Abstract
The most abundant form of estrogen circulating in fetal plasma is sulfo-conjugated estrogen; for example, estradiol-3-sulfate (E2SO4) is more highly abundant than estradiol (E2). The present study investigated the ontogeny of the deconjugating (steroid sulfatase [STS]) and conjugating (estrogen sulfotransferase [STF]) enzymes in ovine fetal brain and tested the hypothesis that treatment with E2SO4 would alter the expression of one or both enzymes. Steroid sulfatase was more highly expressed than STF, and both changed as a function of gestational age. Estradiol-3-sulfate infused intracerebroventricularly (icv) significantly increased plasma adrenocorticotropic hormone (ACTH) and cortisol concentrations. Plasma E2 and E2SO4 were increased, and brain expression of estrogen receptor α was decreased. The proteins STS and STF were up- and downregulated, respectively. Pituitary proopiomelanocortin (POMC) and follicle-stimulating hormone (FSH) and hypothalamic corticotrophin-releasing hormone (CRH) messenger RNA (mRNA) was decreased. We conclude that E2SO4 has complex actions on the fetal brain, which might involve deconjugation by STS, but that the net result of direct E2SO4 icv infusion is more complex than can be accounted for by infusion of E2 alone.
Keywords: ACTH, cortisol, estrogen receptor, prostaglandin synthase, cyclooxygenase, FSH, LH, prolactin, steroid sulfatase, estrogen sulfotransferase
Introduction
In the late gestation fetal sheep, the development of the fetal brain governs fetal homeostasis and preparation for ex utero survival. In the sheep, the fetal hypothalamus-pituitary-adrenal (HPA) neuroendocrine axis initiates parturition, although this is somewhat controversial in the human beings and nonhuman primates. A longstanding view is that the placenta synthesizes and releases into the fetal blood signaling molecules that modify fetal central nervous system (CNS) development and/or function. One modulator of fetal CNS activity is estrogen, released by the placenta and circulating in relatively high plasma concentrations.
Estrogen is secreted in large quantities by the ovine placenta at the end of gestation.1 Infusion of estradiol (E2) into late gestation fetal sheep at rates that increase circulating concentrations within the range produced by placental secretion increases neuronal activity within fetal brain2 and increases fetal adrenocorticotropic hormone (ACTH) and cortisol secretion.3 At the same time, there is a maturation of estrogen receptor (ER) expression in the brain and pituitary. The late gestation ovine fetal brain and pituitary express both estrogen receptors α (ER-α) and β (ER-β).4 The expression of ER-α in medullary brain stem increases in the postnatal animal, and the expression of ER-β increases starting before birth.4 In pituitary, ER-α increases in late gestation, and the ratio of ER-α/ER-β increases as the fetus nears spontaneous parturition.4
Estrogen circulates in ovine fetal plasma as both unconjugated and sulfo-conjugated estrogen.5,6 Sulfo-conjugated estrogen is not thought to be directly biologically active because it does not directly bind to the ER.7,8 Nevertheless, the circulating concentrations of sulfo-conjugated estrogens in fetal plasma—especially the steroids sulfo-conjugated in the 3-position—are far higher than those of the unconjugated estrogens.9–11 We have previously reported ovine fetal plasma concentrations of estradiol-3-sulfate (E2SO4) between 1 and 5 ng/mL and of E2 of approximately 50 pg/mL,9 similar to previous measurements by Carnegie and Robertson for E2SO4 and E2.10 Concentrations of estrone-3-sulfate and estrone are similar.10 The fetal brain has a mechanism for uptake of the sulfo-conjugated estrogens.9 Sulfo-conjugated estrogen uptake by the fetal brain is substantial.9 Although the percentage uptake of conjugated estrogen is low compared to unconjugated estrogen, the marked concentration difference in plasma between conjugated and unconjugated forms dictates greater molar uptake of the sulfo-conjugated estrogen.9 In a previous study, we found that the uptake of estrone was 5 to 11 times as efficient as that of estrone-3-sulfate, but the 20-fold higher concentration of estrone-3-sulfate in fetal plasma predicted an overall greater uptake of estrone-3-sulfate into the fetal brain.9
As the sulfo-conjugated estrogen does not directly bind the ER, biological activity theoretically requires interconversion of conjugated and unconjugated forms at the site of uptake or action.12 The fetal brain expresses the enzymes responsible for the bidirectional interconversion of these steroids,13,14 providing a possible explanation for the biological activity of infused E2SO4.9 This study was designed to test the hypothesis that both steroid sulfatase (STS) and estrogen sulfotransferase (STF) are expressed in fetal brain regions important for HPA axis control, that the relative expression of STS is greater than that of STF, and that the expression of STS increases at the time that the fetal HPA axis is increasing in activity. The brain regions that we have specifically included are pituitary and hypothalamus, as well as regions with known influences on the hypothalamic control of ACTH secretion in adult animals (hippocampus, medullary brain stem, and cerebral cortex), plus cerebellum (because of the rich pattern of estrogen signaling in this brain region). We have also tested the hypothesis that STS and STF expression in the fetal brain are influenced by an increase in E2SO4 availability.
