adenosine monophosphate-activated protein kinase (AMPK) signaling is critical in regulating mitochondrial content and function in a PGC-1α -dependent pathway in different tissues and in response to various stimuli [47]; furthermore AMPK signaling is important in preventing the mitochondrial dysfunction/impairment increasing ROS leakage and accompanying sarcopenia, disuse muscle atrophy, and other degenerative muscle disorders, in such a way that it can be considered as an indirect antioxidant cellular setting. Ceddia and Sweeney [48] firstly demonstrated that Cr supplementation may improve cellular bioenergetics by activating AMPK to improve overall mitochondrial content and/or function. It is currently unknown whether Cr supplementation exerts similar AMPK effects in oxidatively injured muscle tissue. At this regard, we recently observed in either control or oxidatively challenged differentiating myoblasts that a 24 h Cr preloading [0.3 mM] is an adequate stimulus to activate the AMPK pathway (unpublished observation).
This observation, along with others showing that Cr also acts as a direct antioxidant [5, 44, 49, 50] and protects differentiating myoblasts from H2O2-dependent differentiative arrest [5], suggests that in oxidative stressing conditions Cr treatment might confer myoblasts an enhanced adaptive capacity resulting in increased mitochondrial functionality and biogenesis and reduction of oxidative damage during myogenesis. Thus, besides established antioxidants, Cr might represent a skeletal-muscle-directed endogenous molecule capable of exerting multiple, pleiotropic actions which collectively help counteracting excessive ROS pressure. Consistently, beneficial effects of Cr supplementation have been reported for a large number of muscular, neurological, and cardiovascular diseases as well as in sarcopenia and aging [44, 51–56]. On the contrary, although great attention has been—and is still—paid to the administration of established antioxidants including polyphenols and vitamins in order to reduce the potential risk of the sustained and persistent action of ROS on skeletal muscle, there is no clear consensus on the benefits of these supplements [40, 57–59]. Thus, exploiting the efficacy of “atypical” and pleiotropic antioxidants such as Cr deserves consideration.
The integrity of the antioxidant network is particularly important in aging. Indeed, it is well-established that aging is associated with increased free radical generation and the resulting oxidative damage accumulated in organisms are likely to be involved, at least at a concausal level, in the progression of numerous diseases [60, 61]. It has long been suspected that senescent skeletal muscle may progressively loose its ability to adapt to oxidative stress [62, 63]. However, at the present, there is no clear consensus about how and whether senescent skeletal muscle becomes more susceptible to ROS pressure. For example, although in skeletal muscle antioxidant enzyme activities are increased with old age [64, 65], protein and mRNA levels of CuZnSOD, MnSOD, and GPx were found to be decreased or unaltered in the aged muscle [66, 67]. More importantly, aged muscle exhibited reduced antioxidant adaptation compared to training young muscle [62]. The reduced ability to rapidly activate an antioxidant adaptation program may render the senescent muscle more prone to oxidative damage. Notably, it has been hypothesized that the lack of adaptive capacity in aging muscle may depend on the impairment of signal transduction of antioxidant gene expression in response to oxidative stress [68]. At this regard, as discussed above, NF-κB and AP-1 are known to play an important role in MnSOD gene expression [41]. The decreased binding of these nuclear factors, despite increased ROS generation found in aged muscles, would suggest that aging slows down molecular signaling of antioxidant gene expression. Thus aging seems to decrease the ability of aged muscle to express at least MnSOD as demonstrated by lower nuclear protein binding, mRNA levels, and unaltered enzyme protein [62]. The observed increase in MnSOD activity in the same setting might depend on a posttranslational modification (activation) of the enzyme molecules in aged muscle. In contrast to MnSOD, CuZnSOD showed increased protein content and activity with age in type II muscle in the absence of mRNA changes [66]. On the whole, these data suggest that the widely reported increase in antioxidant enzyme activities in aging skeletal muscle do not depend on enhanced gene transcription, but can rather derive from translational and/or posttranslational mechanisms. Since aged skeletal muscles are affected by augmented levels of lipid peroxidation, protein oxidation, and DNA damage, these compensatory increases in antioxidant enzyme activity are ineffective in counteracting increased ROS generation.
4. ROS, Mitochondrial Biogenesis and Function
It is well known that oxidative signals affect mitochondrial biogenesis, morphology, and function in skeletal muscle cells [69–71]: again, the effect of ROS seems to be bifaceted and controversial. Indeed, ROS may be important either in eliciting pathological effects leading to mitochondrial dysfunction and cell death ([72, 73], see also below “Mitochondrial ROS Mediate Autophagy and Apoptosis”), or play physiological roles promoting positive responses in mitochondrial biogenesis and function. Mitochondrial biogenesis is dependent on the expression of the mitochondrial genome and the nuclear genes that encode mitochondrial proteins [74]. An important pathway triggered by ROS is that leading to the upregulation of the mitochondrial biogenesis master gene PGC-1α. The PGC-1α transcriptional coactivator is a major regulator of energy metabolism [75]. It controls many aspects of oxidative metabolism, including mitochondrial adaptations, insulin-sensitizing via the upregulation of selected genes involved in fatty acid β-oxidation, glucose transport, and oxidative phosphorilation [76–79]. The mitochondrial biogenesis signaling activated by PGC-1 members family involves the transcription factors that regulate expression of nuclear genes such as nuclear respiratory factor (NRF) 1/2 and estrogen-related receptor-α (ERR-α). These three latter genes control the expression of nuclear genes encoding mitochondrial proteins and induce expression of mitochondrial transcription factor A (T-fam), which regulates mtDNA replication and transcription, thus activating the coordinated expression of mitochondrial proteins [80, 81].
Several signaling kinases have been involved in mediating PGC-1α transcriptional activation in response to a variety of stimuli among which the most important are calcium/calmodulin-dependent protein kinase (CaMK) type IV [82], AMPK [83], and p38 mitogen-activated protein kinase [84]. Their activation induces the PGC-1α promoter transcriptional regulation [69]. Recently, it has been demonstrated that mitochondrial biogenesis in skeletal muscle is controlled, at least in part, by a redox-sensitive mechanism and that physical exercise, increasing the ROS production over the physiological level, stimulates the muscle PGC-1α/NRF-1/T-fam signaling [85]. Irrcher and colleagues have evaluated the link between ROS levels and PGC-1α gene expression [69] in C2C12 cells. They found that endogenously produced ROS, at least within skeletal muscle cells, are important for the maintenance of PGC-1α expression levels within a normal physiological range. Indeed, quenching basal endogenous ROS with N-acetylcysteine (NAC) results in reduced PGC-1α mRNA expression, an effect which is unrelated to any inhibition of PGC-1α promoter activity, but probably dependent on the enhanced instability of PGC-1α mRNA occurring in a low ROS environment. On the contrary, increasing ROS levels with exogenous H2O2 augments PGC-1α transcription indirectly via the AMPK activation caused by the oxidatively-induced ATP depletion. This stimulates the binding of USF-1 to an Ebox within the PGC-1α promoter, increases transcription and results in the induction of PGC-1α mRNA expression, whose stability would also be restored in a more ROS-rich environment. The interplay of PGC-1α and ROS is further strengthened by the fact that, besides being a key modulator of mitochondrial biogenesis, it is important in regulating the expression level of protective enzymes acting against ROS generation and damage [86]. Indeed, experiments with either genetic knockouts (KOs) or using RNA interference for PGC1α show that the ability of ROS to induce a ROS-scavenging program depends largely on PGC-1α activity [86]. This response includes genes encoding for antioxidant enzymes localized either within mitochondria (MnSOD) or cytosol (catalase and GPxs). Indeed, cells lacking PGC-1α are more susceptible to the toxicity induced by oxidative stress caused by H2O2 [86]. These latter effects of PGC-1α are likely to represent a compensatory response where it plays a central role in the adaptation of cellular energy metabolism, mitochondrial biogenesis and antioxidant capacity in response to oxidative challenge. At this regard and extending previous research from our group [5], we have recently addressed the problem of the role of PGC-1α in C2C12 myoblasts subjected to oxidative stress during the early stages of differentiation. In particular, we examined the effect of a mildly toxic concentration of exogenously added H2O2 [0.3 mM] on the regulation of PGC-1α expression and its relationship with AMPK activation (unpublished observations). According to Kang and Irrcher [69, 85], we found that 1 h treatment with H2O2 markedly increased PGC-1α mRNA expression. It is of worth that, concurrently, we also found an increased phosphorylation of AMPK as compared to untreated cells, suggesting that oxidative stress induces PGC-1α through the AMPK signaling pathway. However, despite the fact that challenged C2C12 myoblasts rapidly activate a defense-oriented signaling cascade, they displayed a 30–40% reduction of their viability as well as a survivors' reduced differentiative efficiency during the post-challenge incubation stage (up to 7 days of culture). This observation would imply that, besides probably being an obligatory and physiological response to ROS, activation of AMPK and of PGC-1α may not be sufficient to afford a complete protection to cells against an overwhelming oxidative stress. Accumulating or excessive oxidative stress is known to be detrimental for mitochondria: for instance mtDNA represents a critical target for oxidative damage [49]. Indeed, mtDNA mutations are known as being an etiological factor in oxidative stress-related disorders including cardiovascular diseases and inherited or acquired neurodegenerative disorders, mitochondrial myopathies, and the normal aging process.
