Abstract
The mammalian p38 mitogen-activated protein kinases (MAPKs) family is composed of four members (p38α, p38β, p38γ, and p38δ), which are very similar in amino acid sequence but differ in their expression patterns. This suggests that they may have specific functions in different organs. In the last years most of the effort has been centred on the study of the function of the p38α isoform, which is widely referred to as p38 in the literature. However, the role that other p38 isoforms play in cellular functions and their implication in some of the pathological conditions have not been precisely defined so far. In this paper we highlight recent advances made in defining the functions of the two less studied alternative p38MAPKs, p38γ and p38δ. We describe that these p38MAPKs show similarities to the classical p38α isoform, although they may play central and distinct role in certain physiological and pathological processes.
1. Introduction
To preserve the homeostasis and health of the organism cells are constantly responding to changes in the physical and chemical properties of the environment by altering many of their cellular functions. The activation of Mitogen Activated Protein Kinases (MAPKs) is involved in the transduction of most extracellular signals, and it is one of the major signal transduction mechanism by which the cell adapts to changes in the surrounding medium. In mammalian cells there are four well-characterised MAPK families: ERK1/2, ERK5, JNK, and p38MAPK, which are serine/threonine kinases that catalyze the reversible phosphorylation of proteins.
The p38MAPK family comprises four members, p38α, p38β, p38γ, and p38δ. The isoform p38α was identified in 1994 by four groups as a 38 kDa polypeptide that is activated in response to endotoxin treatment, cell stress, or cytokines [1]. Two to three years later, three additional isoforms were described: p38β [2–4], p38γ [5, 6], and p38δ [7, 8]. These kinases share highly similar protein sequences; p38α and p38β are 75% identical, whereas p38γ and p38δ are 62% and 61% identical to p38α, respectively. In turn, p38γ, and p38δ are ~70% identical to each other. The four p38MAPK isoforms are widely expressed, although p38β, p38γ and p38δ expression appear to be higher in specific tissues; for example, p38β is abundant in brain, p38γ in skeletal muscle, and p38δ in endocrine glands [1, 9]. In general, all p38MAPKs are strongly activated by a wide variety of environmental and cellular stresses or by inflammatory cytokines and are poorly activated by serum or growth factors [1]. The p38MAPK family can be further divided into two subsets based on sequence homology, substrate specificities, and sensitivity to chemical inhibitors, with p38α and p38β in one group and p38γ and p38δ in the other. In this paper, we provide an overview of the less known p38MAPK isoforms, the p38γ and p38δ MAPK pathways, which are strongly activated by stress, but also play important roles in tissue regeneration, differentiation, metabolic diseases, and cancer.
2. General Features of p38γ and p38δ MAPKs
Human p38γ and p38δ isoforms are serine/threonine protein kinase of 367 and 365 amino acids with a predicted molecular mass of 42–45 kDa and are encoded by different genes located on chromosomes 22q13.3 and 6p21.31, respectively [1, 7, 10]. p38γ is also known as ERK6, SAPK3, and MAPK12. It was first described by three independent studies as either a MAPK involved in myoblast differentiation [5], a stress-activated protein kinase (SAPK) highly expressed in skeletal muscle [6], or a new member of the p38MAPK family [11]. p38δ, also known as SAPK4 and MAPK13, was cloned as the fourth member of the p38MAPK family by two different groups [7, 8].
The structure of doubly phosphorylated, active p38γ in complex with an ATP analog has been determined by X-ray crystallography [12]. The global structure of p38γ is similar to other enzymes of the MAPK family and is characterized by two domains separated by a deep channel where potential substrates might bind. The dually phosphorylated p38MAPK goes through characteristic global conformational changes that alters the alignment of the two kinase halves (N-terminal and C-terminal domains) of the folded protein and enhances access to substrate. In addition, the interaction between MAPKs and their upstream activators seems to work allosterically to make the MAPKs activation loop available for processing by kinases and phosphatases, which further increases enzymatic activity [12–14]. Although the conformation of p38γ activation loop is almost identical to that observed in the structure of activated ERK2, contrary to ERK2, the crystal structure of activated p38γ exists as a monomer, suggesting that not all activated MAPKs form dimers [12]. A feature that makes p38γ unique among other MAPKs is its short C-terminal sequence-KETXL, an amino acid sequence which docks directly to PDZ domains of proteins, such as α1-syntrophin, SAP (synapse-associated protein) 90/PSD (postsynaptic density) 95 and SAP97/hDlg (human disc large), and phosphorylation of these proteins by p38γ is dependent on its binding to the PDZ domains [15–17].
The information about p38γ and p38δ biological role is limited compared to the extensive knowledge of p38α and p38β functions. This is at least in part due to the lack of specific inhibitors for p38γ and p38δ. In vitro and in vivo assays demonstrated that only p38α and p38β are inhibited by certain compounds, such as SB203580 and other pyridinyl imidazoles, whereas p38γ and p38δ are completely unaffected by these drugs [7, 18, 19]. This is mainly due to the differences, between p38γ and p38δ compared to p38α and p38β, in the amino acid sequence of the ATP-binding pocket, the site where most protein kinase inhibitors bind and directly compete with ATP [1].