Materials and Methods
All of the experiments reported were approved by the University of Florida Institutional Animal Care and Use Committee and were performed in accordance with the Guiding Principles for Research Involving Animals and Humans.15
In study 1, we used 46 time-dated fetuses and neonates, none of which had been subjected to catheterization or experimentation. Fetal sheep of known gestational age (80-145 days, n = 3-5 per group), and lambs were euthanized with an overdose of sodium pentobarbitol. Spontaneous parturition in the sheep occurs between days 147 and 150.16 Brains were rapidly removed, dissected into distinct regions using defined parameters, and snap frozen in liquid nitrogen. The following brain tissues were collected: (1) medullary brain stem, (2) hippocampus, (3) frontal cortex, (4) cerebellum, (5) pituitary, and (6) hypothalamus. The medullary brain stem tissue was sectioned ~1 mm rostral to the obex and at the caudal medulla–rostral spinal cord border, which we identify as being caudal to the hypoglossal nerve (cranial nerve XII). Frontal cortex consisted of the most rostral 1 cm block of prefrontal cortex. Whole hippocampus was dissected bilaterally. Whole cerebellum was detached from the brain stem after sectioning the cerebellar peduncles. Hypothalamus was removed as a single block of tissue, bounded on the rostral edge by the rostral edge of the optic chiasm, on the caudal side by the caudal edge of the median eminence, and on the sides by the edges of the median eminence. Tissues were stored at −80°C until processed for mRNA or protein. Entire pituitaries and hypothalami were processed for mRNA extraction, while other areas were divided for simultaneous mRNA and protein analysis. Pituitary was not used in the present study. Gene expression studies for ER and prostaglandin synthase isoforms and for components of the fetal HPA axis have been previously performed on these tissues and previously reported.4,17,18
In study 2, 4 time-dated ewes each with chronically catheterized twin fetuses were used in this study (8 fetuses, 120-127 days gestation). One fetus served as the experimental fetus, while its twin served as an internal age-matched control. There were 2 experimental groups: E2SO4 infused intracerebroventricularly ([icv] 1 mg/d, n = 4) versus untreated control (n = 4). Twin fetuses were randomly assigned to the 2 groups at the time of surgery. All animals were housed in individual pens located in the Animal Resources Department at the University of Florida, and all of these experiments were approved by the University of Florida Institutional Animal Care and Use Committee. The rooms maintained controlled lighting and temperature and sheep were given food and water ad libitum.
Food was withheld from the pregnant ewes for 24 hours before surgery in study 2. Ewes were intubated and anesthetized with halothane (0.5%-2%) in oxygen before and during surgical preparation, as previously described.9 Surgery and arterial catheter placement for all fetuses was performed using aseptic technique as previously described, with lateral cerebral ventricle, femoral arterial, and venous catheters as well as amniotic fluid catheters.9,19 For placement of the catheter into the lateral cerebral ventricle, the scalp was retracted and a small catheter (outside diameter, 0.05 inch; inside diameter, 0.03 inch) attached to an osmotic minipump (size 2 mL2 Durect Corp, Cupertino, California) was inserted through a hole made in the skull. The catheter was held in place using VetBond (3M Corp, St. Paul, Minnesota). The exposed catheter and osmotic minipump were placed subcutaneously before closing the incision on the head. All minipumps in the treated fetuses were filled with E2SO4 (Sigma Aldrich, St. Louis, Missouri) in vehicle (water) and minipumps in the control fetuses were filled with vehicle only. These minipumps deliver a constant infusion of 5 μL/h; the concentration of E2SO4 in infusates was therefore 8.33 μg/μL. The position of the catheters and the function of the pumps was verified by visual inspection at the time of sacrifice and tissue collection. At the end of the surgery, antibiotics (750 mg ampicillin) were administered into the amniotic cavity via direct injection. Vascular catheters were exteriorized through the flank of the ewe using a trochar, where they were maintained in a cloth pocket. Ewes were given 1 mg/kg flunixin meglumine (Webster Veterinary, Sterling, Massachusetts) for analgesia and returned to their pens where they were monitored until they could stand on their own. Twice daily during a 5-day recovery period, ewes were treated with antibiotic (ampicillin, 750 mg intramuscularly [im]: Polyflex, Fort Dodge Laboratories, Fort Dodge, Iowa) and rectal temperatures were monitored for indication of postoperative infection.
In study 1, blood samples were not drawn from either ewe or fetus either before or at the time of sacrifice. Following the recovery period in study 2, fetal blood samples (3 mL) were drawn from the arterial catheter every other morning (between 0800 and 1000 hours) until day 131 to 134 of gestation for use in hormone assays. The actual number of blood samples varied from 1 to 5 pairs of samples. Samples were kept on ice until centrifuged at 3000g for 15 minutes at 4°C to separate red blood cells and plasma. Plasma was stored at −20°C until analysis. Blood gases were measured at the time of blood sampling in a separate 1 mL blood sample using an ABL 77 Radiometer (Radiometer America Inc, Cleveland, Ohio) blood gas analyzer. At the end of the blood sampling period, twin fetuses in study 2 (131-134 days) were euthanized with an overdose of sodium pentobarbital. These fetuses were euthanized 7, 7, 12, and 12 days after surgery and the start of the icv infusion of E2SO4 or vehicle. Brains were rapidly removed, dissected into distinct regions, and snap frozen in liquid nitrogen. The following tissues were collected: brain stem, hippocampus, frontal cortex, cerebellum, hypothalamus, and pituitary. Tissues were stored at −80°C until processed for mRNA or protein.
