Abstract
We report a 4-generation Turkish family with 10 affected members presenting with myotonia and potassium- and exerciseinduced paralytic attacks. The clinical presentation was neither typical for the chloride channel myotonias Thomsen and Becker nor for the separate sodium channel myotonia entities potassiumaggravated myotonia, paramyotonia congenita, and hyperkalemic periodic paralysis. It is best described by a combination of potassium-aggravated myotonia and hyperkalemic periodic paralysis. We excluded exonic chloride channel mutations including CLCN1 exon deletion/duplication by MLPA. Instead we identified a novel p.N440K sodium channel mutation that is located at the inner end of segment S6 of repeat I. We discuss the genotype phenotype relation.
Key words: chloride channel myotonia Thomsen and Becker, sodium channel myotonia, hyperkalemic periodic paralysis, potassiumaggravated myotonia, paramyotonia congenita, CLCN1, MLPA
Recurring episodes of muscle stiffness or weakness are usually triggered by typical circumstances such as rest, exercise, oral K+ load, or cold environment.
Introduction
In the classical Thomsen myotonia and the more frequent Becker myotonia, muscle stiffness is generalized, most severe after rest and improves with continued exercise (warm-up phenomenon). Myotonic stiffness can be associated with weakness (transient weakness) but is not aggravated by cold (1) although many authors believe that any kind of myotonia worsens with exposure to cold. Both types are caused by CLCN1 mutations (2).
In paramyotonia congenita (PMC), myotonia is induced by cold (Table 1) and aggravated by repeated contractions (paradoxical myotonia). It is usually followed by flaccid weakness that lasts for several hours. This type of myotonia is caused by a sodium channel defect (3, 4).
Table 1.
Clinical features of the various forms of myotonia.
| Features | Chloride channel myotonia | Sodium channel myotonia | ||
|---|---|---|---|---|
| Becker and Thomsen | PAM | PMC | HyperPP | |
| Trigger for myotonia | Rest | Potassium, exercise | Cold, exercise | Potassium, exercise |
| Trigger for flaccid weakness | Fasting, exercise | -- | Cold | Potassium, exercise, cold |
| Warm-up phenomenon | ++ | + | + in warmth, - in the cold | + |
| Paradoxical myotonia | -- | Only in eyelid muscles | Always in the cold | - |
| Therapy | Antiarrhythmics, acetazolamide? | Antiarrhythmics, acetazolamide | Antiarrhythmics | Hydrochlorothiazide, acetazolamide |
In potassium-aggravated myotonia (PAM), episodes of weakness do not occur (5). This myotonia is exacerbated by rest following exercise (delayed myotonia) (6, 7). The potassium dependence can be used to differentiate PAM from chloride channel myotonias. The variability of PAM is large, ranging from mild myotonia fluctuans to severe myotonia permanens (6-11). As in the other forms of myotonia, myotonia in PAM results from uncontrolled repetitive action potentials.
The contrary, potassium-induced lack of action potentials resulting in muscle weakness, occurs in hyperkalemic periodic paralysis (HyperPP). HyperPP is characterized by episodes of flaccid muscle weakness which are not only produced by ingestion of potassium-rich food but are often triggered by rest, particularly after exercise (12). The spontaneous attacks usually begin early in the day (before breakfast) and can last for minutes to hours. Clinical myotonia is not common in HyperPP but may precede an attack, and myotonic activity is usually detected at rest with needle EMG (latent myotonia) (13).
Here we report a myotonic family with an unusual clinical phenotype consisting of PAM with potassiumand exercise-induced weakness. This is caused by a novel mutation in a non-hotspot area of the sodium channel protein.
Materials and methods
Members of the family participating in the study gave their informed consent. Experiments were approved of by the Ethics Committee of Ulm University and were in concordance with the Declaration of Helsinki. The affected individuals were neurologically examined by one of the authors. Electromyography (EMG) and nerve conduction velocities were determined in several members as well as molecular genetics. In the proband, potassium provocation, bicycle exercise, and cooling were performed as provocation tests.
