Abstract
A novel, interlocked, self-assembled (M2L2)2 molecular architecture (3) was constructed from arene-Ru acceptor 1 and 1,4-di(pyridine-4-yl)buta-1,3-diyne donor 2. Two M2L2 units, with cavities of ~7.21 Å, spontaneously interlock with one unit encapsulating a twin in a non-catenane fashion. The dimeric host-guest complex thus formed is unique among two dimensional self-assemblies and is stabilized by π-π interactions between the M2L2 units.
Self-assembly is a ubiquitous phenomenon in natural systems and is at the heart of many biological processes,1 including protein synthesis and DNA formation. These processes have inspired chemists to adopt approaches which take advantage of the molecular principles employed by nature to develop intricate abiological supramolecular systems2 with respect to both structure and function, all the while maintaining synthetic ease and versatility. For example, the synthesis of various complex polygons utilizing coordination-driven self-assembly has garnered a significant amount of interest as a route towards nano-scale machinery.3 From its origins in exploring molecular self-recognition and generating simple 2D shapes, coordination-driven self-assembly has grown to afford a level of control and rational design which enables a myriad of 2D and 3D metallacycles and metallacages.4,5 These constructs use a variety of metal acceptors and organic donors and have extended beyond a simple two-component paradigm to encompass selective self-assembly, in which multiple assemblies form from a mixture of subunits, to multicomponent assemblies.6 Non-covalent interactions7 between coordination-driven self-assemblies can result in increased complexity, leading to architectures such as nano-capsules,8 nano-reactors9 and multi-component selfassemblies. 10 In this context, complex, interwoven structures11 such as catenanes, pseudorotaxanes, and rotaxanes, have attracted considerable attention, not only for their interesting topological properties but also for their function as molecular machines.12 The prevalent molecular phenomena behind the formation of interlocked structures are metal-ligand coordinations and H-bonding interactions.13 Among these, there are but a few examples which invoke the importance of π-π interactions between subunits.14 Herein, we report for the first time the preparation of a self-assembled metalla-rectangle which encapsulates a second, identical rectangle through π-π interactions. This ensemble results from the coordination-driven self-assembly of arene-Ru acceptor 1 and 1,4-di(pyridine-4-yl)buta-1,3-diyne donor 2, and may represent a new paradigm for the formation of interlocked supramolecular species which form with π-π interactions as the impetus.
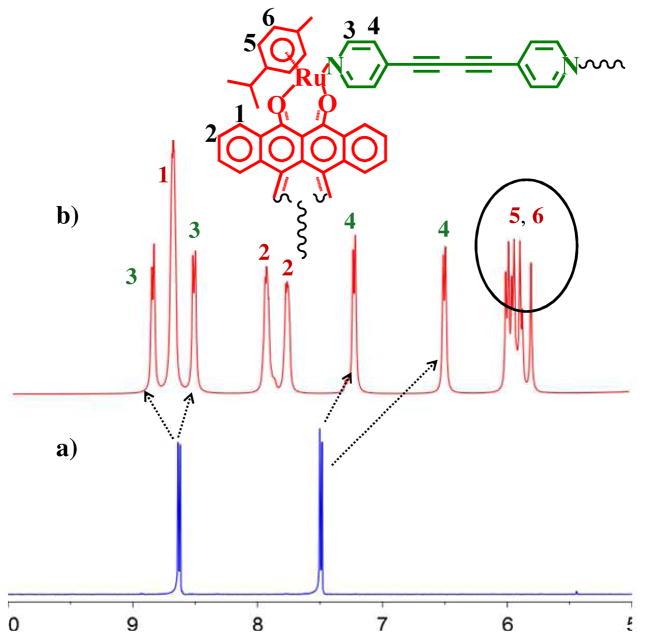
The first indication of the dimeric nature of 3 was found in the 1H NMR spectrum of the reaction mixture. The treatment of an equimolar amount of arene-Ru acceptor 1 with 1,4-di(pyridin-4-yl)buta-1,3-diyne (2) in CD3NO2-CD3OD (1:1) gave a complex 1H NMR spectrum after stirring for 24 h at room temperature. Initially interpreted as resulting from incomplete assembly, the reaction time was increased to three days with no noticeable change in the 1H NMR spectrum throughout the course of the experiment. Hence, the product was isolated by the addition of diethyl-ether. The 1H NMR spectrum of the isolated product supported a dimeric structure as all the protons resonances were split into two signals, rather than the simple resonances previously observed for metalla-rectangles of the M2L2-type.