Plasma Hormone Assays
Assays for E2, E2SO4, cortisol, dehydroepiandrosterone sulfate (DHEAS), progesterone, ACTH, ACTH1-39, and proopiomelanocortin (POMC) were measured using enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA). Plasma E2 concentration was measured using a commercially available ELISA kit (Oxford Biomedical Research, Rochester Hills, MI, cat # EA70) after extraction with hexane/ethyl acetate (3:2 vol/vol). Cross-reactivity with 17β-estradiol, estriol, and estrone in this kit is 100%, 0.41%, and 0.10%, respectively, as reported by the manufacturer. Plasma E2SO4 concentration was measured using the same E2 ELISA (Oxford Biomedical Research, cat # EA70) but after deproteinization with ethanol, as previously reported.9 Plasma cortisol concentration was measured using the cortisol ELISA kit from Oxford Biomedical Research (cat # EA65) after deproteinization with ethanol, as described previously.20 Cross-reactivity with cortisone, 11-deoxycortisol, and corticosterone in this kit is 15.77%, 15%, and 4.81%, respectively, as reported by the manufacturer. Plasma DHEAS concentration was measured with the 125I-DHEA-SO4 Coat-A-Count assay from Diagnostic Products Corporation (Los Angeles, California; cat # TKDS5). Percentage of cross-reactivity with DHEA, and estrone-3-SO4 was 0.57 and 0.25. respectively, as reported by the manufacturer. Plasma progesterone concentration was measured with a 125I-Progesterone Coat-A-Count kit from Diagnostic Products Corporation (cat # TKPG5). Percentage cross-reactivity with 17α-hydroxyprogesterone, medroxyprogesterone, and pregnenolone was 3.4, 0.3, and 0.1, respectively, as reported by the manufacturer. ACTH1-39 was measured using a 2-site immunoradiometric assay kit purchased from DiaSorin (Stillwater, Minnesota, cat # 27130). This assay has been previously validated for use in fetal sheep plasma by Myers and Ducsay.21 Proopiomelanocortin was measured using an ELISA kit from Immunodiagnostic Systems Ltd (cat # AC-71F1), according to the manufacturer’s instructions. Cross-reactivity with POMC and Pro-ACTH is 100%, as reported by the manufacturer. However, this assay does not recognize ACTH1-39.
Tissue mRNA and Protein Measurements
For mRNA and protein isolation, tissues were individually pulverized using Bio-Pulverizer (Bio-Spec Products, Bartlesville, Oklahoma), a trigger-style mortar-and-pestle device. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, California), according to the manufacturer’s instructions. A high-speed Polytron homogenizer (Tekmar, Janke and Kunkel, West Germany) was used for homogenization. RNA pellets were resuspended in 200 µL RNAsecure (Ambion, Austin, Texas) preheated to 60°C. The pellets were incubated in a 60°C water bath for 10 minutes in order to inactivate RNAses. RNA concentration in each sample was quantified by spectrophotometry. RNA samples were converted into 4 µg stable complementary DNA (cDNA) by reverse transcription using a High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, California), according to the manufacturer’s instructions. Reverse transcription reactions were performed in RNase/DNase free microcentrifuge tubes on a thermocycler (Biometra, Ltd, Kent, Maine) using a thermal profile that ran for 10 minutes at 25°C, followed by 120 minutes at 37°C. The resulting cDNA samples were stored at −20°C until real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed. With the exception of STF and STS, the specific probes and primers in the real-time RT-PCR assays have been described elsewhere.19 We measured mRNA abundance for hypothalamic corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) and pituitary POMC, prohormone convertase 1 (PC-1), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin (PRL). In pituitary plus the 5 brain regions studied in study 1, we also measured mRNA abundance for prostaglandin endoperoxide synthase 1 and 2 (PGHS-1 and -2) and ERα and ERβ because of known changes in expression patterns with alterations in estrogen treatment or ER blockade.19,22,23 Steroid sulfatase primers were CACCAGCAACATGGACATCTTT forward, GTCTTGCGGCAGAGGAGATC reverse, and CCACGGTGGCCAAACTCGCG probe. Estrogen sulfotransferase primers were designed from the bovine STF mRNA sequence (accession number NM_177488). The forward and reverse primers were TTGGATTACCCAGTTGCTTTCA and AAAAGGATGGTTTGGAAGAACTCA, respectively. This assay was run using Sybr Green (Applied Biosystems). A portion of the ovine Steroid Sulfatase(oSTS) mRNA was sequenced and the resultant sequence was used for design of primers and Taqman probe for oSTS. The partial sequence of oSTS is reported in Table 1 .
Table 1.
Partial Sequence of ovine Steroid Sulfatase(oSTS)
| 1 | CCTTCCGGCT | GCTGGTGTTC | GTGCCCCTGC | AGCTCATCGC | CGTGGCCCTG |
| 51 | CTGACCCTGG | CCATGCTCAA | GTGGCTGCGG | CTCTGCCAGG | CGCCCCCCTG |
| 101 | NGTCTTCCTC | TTCCTCTTCC | TCCTGGCCGC | CCTACTCCTC | GGCCTCCTGC |
| 151 | TCGGCTTCCT | GCACTATTTC | CGGCCGCTCA | ACTGCTTCCT | GATGCGGAAC |
| 201 | CGTGACGTCA | CGCAGCAGCC | CATGTCCTAC | GACAACCTCA | CGCAGAGGCT |
| 251 | CACGGCGGAC | GCCGCCCGCT | TCTTACGTCG | GAATGCTGAG | ACCCCTTTCC |
| 301 | TGCTTGTCCT | GTCTTACCTG | CATGTACACA | CGGCTCNCTT | CTCTTCGAAG |
| 351 | GACTTTGCCG | GCAAAAGTCA | ACACGGCTCC | TACGGGGATG | CCGCTGAGGA |
| 401 | GATGGATTGG | AGCGTCGGGC | AGATCTTGGA | TGTCCTCGAN | GAGCTGAAAC |
| 451 | TGGCNAATAA | CACCCTGGTG | TACTTCTCCT | CNGACCAAGG | AGCTCACGTA |
| 501 | GAGGAGGTGA | CNGTCAAAGG | GGAAGTTCAA | GGAGGCAGTA | ACGGCATCTA |
| 551 | CAAAGGGGGA | AAAGCCAACA | ACTGGGAAGG | AGGCATCCGG | GTTCCCGGCA |
| 601 | TCTTCCGGTG | GCCGGGAGTG | ATCCGGGCTG | GCTTGGAGAT | TGATGAGCCC |
| 651 | ACCAGCAACA | TGGACATCTT | TCCCACGGTG | GCCAAACTCG | CGGGATCTCC |
| 701 | TCTGCCGCAA | GACAGAGTCA | TCGACGGCCG | GGACCTGATG | CCCTTGCTGC |
| 751 | AGGGGCGGAC | CCAGCGATCG | GAAGCACGA |
In order to estimate the ratio of expression of STS/STF mRNA, we synthesized long oligonucleotides (Gene Link, Inc, Hawthorne, New York) that could be used as standards in the STS Taqman and STF Sybr Green assays. Complementary DNA from each brain region at 145 days' gestation was then analyzed relative to the standard curves and transcript abundance (quantified as transcript number/mass cDNA) was calculated for STS and STF for each fetus. From the transcript number, STS/STF expression ratios were calculated for each region in each fetus. The sequences of the STS and STF standards were CCACCAGCAACATGGACATCTTTCCCACGGTGGCCAAACTCGCGGGATCTCCTCTGCCGCAAGAC and TTGGATTACCCAGTTGCTTTCACAGGATCATCTGGACAGTGTACCACTCCACGATGAGTTCTTCCAAACCATCCTTTTC, respectively. Standard curves were run in a range of 102 to 1010 transcripts/reaction. The lower limit of detection for STF was 104 transcripts/reaction and for STS was 102 transcripts/reaction. Because the abundance of each transcript was quantified on the basis of the number of transcripts per unit mass cDNA, the difference between Taqman and Sybr methodology did not introduce bias into the calculated ratios.