5. ROS Mediate Autophagy and Apoptosis
ROS may trigger either autophagy or apoptosis: whether these two pathways will be activated depends on the cell context and on the availability of specific modulators of ROS activity [87]. Autophagy is one of the cellular defense mechanisms activated in response to an excessive ROS production. Indeed, ROS act as signaling molecules in the early events of autophagy induction [87]. Phosphoinositide 3 kinase (PI3K) is known to mediate, at least in part, ROS effects. If the prosurvival effort fails, ROS induce cell death which may involve either the autophagic or the apoptotic pathway, or both [72, 88].
ROS signaling pathways play an important role in the induction of autophagy under physio- and pathological conditions. In healthy cells, autophagy is routinely involved in organelles and proteins turnover as well as in cellular energetic balance [89]. One of its strongest and better-characterized stimuli is starvation, where mitochondrial ROS production is enhanced and autophagy increased [87]. Increased ROS generation in the mitochondria under starvation is known to depend, at least in part, by class III PI3K: this event is essential for the induction of autophagy [90]. Indeed, upon starvation, ROS, and in particular H2O2, oxidize and inhibit Atg4, a protease responsible for microtubule-associated protein (MAP) light chain 3 (LC3) delipidation, that is, a condition resulting in the stabilization of the lipidated forms of LC3 and promoting the autophagosome maturation [87]. Notably, the same authors reported that addition of antioxidants inhibits these effects, preventing autophagosome biogenesis [91].
Thus, autophagy induced by starvation, where ROS participate in a feedforward manner, plays a prosurvival role since it contributes to the mobilization and reutilization of diverse cellular energy stores [89].
In a different direction, it is also known that when autophagy is prolonged, it could lead to cell death independently from apoptosis [92]. Indeed in nonmuscle tissues and in specific pathological conditions, ROS-induced autophagy was often linked to cell demise and death. As to skeletal muscle, ROS have been implicated in the induction of autophagy in muscle atrophy, disuse, and aging [72, 93]. Important new evidence on the wasting effect induced by increased oxidative stress on muscle phenotype was obtained by targeting a mutant SOD variant found in human amyotrophic lateral sclerosis myopathy [93, 94]. Indeed, these authors created a mouse model with a G93A mutation of SOD1 restricted to skeletal muscle [93]: accumulation of ROS in the muscles of these mice induced progressive atrophy associated with increased autophagy and forkhead transcription factors O (FoxO3) expression, a transcription factor which controls the transcription of autophagy-related genes and is required for the induction of autophagy through the lysosomal pathway in skeletal muscle in the absence of AKT repression [95–97]. In addition, NF-κB signaling has been proposed as an alternative pathway linked to ROS-mediated skeletal muscle atrophy [98]: indeed NF-κB was found to induce muscle atrophy and wasting via the lysosomal enzyme cathepsin L [93, 99] upregulation. Since cathepsin L is typically upregulated by FoxO3, it might be speculated that ROS-induced NF-κB converges on the FoxO3 autophagic pathway.
Increasing evidence suggests that autophagy of mitochondria is a selective and defense-oriented response against ROS, mitochondrial dysfunction and the accumulation of somatic mutations of mtDNA with aging [72, 100]. For this reason it has been recently proposed the term “mitophagy” to emphasize the nonrandom nature of this process [100]. Damaged mitochondria are removed by mitophagy by Binp3, a BH3 proapoptotic member of the Bcl-2 family and fis 1, a pro-fission mitochondrial protein that induces mitochondrial fragmentation and enhances the extent of mitophagy. Notably, inhibition/alteration of mitophagy can contribute to myofiber degeneration and weakness in muscle disorders characterized by accumulation of abnormal mitochondria and inclusions [101, 102].
ROS may have various and important roles in apoptotic cell death: direct actions such as oxidation of cellular proteins and lipids, damage of nucleic acids and functional alteration of organelles; ROS may also modulate cell death processes affecting various signaling cascades [103]. Indeed, ROS participate in early and late steps of the regulation of apoptosis, affecting different apoptotic signaling cascades in both intrinsic or extrinsic pathways.
The extrinsic path, which involves stimulation of receptor-mediated apoptotic pathways, can be initiated by ligand-induced (e.g., TNFα and Fas-L and TNF-related apoptosis-inducing ligand, TRAIL) binding, which promotes the activation of caspase-3 and subsequent degradation of genomic DNA [103]. Recent evidence suggests possible direct roles for ROS in mediating death receptors activation and subsequent induction of apoptosis [104]. Indeed, apoptotic signaling is induced by NOX-derived ROS at the plasma membrane level, which lead to lipid raft formation and death receptor clustering activation [104]. The physiological relevance and significance of ROS-dependent receptor-mediated apoptosis as compared to the classical receptor/ligand-induced apoptotic signaling is, at present, incompletely understood and warrants further investigation.
ROS may act as intracellular intermediates directly dysregulating the sarcoplasmic reticulum Ca++ flux and handling, which results in caspase-7 and calpain activation. Furthermore, ROS may cause mitochondrial swelling and fragmentation, and/or alter the conformation of the mitochondrial permeability transition pores (MPTPs), thus facilitating their opening and the release of proapoptotic proteins such as cytochrome c (Cyt C). Independently of caspase activity, apoptosis may follow the intrinsic path, where ROS may directly cause the release of mitochondrial endonuclease G (Endo G), and/or of apoptosis inducing factor (AIF), which is capable of promoting DNA fragmentation in skeletal muscle myonuclei [105].
Another protein coupled with ROS-induced apoptosis is the voltage-dependent anion selective channel protein 1 (VDAC1). This transmembrane protein has been defined a ROS sensor [106] that triggers opening of the MPTP complex under conditions of oxidative stress. Indeed VDAC1 is the main channel within the mitochondrial outer membrane and upon ROS accumulation exhibits an increased conductance associated with MPTP opening and dissipation of ΔΨ, thus favouring the efflux of apoptotic proteins located in the intermembrane space and finally cell death [107]. Notably, the pro- and antiapoptotic Bcl2-family proteins are released via VDAC1 action and ROS may further affect these responses as they are known, in nonmuscle cells, to down-regulate the endogenous levels of the antiapoptotic protein Bcl-2 [108]. The mechanism through which Bcl-2 levels are affected by ROS has been studied by Azad et al. in nonmuscle cell types and seems to depend on superoxide anion-related degradation of Bcl-2 protein through the ubiquitin-proteasomal pathway [109].
Furthermore, under oxidative stressing conditions, ROS activate a signaling cascade involving the protein kinase C (PKC) b-dependent phosphorylation of the Shc adaptor protein p66shc and its translocation to the mitochondrial matrix. In particular, the mitochondrially translocated fraction of p66shc behaves as redox enzyme that utilizes reducing equivalents derived from the mitochondrial electron transport chain to produce H2O2 in the intermembrane space, an event which is known to trigger apoptosis [110, 111].
The accumulation of ROS within the mitochondrial matrix, as well as their capacity of triggering apoptosis, is counteracted/regulated by mitochondrial antioxidant enzymes, namely, phospholipids hydroperoxide glutathione peroxidase, GPx, and Mn-SOD [3, 112].
Thus increased mitochondrial production of ROS is involved at multiple levels in promoting apoptosis in skeletal muscle cells, an event which participates in the aetiology and progression of numerous pathologies including sarcopenia and disuse muscle atrophy as well as in aging [71, 113].
Physical training and exercise are known to increase mitochondrial biogenesis and density as well as mitochondrial ROS production especially during repeated contractions [85]. Therefore and unless other determinants are considered, it might appear paradoxical that although a routine of regular exercise is associated with numerous health benefits, physical exercise might potentially promote oxidative stress and ROS-associated apoptosis of skeletal muscle cells [17]. Indeed, chronic contractile activity (CCA) and endurance training induce an adaptive response in skeletal muscle cells leading to increased mitochondrial biogenesis [114] and—theoretically—an obligatory increase in a number of proapoptotic mitochondrial proteins and byproducts such as ROS. However, as a matter of fact recent evidence indicates that mitochondria isolated from rat skeletal muscle subjected to CCA seem to acquire an antiapoptotic, rather than proapoptotic, behaviour [114]. The study also addressed the problem of the relative antiapoptotic role acquired by different mitochondrial subpopulations from CCA-trained muscles, namely, the intramyofibrillar (IMF) and the subsarcolemmal (SS) mitochondria. The release of both Cyt C and AIF caused by exogenous H2O2 from CCA-isolated IMF and SS mitochondria was decreased; CCA augmented the expression of antiapoptotic HSP70 and caspase recruitment domain protein in either SS or IMF and caused a decreased ROS generation in IMF mitochondria. On the contrary, states III and IV respiring SS mitochondria showed a modestly increased rate of ROS generation as well as an increased resilience of MPTP opening. It was then hypothesized that these effects might collectively reflect the overall reduced apoptogenic capacity acquired by mitochondria following CCA training of skeletal muscles and that, in particular, the slight increase of ROS generated by SS would contribute to the activation of redox-sensitive transcription factors promoting muscle fiber plasticity and adaptation, rather than to function as proapoptotic triggers. Again, such a scenario is indicative of the diverging effects that ROS may assume depending on specific situations of cells' life, rather than on their net concentration and site of generation.
6. ROS Signaling and Myogenic Differentiation
Increasing evidence indicates that ROS are capable of affecting—mostly reducing—the efficiency of myogenic differentiation. The integrity/alteration of myogenic differentiation is central to many physiological and pathological processes. Successful differentiation of satellite-derived myoblasts into functioning and integrated myotubes is a fundamental prerequisite for muscle regeneration, a repair process which is of primary importance in maintaining muscle function [115]. Notably, oxidative stress is known to play a concausal and detrimental role in a variety of multifactorial muscular pathologies characterized by proliferation/differentiation imbalance such as Duchenne dystrophy [116], myotonic dystrophy [117], sarcopenia [118], and cachexia [119].