Provocation tests: Potassium (60 mmol) was orally administered in an unsweetened solution in the fasting state. Serum potassium levels were determined approximately in intervals of 20 minutes prior to and after ingestion, the ECG monitored and an anaesthetist available in case of a wide-spread paralysis involving respiratory muscles. After the ingestion, the patient avoided all muscle activity. In addition, exercise on a bicycle was performed for 25 minutes with a load that increased the pulse rate to 120-160 beats/min. This test was followed by absolute rest. In the cooling test, one forearm was cooled for 30 minutes in water of 15°C. Subsequently the patient performed repetitive strong fist closures and openings. Muscle strength was always scored according to the MRC grading scale.
Molecular genetics. DNA was extracted from whole blood in EDTA using standard methods. Exons 1-24 of SCN4A, the gene encoding the major sodium channel protein Nav1.4 of skeletal muscle, were amplified by polymerase chain reaction (PCR), and the products examined for mutations by direct PCR sequencing using an automated 373A sequencer (Applied Biosystems). Samples were bidirectionally sequenced. The primers and the cycling conditions were reported in Heine et al. (5). Particularly, the primers used for exon 9 in which an alteration was identified were atccgtttggtttatgtggt (forward) and ccctggggtgccttttctgc (reverse). This exon was sequenced in 200 control individuals for comparison. To find out whether the genetic alteration is disease-causing or just a benign polymorphism, LOD scores were calculated with MLINK of the linkage package using an assumed penetrance of 99% and an allele frequency of 1:100,000. In addition, exons 1-23 of CLCN1, the gene encoding the major chloride channel ClC1 of skeletal muscle were sequenced in the same way by use of primers reported by Lehmann-Horn et al. (14). To test for an exon-wide deletion or duplication on one allele that would not be identified by the method above, multiplex ligation-dependent probe amplification (MLPA) analysis was performed. We used a commercially available kit (SALSA MLPA kit P350-A1 CLCN1) according to the manufacturer's guidelines (MRC-Holland, Amsterdam). This kit contained probes for all 23 CLCN1 exons. A patient vs. control product ratio of > 1.25 suggests a duplication and a ratio of < 0.75 a deletion.
Results
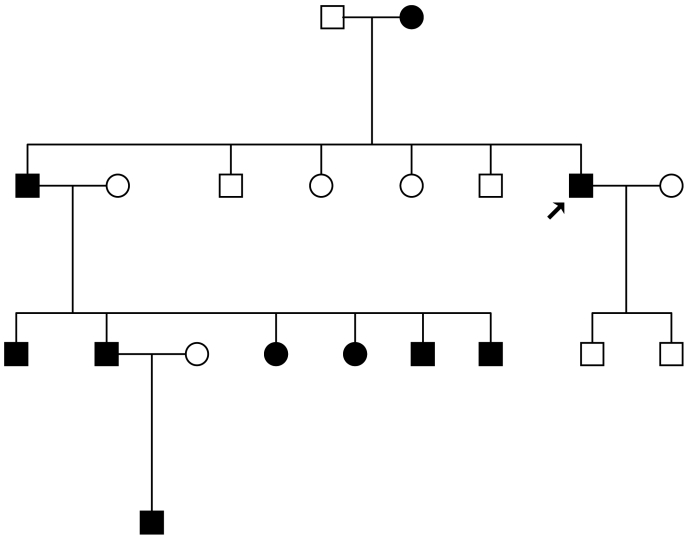
The proband is a 47 year old Turkish man (Fig. 1, arrow), presenting with myotonia, generalized muscle hypertrophy, and weakness episodes. Muscle stiffness had already been observed in infancy. Once in childhood, swimming in warm water caused a life-threatening weakness of the exercised muscles. Cold sensitivity of the musculature was denied. On average once a month, an episode of weakness provoked by exercise occurs in distal muscles and can last for 2 to 3 days. In his family there are 9 additional affected members (Fig. 1).
Figure 1.