Both STS and STF protein abundances were measured using Western blot analysis, as previously described.13,14 These assays have been previously validated for specificity, using polyclonal antisera that were custom designed from known amino acid sequences.13,14 For each sample, the data were normalized to the abundance of β-actin. In study 1, the complete ontogeny of STS or STF abundance for each tissue was accomplished using two 18-well Criterion gels (BioRad Co, Hercules, California). In study 2, comparison of treated and untreated fetuses was performed using a single gel for each tissue.
Statistical Analysis
Ontogeny data (study 1) were analyzed by 1-way analysis of variance (ANOVA) followed by pairwise comparison using the Student-Newman Keuls multiple range test.24 The analysis of the protein data from study 1 included an additional factor in the ANOVA to control the variance introduced by the individual films, as previously described.25 Messenger RNA data from study 2 were analyzed by 2-way ANOVA, with brain region and E2SO4 treatment as the 2 main factors. Protein data from study 2 were logarithmically transformed prior to analysis to correct for heteroscedascity.24 Post hoc analysis was performed using the Student-Newman Keuls test.24 For specific pituitary and hypothalamic genes, the data were analyzed by paired t test.26 Fetal plasma hormone concentrations were also analyzed by 2-way ANOVA using values from plasma samples for each fetus (we collected 2-5 plasma samples per fetus). Plasma hormone concentrations are reported as mean values. SPSS 17.0 (SPSS Corp, Chicago, Illinois) was used for data analysis. A significance level of P < .05 was used to reject the null hypothesis. Values are reported as mean ± SEM.
Results
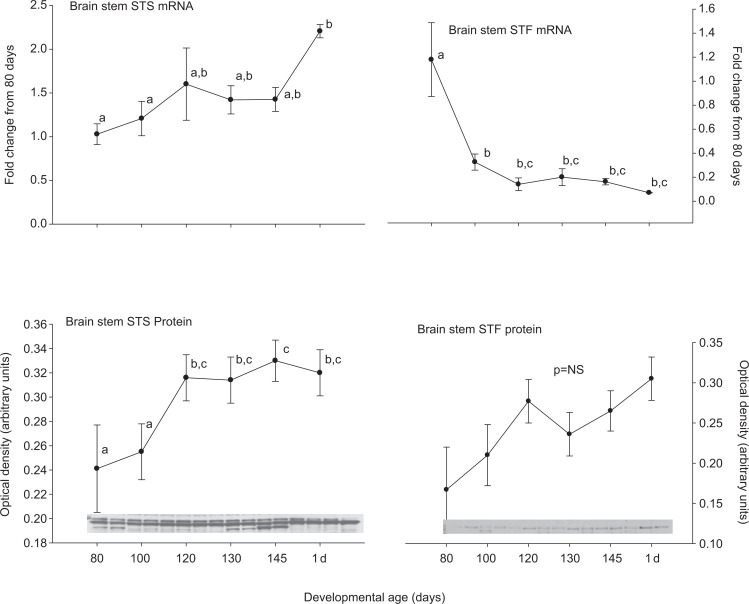