The role of ROS in this context has been extensively documented. Ardite et al. [120] showed that ROS induced a strong depletion of the intracellular GSH pool: notably depletion of GSH causes further intracellular accumulation of ROS which favors NF-κB activation, thus contributing to the lower expression of MyoD and impaired myogenesis (see below).
According to Ardite et al. [120], data from our group [5] indicate that a mildly toxic H2O2 treatment during the early stages of C2C12 myoblast differentiation results in GSH depletion and strongly impairs the differentiative outcome. This effect is unlikely to be a mere result of ROS-induced cell demise: indeed, the cells surviving H2O2, although exhibiting a partial and late recovery of protein synthesis and of viability, were unable to continue and execute the differentiative task. These cells also displayed a strong and long-lasting reduction of the mRNA levels of MyoD, which is involved in early stem cell commitment, and of myogenin and MRF4, both recruited at later differentiation times [121, 122]. Whether the transcription of these muscle regulatory factors (MRFs) is a result of a specific signaling promoted by ROS or of a cell suffering is still to be understood. Under the same conditions depressing these MRFs, insulin-like growth factor 1 (IGF-1) which plays a pivotal role in controlling muscle growth [123], was inhibited to an even greater extent (see also “ROS and IGF-1 signaling”). Interestingly, H2O2-injured cells showed signs of extensive mitochondrial degeneration (swelling and disruption) and lower mitochondrial density, suggesting that these organelles are specifically targeted by—or particularly sensitive to—exogenous ROS. Loss of mitochondria is a clearly detrimental event in a process typically requiring active mitochondriogenesis such as muscle differentiation [4, 124].
ROS generated by the inflammatory cytokine TNFα are known to inhibit myogenesis, and this effect is widely attributed to oxidative activation of NF-κB and subsequent gene expression [125–127]. However, the effect of TNFα is likely to be more complex since Langen et al. [9] showed that TNFα causes loss of myogenic capacity of C2C12 cells via NF-κB-dependent and-independent and oxidative-sensitive and-insensitive pathways. In particular they hypothesized that an oxidative-sensitive, NF-κB-independent mechanism might involve the blockage of the formation of functional catenin-adherin complexes proximate to the cell membrane [128]. Potentially, disruption of these complexes and the resulting alteration of cell-matrix and cell-cell interactions, might be responsible for the inhibition of myotube formation independently of NF-κB.
The redox regulation of the NF-κB family of transcriptional activators plays a central role in differentiation, adaptation, and death of muscle cells. This role is extremely complex: indeed the effects promoted by NF-κB are sometimes contradicting. As an example, although ROS can directly stimulate NF-κB, oxidized NF-κB has a diminished DNA-binding activity [17]. NF-κB has been mostly associated with a negative regulation of skeletal muscle differentiation [119, 129, 130]. NF-κB is constitutively active in proliferating myoblasts and can inhibit myogenesis by promoting a mitogenic activity via cyclin D1 or by inhibiting the synthesis of MyoD, a muscle-specific helix-loop-helix transcription factor operating in muscle development and repair [131–133]. More recently, NF-κB was shown to suppress myofibrillar gene expression through the regulation of the myogenic transcriptional repressor Yin Yiang 1 [134]. Moreover, treatment of primary myoblasts with the NF-κB inhibitor curcumin stimulates myoblast fusion thereby enhancing myogenesis and repair [125]. In line with these in vitro findings, activation of the TNFα pathway by muscle gene transfer inhibits regeneration in vivo, while muscle-specific deletion of the heteromeric kinase complex IKK was recently described to promote secondary myogenesis in response to acute injury signals [126, 127]. Activation of NF-κB downstream ROS formation is also capable of stimulating the activity of inducible nitric oxide synthase (iNOS), whose role in myogenic process is controversial [24]. Some authors found that iNOS activity suppresses muscle differentiation, whereas others reported that stimulation of iNOS via NF-κB represents a positive and necessary stimulus for muscle differentiation, that iNOS activity paralleled myogenesis from the early to later stages in H9C cells and that ROS formed by NOX 2 were the basic trigger leading to iNOS stimulation via NF-κB recruitment [24, 135, 136]. Blockage of this pathway, or inhibition of iNOS with specific inhibitors, led to differentiative arrest. Also, a recent article by Lee et al. [137] indicates that complex-I-derived superoxide anions, produced through reverse electron transport, were dismutated into H2O2 by MnSOD induced via NF-κB activation and that H2O2 stimulated muscle differentiation as a signaling messenger. Thus the scenario arising from these results would indicate that ROS negatively or positively regulate muscle differentiation via the signaling pathways involving NF-κB activation.
Another evidence which lends support to the detrimental role of ROS in muscle differentiation comes from the studies on the role of p66Shc in skeletal muscle ischemic injury. p66Shc, along with its isoforms p46 and p52, constitutes the mammalian Shc adaptor protein group. The three isoforms share a common structure, but p66ShcA has the unique feature of an additional domain at the N terminus which contains a serine residue at position 36 (Ser-36) that is phosphorylated in response to several stimuli, including H2O2. Due to this feature p66 isoform regulates ROS metabolism and apoptosis [138, 139]; indeed, a fraction of p66ShcA is localized in the mitochondria where, as discussed above, it produces mitochondrial ROS as signaling molecules for apoptosis [110, 111]. Interestingly, both p66ShcA KO cells and mice display lower levels of intracellular ROS [139–141] and are less prone to apoptosis induced by an array of different stimuli. Also, p66Shc KO mice are resistant to ischemia-induced apoptosis and show decreased muscle damage in response to hind limb ischemia [142]. More recently, Zaccagnini et al. [143] unravelled the role of p66Shc and ROS in muscular damage and regeneration following acute hind limb ischemia in both WT and p66Shc KO mice. WT mice showed detectable levels of oxidative stress markers during the postischemic and regenerative stages; on the contrary, the same markers were undetectable in KO mice. More interestingly, although the initial ischemic damage was identical and no advantage in terms of muscle vascularization and perfusion was observed in KO mice, their regenerative capacity was significantly higher as compared to WT. Satellite cell populations were similar in both groups, but those from KO mice showed a higher proliferation rate at first and spontaneous differentiation when cultured under prodifferentiative conditions. Finally, p66Shc KO satellite cells were resistant to the myogenic inhibition induced by H2O2 acute challenge or hypoxia. The authors proposed different and possible explanations for the above effects. The first one involves the different availability of NO—whose promyogenic role has been discussed above—in KO mice: since active p66Shc generates superoxide anions, which consume available NO forming the toxic species peroxynitrite, p66Shc KO mice would benefit of higher NO availability and would not suffer of peroxynitrite toxicity, two effects favouring myogenesis and muscle regeneration. Another plausible mechanism involves the NAD+-dependent histone deacetylase Sir2. Sir2 deacetylase activity is dependent on the fluctuation of cytosolic NAD+/NADH ratio, that is, the cellular redox state [144]. Under conditions of high ROS concentrations, NAD levels increase and promote Sir2 activation, which in turn inhibits MyoD-dependent transcription. p66Shc KO mice are characterized by lower levels of ROS and, as a result, decreased Sir2 activity, that is a condition which affects MyoD functions to a lesser extent. Finally, since oxidative DNA damage may trigger a differentiation checkpoint and cause a reversible inhibition of myogenic differentiation targeting MyoD phosphorylation, such a checkpoint activation may be attenuated by p66ShcA deletion, which results in decreased intracellular ROS levels.
With regard to prodifferentiative effects induced by ROS in this context, in addition to the already cited report by Lee et al. [137], it has been recently demonstrated that in a non skeletal-muscle cell, that is, vascular smooth muscle cells (VSMC), ROS increase their differentiation rate after quiescence through a p38 MAPK-dependent pathway [145]. Similarly, other studies focusing on ROS and muscle metabolism, differentiation, and growth unravelled some positive interactions with IGF-1 signaling (see below).
Again, the most likely explanation for these opposite effects is that cell fate may depend on the intracellular ROS type (i.e., which is the prevailing reactive species) and level. In fact, it is well known that ROS elicit a wide spectrum of cellular responses, depending on their intracellular level [146]. A low dose of ROS controls normal cellular signaling pathways while an intermediate dose results in either temporary or permanent growth arrest [147]. Obviously, a high dose of ROS causes cell death via either apoptotic or necrotic mechanisms [142].
7. ROS and IGF-1 Signaling
Growing evidence suggests that oxidative stress is responsible, as a causal or a concausal factor, for the pathogenesis of many muscle diseases and muscle wasting [148, 149]. In muscle cells, IGF-1 is known to promote muscle welfare inducing muscle hypertrophy and stimulate muscle-cell proliferation, differentiation, and survival [123]. IGF-1 has also been found to contribute to oxidative balance and to mediate protective responses against iron-induced-lipid oxidative stress in vivo [150]. Accordingly, Yang and colleagues [151] demonstrated that IGF-1 displayed protective effects on muscle cells after oxidative stress: indeed, pretreatment with IGF-1 protected muscle cells from H2O2-induced cell death and enhanced their survival through promotion of the antiapoptotic protein Bcl2. The same authors showed that protection was via an IGF-1 subpathway: PI3K/Akt and ERK1/2 MAPK pathways [151].
IGF-1 is a peptide hormone with a complex post-trascriptional regulation, generating distinct isoforms, namely, IGF-1Ea, IGF-1Eb, and IGF-1Ec (this latter also known as mechano growth factor, MGF) [152]. Mouse models have provided insights into the tissue-specific functions and responses to ROS of the different IGF-1 isoforms [152–155]. For example, in murine models, the local muscle isoform of IGF-1 (mIGF-1, the orthologue of human MGF) has been shown not only to activate proliferation of myoblasts [156], but also to protect cardiomyocytes from oxidative stress via the Sirtuin 1 deacetylase activity [157].