Pedigree of the Turkish family. The affected members harbor the N440K sodium channel mutation while the non-affected do not.
The neurological examination of the proband revealed percussion myotonia and myotonic stiffness of distal muscles and – after strenuous contraction – stiffness of proximal and neck muscles. Muscle relaxation lasted several seconds up to 30 seconds. Both eyelid and limb muscles revealed a warm-up phenomenon. The EMG exhibited marked myotonic activity in all muscles tested. Nerve conduction velocities of the median nerve and distal latencies were normal. Bicycle exercise (75 W) caused an increase in myotonic stiffness of most muscles and weakness of the flexors of the hands (MRC 4) without a pathological serum potassium deviation in the postexercise phase. The cooling test did not result in weakness while exercise of the cooled muscles revealed the same myotonic stiffness as observed in ambient temperature.
Examination of affected family members allowed to collect additional information: One nephew reported provocation of flaccid weakness and muscle cramps due to potassium-rich food in addition to exercise-induced weakness. He exhibited grip myotonia and a disproportionate figure with poorly developed and weak muscles of the neck and the upper limb girdle whereas the lower limble girdle muscles were hypertrophied. The muscle reflexes were absent in the upper extremities and normal in the lower extremities. The clinical presentation was thus reminiscent of Becker myotonia. The EMG displayed many myotonic runs and normal motor nerve conduction velocities. Potassium provocation resulted in muscle weakness (MRC 4). Two other nephews reported spells of weakness following fasting and exercise, difficulties to relax the fist but no substantial sensitivity to cold. In the affected family members, CK was often elevated to values up to twice the upper normal limit. Several family members responded well to hydrochlorothiazide and acetazolamide. However, they chose not to take the drugs regularly but preferred using them as needed in particular when they noticed the beginning of an episode of weakness. They reported that with the drugs the severity of weakness could be mitigated.
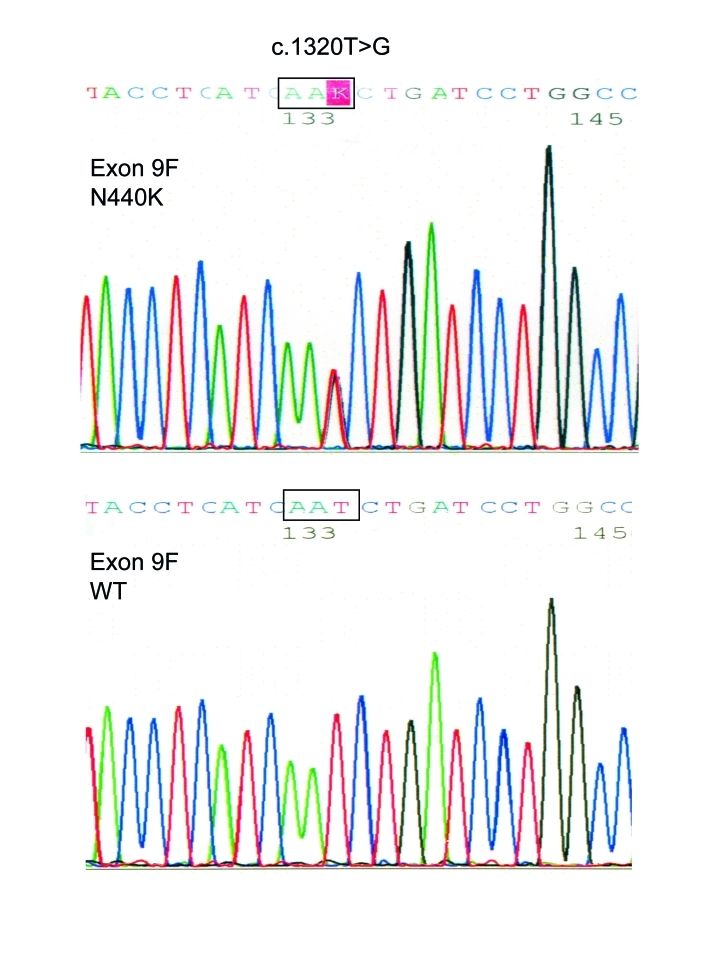
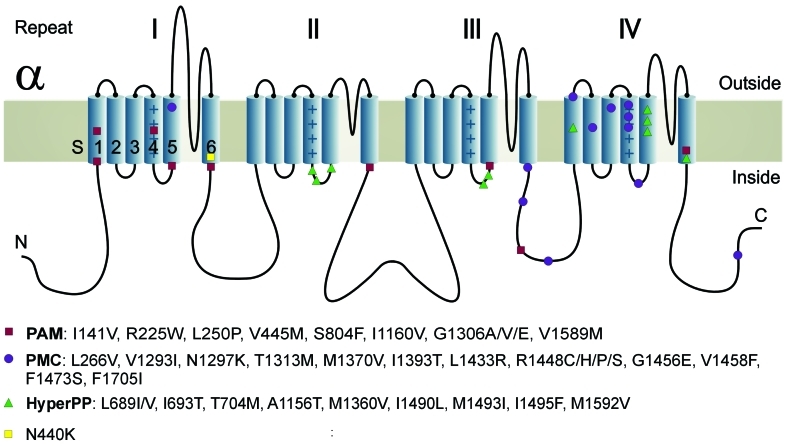
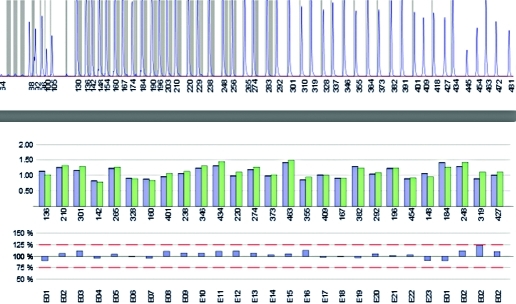