3. Regulation of p38γ and p38δ
The canonical activation of p38MAPKs occurs via dual phosphorylation of tyrosine and threonine residues in a conserved TGY motif, located in the activation loop of kinase subdomain VIII. MAPK phosphatases reverse this phosphorylation and return the p38MAPK to their inactive state. Phosphorylation of p38MAPKs is catalysed by the dual specificity kinases (MKK or MAP2Ks), MKK3 and MKK6, which are in turn activated upon phosphorylation of serine/threonine residues by phosphorylation by a MAPK kinase kinase (MAP3K) (Figure 1). The MAP3K responsible for activating the p38MAPK pathways appears to be cell type and stimulus specific. Several MAP3Ks have been implicated in the regulation of p38MAPK signalling, these include MLKs (mixed-lineage kinases), ASK1 (apoptosis signal-regulating kinase-1), TAO (thousand and one amino acid) 1 and 2, TAK1 (TGF β-activated kinase 1), and some members of the MEKK (MAPK/ERK kinase kinase) family [20]. The diversity of MAP3Ks and their ability to activate also other MAPKs provide a mechanism to respond to many stimuli and to integrate different signalling pathways. It has been shown that MAP3K of the p38MAPK pathway are regulated by binding to low molecular weight GTP-binding proteins, ubiquitination or phosphorylation by STE20 family members [1, 20].
Figure 1.

The p38MAPK pathway. p38γ and p38δ MAPK substrates identified so far are shown.
MKK3 and MKK6 are highly selective for p38MAPKs and do not activate other MAPKs [1]. The major MKK required for the activation of specific p38MAPK may be determined by several factors: one is the cell type as the level of expression varies [21, 22]; another is the nature and also the strength of the stimuli. Since MKK6 can activate all p38 isoforms in vitro, it has been suggested that the pattern of downstream p38MAPK activation in the particular response may be determined by the level of MKK6 activity triggered by a given stimulus [23]. Moreover, there are two important structural requirements for selective activation of p38MAPK isoforms by MKKs: docking sequences in the N-terminus of the MKK and isoform-specific sequences of the p38MAPK isoforms within the activation loop [13, 24, 25]. Using MKK-targeted gene disruption and small interfering RNA (siRNA) approaches, it has been shown that, in response to most stimuli, MKK3 and MKK6 are the main p38α activators but, in some circumstances, such as ultraviolet radiation, MKK4, an activator of JNK, may contribute to p38α activation [26]. Moreover, although it has been shown that in vitro experiments MKK4 also phosphorylates and activates p38γ and p38δ [7, 27], studies utilizing mouse embryonic fibroblasts lacking MKK3 and/or MKK6 indicate that activation of distinct p38MAPK isoforms is regulated by the selective and synchronized action of the two MKKs, in response to cell stress. Thus, both MKK3 and MKK6 are essential for p38γ activation induced by environmental stresses, whereas MKK6 is the major p38γ activator in response to the cytokine tumour necrosis factor-α (TNFα). On the other hand, MKK3 is the major kinase responsible for p38δ activation by ultraviolet radiation, hyperosmotic shock, TNFα or by the protein synthesis inhibitor anisomycin (Figure 1) [17]. Supporting this is the finding that endogenous p38δ activation in response to TGFβ1 is impaired in glomerular mesangial cells from MKK3-deficient mice [22]. Nonetheless, the relative contribution of MKK3 and MKK6 to p38γ and p38δ activation might strongly depend not only on the nature and strength of the stimulus, but also on the cell type.
The magnitude and duration of p38MAPK signal transduction are critical determinants of its biological effects. Termination of p38 kinase catalytic activity involves the activity of several phosphatases that target the activation loop threonine and tyrosine residues. In mammalian cells there are good in vivo pieces of evidence for p38α activity downregulation by several protein phosphatases, including protein serine/threonine phosphatases (PPs) [28, 29], protein tyrosine phosphatases (PTPs) [30], and dual-specificity phosphatases (DUSPs, also known as MAPK phosphatases (MKPs)) [31]. However, their role in p38γ and p38δ dephosphorylation has not been extensively studied, and therefore very little is known about physiological p38γ and p38δ protein phosphatases. Recently, it has been shown in one study that p38γ interacts through its C-terminal binding PDZ motif with the single PDZ domain of the protein tyrosine phosphatase PTPH1. Moreover, PTPH1 can dephosphorylate p38γ, but not p38α, in vitro and in overexpression experiments in cells. This specificity seems to be determined by both p38γ C-terminal PDZ-binding sequence and the conserved TGY motif within the kinase subdomain [32].
4. p38γ and p38δ Substrates and Biological Functions
p38MAPK family members have overlapping substrate specificities, and the genetic ablation of specific p38MAPK family members has also demonstrated the existence of functional redundancy [16]. However, there are some differences, with particular substrates being better phosphorylated by p38α and p38β than p38γ and p38δ or vice versa. For example, MAPK-activated protein kinase 2 (MK2) and MK3 are very good substrates for p38α and p38β, but cannot be phosphorylated by other p38MAPK isoforms [1].
