Abstract
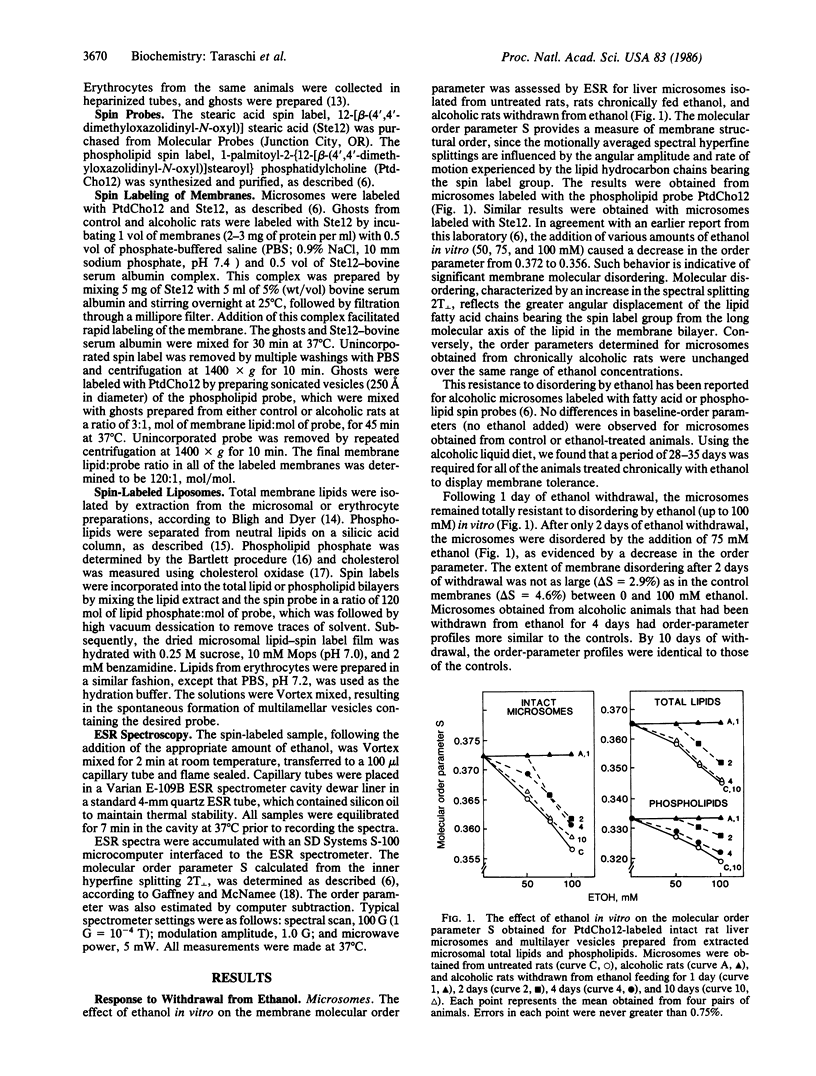
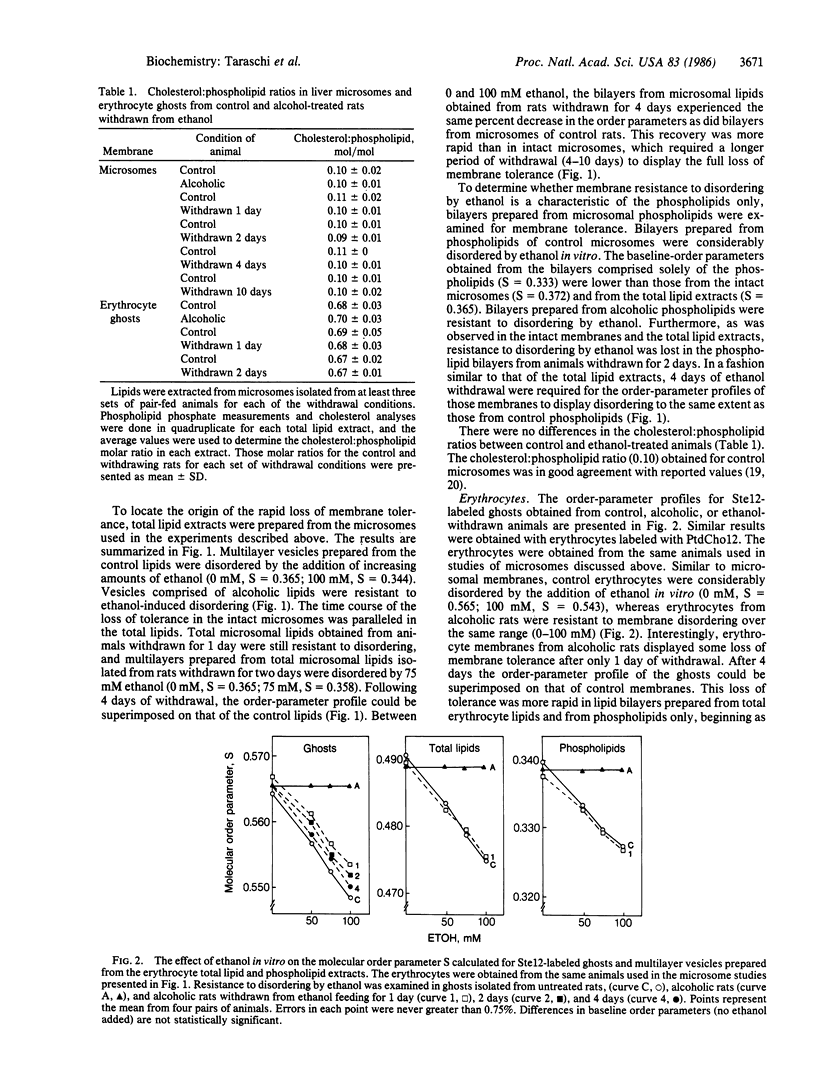
The structural properties of liver microsomes and erythrocytes obtained from rats that had been chronically administered ethanol were examined by electron spin resonance (ESR) following ethanol withdrawal for 1-10 days. Membranes obtained from control animals exhibited considerable molecular disordering upon the addition of ethanol in vitro (50-100 mM). Conversely, microsomal and erythrocyte membranes from alcoholic animals were resistant to this disordering by ethanol (membrane tolerance). These membrane properties were also apparent in lipid bilayers comprised of either total lipids or phospholipids isolated from the control and alcoholic animals. While several weeks of ethanol administration were required for both erythrocytes and microsomes to develop membrane tolerance, erythrocytes from alcoholic animals were disordered by ethanol in vitro after the animals had been withdrawn from ethanol for only 1 day. The same rapid loss of tolerance was observed in microsomes after 2 days of withdrawal. The same time course for the loss of tolerance was observed in lipid bilayers prepared from the total lipid and phospholipid extracts. No significant differences in the cholesterol/phospholipid ratio were observed between the microsomal or erythrocyte membranes isolated before and after withdrawal. Thus, alterations in the microsomal and erythrocyte phospholipids, and not cholesterol content, were responsible for conveying membrane tolerance. Membrane structural properties can be rapidly adjusted in a mammalian system in response to the withdrawal of the external membrane perturbant ethanol. The withdrawal model, which begins with established membrane tolerance and leads to rapid and complete loss of tolerance, provides a model to analyze the compositional changes responsible for this tolerance to disordering by ethanol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alling C., Liljequist S., Engel J. The effect of chronic ethanol administration on lipids and fatty acids in subcellular fractions of rat brain. Med Biol. 1982 Jun;60(3):149–154. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chin J. H., Goldstein D. B. Drug tolerance in biomembranes: a spin label study of the effects of ethanol. Science. 1977 May 6;196(4290):684–685. doi: 10.1126/science.193186. [DOI] [PubMed] [Google Scholar]