Two sets of signals for the α-pyridinyl protons were observed at δ = 8.87 (downfield) and 8.53 ppm (upfield) with significant shifts as compared to those found in the spectrum of 2. Similarly, two sets of doublets were assigned to the β-pyridinyl protons at δ = 7.24 and 6.50 ppm, with noticeable upfield shifts as compared to the spectrum of 2. The large upfield shift of the β–pyridinyl proton resonances is due to the increased shielding by the flanking tetracene moieties. Likewise, the tetracene protons are observed as four multiplets at δ = 8.71 (two multiplets overlapped for H1, 16 H), δ 7.95 and 7.28 ppm (H2, 16H). The p-cymene protons of 3 are observed at δ = 6.00, 5.95, 5.88 and 5.79 ppm as four doublets for 3 (Figure 1).
Figure 1.
Partial 1H NMR spectra (300 MHz) of donor 2 (a), and 3 (b) in d3-nitromethane.
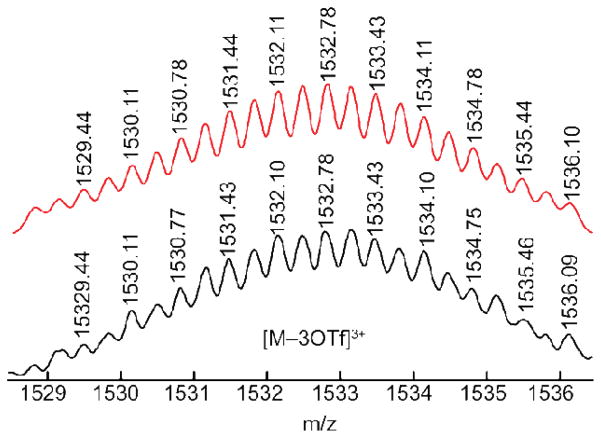
The HR-ESI-MS spectrum of 3 confirmed the (M2L2)2 composition with a prominent signal at m/z = 1532.1 ([M – 3OTf]3+; Figure 2). The theoretical isotopic distribution is in good agreement with the experimental peak. The 1H NMR coupled with the HR-ESI-MS data demonstrates that the (M2L2)2 dimeric structure is present in the solution phase.
Figure 2.
Calculated (red) and experimental (black) HR-ESI-mass spectra of intercalated rectangle dimer 3.
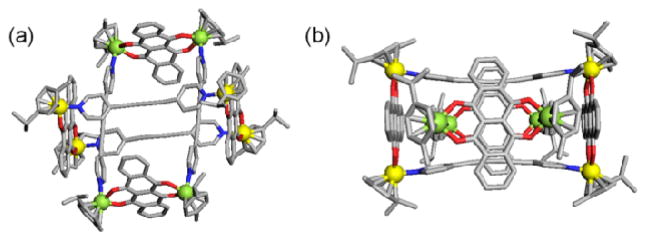
To further investigate the structural features of 3 we performed single-crystal X-ray analysis using synchrotron radiation (Figure 3).15 A single crystal suitable for X-ray diffraction was obtained by slow vapour diffusion of diethyl ether into a nitromethane-methanol solution of 3. The dimeric nature of 3 was confirmed upon structural refinement, clearly revealing one rectangle threaded through the cavity of a second rectangle. A noteworthy feature of the structure is the close contact of the tetracene units of the outer rectangle with the diethynyl-dipyridinyl moieties of the inner rectangle, exhibiting intramolecular π-π interactions with a distance of 3.4 Å throughout the dimer. In order to accommodate the inner rectangle, some distortions are observable in the outer rectangular subunit. The pyridinyl donors of the outer rectangle are bent inwards, resulting in a separation of 7.21 Å between the two pyridinyl units. This is in contrast to the pyridinyl units of the inner rectangle which are bent outward to yield a diameter of 9.41 Å, thus maximizing the π-π interactions. Similarly, the tetracene skeleton of the outer rectangle is bent outward with a distance of 16.33 Å between the intramolecular moieties, while the tetracene frame of the inner rectangle curls inward with a distance of 13.72 Å between moieties, potentially reducing the steric-strain while maximizing the π-π interactions. This macromolecule-in-a-macromolecule motif is rarely observed in supramolecular host-guest chemistry and to the best of our knowledge it is the first example among coordination-driven self-assembled metallacycles.16
Figure 3.