As to physical activity, although its role in regulating the expression of specific IGF-1 isoforms has been widely studied, data in the literature regarding humans are often contradictory and are affected by many uncontrolled variables such as the lack of dietary control, heterogeneity of subjects, their physical fitness, differences in proposed physical exercise, and time course of sampling [158–160].
Similarly to other pathways, ROS may regulate either positively or negatively IGF-1 signaling [161]. Low levels of endogenous ROS—due to their reversible oxidative inhibition of protein tyrosine phosphatases (see also “ROS as multipurpose local regulators of muscle cell functions”)—induce the phosphorylation on specific tyrosine residues of insulin receptor (IR) and IR substrates (IRS) protein(s), thus facilitating the IGF-1 signaling. Indeed, the IRβ chain contains multiple sites for the phosphorylation of tyrosine that are sensitive targets of ROS such as H2O2 [162]. By contrast, higher ROS levels inhibit IGF-1 signaling cascades and recent evidence implicates ROS as downregulators of IGF-1 signaling and inducers of insulin resistance and its pathological sequelae [162].
However, ROS may be also involved in the activation of “insulin-like” metabolic effects by activating other non-insulin-initiated signaling pathways: one of the most important examples is the stimulation of glucose transport in skeletal muscle during exercise [163, 164]. Skeletal muscle contraction stimulates, as well as insulin, glucose transport by up to 50-fold during maximal exercise in humans [165]. Adding exogenous ROS to skeletal muscle in vitro stimulates glucose transport [166] whereas NAC, a potent antioxidant, reduces contraction-mediated glucose uptake by about 50% [167]. This effect of NAC was associated with a similar degree of inhibition of contraction-induced activation of AMPK. This kinase is a fundamental signaling kinase which, besides being involved in mitochondrial biogenesis (see “ROS signaling and myogenic differentiation”), is also known to upregulate the glucose uptake in muscle under conditions of high AMP/ATP ratio, like hypoxia and muscle contraction, forming a non-insulin-dependent pathway to increase muscle glucose utilization [158, 168–172]. Thus the proposed role of ROS in mediating the stimulation of glucose transport is related to skeletal muscle contraction, that increases superoxide anion production via mitochondrial respiration. Superoxide anion is rapidly converted to H2O2 by SOD, resulting in direct activation of AMPK, Glucose transporter 4 (Glut4) translocation to the plasma membrane, and an increase in glucose transport [173]. Moreover, in muscle cells, NAC antagonized ROS-mediated increase in glucose uptake in response to contraction, but not to insulin. Activation of AMPK in aerobic-exercise-induced glucose uptake is paradigmatic of ROS participation in physiologically-oriented signaling pathways relevant to the homeostasis of the entire organism.
It has also been demonstrated that ROS regulate IGF-1-induced myotube hypertrophy in vitro. It is well known that exercise-induced muscle hypertrophy mostly depends on the increased local production of IGF-1 via activation of the PI3K/Akt pathway [174, 175]; interestingly ROS, which are being overproduced during exercise, contribute in a feedforward manner to stimulate IGF1 net accumulation.
Previous reports show that there are positive and negative interactions between ROS and IGF-1 synthesis in both skeletal and VSMCs [176–178]. Treating VSMCs with H2O2 or XO augments both IGF-1 mRNA and IGF-1 protein secreted into the cultured medium, indicating that ROS enhance the IGF-1 autocrine system in VSMCs [176]. By contrast, we and others [5, 178] found that toxicologically relevant concentrations of H2O2 negatively regulate the IGF-1 mRNA levels in differentiating C2C12 myoblasts. In our experience, oxidative insult significantly decreased IGF-1 mRNA expression levels [5]. Cr, notably, prevented its inhibition: moreover Cr is known to induce hypertrophy of differentiating myoblasts via IGF-1 pathway [123].
Taken collectively, these results suggest that—although ROS enhance IGF-1 signaling—there is a negative feedback regulation of IGF-1 mRNA levels occurring with mildly toxic ROS levels in C2C12 cells. Thus, ROS regulate IGF-1 action via a variety of mechanisms, and the effects are likely, again, to be cell type and dose dependent.
Thus, ROS play a crucial role in the IGF-1 signaling regulation and its biological action in muscle cells. However, additional studies are necessary to better explain the physiological significance of these interactions in humans, with particular regard to the identification of the distinct actions on the IGF-1 propeptide isoforms.
8. ROS as Multipurpose Local Regulators of Muscle Cell Functions
Similarly to other noninflammatory cells, skeletal muscle cells produce transient fluxes of ROS in response to an array of diverse stimuli, such as intense contractile activity [179, 180], heat stress [181], short-term disuse atrophy [182], acute hypoxia [143, 183], acute osmotic stress [184], and stretch [185]. Furthermore, locally produced waves of ROS are also released by skeletal muscle in response to cell surface receptor activation via cytokines, hormones, growth factors [186–188], or nuclear receptor activation [189, 190]. Considering the large variety of different stimuli converging to ROS production along with their lack of chemical specificity, it is hard to formulate a unitary explanation of the physiological significance of ROS in the responses triggered by such divergent signals [188]. At this regard, data published by Wright et al. [191] prompted these authors to draw an attractive hypothesis which involves the regulation of the protein phosphatases (PPases) “tone” in muscle cells and tissue. PPases belong to two broad families, the protein tyrosine PPases (about 112 human proteins) and the serine/threonine PPases (about 31 proteins). These two families are divided into further subclasses according to their specificity (only tyrosine targets or tyrosine plus serine/threonine targets) or, with regard to the second family, the subclasses characterized by a Zn2+/Fe2+ complex at the catalytic site or by the Mn2+/Mg2+ dependence [191].
The redox sensitivity of the protein tyrosine PPases and its potential biological importance is well documented in vitro and in cell culture systems since the early 1990s [192, 193]. As to Ser/Thr PPases, their sensitivity to oxidants is more controversial: calcineurin is the first whose sensitivity to oxidants has been clearly identified [194–196].
Interestingly, Wright et al. [191] found that not only protein tyrosine PPases, but also Ser/Threo PPases are inhibited by exposure to ROS or ROS generating agents (namely, H2O2 and DMNQ, resp.). The relative sensitivity of different PPases to oxidation in the above scenario has not yet been addressed. The mechanism by which PPases are oxidized is likely to involve the vulnerability of their ubiquitous and conserved cysteine-based active site; more surprising and still unexplained is the observed inhibition of ser/threo PPases which—with the exception of calcineurin—are best known in literature as “relatively immune to oxidation”.
Indeed, the same study by Wright et al. [191] shows that in muscle tissue even minimal, physiologically relevant concentrations of oxidants, lead to an overall inhibition of PPases' activity. Notably, the concentrations used neither affected contractile function nor resulted in clear oxidative stress. Consistently, the level of net phosphorylation of a wide range of functionally diverging proteins was correspondingly higher in treated muscle preparations. This latter data suggests that oxidants are capable of affecting a broad range of PPases. Interestingly the majority of kinases are equally sensitive to oxidants but, contrary to PPases, oxidants promote their activation. Since kinases operate sequentially as amplification chains, it is likely that the observed increase in the net protein phosphorylation level under low-oxidative stressing conditions is the result of a lower PPases activity along with an increased kinases activity. These two combined events promoted by ROS would obviously trigger and/or affect many different signaling pathways, contributing to orchestrate the final cellular responses.
In summary, oxidants could function to regulate in vivo global “phosphatase and kinase tone” and thus influence the kinetics and amplification of many kinase signaling pathways. With respect to skeletal muscle, such a scenario would be of great biological and physiopathological relevance, since muscle cells typically and continually produce ROS fluxes of different duration, intensity, and localization, depending on either intrinsic and extrinsic variables. Notably, such a hypothesis fits well with the hormetic nature of ROS.
9. Conclusion
The picture arising from this review indicates that ROS activate and/or participate in many signaling pathways promoting complex and diverging effects in skeletal muscle cells, ranging from positive to detrimental. As an example, many studies have concluded that inactivity-induced ROS production in skeletal muscle contributes to disuse muscle atrophy [197, 198]. On the contrary, growing evidence also suggests that intracellular ROS production is a required signal for the normal remodelling that occurs in skeletal muscle in response to repeated bouts of endurance exercise [40, 199, 200]. How can the same trigger promote such opposite effects? Based upon current knowledge, it appears that the mode and the situation characterizing skeletal muscle cells exposure to ROS may account, at least in part, for this apparent paradox. Transiently increased, moderate levels of oxidative stress might represent a potentially health-promoting process, whereas its uncontrolled persistence and/or propagation might result in overwhelming cell damage thus turning into a pathological event: for instance, the role of ROS in inflammation fits well with this model. In addition, the complexity, the variety, the interplay, and the functionally diverging roles of the signaling pathways activated or modulated by ROS contribute to further complicate this scenario. Thus, a gradual and variable, rather than a sharp, boundary is likely to characterize the transition between the two types of ROS actions. Such a variable “greyscaling” of ROS effects may depend on extrinsic and intrinsic situations such as, at least, (i) the concentration of ROS, (ii) the type of reactive species involved, (iii) the persistence of ROS activity, (iv) the localization of ROS source, (v) the antioxidant capacity and the energy status of muscle cells, (vi) their ability to adapt to oxidative stress (which in vivo also depends on ageing and/or physical training), (vii) the differentiative status, for example, myoblasts versus integrated myotubes, (viii) the absence/presence of an inflammatory process, and (ix) the plasticity of the signaling pathways triggered/affected by ROS. The balance between these factors will ultimately determine which type of signal(s) and effect(s) will prevail within the cell. Again, the hormetic nature of ROS emerges as the key feature of these species in many tissues, including skeletal muscle. Careful titration of ROS levels within skeletal muscle cell may therefore lie at the cross between the initiation and progression of disease and cell death, the induction of mitochondrial biogenesis, repair, and more generally cellular metabolic health. Supplementation with exogenous antioxidants is being widely studied to attain and maintain an “ideal titration” of ROS within skeletal muscle: unfortunately, at the present, no clear indication of the benefits arising from supplemental antioxidant intake emerges from literature. This reflects the need for further studies aimed at clarifying how to regulate ROS levels to exploit their physiological effects and avoid their damages.