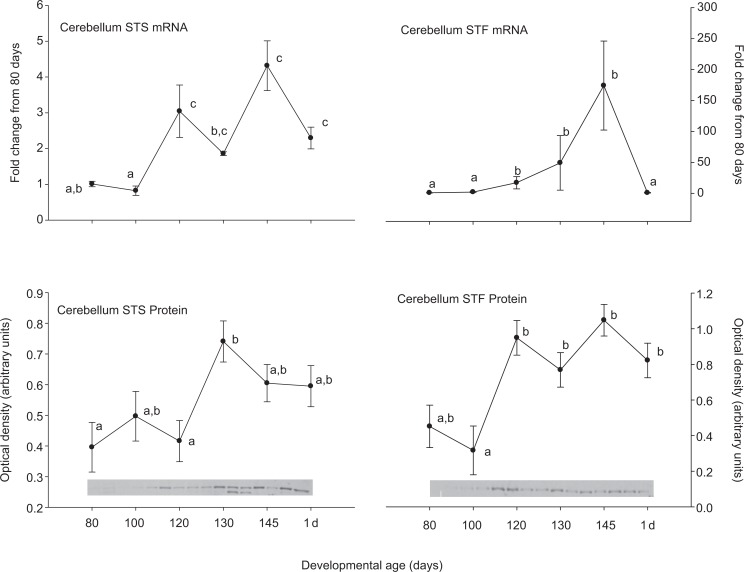
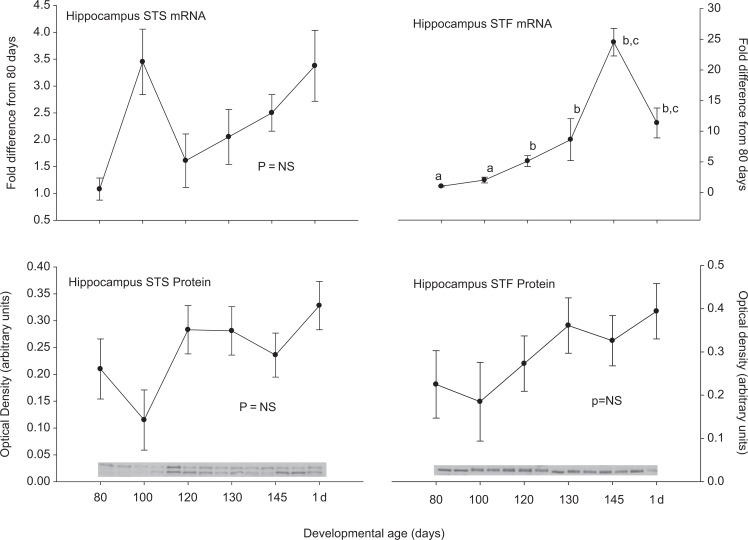
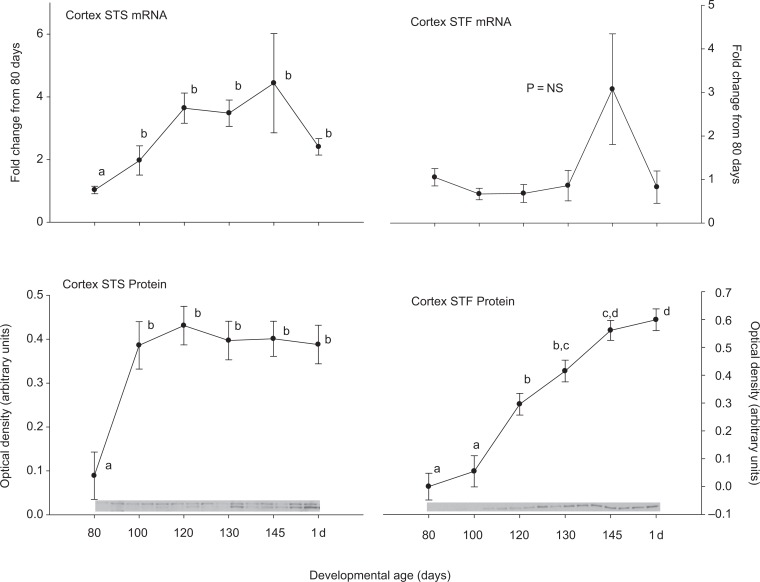
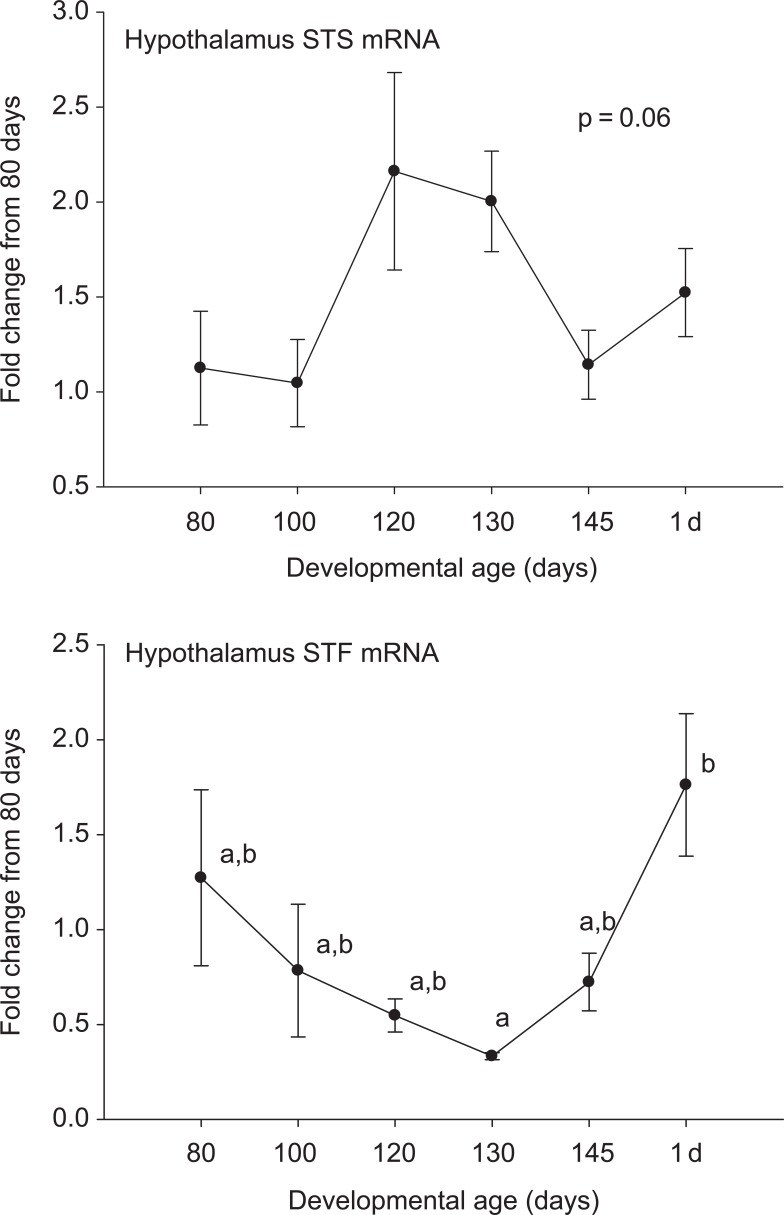
Steroid sulfatase and STF were expressed in all of the tested brain regions at both the mRNA and protein levels. In medullary brain stem, there was an increase in STS and a decrease in STF expression at the mRNA level after 80 days gestation (Figure 1). These patterns were not both repeated at the protein level. Steroid sulfatase protein abundance did increase similar to the STS mRNA, but the STF protein abundance did not change significantly. In the cerebellum (Figure 2), hippocampus (Figure 3), and cerebral cortex (Figure 4), there were increases in the expression of both STS and STF, although the timing of the increases was not the same in the different brain regions. Estrogen sulfotransferase mRNA peaked in all the 3 brain regions at 145 days. While the STS mRNA patterns were more complex (eg, there was a statistically significant nadir in expression at 120 days in hippocampus followed by a progressive rise in expression), there was a statistically significant decrease in expression at 130 days in cerebellum followed by a progressive rise. Estrogen sulfotransferase protein in hippocampus and STS protein in cerebellum did not change significantly but in cortex increased in the latter half of gestation. The pattern of increase did not, however, correspond to that in the respective mRNA. In hypothalamus, we did not have measurements at the protein level, but the mRNA expression pattern for STF decreased to a nadir at 130 days (Figure 5). Steroid sulfatase mRNA appeared to peak at 120 days in hypothalamus, but the apparent change was not statistically significant (Figure 5).