X-ray crystal structure of 3 with a top view (a) and side view (b). Hydrogen atoms, counter-ions and solvents of crystallization are omitted for clarity.
The 1H NMR, HR-ESI-MS and crystal structure of 3 provide collective proof for the formation of a unique, intercalated structure driven by π-π interactions which exist in both solution and the solid state.
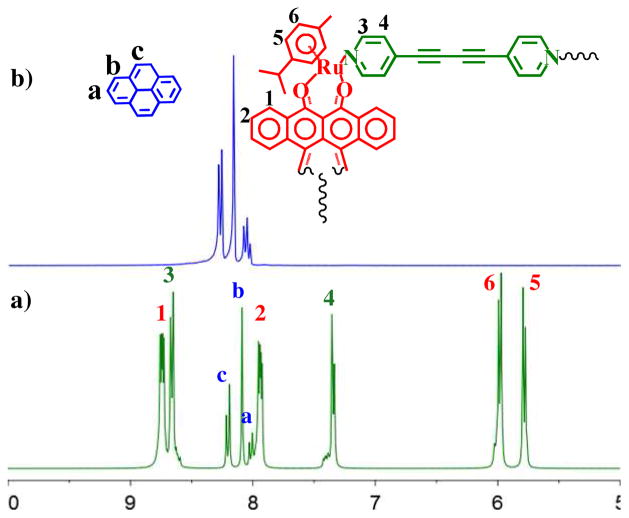
In order to probe the importance of π-π interactions in the formation of the dimeric structure 3, experiments were carried out in the presence of 2.0 equiv of pyrene, included to inhibit intramolecular π-π interactions between the rectangles. Pyrene was added to CD3NO2-CD3OD solutions of 3 which were stirred for 24 h at room temp and 60°C. No change was observed in 1H NMR spectra of these solutions, attesting to the stability of the (M2L2)2 dimer. A second experiment involved the addition of pyrene prior to self-assembly: the 1H NMR spectrum of a solution of arene-Ru acceptor 1, donor 2 and 2.0 equiv of pyrene in nitromethane-methanol suggests the formation of singular M2L2 rectangles (4) after 24 h of stirring at room temperature. The presence of pyrene is expected to hinder the formation of 3, thus yielding a M2L2type metalla-rectangle 4 in high yield (77%). The simpler 1H NMR spectrum obtained (Figure 4) clearly supports the formation of 4. Two doublets (δ = 8.67 and 7.35 ppm) for the pyridinyl protons are observed in the reaction mixture. The p-cymene protons are observed at δ = 5.99 and 5.79 ppm and the tetracene protons are observed as two multiplets at δ = 8.73 and 7.95 ppm. The peaks associated with pyrene are shifted in the spectrum of 4 versus free pyrene (Figure 4). These shifts support that the formation of 3 is disrupted by π-π interactions between pyrene and the metalla-rectangles.
Figure 4.
Partial 1H NMR spectra (300 MHz) of 4 (a), and free pyrene (b) in d6-acetone.