Genetics: Exon 9 of all affected family members revealed a c.1320T>G base exchange predicting a p.N440K substitution (Fig. 2). The replaced amino acid N440 is situated in segment 6 of repeat I (Fig. 3). N440 is conserved among all human voltage-gated sodium channels. No other SCN4A mutation was found in the proband. No CLCN1 alteration was identified in the coding sequence and the exon-intron boundaries. MLPA identified neither an exon-wide deletion nor an exon duplication (Fig. 4).
Figure 2.
A comparison of the SCN4A sequence of a forward (F) fragment of exon 9 of the proband with the c.1320T > G base exchange (p.N440K) and from a control (WT). This alteration was absent in all 200 control individuals.
Figure 3.
Cartoon of the alpha-subunit of the voltage-gated sodium channel of skeletal muscle, NaV1.4 indicates the mutations responsible for potassium-sensitive myotonia (PAM), paramyotonia (PMC), and hyperkalemic periodic paralysis (HyperPP). The subunit is composed of four highly homologous repeats (I–IV) each consisting of six transmembrane segments (S1–S6). When inserted in the membrane, the four repeats form a central pore with segments 5 and 6 lining its wall. The repeats are connected by intracellular loops; one of them, the III-IV linker, contains the fast-inactivation particle of the channel next to the PC mutation T1313M. Also repeat IV in which the PC mutation R1448H/C/P mutations are situated is important for the fast-inactivation process. HyperPP and PAM mutations are usually located at the inner end of the segments which bind the fast-inactivation particle in a voltage-dependent manner and also are involved in the slow-inactivation process. The novel N440K mutation is situated next to the PAM mutation V445M. Modified after Jurkat-Rott et al. (15)
Figure 4.
Multiplex ligation-dependent probe amplification (MLPA) analysis of all 23 CLCN1 exons. The upper panel shows the peaks whose area and amplitude correspond to the efficiency of the amplification which usually is smaller at the right end; particularly the fifth peak from the right is always small. The numbering corresponds to the length (in basepairs) of the products. The middle panel shows the histograms (blue: average of control products, green: products of the patient's sample) for the 23 exons. The lower panel displays the ratio patient vs. controls. As all ratios were between 0.75 and 1.25, an exon duplication or deletion is excluded. The analysis also contains an exon-1 probe and 3 exon-2 probes for KCNJ2, another gene analyzed by this commercial kit (on the right of the middle and the lower panels).