- Chin J. H., Parsons L. M., Goldstein D. B. Increased cholesterol content of erythrocyte and brain membranes in ethanol-tolerant mice. Biochim Biophys Acta. 1978 Nov 16;513(3):358–363. doi: 10.1016/0005-2736(78)90204-3. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- DeCarli L. M., Lieber C. S. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967 Mar;91(3):331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- Gaffney B. J., McNamee C. M. Spin-label measurements in membranes. With appendix: a use of computers in EPR spectroscopy. Methods Enzymol. 1974;32:161–198. [PubMed] [Google Scholar]
- Goldstein D. B., Zaechelein R. Time course of functional tolerance produced in mice by inhalation of ethanol. J Pharmacol Exp Ther. 1983 Oct;227(1):150–153. [PubMed] [Google Scholar]
- Harris R. A., Baxter D. M., Mitchell M. A., Hitzemann R. J. Physical properties and lipid composition of brain membranes from ethanol tolerant-dependent mice. Mol Pharmacol. 1984 May;25(3):401–409. [PubMed] [Google Scholar]
- Holloway C. T., Garfield S. A. Effect of diabetes and insulin replacement on the lipid properties of hepatic smooth endoplasmic reticulum. Lipids. 1981 Jul;16(7):525–532. doi: 10.1007/BF02535051. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Lee N. M., Cooke R., Loh H. H. Ethanol-induced fluidization of brain lipid bilayers: required presence of cholesterol in membranes for the expression of tolerance. Mol Pharmacol. 1979 May;15(3):739–746. [PubMed] [Google Scholar]
- Larson L. R., Ellingson J. S. Fatty acid profiles of phospholipids in rabbit and bovine dental pulp. Biochim Biophys Acta. 1977 Mar 25;486(3):437–443. doi: 10.1016/0005-2760(77)90093-5. [DOI] [PubMed] [Google Scholar]
- Nelson G. J. Composition of neutral lipids from erythrocytes of common mammals. J Lipid Res. 1967 Jul;8(4):374–379. [PubMed] [Google Scholar]