Figure 1.
Ontogeny of steroid sulfatase (STS, left) and estrogen sulfotransferase (STF, right) mRNA (top panels) and protein (bottom panels) in medullary brain stem. Data are represented as mean values ± SEM with overlay representing Western blot data. Different superscripts associated with symbols denote statistically significant differences. Values for STS mRNA (F = 6.525; 6,18 df; P = .001), STF mRNA (F = 8.875; 6,20 df; P < .001), STS protein (F = 2.821; 6,14 df; P = .05) changed significantly as a function of developmental age, while STF protein (F = 2.20; 6,13 df; P = .110) did not. mRNA indicates messenger RNA; SEM, standard error of the mean.
Figure 2.
Ontogeny of steroid sulfatase (STS, left) and estrogen sulfotransferase (STF, right) mRNA (top panels) and protein (bottom panels) in cerebellum. Data are represented as mean values ± SEM with overlay representing Western blot data. Different superscripts associated with symbols denote statistically significant differences. Values for STS mRNA (F = 7.76; 6,18 df; P < .001), STF mRNA (F = 3.777; 6,20 df; P = .011), STS protein (F = 4.043; 6,14 df; P = .015), STF protein (F = 5.402; 6,13 df; P = .005) changed significantly as a function of developmental age. mRNA indicates messenger RNA; SEM, standard error of the mean.
Figure 3.
Ontogeny of steroid sulfatase (STS, left) and estrogen sulfotransferase (STF, right) mRNA (top panels) and protein (bottom panels) in hippocampus. Data are represented as mean values ± SEM with overlay representing Western blot data. Different superscripts associated with symbols denote statistically significant differences. Values for STF mRNA (F = 17.998; 6,20 df; P < .001) changed significantly as a function of developmental age, while STS mRNA (F = 2.026; 6,18 df; P = .115), STS protein (F = 1.877; 6,14 df; P=0.156), STF protein (F = 1.034; 6,13 df; P = .447) did not. mRNA indicates messenger RNA; SEM, standard error of the mean.
Figure 4.
Ontogeny of steroid sulfatase (STS, left) and estrogen sulfotransferase (STF, right) mRNA (top panels) and protein (bottom panels) in cerebral cortex. Data are represented as mean values ± SEM with overlay representing Western blot data. Different superscripts associated with symbols denote statistically significant differences. Values for STS mRNA (F = 2.985; 6,18 df; P = .033), STS protein (F = 5.922; 6,14 df; P = .003), STF protein (F = 33.756; 6,13 df; P < .001) changed significantly as a function of developmental age, while STF mRNA (F = 1.665; 6,20 df; P = .182) did not. mRNA indicates messenger RNA; SEM, standard error of the mean.
Figure 5.
Ontogeny of steroid sulfatase (STS, top panel) and estrogen sulfotransferase (STF, bottom panel) mRNA in hypothalamus. Data are represented as mean values ± SEM. Different superscripts associated with symbols denote statistically significant differences. Values for STS mRNA (F = 8.340; 6,18 df; P < .001) and STF mRNA (F = 3.369, 6,20 df; P = .018) changed significantly as a function of developmental age. mRNA indicates messenger RNA; SEM, standard error of the mean.
In all of the studied brain regions, STS mRNA was much more abundant than STF mRNA. Measured at 145 days' gestation, minimum estimates (calculated using the lower limit of detection of STF transcripts) of the ratio of STS/STF mRNA abundance were 68 ± 4, 36 ± 16, 105 ± 38, 11 ± 4, and 3 3± 8 in brain stem, cerebellum, hippocampus, cortex, and hypothalamus, respectively (n = 5/region).
Delivery of E2SO4 directly into the brain altered gene expression in a manner consistent with increased estrogen action at the pituitary and altered HPA axis activity. Plasma hormone concentration responses to the E2SO4 infusion were consistent with our previous data (Table 2). Intracerebroventricular infusion of E2SO4 for 5 to 7 days increased plasma concentrations of E2SO4 (413 ± 55 vs 1139 ± 99 pg/mL in control and E2SO4-infused fetuses, respectively), E2 (29 ± 3 vs 229 ± 32 pg/mL in control and infused fetuses), ACTH (21 ± 1 and 27 ± 2 pg/mL in control and infused fetuses) and cortisol (5.4 ± 3.7 vs 20.3 ± 7.6 ng/mL in control and infused fetuses). The ratio of E2SO4 to E2 was decreased in the E2SO4 infused fetuses (15.43 ± 2.32 vs 5.88 ± 0.58 in control and infused fetuses, respectively). Interestingly, plasma POMC concentrations did not change significantly (33 ± 7 vs 41.6 ± 6.0 nmol/L in control and infused fetuses, respectively).