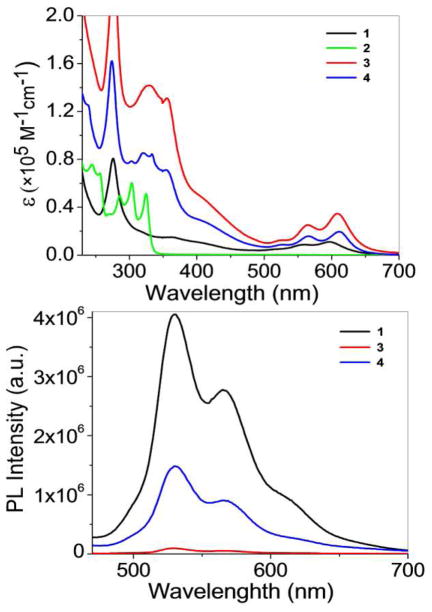
The UV-Vis absorption and emission spectra of 3 and 4, along with their corresponding metal acceptor (1) and donor (2) were also investigated (Figure 5, top). The absorption spectra of 3 and 4 in dichloromethane solution (1.0 × 10−5 M) exhibit intense bands at λabs = 329 nm, 357 nm, 565 nm, 604 nm for 3 and 4. The absorbance bands of 3 and 4 are dominated by π-π* transitions of the tetracene moieties from 550 to 620 nm. The tetracene rings of complex 3 are held parallel to its pyridine groups, resulting in a red-shifted band (Δλ ~ 15 nm) with respect to acceptor 1. Similar red-shifts (Δλ ~ 25 nm) are observed for the bands in 3 which correspond to bands in donor 2.
Figure 5.
UV-vis (top) and Fluorescence (bottom) spectra of 1–4.
The photo-excitation at 390 nm (Figure 5, bottom) of acceptor 1 and complexes 3 ((M2L2)2) and 4 (M2L2) show emission bands at λem= 531 nm and 566 nm, respectively. The fact that the emission wavelengths of 3 and 4 match well with acceptor 1 suggests that the emission originates from the arene-Ru moiety. Interestingly, the fluorescence intensity of 3 and 4 decrease markedly in comparison with that of the acceptor 1 at the same molar concentration of arene-Ru moiety. The occurrence of intramolecular photo-induced electron transfer (PET)17 in 3 and 4 from the arene-Ru fluorophore unit to the 1,4-di(pyridine-4-yl)buta-1,3-diyne moeities is likely to be responsible for the emission quenching. As 1 contains no diyne ligand, this quenching pathway is precluded. The emission features of arene-Ru acceptor 1 indicates that the tetracene ring of the acceptor behaves as a luminophore.18 The further attentuation of emission from 1 to 4 to 3 is due to an increase in quenching associated with the more extensive conjugated system due to the stronger π-π stacking in 3 as compared to 4, which increases the efficienty of the PET pathway from the arene-Ru unit to the diyne ligands.
In summary, we report here a novel example of a non-catenane interlocking of metalla-rectanges stabilized by multiple π-π interactions between the tetracene-containing arene-Ru acceptors and the four π-electron rich 1,4-di(pyridine-4-yl)buta-1,3-diyne donors. This new class of a molecular host with extended aromatic π surfaces holds promise for host-guest properties and can potentially be used to develop new functional materials and sensors.
Supplementary Material
Scheme 1.