The lack of specific inhibitors for p38γ and/or p38δ has slowed down the identification of their in vivo substrates and the elucidation of their biological roles. Nonetheless, this problem can be partly solved by the use of p38 knockout mouse models. p38γ and p38δ and double p38γ/p38δ knock-out mice have been generated, which are viable and fertile [16]. Moreover, the diaryl urea compound BIRB796 is not only a potent inhibitor of p38α and p38β, but also inhibits p38γ and p38δ at higher concentrations in cell-based assays providing a new tool for identifying physiological roles of these two p38MAPK isoforms [19, 33].
Several physiological substrates for p38γ MAPK isoform have been described in the past years (Figure 1). A feature that makes p38γ unique among all MAPKs is its short C-terminal sequence ideal for binding PDZ domains in proteins. p38γ binds to the PDZ domain of a variety of these proteins, such as α1-syntrophin, SAP90/PSD95, and SAP97/hDlg, and under stress conditions is able to phosphorylate them and modulate their activity [15–17]. One valuable tool used in the identification of p38γ substrates has been the cell permeant peptide TatSAPK3C which contains the last nine residues of p38γ fused to the cell-membrane transduction domain of the human immunodeficiency virus-type 1 (HIV-1) Tat protein. This peptide blocks the phosphorylation of PDZ domain-containing proteins by p38γ in intact cells by preventing the association of the kinase with the PDZ domain of the substrate [16, 17]. These PDZ domain-containing proteins are scaffold proteins usually targeted to the plasma membrane cytoskeleton at specialised sites such as the neuromuscular junction and gap junctions through protein-protein interactions. In the case of SAP97/hDlg its phosphorylation by p38γ provides a mechanism of dissociating it from the cytoskeleton [16], which indicates a role of this p38MAPK isoform in modulation of cytoskeletal organization. SAP97/hDlg is the mammalian homologue of the Drosophila tumour suppressor Dlg, a scaffold protein that forms multiprotein complexes with a variety of proteins and is targeted to the cytoskeleton by its association with guanylate kinase-associated protein (GKAP). The p38γ-catalysed phosphorylation of SAP97/hDlg triggers its dissociation from GKAP and therefore releases it from the cytoskeleton (Figure 2). This is likely to regulate the integrity of intercellular complexes, cell shape, and volume as an adaptive mechanism to changes in the environment [16].
Figure 2.
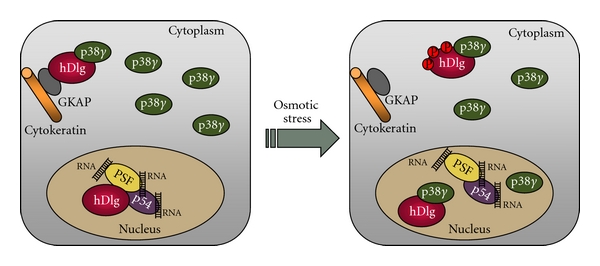
The involvement of p38γ in the regulation of nuclear and cytoplasmic protein complexes. In the nucleus of resting cells SAP97/hDlg complexes with PSF/p54-RNAs, whereas in the cytoplasm it interacts at the cytoskeleton with both the protein GKAP and a fraction of p38γ, which is localized mainly in the cytoplasm. Changes in the osmolarity of the environment causes: (i) p38γ activation in the cytoplasm, which phosphorylates SAP97/hDlg causing its dissociation from GKAP and therefore from the cytoskeleton, (ii) accumulation of p38γ in the nucleus, and (iii) the nuclear interaction of p38γ with SAP97/hDlg, which leads to its dissociation from PSF/p54-RNAs independently of SAP97/hDlg phosphorylation.
SAP97/hDlg also localizes in the nucleus where it forms a complex with the proteins polypyrimidine tract-binding (PTB) protein-associated splicing factor (PSF) and p54nrb, and with various RNAs, [34]. PSF and p54nrb are nucleic acid-binding proteins that associate in vivo and regulate transcription, pre-mRNA processing, nuclear retention of defective RNA, as well as DNA unwinding and repair [35]. p38γ regulates hDlg-PSF complex dissociation in the nucleus independently of hDlg phosphorylation by displacing PSF from hDlg, since both proteins, p38γ and PSF, bind to PDZ1 of hDlg [34]. p38γ accumulates in the nucleus after hyperosmotic stress (Figure 2), but not following other p38γ-activating stimuli such as UV irradiation. This indicates that the nature of the stimulus determines p38γ distribution and that some signals could release p38γ from docking molecules that retain it in the cytosol. Moreover, the nuclear accumulation of p38γ might be a response mechanism to some stimuli facilitating phosphorylation of p38γ targets in the nucleus. A nuclear role for p38γ, including functional interaction with SAP97/hDlg, would not exclude its distinct cytoplasmic role in modulating the SAP97/hDlg-cytoskeleton complex. Indeed, through its ability to shuttle between cytoplasm and nucleus, p38γ-SAP97/hDlg might provide a connection between two processes critical for adaptation to environmental changes: gene expression and cytoskeletal reorganization.