- Omodeo Salè F., Marchesini S., Fishman P. H., Berra B. A sensitive enzymatic assay for determination of cholesterol in lipid extracts. Anal Biochem. 1984 Nov 1;142(2):347–350. doi: 10.1016/0003-2697(84)90475-5. [DOI] [PubMed] [Google Scholar]
- Polokoff M. A., Simon T. J., Harris R. A., Simon F. R., Iwahashi M. Chronic ethanol increases liver plasma membrane fluidity. Biochemistry. 1985 Jun 18;24(13):3114–3120. doi: 10.1021/bi00334a007. [DOI] [PubMed] [Google Scholar]
- Ponnappa B. C., Waring A. J., Hoek J. B., Rottenberg H., Rubin E. Chronic ethanol ingestion increases calcium uptake and resistance to molecular disordering by ethanol in liver microsomes. J Biol Chem. 1982 Sep 10;257(17):10141–10146. [PubMed] [Google Scholar]
- Rottenberg H. Membrane solubility of ethanol in chronic alcoholism. The effect of ethanol feeding and its withdrawal on the protection by alcohol of rat red blood cells from hypotonic hemolysis. Biochim Biophys Acta. 1986 Feb 27;855(2):211–222. doi: 10.1016/0005-2736(86)90167-7. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Waring A., Rubin E. Tolerance and cross-tolerance in chronic alcoholics: reduced membrane binding of ethanol and other drugs. Science. 1981 Jul 31;213(4507):583–585. doi: 10.1126/science.6264608. [DOI] [PubMed] [Google Scholar]
- Ruggiero F. M., Landriscina C., Gnoni G. V., Quagliariello E. Lipid composition of liver mitochondria and microsomes in hyperthyroid rats. Lipids. 1984 Mar;19(3):171–178. doi: 10.1007/BF02534794. [DOI] [PubMed] [Google Scholar]
- Taraschi T. F., Rubin E. Effects of ethanol on the chemical and structural properties of biologic membranes. Lab Invest. 1985 Feb;52(2):120–131. [PubMed] [Google Scholar]
- Taraschi T. F., Wu A., Rubin E. Phospholipid spin probes measure the effects of ethanol on the molecular order of liver microsomes. Biochemistry. 1985 Dec 3;24(25):7096–7101. doi: 10.1021/bi00346a012. [DOI] [PubMed] [Google Scholar]
- Waring A. J., Rottenberg H., Ohnishi T., Rubin E. Membranes and phospholipids of liver mitochondria from chronic alcoholic rats are resistant to membrane disordering by alcohol. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2582–2586. doi: 10.1073/pnas.78.4.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]