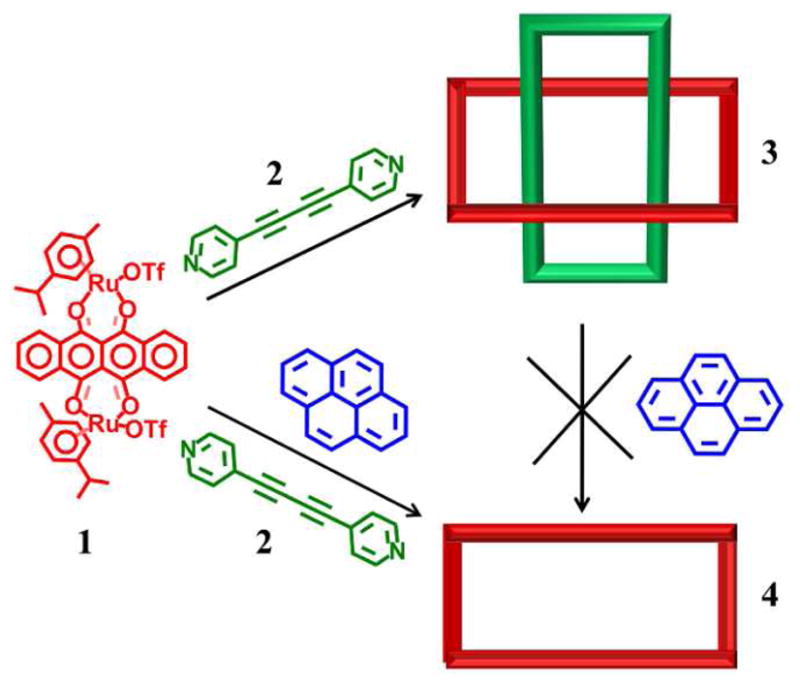
Synthesis of a discrete (M2L2)2 supramolecule.
Acknowledgments
This work was supported by the World Class University (WCU) program (R33-2008-000-10003) and Priority Research Centers program (2009-0093818) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology. We also thank Pohang Accelerator Laboratory in Korea for their help in the X-ray analysis. P.J.S thanks the NIH (GM-57052) for financial support.
Footnotes
Supporting Information. Synthetic details and 1H, 13C NMR spectra for 3 and 4.“This material is available free of charge via the Internet at http://pubs.acs.org.” For instructions on what should be included in the Supporting Information as well as how to prepare this material for publication, check the Instructions for Authors at (http://pubs.acs.org/page/jacsat/submission/authors.html).
References
- 1.(a) Steed JW, Turner DR, Wallace KJ. In Core Concepts in Supramolecular Chemistry and Nano-chemistry. Wiley; Chichester, U.K: 2007. [Google Scholar]; (b) Groll M, Dizel L, Lowe J, Stock D, Bochter M, Bartunik HD, Huber R. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]; (c) Lehn JM. Chem Soc Rev. 2007;36:151–160. doi: 10.1039/b616752g. [DOI] [PubMed] [Google Scholar]; (d) Cann AJ. Principles of Molecular Virology. Academic Press; San Diego: 1993. [Google Scholar]; (e) De S, Mahata K, Schmittel M. Chem Soc Rev. 2010;39:1555–1575. doi: 10.1039/b922293f. [DOI] [PubMed] [Google Scholar]
- 2.(a) Stang PJ, Olenyuk B. Acc Chem Res. 1997;30:502–518. [Google Scholar]; (b) Leininger S, Olenyuk B, Stang PJ. Chem Rev. 2000;100:853–908. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (c) Seidel SR, Stang PJ. Acc Chem Res. 2002;35:972–983. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (d) Holliday BJ, Mirkin CA. Angew Chem, Int Ed. 2001;40:2022–2043. [PubMed] [Google Scholar]; (e) Fujita M, Tominaga M, Hori A, Therrien B. Acc Chem Res. 2005;38:369–378. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (f) Oliver CG, Ulman PA, Wiester MJ, Mirkin CA. Acc Chem Res. 2008;41:1618–1629. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chakrabarty R, Mukherjee PS, Stang PJ. Chem Rev. ASAP; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Okuno E, Hiraoka S, Shionoya M. Dalton Trans. 2010;39:4107–4116. doi: 10.1039/b926154k. [DOI] [PubMed] [Google Scholar]; (b) Kay ER, Leigh DA, Zerbetto F. Angew Chem, Int Ed. 2007;46:72–191. doi: 10.1002/anie.200504313. [DOI] [PubMed] [Google Scholar]; (c) Champin B, Mobian P, Sauvage JP. Chem Soc Rev. 2007;36:358–366. doi: 10.1039/b604484k. [DOI] [PubMed] [Google Scholar]; (d) Balzani V, Credi A, Venturi M. Chem Soc Rev. 2009;38:1542–1550. doi: 10.1039/b806328c. [DOI] [PubMed] [Google Scholar]; (e) Hiraoka S, Shiro M, Shionoya M. J Am Chem Soc. 2004;126:1214–1218. doi: 10.1021/ja036388q. [DOI] [PubMed] [Google Scholar]; (f) Brouwer AM, Frochot C, Gatti FG, Leigh DA, Mottier L, Paolucci F, Roffia S, Wurpel GWH. Science. 