Abbreviations
- AMPK:
Adenosine monophosphate-activated protein kinase
- Bcl-2:
B-cell lymphoma 2
- FoxO:
Forkhead box O
- GLUT4:
Glucose transporter type 4
- MAPK:
Mitogen-activated protein kinase
- NF-κB:
Nuclear factor kappa B
- PGC-1α:
Peroxisome proliferator-activated receptor gamma coactivator 1 alpha
- PPases:
Phosphatases
- ROS:
Reactive oxygen species
- SOD:
Superoxide dismutase.
References
- 1.Murphy ME, Kehrer JP. Activities of antioxidant enzymes in muscle, liver and lung of chickens with inherited muscular dystrophy. Biochemical and Biophysical Research Communications. 1986;134(2):550–556. doi: 10.1016/s0006-291x(86)80455-7. [DOI] [PubMed] [Google Scholar]
- 2.Tidball JG. Inflammatory processes in muscle injury and repair. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2005;288(2):R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 3.Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Experimental Physiology. 2003;88(1):99–107. doi: 10.1113/eph8802505. [DOI] [PubMed] [Google Scholar]
- 4.Rochard P, Rodier A, Casas F, et al. Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression and activity of myogenic factors. Journal of Biological Chemistry. 2000;275(4):2733–2744. doi: 10.1074/jbc.275.4.2733. [DOI] [PubMed] [Google Scholar]
- 5.Sestili P, Barbieri E, Martinelli C, et al. Creatine supplementation prevents the inhibition of myogenic differentiation in oxidatively injured C2C12 murine myoblasts. Molecular Nutrition and Food Research. 2009;53(9):1187–1204. doi: 10.1002/mnfr.200800504. [DOI] [PubMed] [Google Scholar]
- 6.Davies KJA, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochemical and Biophysical Research Communications. 1982;107(4):1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 7.Koren A, Sauber C, Sentjurc M, Schara M. Free radicals in tetanic activity of isolated skeletal muscle. Comparative Biochemistry and Physiology—B Biochemistry and Molecular Biology. 1983;74(3):633–635. doi: 10.1016/0305-0491(83)90241-9. [DOI] [PubMed] [Google Scholar]
- 8.Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. Journal of Applied Physiology. 2007;102(4):1664–1670. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- 9.Langen RCJ, Schols AMWJ, Kelders MCJM, van der Velden JLJ, Wouters EFM, Janssen-Heininger YMW. Tumor necrosis factor-α inhibits myogenesis through redox-dependent and -independent pathways. American Journal of Physiology—Cell Physiology. 2002;283(3):C714–C721. doi: 10.1152/ajpcell.00418.2001. [DOI] [PubMed] [Google Scholar]
- 10.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochemical Journal. 1973;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loschen G, Azzi A, Richter C, Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Letters. 1974;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 12.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. Journal of Bioenergetics and Biomembranes. 1999;31(4):347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 13.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. Journal of Biological Chemistry. 2004;279(47):49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 14.Kanter M. Free radicals, exercise and antioxidant supplementation. Proceedings of the Nutrition Society. 1998;57(1):9–13. doi: 10.1079/pns19980004. [DOI] [PubMed] [Google Scholar]
- 15.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1-2):41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 16.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. Journal of Biological Chemistry. 2002;277(47):44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 17.Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radical Biology and Medicine. 2011;51(5):942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Brand MD, Affourtit C, Esteves TC, et al. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radical Biology and Medicine. 2004;37(6):755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 19.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metabolism. 2005;2(2):85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. American Journal of Physiology—Cell Physiology. 2005;289(4):C994–C1001. doi: 10.1152/ajpcell.00031.2005. [DOI] [PubMed] [Google Scholar]
- 21.Di Meo S, Venditti P. Mitochondria in exercise-induced oxidative stress. Biological Signals and Receptors. 2001;10(1-2):125–140. doi: 10.1159/000046880. [DOI] [PubMed] [Google Scholar]
- 22.Herrero A, Barja G. ADP-Regulation of mitochondrial free radical production is different with complex I- or complex II-linked substrates: implications for the exercise paradox and brain hypermetabolism. Journal of Bioenergetics and Biomembranes. 1997;29(3):241–249. doi: 10.1023/a:1022458010266. [DOI] [PubMed] [Google Scholar]
- 23.Kozlov AV, Szalay L, Umar F, et al. Skeletal muscles, heart, and lung are the main sources of oxygen radicals in old rats. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2005;1740(3):382–389. doi: 10.1016/j.bbadis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Piao YJ, Seo YH, Hong F, et al. Nox 2 stimulates muscle differentiation via NF-κB/iNOS pathway. Free Radical Biology and Medicine. 2005;38(8):989–1001. doi: 10.1016/j.freeradbiomed.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiological Reviews. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo L, Christofi FL, Wright VP, Bao S, Clanton TL. Lipoxygenase-dependent superoxide release in skeletal muscle. Journal of Applied Physiology. 2004;97(2):661–668. doi: 10.1152/japplphysiol.00096.2004. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, Bey EA, Wientjes FB, Cathcart MK. Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity: cPLA2 affects translocation but not phosphorylation of p67phox and p47phox. Journal of Biological Chemistry. 2002;277(28):25385–25392. doi: 10.1074/jbc.M203630200. [DOI] [PubMed] [Google Scholar]
- 28.Nethery D, Callahan LA, Stofan D, Mattera R, DiMarco A, Supinski G. PLA2 dependence of diaphragm mitochondrial formation of reactive oxygen species. Journal of Applied Physiology. 2000;89(1):72–80. doi: 10.1152/jappl.2000.89.1.72. [DOI] [PubMed] [Google Scholar]
- 29.Gong MC, Arbogast S, Guo Z, Mathenia J, Su W, Reid MB. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. Journal of Applied Physiology. 2006;100(2):399–405. doi: 10.1152/japplphysiol.00873.2005. [DOI] [PubMed] [Google Scholar]
- 30.Nethery D, Stofan D, Callahan L, DiMarco A, Supinski G. Formation of reactive oxygen species by the contracting diaphragm is PLA2 dependent. Journal of Applied Physiology. 1999;87(2):792–800. doi: 10.1152/jappl.1999.87.2.792. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Cabrera MC, Borrás C, Pallardo FV, Sastre J, Ji LL, Viña J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. Journal of Physiology. 2005;567, part 1:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gómez-Cabrera MC, Pallardó FV, Sastre J, Viña J, Garcia-del-Moral L. Allopurinol and markers of muscle damage among participants in the Tour de France. Journal of the American Medical Association. 2003;289(19):2503–2504. doi: 10.1001/jama.289.19.2503-b. [DOI] [PubMed] [Google Scholar]
- 33.Baudry N, Laemmel E, Vicaut E. In vivo reactive oxygen species production induced by ischemia in muscle arterioles of mice: involvement of xanthine oxidase and mitochondria. American Journal of Physiology—Heart and Circulatory Physiology. 2008;294(2):H821–H828. doi: 10.1152/ajpheart.00378.2007. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Brakefield D, Pan Y, Hunter D, Myckatyn TM, Parsadanian A. Muscle-derived but not centrally derived transgene GDNF is neuroprotective in G93A-SOD1 mouse model of ALS. Experimental Neurology. 2007;203(2):457–471. doi: 10.1016/j.expneurol.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Ji LL. Antioxidant signaling in skeletal muscle: a brief review. Experimental Gerontology. 2007;42(7):582–593. doi: 10.1016/j.exger.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen BK, Ostrowski K, Rohde T, Bruunsgaard H. The cytokine response to strenuous exercise. Canadian Journal of Physiology and Pharmacology. 1998;76(5):505–511. doi: 10.1139/cjpp-76-5-505. [DOI] [PubMed] [Google Scholar]
- 37.Gutteridge JMC, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Annals of the New York Academy of Sciences. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 38.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Molecular Cell. 2007;26(1):1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Altun M, Edström E, Spooner E, et al. Iron load and redox stress in skeletal muscle of aged rats. Muscle and Nerve. 2007;36(2):223–233. doi: 10.1002/mus.20808. [DOI] [PubMed] [Google Scholar]
- 40.Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radical Biology and Medicine. 2008;44(2):126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Schreck R, Baeuerle A. A role for oxygen radicals as second messengers. Trends in Cell Biology. 1991;1(2-3):39–42. doi: 10.1016/0962-8924(91)90072-h. [DOI] [PubMed] [Google Scholar]
- 42.Bensaad K, Cheung EC, Vousden KH. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO Journal. 2009;28(19):3015–3026. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bensaad K, Tsuruta A, Selak MA, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 44.Sestili P, Martinelli C, Colombo E, et al. Creatine as an antioxidant. Amino Acids. 2011;40(5):1385–1396. doi: 10.1007/s00726-011-0875-5. [DOI] [PubMed] [Google Scholar]
- 45.Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40(5):1271–1296. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young JF, Larsen LB, Malmendal A, et al. Creatine-induced activation of antioxidative defence in myotube cultures revealed by explorative NMR-based metabonomics and proteomics. Journal of the International Society of Sports Nutrition. 2010;7(1, article 9) doi: 10.1186/1550-2783-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zong H, Ren JM, Young LH, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ceddia RB, Sweeney G. Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. Journal of Physiology. 2004;555, part 2:409–421. doi: 10.1113/jphysiol.2003.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guidi C, Potenza L, Sestili P, et al. Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochimica et Biophysica Acta—General Subjects. 2008;1780(1):16–26. doi: 10.1016/j.bbagen.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of creatine. Biochemical and Biophysical Research Communications. 2002;290(1):47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- 51.Gordon A, Hultman E, Kaijser L, et al. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovascular Research. 1995;30(3):413–418. [PubMed] [Google Scholar]