p38γ regulation of the SAP97/hDlg-PSF complex is independent of its kinase activity. This has been shown using cells from knockin mice expressing an endogenous inactive p38γ mutant in combination with cells from mice lacking p38γ [34]. Similarly, experiments in rat intestinal epithelial cells also suggest a phosphorylation-independent role for p38γ in K-Ras transformation, although the precise mechanism for this regulation remains unknown [36]. p38MAPKs act normally by direct phosphorylation of substrates on serine or threonine residues followed by proline. However, there are few examples showing that mammalian p38α- and yeast p38MAPK-related proteins such as Spc1 or Hog1 may also have kinase-independent roles (reviewed in [9]). Like p38α, p38γ seems to have a kinase-independent function by associating to protein targets and modulating their function in the absence of phosphorylation.
p38γ substrates that do not require PDZ domain-binding interactions are the mitochondrial protein Sab [37] and the transcription factor MyoD, whose phosphorylation by p38γ results in a decrease in its transcriptional activity [38].
Some p38δ substrates are proteins involved in the regulation of microtubule dynamics, suggesting that this p38MAPK may play a role in cytoskeletal remodelling. Thus, the protein stathmin and the neuronal microtubule-associated protein Tau are phosphorylated by p38δ in vitro and in transfected cells [39–42]. Tau function is modulated by phosphorylation, and its ability to bind and stabilise microtubules correlates inversely with its phosphorylation which may facilitate its self-assembly. Tau is a good in vitro p38δ and p38γ substrate, and its phosphorylation by these two kinases results in a reduction in its ability to promote microtubule assembly [39, 40]. Using a siRNA approach, p38δ has been reported to be the major Tau kinase in neuroblastoma in response to osmotic shock. p38δ phosphorylates endogenous Tau at residue threonine-50 (Tau-T50), which is phosphorylated in filamentous Tau from Alzheimer's disease brain. It seems that Tau-T50 phosphorylation is an early event after p38δ activation. Surprisingly, this phosphorilation causes an increase in the ability of Tau to promote microtubule assembly and help to the adaptive response of neurons to osmotic shock, whereas subsequent Tau phosphorylation at additional sites by p38δ or/and by other protein kinase(s) may then instead induce the detachment of Tau from the microtubule and destabilize the microtubule network [39].
Finally, it has been shown that p38δ phosphorylates and inactivates the eukaryotic elongation factor 2 (eEF2) kinase and the protein kinase D1 (PDK1) [43–45]. PDK1 controls insulin exocytosis in pancreatic beta cells, which suggests that p38δ plays a role in the regulation of insulin secretion [45].
5. Physiological Roles of p38γ and p38δ MAPK Pathways
Evidence from a number of studies carried out during the past few years suggests that many physiological functions of the p38MAPK isoforms may overlap but may not necessarily be redundant and/or identical [10]. Thus, during the last few years, studies using knock-out mice have provided important information concerning p38γ- and p38δ-functions in vivo. Contrary to p38α, whose constitutive deletion causes death during embryonic development [46–48], p38γ and p38δ deficient mice are viable and have not apparent phenotype [16]. Functional redundancy of all four p38MAPKs may contribute, at least in part, to the lack of evident phenotype of p38γ- and p38δ-deficient mice. Nonetheless, there are recent reports showing the implication of p38γ and p38δ in tissue regeneration, cancer, and metabolic diseases, further strengthening the interest of these pathways for the development of new therapeutics strategies. Thus, p38δ seems to be a regulator of processes related to the pathogenesis of diabetes, such as insulin secretion and β cells death. p38δ-deficient mice have improved glucose tolerance as a result of enhanced insulin exocytosis by pancreatic β cells. Correlating with this, p38δ-deficient mice show higher levels of active PKD1, which is known to positively regulate secretion in neuroendocrine cells, as a result of the lack of p38δ-mediated inhibitory phosphorylation. In addition, p38δ has been suggested as a potential therapeutic target for human diabetes, since p38δ-deficient mice are protected against the insulin resistance induced by a high-fat diet and the oxidative stress-mediated β-cell failure [45].
Using mainly ectopic expression and knock-down model cell lines it has been shown that p38γ and p38δ pathway could be involved in the modulation of some processes implicated in cellular malignant transformation, such as proliferation, cell cycle progression, or apoptosis [1, 10, 49, 50] indicating a potential oncogenic role of these kinases in cancer development and progression. In one study, p38δ promotes the malignant phenotype of squamous cell carcinoma by regulating cell proliferation and invasion [51]. In rat intestinal epithelial cells (IECs) and in human breast cancer, p38γ RNA and protein expression increases during Ras-induced transformation [36, 52]. p38γ knock-down in IEC blocks the Ras transformation activity and results in the significant diminution of the oncogenic characteristics of breast cancer cells [53–55]. Additionally, one recent study shows that p38γ mediates Ras-induced senescence at least partly by stimulating the transcriptional activity of p53 through direct phosphorylation; in contrast p38α appears to regulate senescence in a p53-independent manner [56]. These results indicate that increased p38γ gene expression is required for Ras oncogene activity but the mechanism by which p38γ may promote Ras transformation is not clear. Interestingly, p38δ was recently shown to mediate 12-O-tetradecanoylphorbol-13-acetate- (TPA-) induced epidermal cell proliferation in mice, and mice lacking p38δ show reduced susceptibility to the development of TPA-induced skin carcinomas [57]. All these results indicate the oncogenic function of p38γ and p38δ. Contrary, there is one study that shows pieces of evidence indicating that p38γ and p38δ have a role in the suppression of tumor development using mouse embryonic fibroblasts derived from mice lacking p38γ or p38δ [58]. Lack of either p38γ or p38δ increases cell migration and metalloproteinase-2 secretion, whereas only p38δ deficiency impairs cell contact inhibition. In addition, lack of p38γ in K-Ras-transformed fibroblasts leads to increased cell proliferation as well as tumorigenesis both in vitro and in vivo [58]. These discrepancies between different studies could be due not only to the difference in the experimental model and approaches used, but also to the distinct nature of cell(s) and process(es) that is/are involved in each experimental approach. Opposing roles in tumor development have also been reported for the isoform p38α [1, 59].