2001;291:2124–2128. doi: 10.1126/science.1057886. [DOI] [PubMed] [Google Scholar]; (g) Thordarson P, Bijsterveld EJA, Rowan AE, Nolte RJM. Nature. 2003;424:915–918. doi: 10.1038/nature01925. [DOI] [PubMed] [Google Scholar]
- 4.(a) Wang M, Vajpayee V, Shanmugaraju S, Zheng YR, Zhao Z, Kim H, Mukherjee PS, Chi KW, Stang PJ. Inorg Chem. 2011;50:1506–1512. doi: 10.1021/ic1020719. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chi K-W, Addicott C, Moon M-E, Lee HJ, Yoon SC, Stang PJ. J Org Chem. 2006;71:6662–6665. doi: 10.1021/jo060971i. [DOI] [PubMed] [Google Scholar]; (c) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc Chem Res. 2005;38:349–358. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (d) Raymond KN. Nature. 2009;460:585–586. [Google Scholar]; (e) Pluth MD, Bergman RG, Raymond KN. Acc Chem Res. 2009;42:1650–1659. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]; (f) Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Chem Soc Rev. 2009;38:1450–1459. doi: 10.1039/b807080f. [DOI] [PubMed] [Google Scholar]; (g) Vajpayee V, Yang YJ, Kang SC, Kim H, Kim IS, Wang M, Stang P, Chi KW. Chem Commun. 2011;47:5184–5186. doi: 10.1039/c1cc10167f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Vajpayee V, Song YH, Yang YJ, Kang SC, Kim H, Kim IS, Wang M, Stang PJ, Whan K-W. Organometallics. 2011;30:3242–3245. doi: 10.1021/om200294x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Therrien B, Süss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ. Angew Chem, Int Ed. 2008;47:3773–3776. doi: 10.1002/anie.200800186. [DOI] [PubMed] [Google Scholar]; (j) Barry NPE, Zava O, Furrer J, Dyson PJ, Therrien B. Dalton Trans. 2010;39:5272–5277. doi: 10.1039/c001521k. [DOI] [PubMed] [Google Scholar]; (k) Pitto-Barry A, Barry NPE, Zava O, Deschenaux R, Dyson PJ. Chem–Eur J. 2011;17:1966–1971. doi: 10.1002/chem.201002634. [DOI] [PubMed] [Google Scholar]
- 5.(a) Piotrowski H, Polborn K, Hilt G, Severin K. J Am Chem Soc. 2001;123:2699–2700. doi: 10.1021/ja005804t. [DOI] [PubMed] [Google Scholar]; (b) Granzhan A, Schouwey C, Riis-Johannessen T, Scopelliti R, Severin K. J Am Chem Soc. 2011;133:7106–7115. doi: 10.1021/ja200580x. [DOI] [PubMed] [Google Scholar]; (c) Grote Z, Bonazzi S, Scopelliti R, Severin K. J Am Chem Soc. 2006;128:10382–10383. doi: 10.1021/ja0637806. [DOI] [PubMed] [Google Scholar]; (d) Barry NPE, Furrer J, Therrien B. Helv Chim Acta. 2010;93:1313–1328. [Google Scholar]; (e) Barry NPE, Furrer J, Freudenreich J, Süss-Fink G, Therrien B. Eur J Inorg Chem. 2010:725–728. [Google Scholar]