- 52.Matthews RT, Ferrante RJ, Klivenyi P, et al. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Experimental Neurology. 1999;157(1):142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- 53.Mazzini L, Balzarini C, Colombo R, et al. Effects of creatine supplementation on exercise performance and muscular strength in amyotrophic lateral sclerosis: preliminary results. Journal of the Neurological Sciences. 2001;191(1-2):139–144. doi: 10.1016/s0022-510x(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 54.Stout JR, Eckerson JM, May E, Coulter C, Bradley-Popovich GE. Effects of resistance exercise and creatine supplementation on myasthenia gravis: a case study. Medicine and Science in Sports and Exercise. 2001;33(6):869–872. doi: 10.1097/00005768-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Tarnopolsky MA, Mahoney DJ, Vajsar J, et al. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology. 2004;62(10):1771–1777. doi: 10.1212/01.wnl.0000125178.18862.9d. [DOI] [PubMed] [Google Scholar]
- 56.Vorgerd M, Grehl T, Jäger M, et al. Creatine therapy in myophosphorylase deficiency (McArdle disease): a placebo-controlled crossover trial. Archives of Neurology. 2000;57(7):956–963. doi: 10.1001/archneur.57.7.956. [DOI] [PubMed] [Google Scholar]
- 57.McGinley C, Shafat A, Donnelly AE. Does antioxidant vitamin supplementation protect against muscle damage? Sports Medicine. 2009;39(12):1011–1032. doi: 10.2165/11317890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Nakazato K, Ochi E, Waga T. Dietary apple polyphenols have preventive effects against lengthening contraction-induced muscle injuries. Molecular Nutrition and Food Research. 2010;54(3):364–372. doi: 10.1002/mnfr.200900145. [DOI] [PubMed] [Google Scholar]
- 59.Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Medicine and Science in Sports and Exercise. 2010;43(6):1017–1024. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 60.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mechanisms of Ageing and Development. 2006;127(5):436–443. doi: 10.1016/j.mad.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 62.Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Annals of the New York Academy of Sciences. 2001;928:236–247. doi: 10.1111/j.1749-6632.2001.tb05653.x. [DOI] [PubMed] [Google Scholar]
- 63.McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Research Reviews. 2002;1(1):79–93. doi: 10.1016/s0047-6374(01)00368-2. [DOI] [PubMed] [Google Scholar]
- 64.Ji LL, Dillon D, Wu E. Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. American Journal of Physiology. 1990;258(4, part 2):R918–R923. doi: 10.1152/ajpregu.1990.258.4.R918. [DOI] [PubMed] [Google Scholar]
- 65.Luhtala TA, Roecker EB, Pugh T, Feuers RJ, Weindruch R. Dietary restriction attenuates age-related increases in rat skeletal muscle antioxidant enzyme activities. Journals of Gerontology. 1994;49(5):B231–B238. doi: 10.1093/geronj/49.5.b231. [DOI] [PubMed] [Google Scholar]
- 66.Hollander J, Bejma J, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mechanisms of Ageing and Development. 2000;116(1):33–45. doi: 10.1016/s0047-6374(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 67.Oh-Ishi S, Toshinai K, Kizaki T, et al. Effects of aging and/or training on antioxidant enzyme system in diaphragm of mice. Respiration Physiology. 1996;105(3):195–202. doi: 10.1016/0034-5687(96)00057-6. [DOI] [PubMed] [Google Scholar]
- 68.Ji LL. Exercise-induced modulation of antioxidant defense. Annals of the New York Academy of Sciences. 2002;959:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 69.Irrcher I, Ljubicic V, Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1α transcription in skeletal muscle cells. American Journal of Physiology—Cell Physiology. 2009;296(1):C116–C123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 70.Jackson MJ. Skeletal muscle aging: role of reactive oxygen species. Critical Care Medicine. 2009;37(10):S368–S371. doi: 10.1097/CCM.0b013e3181b6f97f. [DOI] [PubMed] [Google Scholar]
- 71.Musarò A, Fulle S, Fanò G. Oxidative stress and muscle homeostasis. Current Opinion in Clinical Nutrition and Metabolic Care. 2010;13(3):236–242. doi: 10.1097/MCO.0b013e3283368188. [DOI] [PubMed] [Google Scholar]
- 72.Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mechanisms of Ageing and Development. 2010;131(7-8):536–543. doi: 10.1016/j.mad.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marzetti E, Hwang JCY, Lees HA, et al. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochimica et Biophysica Acta—General Subjects. 2010;1800(3):235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. Journal of Experimental Biology. 2006;209, part 12:2265–2275. doi: 10.1242/jeb.02182. [DOI] [PubMed] [Google Scholar]
- 75.Choi SC, Befroy DE, Codella R, et al. Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koves TR, Li P, An J, et al. Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. Journal of Biological Chemistry. 2005;280(39):33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 77.Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. American Journal of Physiology—Advances in Physiology Education. 2006;30(4):145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 78.Michael LF, Wu Z, Cheatham RB, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uguccioni G, Hood DA. The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. American Journal of Physiology—Endocrinology and Metabolism. 2011;300(2):E361–E371. doi: 10.1152/ajpendo.00292.2010. [DOI] [PubMed] [Google Scholar]
- 80.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes and Development. 2004;18(4):357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 81.Barbieri E, Battistelli M, Casadei L, et al. Morphofunctional and biochemical approaches for studying mitochondrial changes during myoblasts differentiation. Journal of Aging Research. 2011;2011:16 pages. doi: 10.4061/2011/845379. Article ID 845379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu H, Kanatous SB, Thurmond FA, et al. Regulation of mitochondrial biogenesis in skeletal muscle by caMK. Science. 2002;296(5566):349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- 83.Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS ONE. 2008;3(10) doi: 10.1371/journal.pone.0003614. Article ID e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akimoto T, Pohnert SC, Li P, et al. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. Journal of Biological Chemistry. 2005;280(20):19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 85.Kang C, O’Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-γ coactivator-1α signaling is redox sensitive. Free Radical Biology and Medicine. 2009;47(10):1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 86.Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Foundation Symposium. 2007;287:60–63. [PubMed] [Google Scholar]
- 87.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO Journal. 2007;26(7):1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nature Reviews Molecular Cell Biology. 2008;9(12):1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metabolism. 2011;13(5):495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kirisako T, Ichimura Y, Okada H, et al. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. Journal of Cell Biology. 2000;151(2):263–275. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scherz-Shouval R, Shvets E, Elazar Z. Oxidation as a post-translational modification that regulates autophagy. Autophagy. 2007;3(4):371–373. doi: 10.4161/auto.4214. [DOI] [PubMed] [Google Scholar]
- 92.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death and Differentiation. 2008;15(1):171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 93.Dobrowolny G, Aucello M, Rizzuto E, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metabolism. 2008;8(5):425–436. doi: 10.1016/j.cmet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 94.Aucello M, Dobrowolny G, Musarò A. Localized accumulation of oxidative stress causes muscle atrophy through activation of an autophagic pathway. Autophagy. 2009;5(4):527–529. doi: 10.4161/auto.5.4.7962. [DOI] [PubMed] [Google Scholar]
- 95.Mammucari C, Milan G, Romanello V, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metabolism. 2007;6(6):458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 96.Sandri M. Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. American Journal of Physiology—Cell Physiology. 2010;298(6):C1291–C1297. doi: 10.1152/ajpcell.00531.2009. [DOI] [PubMed] [Google Scholar]
- 97.Zhao J, Brault JJ, Schild A, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metabolism. 2007;6(6):472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Bar-Shai M, Carmeli E, Reznick AZ. The role of NF-κB in protein breakdown in immobilization, aging, and exercise: from basic processes to promotion of health. Annals of the New York Academy of Sciences. 2005;1057:431–447. doi: 10.1196/annals.1356.034. [DOI] [PubMed] [Google Scholar]
- 99.Deval C, Mordier S, Obled C, et al. Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochemical Journal. 2001;360, part 1:143–150. doi: 10.1042/0264-6021:3600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Research. 2005;8(1):3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 101.Masiero E, Agatea L, Mammucari C, et al. Autophagy is required to maintain muscle mass. Cell Metabolism. 2009;10(6):507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 102.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. International Journal of Biochemistry and Cell Biology. 2004;36(12):2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Primeau AJ, Adhihetty PJ, Hood DA. Apoptosis in heart and skeletal muscle. Canadian Journal of Applied Physiology. 2002;27(4):349–395. doi: 10.1139/h02-020. [DOI] [PubMed] [Google Scholar]
- 104.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biology and Medicine. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dupont-Versteegden EE, Strotman BA, Gurley CM, et al. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2006;291(6):R1730–R1740. doi: 10.1152/ajpregu.00176.2006. [DOI] [PubMed] [Google Scholar]