p38δ has been suggested to play an important role in inducing keratinocyte differentiation by regulating the expression of involucrin, which is a protein expressed during keratinocyte differentiation [60]. p38δ expression is detected in mouse and human epidermis [57, 61]. It has been shown that activation of exogenously expressed p38δ by differentiation-inducing agents correlates with increased involucrin promoter activity in keratinocytes [61, 62]. This occurs in a p38α/β-independent manner, and what is more, p38γ is poorly expressed in keratinocytes [63]. More data supporting the idea that p38δ may play a role in keratinocyte differentiation come from a study carried out in lesional psoriasis skin. Psoriasis is a chronic inflammatory skin disorder characterised by keratinocytes hyperproliferation and differentiation. It has been shown that the activity of p38α, p38β, and p38δ is augmented in lesional psoriasis skin compared with nonlesional psoriasis skin [64]. Additionally, p38δ may have a dual role in keratinocytes contributing not only to the differentiation process, but also to their apoptosis in a PKCδ-dependent manner, though the exact mechanisms by which p38δ may regulate keratinocyte differentiation or apoptosis are still unknown [65, 66]. It is important to notice that most of the pieces of evidence involving p38δ in regulating keratinocyte differentiation or apoptosis are based in overexpression experiments and require verification using other tools to both inhibit the activity or the expression of different p38MAPKs.
A possible p38δ and p38γ role in primary human erythroid cells differentiation has been suggested. Analysis of the mRNA expression pattern of each p38 isoform during erythroid differentiation of primary human erythroid progenitors shows that p38α and p38γ are expressed in early and late stages, whereas p38δ mRNA is expressed only at terminal stages of differentiation, indicating a possible role of p38γ in hematopoiesis and of p38δ during the terminal phase of differentiation [67].
Since p38γ expression is very high in skeletal muscle in comparison to other tissues, it is not surprising that it may play a fundamental role in skeletal muscle differentiation. Thus, endogenous p38γ protein level increases when myoblast differentiates into myotubes [68, 69]. Moreover, it has been shown that overexpression of p38γ in skeletal muscle cells leads to differentiation from myoblast to myotubes and that a dominant-negative mutant of p38γ prevented this differentiation process [5]. Recently, studies in p38γ null mice reported that p38γ plays a cardinal role in blocking the premature differentiation of skeletal muscle stem cells, the satellite cells that participate in adult muscle regeneration. p38γ phosphorylates the transcription factor MyoD and promotes MyoD association to the histone methyltransferase KMT1A. This complex acts repressing transcription and the premature expression of myogenin [38]. This is in contrast with the essential role of p38α in muscle differentiation [1, 70]. Moreover, p38γ is involved in muscle-specific exercise-induced skeletal muscle adaptation, and it seems to be required for the upregulation of PGC-1α (peroxisome proliferator-activated receptor-γ (PPARγ) coactivator-1α) in mitochondrial biogenesis and angiogenesis in response to exercise and nerve stimulation in mice [71].
6. Conclusion
Most of the studies to date have focused on the role of the p38α isoform and report the implication of this p38MAPK isoform in numerous biological and physiological processes. However, the in vivo functions of other alternative p38 isoforms or the molecular mechanism by which these kinases regulate particular cell processes remain largely unknown, and several important questions remain to be answered to address why a variety of p38MAPK isoforms is needed in mammalian cells are: for example, (i) how the p38MAPK isoforms are differentially activated by certain stimuli to mediate specific nonredundant signals, (ii) the identification of specific physiological substrates and how they are modulated by each p38 isoform, and (iii) the elucidation of new in vivo roles. The use of a combination of genetically modified mice, such as mouse lacking one or more p38 isoforms, tissue-specific knock-out mice, and knock-in mice expressing inactive p38MAPK will be a powerful tool to elucidate in vivo functions. Furthermore, high throughput genomic and proteomic technologies will also help to answer these questions and to generate enough knowledge that hopefully could be translated in therapeutics strategies by targeting the alternative, p38γ and p38δ MAPK isoforms.
Acknowledgment
The work in the authors' laboratory is supported by grants from the Ministerio de Ciencia e Innovación (MICINN), Spain (BFU2010-19734).