Table 2.
Plasma Hormone Concentrations in Study 2
| Hormone | Vehicle Treated | E2SO4 Treated | Statistics |
|---|---|---|---|
| ACTH (pg/mL) | 21 ± 1 | 27 ± 2 | F = 13.1; P = .001 |
| POMC (nmol/L) | 33 ± 7 | 42 ± 6 | F = 0.79; P = .38 |
| Cortisol (ng/mL) | 5.4 ± 3.7 | 20.3 ± 7.6 | F = 5.88; P = .02 |
| DHEAS (pg/mL) | 12 ± 5 | 17 ± 2 | F = .818; P = .38 |
| Progesterone (ng/mL) | 5.8 ± 0.5 | 4.8 ± 0.3 | F = 2.7; P = .11 |
| Estradiol (pg/mL) | 29 ± 3 | 229 ± 32 | F = 37.9; P < .001 |
| Estradiol-3-sulfate (pg/mL) | 413 ± 55 | 1139 ± 99 | F = 40.9; P < .001 |
| ACTH/POMC ratio | 1.2 ± 0.4 | 1.2 ± 0.4 | F = 0.03; P = .87 |
Abbreviations: ACTH, adrenocorticotropic hormone; POMC, proopiomelanocortin; DHEAS, dehydroepiandrosterone sulfate; E2SO4, estradiol-3-sulfate.
Surprisingly, analysis of mRNA abundances of pituitary hormone transcripts in the fetal pituitaries revealed a reduction in POMC mRNA along with a reduction in FSH mRNA abundance (Table 3). There was no statistically significant effect of the E2SO4 on the mRNA abundance of PC-1, suggesting that there is not a change in POMC processing efficiency. In the hypothalamus, the mRNA abundance of CRH was significantly decreased (treated fetus was 56% ± 7% as abundant as control fetus), while the mRNA abundance of AVP was not (treated fetus was 65% ± 37% as abundant as control fetus).
Table 3.
Gene Expression in Pituitaries and Hypothalami of Twin Fetuses Treated With icv E2SO4 Relative to Fetuses Treated With Vehicle a
| Gene | E2SO4 Treated (% Control Fetus) |
|---|---|
| CRH (hypothalamus) | 56 ± 7 b |
| AVP (hypothalamus) | 65 ± 37 |
| POMC (pituitary) | 42 ± 9 b |
| PC-1 (pituitary) | 146 ± 40 |
| LH (pituitary) | 96 ± 40 |
| FSH (pituitary) | 8 ± 3 b |
| PRL (pituitary) | 111 ± 20 |
Abbreviations: AVP, arginine vasopressin; CRH, corticotrophin-releasing hormone; icv, intracerebroventricularly; mRNA, messenger RNA; POMC, proopiomelanocortin; PC-1, prohormone convertase 1; LH, luteinizing hormone; FSH, follicle-stimulating hormone; PRL, prolactin; E2SO4, estradiol-3-sulfate.
a Data are reported as the percentage level of control fetus mRNA expression levels.
b Statistical significance P < .05.
As presented in Table 4 , E2SO4 infusion stimulated a pattern of gene expression that was only partially similar to that in previous experiments in which we tested gene expression in response to E2.22,23 Estradiol sulfate significantly decreased the mRNA abundance for ER-α (F = 32.06; 1,3 df; P = .01) but did not change ER-β(F = 3.46; 1,3 df; P = .16), when analyzed in 5 brain regions plus pituitary (2-way ANOVA for repeated measures). This overall significant effect of E2SO4 was mostly accounted for by decreases in ER-α in fetal pituitary and hippocampus. Estradiol sulfate infusion did not significantly alter the expression of PGHS-1 (F = 4.57; 1,3 df; P = .122) or PGHS-2 (F = 4.273; 1,3 df; P = .131; Table 4); this is in contrast to the effect of E2, which we reported stimulated PGHS-2 mRNA in medullary brain stem and cerebellum,22 or blockade of ERs, which decreased PGHS-2 mRNA in pituitary, hypothalamus, hippocampus, brain stem, and hippocampus.19
Table 4.
Estrogen Receptors-α, -β, PGHS-1, and PGHS-2 mRNA Abundance in Pituitary and Brain Regions of Twin Fetuses Treated With icv E2SO4 or Vehicle a
| Tissue | ER-α (% Control) | ER-β (% Control) | PGHS-1 (% Control) | PGHS-2 (% Control) |
|---|---|---|---|---|
| Pituitary | 41 ± 8 b | 128 ± 50 | 64 ± 19 | 136 ± 57 |
| Hypothalamus | 72 ± 14 | 59 ± 16 | 118 ± 35 | 262 ± 159 |
| Medullary Brain stem | 184 ± 90 | 86 ± 17 | 93 ± 33 | 102 ± 28 |
| Hippocampus | 59 ± 2 c | 73 ± 19 | 79 ± 6 | 85 ± 7 |
| Cerebellum | 126 ± 56 | 76 ± 15 | 71 ± 25 | 83 ± 21 |
| Cerebral cortex | 72 ± 14 | 88 ± 25 | 120 ± 30 | 62 ± 14 |
Abbreviations: E2SO4, estradiol-3-sulfate; PGHS, prostaglandin endoperoxide synthase; mRNA, messenger RNA; icv, intracerebroventricularly;
a Data are reported as the percentage level of control fetus mRNA expression levels.
b Statistical significance P < .05.