- 106.Madesh M, Hajnóczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. Journal of Cell Biology. 2001;155(6):1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tomasello F, Messina A, Lartigue L, et al. Outer membrane VDAC1 controls permeability transition of the inner mitochondrial membrane in cellulo during stress-induced apoptosis. Cell Research. 2009;19(12):1363–1376. doi: 10.1038/cr.2009.98. [DOI] [PubMed] [Google Scholar]
- 108.Hildeman DA, Mitchell T, Aronowt B, Wojciechowski S, Kappler J, Marrack P. Control of Bcl-2 expression by reactive oxygen species. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):15035–15040. doi: 10.1073/pnas.1936213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Azad N, Iyer A, Vallyathan V, et al. Role of oxidative/nitrosative stress-mediated Bcl-2 regulation in apoptosis and malignant transformation. Annals of the New York Academy of Sciences. 2010;1203:1–6. doi: 10.1111/j.1749-6632.2010.05608.x. [DOI] [PubMed] [Google Scholar]
- 110.Giorgio M, Migliaccio E, Orsini F, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122(2):221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 111.Pinton P, Rimessi A, Marchi S, et al. Protein kinase C β and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315(5812):659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 112.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovascular Research. 2000;47(3):446–456. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 113.Pellegrino MA, Desaphy J-F, Brocca L, Pierno S, Camerino DC, Bottinelli R. Redox homeostasis, oxidative stress and disuse muscle atrophy. Journal of Physiology. 2011;589(9):2147–2160. doi: 10.1113/jphysiol.2010.203232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adhihetty PJ, Taivassalo T, Haller RG, Walkinshaw DR, Hood DA. The effect of training on the expression of mitochondrial biogenesis- and apoptosis-related proteins in skeletal muscle of patients with mtDNA defects. American Journal of Physiology—Endocrinology and Metabolism. 2007;293(3):E672–E680. doi: 10.1152/ajpendo.00043.2007. [DOI] [PubMed] [Google Scholar]
- 115.Forcales SV, Puri PL. Signaling to the chromatin during skeletal myogenesis: novel targets for pharmacological modulation of gene expression. Seminars in Cell and Developmental Biology. 2005;16(4-5):596–611. doi: 10.1016/j.semcdb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 116.Messina S, Altavilla D, Aguennouz M, et al. Lipid peroxidation inhibition blunts nuclear factor-κB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. American Journal of Pathology. 2006;168(3):918–926. doi: 10.2353/ajpath.2006.050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Toscano A, Messina S, Campo GM, et al. Oxidative stress in myotonic dystrophy type 1. Free Radical Research. 2005;39(7):771–776. doi: 10.1080/10715760500138932. [DOI] [PubMed] [Google Scholar]
- 118.Fulle S, Protasi F, Di Tano G, et al. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Experimental Gerontology. 2004;39(1):17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 119.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO Journal. 1996;15(8):1753–1765. [PMC free article] [PubMed] [Google Scholar]
- 120.Ardite E, Barbera JA, Roca J, Fernández-Checa JC. Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-κB activation. American Journal of Pathology. 2004;165(3):719–728. doi: 10.1016/s0002-9440(10)63335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dedieu S, Mazères G, Cottin P, Brustis JJ. Involvement of myogenic regulator factors during fusion in the cell line C2C12. International Journal of Developmental Biology. 2002;46(2):235–241. [PubMed] [Google Scholar]
- 122.Ishibashi J, Perry RL, Asakura A, Rudnicki MA. MyoD induces myogenic differentiation through cooperation of its NH 2- and COOH-terminal regions. Journal of Cell Biology. 2005;171(3):471–482. doi: 10.1083/jcb.200502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Louis M, Van Beneden R, Dehoux M, Thissen JP, Francaux M. Creatine increases IGF-I and myogenic regulatory factor mRNA in C 2C12 cells. FEBS Letters. 2004;557(1–3):243–247. doi: 10.1016/s0014-5793(03)01504-7. [DOI] [PubMed] [Google Scholar]
- 124.Pawlikowska P, Gajkowska B, Hocquette JF, Orzechowski A. Not only insulin stimulates mitochondriogenesis in muscle cells, but mitochondria are also essential for insulin-mediated myogenesis. Cell Proliferation. 2006;39(2):127–145. doi: 10.1111/j.1365-2184.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thaloor D, Miller KJ, Gephart J, Mitchell PO, Pavlath GK. Systemic administration of the NF-κB inhibitor curcumin stimulates muscle regeneration after traumatic injury. American Journal of Physiology—Cell Physiology. 1999;277, part 1(2):C320–C329. doi: 10.1152/ajpcell.1999.277.2.C320. [DOI] [PubMed] [Google Scholar]
- 126.Coletti D, Moresi V, Adamo S, Molinaro M, Sassoon D. Tumor necrosis factor-α gene transfer induces cachexia and inhibits muscle regeneration. Genesis. 2005;43(3):120–128. doi: 10.1002/gene.20160. [DOI] [PubMed] [Google Scholar]
- 127.Mourkioti F, Kratsios P, Luedde T, et al. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. Journal of Clinical Investigation. 2006;116(11):2945–2954. doi: 10.1172/JCI28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goichberg P, Shtutman M, Ben-Ze’ev A, Geiger B. Recruitment of β-catenin to cadherin-mediated intercellular adhesions is involved in myogenic induction. Journal of Cell Science. 2001;114, part 7:1309–1319. doi: 10.1242/jcs.114.7.1309. [DOI] [PubMed] [Google Scholar]
- 129.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen- mediated NF-κB activation in response to tumor necrosis factor α . FASEB Journal. 1998;12(10):871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 130.Langen RCJ, Schols AMWJ, Kelders MCJM, Wouters EFM, Janssen-Heininger YMW. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-κB. FASEB Journal. 2001;15(7):1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- 131.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Molecular and Cellular Biology. 1999;19(8):5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr. NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289(5488):2363–2365. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 133.Parker MH, Perry RLS, Fauteux MC, Berkes CA, Rudnicki MA. MyoD synergizes with the E-protein HEBβ to induce myogenic differentiation. Molecular and Cellular Biology. 2006;26(15):5771–5783. doi: 10.1128/MCB.02404-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang H, Hertlein E, Bakkar N, et al. NF-κB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Molecular and Cellular Biology. 2007;27(12):4374–4387. doi: 10.1128/MCB.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee KH, Kim DG, Shin NY, et al. NF-κB-dependent expression of nitric oxide synthase is required for membrane fusion of chick embryonic myoblasts. Biochemical Journal. 1997;324, part 1:237–242. doi: 10.1042/bj3240237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kaliman P, Canicio J, Testar X, Palacín M, Zorzano A. Insulin-like growth factor-II, phosphatidylinositol 3-kinase, nuclear factor-κB and inducible nitric-oxide synthase define a common myogenic signaling pathway. Journal of Biological Chemistry. 1999;274(25):17437–17444. doi: 10.1074/jbc.274.25.17437. [DOI] [PubMed] [Google Scholar]
- 137.Lee S, Tak E, Lee J, et al. Mitochondrial H2O2 generated from electron transport chain complex i stimulates muscle differentiation. Cell Research. 2011;21(5):817–834. doi: 10.1038/cr.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Migliaccio E, Giogio M, Mele S, et al. The p66(shc) adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402(6759):309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 139.Trinei M, Giorgio M, Cicalese A, et al. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21(24):3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 140.Francia P, Delli Gatti C, Bachschmid M, et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110(18):2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 141.Napoli C, Martin-Padura I, de Nigris F, et al. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zaccagnini G, Martelli F, Fasanaro P, et al. p66ShcA modulates tissue response to hindlimb ischemia. Circulation. 2004;109(23):2917–2923. doi: 10.1161/01.CIR.0000129309.58874.0F. [DOI] [PubMed] [Google Scholar]
- 143.Zaccagnini G, Martelli F, Magenta A, et al. p66ShcA and oxidative stress modulate myogenic differentiation and skeletal muscle regeneration after hind limb ischemia. Journal of Biological Chemistry. 2007;282(43):31453–31459. doi: 10.1074/jbc.M702511200. [DOI] [PubMed] [Google Scholar]
- 144.Fulco M, Schiltz RL, Iezzi S, et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Molecular Cell. 2003;12(1):51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 145.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovascular Research. 2006;71(2):216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. Journal of Cellular Physiology. 2002;192(1):1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 147.Hye SP, Seung HL, Park D, et al. Sequential activation of phosphatidylinositol 3-kinase, βPix, Rac1, and Nox1 in growth factor-induced production of H2O2 . Molecular and Cellular Biology. 2004;24(10):4384–4394. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arthur PG, Grounds MD, Shavlakadze T. Oxidative stress as a therapeutic target during muscle wasting: considering the complex interactions. Current Opinion in Clinical Nutrition and Metabolic Care. 2008;11(4):408–416. doi: 10.1097/MCO.0b013e328302f3fe. [DOI] [PubMed] [Google Scholar]
- 149.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle and Nerve. 2007;35(4):411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 150.Kokoszko A, Dabrowski J, Lewiński A, Karbownik-Lewińska M. Protective effects of GH and IGF-I against iron-induced lipid peroxidation in vivo. Experimental and Toxicologic Pathology. 2008;60(6):453–458. doi: 10.1016/j.etp.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 151.Yang SY, Hoy M, Fuller B, Sales KM, Seifalian AM, Winslet MC. Pretreatment with insulin-like growth factor i protects skeletal muscle cells against oxidative damage via PI3K/Akt and ERK1/2 MAPK pathways. Laboratory Investigation. 2010;90(3):391–401. doi: 10.1038/labinvest.2009.139. [DOI] [PubMed] [Google Scholar]