Discussion
The exact clinical diagnosis in this family was difficult to make as the features did not fit into the current classification of myotonias (Tab. I). The phenotype resembled a combination of PAM because of the myotonia, and HyperPP because of the potassium- and exercise-induced weakness in two family members. However PAM is not associated with weakness, and HyperPP shows no exercise-induced muscle stiffness.
Careful clinical analysis is important because the various symptoms need to be treated appropriately. Myotonia can be best treated by the lidocaine-like, orally administered tocainide and mexiletine. Unfortunately, these agents have been taken of the market. Nevertheless myotonic symptoms and the cold-induced weakness of paramyotonia respond to the modern antiarrhythmic drugs flecainide and propafenone (16). However, these agents do not prevent spontaneous and hyperkalemia-induced weakness episodes. These features respond to potassium-sparing diuretics such as hydrochlorothiazides and acetazolamide. In addition, frequent meals rich in carbohydrates and a low-potassium diet may also prevent weakness episodes like in HyperPP.
In the Turkish family reported here, we have performed CLCN1 studies since the myotonia was predominant in all family members and the recessive Becker myotonia is unusually frequent in this population, probably because of the high incidence of consanguineous marriages. We excluded CLCN1 mutations by conventional PCR and direct sequencing as well as, to our knowledge for the first time, CLCN1 exon deletions and duplications by MLPA analysis.
We have identified a novel SCN4A alteration that fulfills the criteria of a disease-causing mutation: i) the base exchange perfectly segregates with the phenotype; ii) it does not occurr in a high number of healthy controls; iii) it predicts an amino acid exchange in a highly conserved area of functional importance, and finally the calculated LOD score of 2.99 is high enough. The N440K amino acid substitution N440K is situated deeply in the S6 segment of repeat I, and it is in the vicinity of V445M, a PAM mutation (17). V445M is located in the adjacent N-terminal area of the loop connecting repeats I and II (Fig. 3). Although we have not yet performed functional expression of the N440K mutation, its location at the inner end of the S6 segment allows us to anticipate that the mutant channel will resemble both the HyperPP mutation M1592V (18) and the PAM mutation V1589M (5), both residing at the inner end of the S6 segment of repeat IV. Both mutations caused a slight permanent inward current through the central channel pore, slightly shifted the steady-state activation curve, and destabilized the fast inactivation state without slowing fast inactivation (8, 19) and without impairing slow inactivation (20). This pattern is different from PMC mutations which usually markedly slow fast inactivation.
Acknowledgements
We thank the Deutsche Gesellschaft für Muskelkranke e.V. (DGM) for the support. The corresponding author is Senior Research Professor for Neurosciences of the non-profit Hertie Foundation.