- 6.(a) Schmittel M, He B, Mal P. Org Lett. 2008;10:2513, 2516. doi: 10.1021/ol800796h. [DOI] [PubMed] [Google Scholar]; (b) Schmittel M, Saha ML, Fan J. Org Lett. 2011;13:3916–3919. doi: 10.1021/ol201440a. [DOI] [PubMed] [Google Scholar]
- 7.(a) Lehn JM. Science. 1985;227:849–856. doi: 10.1126/science.227.4689.849. [DOI] [PubMed] [Google Scholar]; (b) Lehn JM. Supramolecular Chemistry: Concepts and Perspectives. WILEY-VCH; Weinheim, Germany: 1995. [Google Scholar]; (c) Lehn JM. Science. 1993;260:1762–1763. doi: 10.1126/science.8511582. [DOI] [PubMed] [Google Scholar]
- 8.(a) Caulder DL, Powers RE, Parac TN, Raymond KN. Angew Chem, Int Ed. 1998;37:1840–1842. [Google Scholar]; (b) Umemoto K, Yamaguchi K, Fujita M. J Am Chem Soc. 2000;122:7150–7151. [Google Scholar]; (c) Kishi N, Li Z, Yoza K, Akita M, Yoshizawa M. J Am Chem Soc. 2011;133:11438–11441. doi: 10.1021/ja2037029. [DOI] [PubMed] [Google Scholar]
- 9.(a) Hou J–L, Ajami D, Rebek J., Jr J Am Chem Soc. 2008;130:7810–7811. doi: 10.1021/ja802288k. [DOI] [PubMed] [Google Scholar]; (b) Yoshizawa M, Klosterman JK, Fujita M. Angew Chem, Int Ed. 2009;48:3418–3438. doi: 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]
- 10.(a) Lee J, Ghosh K, Stang PJ. J Am Chem Soc. 2009;131:12028–12029. doi: 10.1021/ja903330j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang M, Lan WJ, Zheng YR, Cook TR, White HS, Stang PJ. J AM Chem Soc. 2011;133:10752–10755. doi: 10.1021/ja204155r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang M, Zheng YR, Ghosh K, Stang PJ. J Am Chem Soc. 2010;132:6282–6283. doi: 10.1021/ja100889h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mahata K, Schmittel M. J Am Chem Soc. 2009;131:16544–16554. doi: 10.1021/ja907185k. [DOI] [PubMed] [Google Scholar]; (e) Mahata K, Saha ML, Schmittel M. J Am Chem Soc. 2010;132:15933–15935. doi: 10.1021/ja108419k. [DOI] [PubMed] [Google Scholar]
- 11.(a) Leigh DA, Wong JKY, Dehez F, Zerbetto F. Science. 2003;424:174–179. doi: 10.1038/nature01758. [DOI] [PubMed] [Google Scholar]; (b) Badjiæ JD, Balzani V, Credi A, Silvi S, Stoddart JF. Science. 2004;303:1845–1849. doi: 10.1126/science.1094791. [DOI] [PubMed] [Google Scholar]; (c) Raymo FM, Stoddart JF. Chem Rev. 1999;99:1643–1663. doi: 10.1021/cr970081q. [DOI] [PubMed] [Google Scholar]; (d) Evans NH, Serpell CJ, Beer PD. Angew Chem, Int Ed. 2011;50:1–5. doi: 10.1002/anie.201007741. [DOI] [PubMed] [Google Scholar]
- 12.(a) Balzani V, Credi A, Raymo FM, Stoddart JF. Angew Chem. 2000;112:3484–3530. doi: 10.1002/1521-3773(20001002)39:19<3348::aid-anie3348>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2000;39:3348–3391. doi: 10.1002/1521-3773(20001002)39:19<3348::aid-anie3348>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]; (b) Schalley CA, Lhtzen A, Albrecht M. Chem Eur J. 2004;10:1072–1080. doi: 10.1002/chem.200305150. [DOI] [PubMed] [Google Scholar]