References
- 1.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochimica et Biophysica Acta. 2007;1773(8):1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. Journal of Biological Chemistry. 1998;273(3):1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Chen C, Li Z, et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β) Journal of Biological Chemistry. 1996;271(30):17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 4.Stein B, Yang MX, Young DB, et al. p38-2, a novel mitogen-activated protein kinase with distinct properties. Journal of Biological Chemistry. 1997;272(31):19509–19517. doi: 10.1074/jbc.272.31.19509. [DOI] [PubMed] [Google Scholar]
- 5.Lechner C, Zahalka MA, Giot JF, Møller NPH, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(9):4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertens S, Craxton M, Goedert M. SAP kinase-3, a new member of the family of mammalian stress-activated protein kinases. FEBS Letters. 1996;383(3):273–276. doi: 10.1016/0014-5793(96)00255-4. [DOI] [PubMed] [Google Scholar]
- 7.Goedert M, Cuenda A, Craxton M, Jakes R, Cohen P. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO Journal. 1997;16(12):3563–3571. doi: 10.1093/emboj/16.12.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Y, Gram H, Zhao M, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38δ . Journal of Biological Chemistry. 1997;272(48):30122–30128. doi: 10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- 9.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochemical Journal. 2010;429(3):403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 10.Iñnesta-Vaquera F, Sabio G, Kuma Y, Cuenda A. Stress-Activated Protein Kinases. 2008. Alternative p38 MAPK pathways. [Google Scholar]
- 11.Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38γ: a new member of p38 group of MAP kinases. Biochemical and Biophysical Research Communications. 1996;228(2):334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- 12.Bellon S, Fitzgibbon MJ, Fox T, Hsiao HM, Wilson KP. The structure of phosphorylated P38γ is monomeric and reveals a conserved activation-loop conformation. Structure. 1999;7(9):1057–1065. doi: 10.1016/s0969-2126(99)80173-7. [DOI] [PubMed] [Google Scholar]
- 13.Chang CI, Xu BE, Akella R, Cobb MH, Goldsmith EJ. Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Molecular Cell. 2002;9(6):1241–1249. doi: 10.1016/s1097-2765(02)00525-7. [DOI] [PubMed] [Google Scholar]
- 14.Limardo RGR, Ferreiro DN, Roitberg AE, Marti MA, Turjanski AG. P38γ activation triggers dynamical changes in allosteric docking sites. Biochemistry. 2011;50(8):1384–1395. doi: 10.1021/bi1007518. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa M, Cuenda A, Spillantini MG, et al. Stress-activated protein kinase-3 interacts with the PDZ domain of α1-syntrophin: a mechanism for specific substrate recognition. Journal of Biological Chemistry. 1999;274(18):12626–12631. doi: 10.1074/jbc.274.18.12626. [DOI] [PubMed] [Google Scholar]
- 16.Sabio G, Arthur JSC, Kuma Y, et al. p38γ regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO Journal. 2005;24(6):1134–1145. doi: 10.1038/sj.emboj.7600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabio G, Reuver S, Feijoo C, et al. Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38γ and ERK1/ERK2. Biochemical Journal. 2004;380(1):19–30. doi: 10.1042/BJ20031628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochemical Journal. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuma Y, Sabio G, Bain J, Shpiro N, Márquez R, Cuenda A. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. Journal of Biological Chemistry. 2005;280(20):19472–19479. doi: 10.1074/jbc.M414221200. [DOI] [PubMed] [Google Scholar]
- 20.Cuevas BD, Abell AN, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26(22):3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka N, Kamanaka M, Enslen H, et al. Differential involvement of p38 mitogen-activated protein kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Reports. 2002;3(8):785–791. doi: 10.1093/embo-reports/kvf153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Ma R, Flavell RA, Choi ME. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38α and p38δ MAPK isoforms by TGF-β1 in murine mesangial cells. Journal of Biological Chemistry. 2002;277(49):47257–47262. doi: 10.1074/jbc.M208573200. [DOI] [PubMed] [Google Scholar]
- 23.Alonso G, Ambrosino C, Jones M, Nebreda AR. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. Journal of Biological Chemistry. 2000;275(51):40641–40648. doi: 10.1074/jbc.M007835200. [DOI] [PubMed] [Google Scholar]