References
- 1.Ricker K, Hertel G, Langscheid K, et al. Myotonia not aggravated by cooling. Force and relaxation of the adductor pollicis in normal subjects and in myotonia as compared to paramyotonia. J Neurol. 1977;216:9–20. doi: 10.1007/BF00312810. [DOI] [PubMed] [Google Scholar]
- 2.Koch MC, Steinmeyer K, Lorenz C, et al. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann-Horn F, Rüdel R, Ricker K. Membrane defects in paramyotonia congenita (Eulenburg) Muscle Nerve. 1987;10:633–641. doi: 10.1002/mus.880100709. [DOI] [PubMed] [Google Scholar]
- 4.McClatchey AI, Bergh P, Pericak-Vance MA, et al. Temperature- sensitive mutations in the III-IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell. 1992;68:769–774. doi: 10.1016/0092-8674(92)90151-2. [DOI] [PubMed] [Google Scholar]
- 5.Heine R, Pika U, Lehmann-Horn F. A novel SCN4A mutation causing myotonia aggravated by cold and potassium. Hum Mol Genet. 1993;2:1349–1353. doi: 10.1093/hmg/2.9.1349. [DOI] [PubMed] [Google Scholar]
- 6.Ricker K, Lehmann-Horn F, Moxley RT., 3rd Myotonia fluctuans. Arch Neurol. 1990;47:268–272. doi: 10.1001/archneur.1990.00530030034012. [DOI] [PubMed] [Google Scholar]
- 7.Ricker K, Moxley RT, 3rd, Heine R, et al. Myotonia fluctuans. A third type of muscle sodium channel disease. Arch Neurol. 1994;51:1095–1102. doi: 10.1001/archneur.1994.00540230033009. [DOI] [PubMed] [Google Scholar]
- 8.Mitrovic N, George AL, Heine R, et al. Potassium-aggravated myotonia: The V1589M mutation destabilizes the inactivated state of the human muscle sodium channel. J Physiol (Lond) 1994;478:395–402. doi: 10.1113/jphysiol.1994.sp020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubota T, Kinoshita M, Sasaki R, et al. New mutation of the Na channel in the severe form of potassium-aggravated myotonia. Muscle Nerve. 2009;39:666–973. doi: 10.1002/mus.21155. [DOI] [PubMed] [Google Scholar]
- 10.Stunnenberg BC, Ginjaar HB, Trip J, et al. Isolated eyelid closure myotonia in two families with sodium channel myotonia. Neurogenetics. 2010;11:257–260. doi: 10.1007/s10048-009-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota T, Roca X, Kimura T, et al. A mutation in a rare type of intron in a sodium-channel gene results in aberrant splicing and causes myotonia. Hum Mutat. 2011;32:773–782. doi: 10.1002/humu.21501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricker K, Camacho L, Grafe P, et al. Adynamia episodica hereditaria: what causes the weakness? Muscle Nerve. 1989;10:883–891. doi: 10.1002/mus.880121103. [DOI] [PubMed] [Google Scholar]
- 13.Jurkat-Rott K, Lehmann-Horn F. Hyperkalemic periodic paralysis type 1. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews [Internet] Seattle (WA): University of Washington; 1993. Gene Rev 2011. http://www.ncbi.nlm.nih.gov/pubmed/20301669. [Google Scholar]
- 14.Lehmann-Horn F, Mailänder V, Heine R, et al. Myotonia levior is a chloride channel disorder. Hum Mol Genet. 1995;4:1397–1402. doi: 10.1093/hmg/4.8.1397. [DOI] [PubMed] [Google Scholar]
- 15.Jurkat-Rott K, Holzherr B, Fauler M, et al. Sodium channelopathies of skeletal muscle result from gain or loss of function. Pflügers Arch – Europ J Physiol. 2010;460:239–248. doi: 10.1007/s00424-010-0814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfonsi E, Merlo IM, Tonini M, et al. Efficacy of propafenone in paramyotonia congenita. Neurology. 2007;68:1080–1081. doi: 10.1212/01.wnl.0000257825.29703.e8. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld J, Sloan-Brown K, George AL., Jr A novel muscle sodium channel mutation causes painful congenital myotonia. Ann Neurol. 1997;42:811–814. doi: 10.1002/ana.410420520. [DOI] [PubMed] [Google Scholar]
- 18.Rojas CV, Wang JZ, Schwartz LS, et al. A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature. 1991;354:387–389. doi: 10.1038/354387a0. [DOI] [PubMed] [Google Scholar]
- 19.Rojas CV, Neely A, Velasco-Loyden G, et al. Hyperkalemic periodic paralysis M1592V mutation modifies activation in human skeletal muscle Na+ channel. Am J Physiol. 1999;276:C259–C266. doi: 10.1152/ajpcell.1999.276.1.C259. [DOI] [PubMed] [Google Scholar]
- 20.Hayward LJ, Sandoval GM, Cannon SC. Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology. 1999;52:1447–1453. doi: 10.1212/wnl.52.7.1447. [DOI] [PubMed] [Google Scholar]