- 152.Wallis M. New insulin-like growth factor (IGF)-precursor sequences from mammalian genomes: the molecular evolution of IGFs and associated peptides in primates. Growth Hormone and IGF Research. 2009;19(1):12–23. doi: 10.1016/j.ghir.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 153.Barton ER, Demeo J, Lei H. The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. Journal of Applied Physiology. 2010;108(5):1069–1076. doi: 10.1152/japplphysiol.01308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dobrowolny G, Giacinti C, Pelosi L, et al. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. Journal of Cell Biology. 2005;168(2):193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Musaró A, Giacinti C, Borsellino G, et al. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(5):1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kravchenko IV, Furalyov VA, Lisitsina ES, Popov VO. Stimulation of mechano-growth factor expression by second messengers. Archives of Biochemistry and Biophysics. 2011;507(2):323–331. doi: 10.1016/j.abb.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 157.Satoh A, Brace CS, Ben-Josef G, et al. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. Journal of Neuroscience. 2010;30(30):10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Berg U, Bang P. Exercise and circulating insulin-like growth factor I. Hormone Research. 2004;62(1):50–58. doi: 10.1159/000080759. [DOI] [PubMed] [Google Scholar]
- 159.Eppler E, Zapf J, Bailer N, Falkmer UG, Reinecke M. IGF-I in human breast cancer: low differentiation stage is associated with decreased IGF-I content. European Journal of Endocrinology. 2002;146(6):813–821. doi: 10.1530/eje.0.1460813. [DOI] [PubMed] [Google Scholar]
- 160.Philip Karl J, Alemany JA, Koenig C, et al. Diet, body composition, and physical fitness influences on IGF-I bioactivity in women. Growth Hormone and IGF Research. 2009;19(6):491–496. doi: 10.1016/j.ghir.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 161.Papaconstantinou J. Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Molecular and Cellular Endocrinology. 2009;299(1):89–100. doi: 10.1016/j.mce.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiological Reviews. 2009;89(1):27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 163.Coderre L, Kandror KV, Vallega G, Pilch PF. Identification and characterization of an exercise-sensitive pool of glucose transporters in skeletal muscle. Journal of Biological Chemistry. 1995;270(46):27584–27588. doi: 10.1074/jbc.270.46.27584. [DOI] [PubMed] [Google Scholar]
- 164.Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(13):5817–5821. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Katz A, Broberg S, Sahlin K, Wahren J. Leg glucose uptake during maximal dynamic exercise in humans. American Journal of Physiology. 1986;251(1, part 1):E65–E70. doi: 10.1152/ajpendo.1986.251.1.E65. [DOI] [PubMed] [Google Scholar]
- 166.Higaki Y, Mikami T, Fujii N, et al. Oxidative stress stimulates skeletal muscle glucose uptake through a phosphatidylinositol 3-kinase-dependent pathway. American Journal of Physiology—Endocrinology and Metabolism. 2008;294(5):E889–E897. doi: 10.1152/ajpendo.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sandström ME, Zhang SJ, Bruton J, et al. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. Journal of Physiology. 2006;575, part 1:251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology. 2006;21(1):48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- 169.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5’AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47(8):1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 170.Holloszy JO. A forty-year memoir of research on the regulation of glucose transport into muscle. American Journal of Physiology—Endocrinology and Metabolism. 2003;284(3):E453–E467. doi: 10.1152/ajpendo.00463.2002. [DOI] [PubMed] [Google Scholar]
- 171.Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5’ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48(8):1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- 172.Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. American Journal of Physiology—Endocrinology and Metabolism. 2006;291(5):E867–E877. doi: 10.1152/ajpendo.00207.2006. [DOI] [PubMed] [Google Scholar]
- 173.Katz A. Modulation of glucose transport in skeletal muscle by reactive oxygen species. Journal of Applied Physiology. 2007;102(4):1671–1676. doi: 10.1152/japplphysiol.01066.2006. [DOI] [PubMed] [Google Scholar]
- 174.Latres E, Amini AR, Amini AA, et al. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. Journal of Biological Chemistry. 2005;280(4):2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- 175.Solomon AM, Bouloux PMG. Modifying muscle mass—The endocrine perspective. Journal of Endocrinology. 2006;191(2):349–360. doi: 10.1677/joe.1.06837. [DOI] [PubMed] [Google Scholar]
- 176.Delafontaine P, Ku L. Reactive oxygen species stimulate insulin-like growth factor I synthesis in vascular smooth muscle cells. Cardiovascular Research. 1997;33(1):216–222. doi: 10.1016/s0008-6363(96)00179-4. [DOI] [PubMed] [Google Scholar]
- 177.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arteriosclerosis, Thrombosis and Vascular Biology. 2004;24(3):435–444. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 178.Handayaningsih A-E, Iguchi G, Fukuoka H, et al. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology. 2011;152(3):912–921. doi: 10.1210/en.2010-0981. [DOI] [PubMed] [Google Scholar]
- 179.Diaz PT, She ZW, Davis WB, Clanton TL. Hydroxylation of salicylate by the in vitro diaphragm: evidence for hydroxyl radical production during fatigue. Journal of Applied Physiology. 1993;75(2):540–545. doi: 10.1152/jappl.1993.75.2.540. [DOI] [PubMed] [Google Scholar]
- 180.Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. Journal of Applied Physiology. 1992;73(5):1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 181.Zuo L, Christofi FL, Wright VP, et al. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. American Journal of Physiology—Cell Physiology. 2000;279(4):C1058–C1066. doi: 10.1152/ajpcell.2000.279.4.C1058. [DOI] [PubMed] [Google Scholar]
- 182.Falk DJ, DeRuisseau KC, Van Gammeren DL, Deering MA, Kavazis AN, Powers SK. Mechanical ventilation promotes redox status alterations in the diaphragm. Journal of Applied Physiology. 2006;101(4):1017–1024. doi: 10.1152/japplphysiol.00104.2006. [DOI] [PubMed] [Google Scholar]
- 183.Zuo L, Clanton TL. Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. American Journal of Physiology—Cell Physiology. 2005;289(1):C207–C216. doi: 10.1152/ajpcell.00449.2004. [DOI] [PubMed] [Google Scholar]
- 184.Martins AS, Shkryl VM, Nowycky MC, Shirokova N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. Journal of Physiology. 2008;586(1):197–210. doi: 10.1113/jphysiol.2007.146571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cheng W, Li B, Kajstura J, et al. Stretch-induced programmed myocyte cell death. Journal of Clinical Investigation. 1995;96(5):2247–2259. doi: 10.1172/JCI118280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Espinosa A, García A, Härtel S, Hidalgo C, Jaimovich E. NADPH oxidase and hydrogen peroxide mediate insulin-induced calcium increase in skeletal muscle cells. Journal of Biological Chemistry. 2009;284(4):2568–2575. doi: 10.1074/jbc.M804249200. [DOI] [PubMed] [Google Scholar]
- 187.Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(18):8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2000;279(6):L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 189.Felty Q, Xiong WC, Sun D, et al. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005;44(18):6900–6909. doi: 10.1021/bi047629p. [DOI] [PubMed] [Google Scholar]
- 190.Zhang A, Jia Z, Guo X, Yang T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. American Journal of Physiology—Renal Physiology. 2007;293(3):F723–F731. doi: 10.1152/ajprenal.00480.2006. [DOI] [PubMed] [Google Scholar]
- 191.Wright VP, Reiser PJ, Clanton TL. Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. Journal of Physiology. 2009;587, part 23:5767–5781. doi: 10.1113/jphysiol.2009.178285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hecht D, Zick Y. Selective inhibition of protein tyrosine phosphatase activities by H2O2 and vanadate in vitro. Biochemical and Biophysical Research Communications. 1992;188(2):773–779. doi: 10.1016/0006-291x(92)91123-8. [DOI] [PubMed] [Google Scholar]
- 193.Heffetz D, Bushkin I, Dror R, Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. Journal of Biological Chemistry. 1990;265(5):2896–2902. [PubMed] [Google Scholar]
- 194.Agbas A, Hui D, Wang X, Tek V, Zaidi A, Michaelis EK. Activation of brain calcineurin (Cn) by Cu-Zn superoxide dismutase (SOD1) depends on direct SOD1-Cn protein interactions occurring in vitro and in vivo. Biochemical Journal. 2007;405(1):51–59. doi: 10.1042/BJ20061202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Namgaladze D, Hofer HW, Ullrich V. Redox control of calcineurin by targeting the binuclear Fe2+-Zn2+ center at the enzyme active site. Journal of Biological Chemistry. 2002;277(8):5962–5969. doi: 10.1074/jbc.M111268200. [DOI] [PubMed] [Google Scholar]
- 196.Namgaladze D, Shcherbyna I, Kienhöfer J, Hofer HW, Ullrich V. Superoxide targets calcineurin signaling in vascular endothelium. Biochemical and Biophysical Research Communications. 2005;334(4):1061–1067. doi: 10.1016/j.bbrc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 197.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2005;288(2):R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 198.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. Journal of Applied Physiology. 2007;102(6):2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 199.Hamilton KL, Staib JL, Phillips T, Hess A, Lennon SL, Powers SK. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radical Biology and Medicine. 2003;34(7):800–809. doi: 10.1016/s0891-5849(02)01431-4. [DOI] [PubMed] [Google Scholar]
- 200.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]