- 24.Biondi RM, Nebreda AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochemical Journal. 2003;372(1):1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enslen H, Brancho DM, Davis RJ. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO Journal. 2000;19(6):1301–1311. doi: 10.1093/emboj/19.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brancho D, Tanaka N, Jaeschke A, et al. Mechanism of p38 MAP kinase activation in vivo. Genes and Development. 2003;17(16):1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuenda A, Cohen P, Buée-Scherrer V, Goedert M. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); Comparison of the specificities of SAPK3 and SAPK2 (RK/p38) EMBO Journal. 1997;16(2):295–305. doi: 10.1093/emboj/16.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.le Guezennec X, Bulavin DV. WIP1 phosphatase at the crossroads of cancer and aging. Trends in Biochemical Sciences. 2010;35(2):109–114. doi: 10.1016/j.tibs.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Takekawa M, Maeda T, Saito H. Protein phosphatase 2Cα inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO Journal. 1998;17(16):4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAlees JW, Sanders VM. Hematopoietic protein tyrosine phosphatase mediates β2-adrenergic receptor-induced regulation of p38 mitogen-activated protein kinase in B lymphocytes. Molecular and Cellular Biology. 2009;29(3):675–686. doi: 10.1128/MCB.01466-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26(22):3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 32.Hou SW, Zhi HY, Pohl N, et al. PTPH1 dephosphorylatesates and cooperates with p38y MAPK to increase ras oncogenesis through PDZ-mediated interaction. Cancer Research. 2010;70(7):2901–2910. doi: 10.1158/0008-5472.CAN-09-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long DL, Loeser RF. P38γ mitogen-activated protein kinase suppresses chondrocyte production of MMP-13 in response to catabolic stimulation. Osteoarthritis and Cartilage. 2010;18(9):1203–1210. doi: 10.1016/j.joca.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabio G, Cerezo-Guisado MI, Del Reino P, et al. p38γ regulates interaction of nuclear PSF and RNA with the tumour-suppressor hDlg in response to osmotic shock. Journal of Cell Science. 2010;123(15):2596–2604. doi: 10.1242/jcs.066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shav-Tal Y, Zipori D. PSF and p54nrb/NonO—multi-functional nuclear proteins. FEBS Letters. 2002;531(2):109–114. doi: 10.1016/s0014-5793(02)03447-6. [DOI] [PubMed] [Google Scholar]
- 36.Tang J, Qi X, Mercola D, Han J, Chen G. Essential role of p38γ in K-Ras transformation independent of phosphorylation. Journal of Biological Chemistry. 2005;280(25):23910–23917. doi: 10.1074/jbc.M500699200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Court NW, Kuo I, Quigley O, Bogoyevitch MA. Phosphorylation of the mitochondrial protein Sab by stress-activated protein kinase 3. Biochemical and Biophysical Research Communications. 2004;319(1):130–137. doi: 10.1016/j.bbrc.2004.04.148. [DOI] [PubMed] [Google Scholar]
- 38.Gillespie MA, le Grand F, Scimè A, et al. p38-γ-dependent gene silencing restricts entry into the myogenic differentiation program. Journal of Cell Biology. 2009;187(7):991–1005. doi: 10.1083/jcb.200907037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feijoo C, Campbell DG, Jakes R, Goedert M, Cuenda A. Evidence that phosphorylation of the microtubule-associated protein Tau by SAPK4/p38δ at Thr50 promotes microtubule assembly. Journal of Cell Science. 2005;118(2):397–408. doi: 10.1242/jcs.01655. [DOI] [PubMed] [Google Scholar]
- 40.Goedert M, Hasegawa M, Jakes R, Lawler S, Cuenda A, Cohen P. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Letters. 1997;409(1):57–62. doi: 10.1016/s0014-5793(97)00483-3. [DOI] [PubMed] [Google Scholar]
- 41.Parker CG, Hunt J, Diener K, et al. Identification of stathmin as a novel substrate for p38 delta. Biochemical and Biophysical Research Communications. 1998;249(3):791–796. doi: 10.1006/bbrc.1998.9250. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida H, Goedert M. Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38δ or JNK2 in the presence of heparin generates the AT100 epitope. Journal of Neurochemistry. 2006;99(1):154–164. doi: 10.1111/j.1471-4159.2006.04052.x. [DOI] [PubMed] [Google Scholar]
- 43.Knebel A, Haydon CE, Morrice N, Cohen P. Stress-induced regulation of eukaryotic elongation factor 2 kinase by SB 203580-sensitive and -insensitive pathways. Biochemical Journal. 2002;367(2):525–532. doi: 10.1042/BJ20020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knebel A, Morrice N, Cohen P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38δ . EMBO Journal. 2001;20(16):4360–4369. doi: 10.1093/emboj/20.16.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumara G, Formentini I, Collins S, et al. Regulation of PKD by the MAPK p38δ in insulin secretion and glucose homeostasis. Cell. 2009;136(2):235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams RH, Porras A, Alonso G, et al. Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Molecular Cell. 2000;6(1):109–116. [PubMed] [Google Scholar]
- 47.Mudgett JS, Ding J, Guh-Siesel L, et al. Essential role for p38α mitogen-activated protein kinase in placental angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(19):10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura K, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M. Requirement for p38α in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell. 2000;102(2):221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 49.Kukkonen-Macchi A, Sicora O, Kaczynska K, et al. Loss of p38γ MAPK induces pleiotropic mitotic defects and massive cell death. Journal of Cell Science. 2011;124(2):216–227. doi: 10.1242/jcs.068254. [DOI] [PubMed] [Google Scholar]