- 13.(a) Kay ER, Leigh DA. Top Curr Chem. 2005;262:133–177. [Google Scholar]; (b) Hunter CA. J Am Chem Soc. 1992;114:5303–5311. [Google Scholar]; (c) West KR, Ludlow RF, Corbett PT, Besenius P, Mansfeld FM, Cormack PAG, Sherrington DC, Goodman JM, Stuart MCA, Otto S. J Am Chem Soc. 2008;130:10834–10835. doi: 10.1021/ja801508q. [DOI] [PubMed] [Google Scholar]
- 14.(a) Lu J, Turner DR, Harding LP, Byrne LT, Baker MV, Batten SR. J Am Chem Soc. 2009;131:10372–10373. doi: 10.1021/ja9041912. [DOI] [PubMed] [Google Scholar]; (b) Fujita M, Aoyagi M, Ibukuro F, Ogura K, Yamaguchi K. J Am Chem Soc. 1998;120:611. [Google Scholar]; (c) Liu Y, Bruneau A, He J, Abliz Z. Org Lett. 2008;10:765–768. doi: 10.1021/ol702830p. [DOI] [PubMed] [Google Scholar]; (d) Vignon SA, Wong J, Tseng H-R, Stoddart JF. Org Lett. 2004;6:1095–1098. doi: 10.1021/ol0364881. [DOI] [PubMed] [Google Scholar]; (e) Coskun A, Saha S, Aprahamian I, Stoddart JF. Org Lett. 2008;10:3187–3190. doi: 10.1021/ol800931z. [DOI] [PubMed] [Google Scholar]
- 15.X-ray data for 3: C216H176F24N8O40Ru8S8, M = 5044.69, triclinic, P-1 (No. 2), a = 21.535(4) Å, b = 22.948(5) Å, c = 25.314(5) Å, α = 97.01(3) °, β = 101.86(3) °, γ = 92.81(3) °, V = 12116(4) Å3, Z = 2, T = 100 K, μ(synchrotron) = 1.191 mm−1, ρcalc = 1.383 g·cm−3, R1(I > 2σ(I)) = 0.0939, wR2 (all data) = 0.3001 (all data), GOF = 1.209. The contributions of the disordered solvent molecules were removed from the diffraction data using the SQUEEZE of PLATON software (ca. 482 e/u.c); Spek AL. A Multipurpose Crystallographic Tool. Utrecht University; Utrecht, The Netherlands: 2001. PLATON.
- 16.(a) Kim SY, Jung IS, Lee E, Kim J, Sakamoto S, Yamaguchi K, Kim K. Angew Chem Int Ed. 2001;40:2119–2121. doi: 10.1002/1521-3773(20010601)40:11<2119::AID-ANIE2119>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Day AI, Blanch RJ, Arnold AP, Lorenzo S, Lewis GR, Dance I. Angew Chem Int Ed. 2002;41:275–277. doi: 10.1002/1521-3773(20020118)41:2<275::aid-anie275>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]; (c) Chiu SH, Pease AR, Stoddart JF, White AJP, Williams DJ. Angew Chem Int Ed. 2002;41:270–274. doi: 10.1002/1521-3773(20020118)41:2<270::aid-anie270>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Vajpayee V, Song YH, Lee MH, Kim H, Wang M, Stang PJ, Whan K-W. Chem Eur J. 2011;17:7837–7844. doi: 10.1002/chem.201100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Nguyen M-H, Yip JHK. Organometallics. 2010;29:2422–2429. [Google Scholar]; (b) Zhang J, Sarrrafpour S, Pawle RH, Thomas SW., III Chem Commun. 2011;47:3445–3447. doi: 10.1039/c0cc05770c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.