- 50.Wu CC, Wu X, Han J, Sun P. p38γ regulates UV-induced checkpoint signaling and repair of UV-induced DNA damage. Protein and Cell. 2010;1(6):573–583. doi: 10.1007/s13238-010-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Junttila MR, Ala-Aho R, Jokilehto T, et al. p38α and p38δ mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene. 2007;26(36):5267–5279. doi: 10.1038/sj.onc.1210332. [DOI] [PubMed] [Google Scholar]
- 52.Loesch M, Chen G. The p38 MAPK stress pathway as a tumor suppressor or more? Frontiers in Bioscience. 2008;13(9):3581–3593. doi: 10.2741/2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng F, Zhang H, Liu G, et al. p38γ mitogen-activated protein kinase contributes to oncogenic properties maintenance and resistance to poly (ADP-ribose)-polymerase-1 inhibition in breast cancer. Neoplasia. 2011;13(5):472–482. doi: 10.1593/neo.101748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qi X, Pohl NM, Loesch M, et al. p38α antagonizes p38γ activity through c-jun-dependent ubiquitin-proteasome pathways in regulating ras transformation and stress response. Journal of Biological Chemistry. 2007;282(43):31398–31408. doi: 10.1074/jbc.M703857200. [DOI] [PubMed] [Google Scholar]
- 55.Qi X, Tang J, Loesch M, Pohl N, Alkan S, Chen G. p38γ mitogen-activated protein kinase integrates signaling crosstalk between Ras and estrogen receptor to increase breast cancer invasion. Cancer Research. 2006;66(15):7540–7547. doi: 10.1158/0008-5472.CAN-05-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwong J, Hong L, Liao R, Deng Q, Han J, Sun P. P38α and p38 mediate oncogenic ras-induced senescence through differential mechanisms. Journal of Biological Chemistry. 2009;284(17):11237–11246. doi: 10.1074/jbc.M808327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schindler EM, Hindes A, Gribben EL, et al. p38δ mitogen-activated protein kinase is essential for skin tumor development in mice. Cancer Research. 2009;69(11):4648–4655. doi: 10.1158/0008-5472.CAN-08-4455. [DOI] [PubMed] [Google Scholar]
- 58.Cerezo-guisado MI, del Reino P, Remy G, et al. Evidence of p38γ and p38δ involvement in cell transformation processes. Carcinogenesis. 2011;32(7):1093–1099. doi: 10.1093/carcin/bgr079. [DOI] [PubMed] [Google Scholar]
- 59.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nature Reviews Cancer. 2009;9(8):537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 60.Eckert RL, Efimova T, Balasubramanian S, Crish JF, Bone F, Dashti S. p38 mitogen-activated protein kinases on the body surface-a function for p38δ . Journal of Investigative Dermatology. 2003;120(5):823–828. doi: 10.1046/j.1523-1747.2003.12120.x. [DOI] [PubMed] [Google Scholar]
- 61.Efimova T, Broome AM, Eckert RL. A regulatory role for p38δ MAPK in keratinocyte differentiation: evidence for p38δ-ERK1/2 complex formation. Journal of Biological Chemistry. 2003;278(36):34277–34285. doi: 10.1074/jbc.M302759200. [DOI] [PubMed] [Google Scholar]
- 62.Balasubramanian S, Efimova T, Eckert RL. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. Journal of Biological Chemistry. 2002;277(3):1828–1836. doi: 10.1074/jbc.M110376200. [DOI] [PubMed] [Google Scholar]
- 63.Dashti SR, Efimova T, Eckert RL. MEK6 regulates human involucrin gene expression via a p38α- and p38δ-dependent mechanism. Journal of Biological Chemistry. 2001;276(29):27214–27220. doi: 10.1074/jbc.M100465200. [DOI] [PubMed] [Google Scholar]
- 64.Johansen C, Kragballe K, Westergaard M, Henningsen J, Kristiansen K, Iversen L. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. British Journal of Dermatology. 2005;152(1):37–42. doi: 10.1111/j.1365-2133.2004.06304.x. [DOI] [PubMed] [Google Scholar]
- 65.Kraft CS, LeMoine CMR, Lyons CN, Michaud D, Mueller CR, Moyes CD. Control of mitochondrial biogenesis during myogenesis. American Journal of Physiology. 2006;290(4):C1119–C1127. doi: 10.1152/ajpcell.00463.2005. [DOI] [PubMed] [Google Scholar]
- 66.Adhikary G, Chew YC, Reece EA, Eckert RL. PKC-δ and-η, MEKK-1, MEK-6, MEK-3, and p38-δ are essential mediators of the response of normal human epidermal keratinocytes to differentiating agents. Journal of Investigative Dermatology. 2010;130(8):2017–2030. doi: 10.1038/jid.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 67.Uddin S, Ah-Kang J, Ulaszek J, Mahmud D, Wickrema A. Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):147–152. doi: 10.1073/pnas.0307075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. Journal of Biological Chemistry. 1999;274(7):4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- 69.Tortorella LL, Lin CB, Pilch PF. ERK6 is expressed in a developmentally regulated manner in rodent skeletal muscle. Biochemical and Biophysical Research Communications. 2003;306(1):163–168. doi: 10.1016/s0006-291x(03)00936-7. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz-Bonilla V, Perdiguero E, Gresh L, et al. Efficient adult skeletal muscle regeneration in mice deficient in p38β, p38γ and p38δ MAP kinases. Cell Cycle. 2008;7(14):2208–2214. doi: 10.4161/cc.7.14.6273. [DOI] [PubMed] [Google Scholar]
- 71.Pogozelski AR, Geng T, Li P, et al. p38γ mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS One. 2009;4(11) doi: 10.1371/journal.pone.0007934. Article ID e7934. [DOI] [PMC free article] [PubMed] [Google Scholar]