c Statistical significance p = 0.001
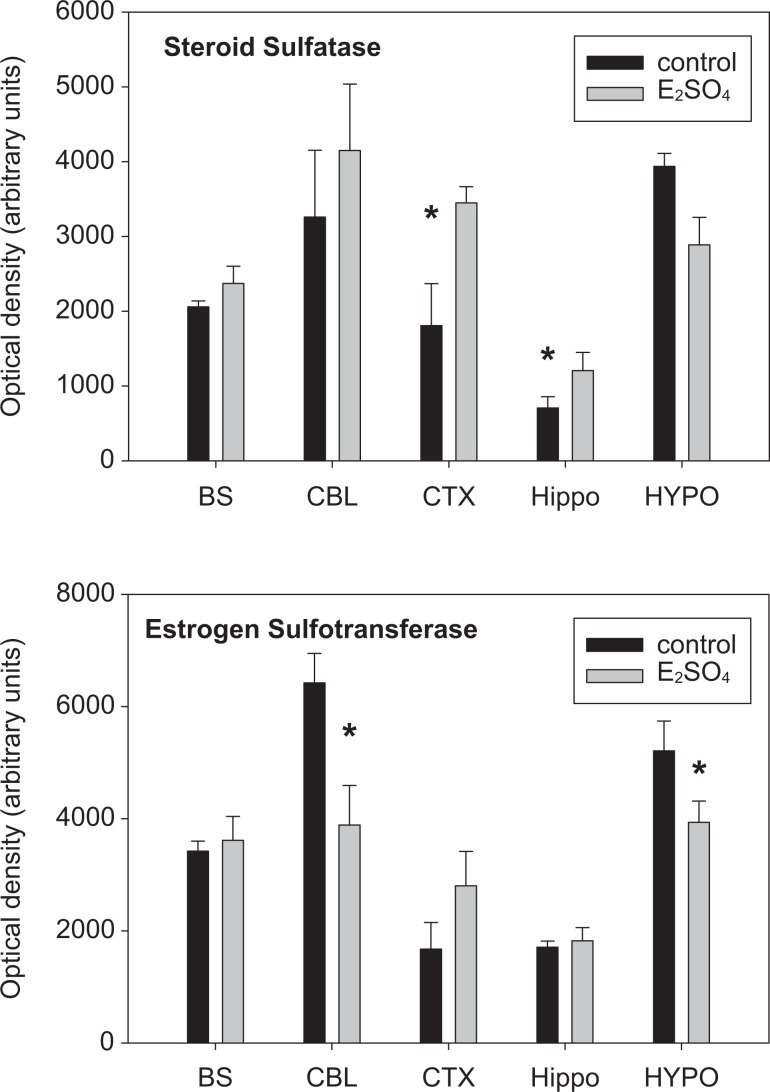
The abundance of STS and STF at the protein level were significantly altered in fetuses infused with E2SO4. The abundance of STS was increased significantly in the fetal cerebral cortex and hippocampus and the abundance of STF was significantly decreased in the fetal cerebellum and hypothalamus (Figure 6).
Figure 6.
Protein abundance for steroid sulfatase (top panel) and estrogen sulfotransferase (bottom panel) in control and estradiol-3-sulfate (E2SO4)-treated fetuses. Data are reported in medullary brain stem (BS), cerebellum (CBL), cerebral cortex (CTX), hippocampus (Hippo), and hypothalamus (HYPO). Values are reported as mean values ± SEM. * represents statistically significant difference between control and E2SO4-treated fetuses. Overall, E2SO4 significantly altered the abundance for STS (F = 4.966; 1,39 df; P = .03) but not for STF (F = .253; 1,39 df; P = .619).
Discussion
The fetal brain has, throughout the latter half of gestation, the ability to deconjugate and conjugate steroids. The influence of steroids on the development of the fetus, the preparation for birth, and survival after birth is now well known and provides the basis for successful antenatal treatment of fetuses that are at risk for premature birth. While the changes in fetal adrenal secretion of cortisol before birth are most dramatic and most useful for designing the treatment of fetuses at risk of premature birth, the fetus develops in an estrogen-rich environment. The maturation of fetoplacental endocrinology in several species (including both ruminants and human beings) features an increase in the secretion of estrogen by the placenta at the end of gestation.
Regulation of the estrogenization of the fetal brain to estrogen can theoretically both protect the fetal brain from excess estrogen and, at appropriate times, allow stimulation of specific neuroendocrine functions. For example, estrogen actions within the developing fetal brain can alter postnatal reproductive behavior.27 On the other hand, blockade of ERs specifically in the fetal brain blocks the prenatal rise in fetal ACTH secretion that is a hallmark of late gestation development in the sheep fetus.19 The blockade of ERs also results in an alteration in fetal neurochemistry that is consistent with the factors known to be important for the correct timing of parturition.19
The results of the present study demonstrate the presence of sulfoconjugating and deconjugating enzymes in the fetal brain throughout the latter half of gestation, indicating that the balance of conjugation and deconjugation shifts as the fetus nears spontaneous parturition. The general pattern of expression for both STS and estrogen STF is a tendency to increase toward the normal time of spontaneous parturition. This suggests an increased ability for enzymatic interconversion of unconjugated and sulfo-conjugated estrogens within the brain. Strikingly, there is a dramatic increase in the expression of sulfotransferase in hippocampus and cerebellum around the time of spontaneous parturition. Whether this is mechanistically related to the timing of parturition or to stress responsiveness at the time of birth is unknown and unexplored in the present investigation. Recent evidence indicates that transcription of the SULT1E1 gene (encoding STF) is increased by glucocorticoid.28 The increasing pattern of STF expression in various brain regions might be at least partially the result of increasing glucocorticoid action secondary to increasing plasma glucocorticoid concentrations as the fetus nears the time of spontaneous parturition. In hippocampus, for example, the abundance of both glucocorticoid and mineralocorticoid receptors increase in late gestation, as does the expression of serum and glucocorticoid-regulated kinase 1 (sgk-1), a molecular response to glucocorticoid action.18
The STS/STF mRNA ratio, favoring deconjugation in the fetal brain, is consistent with the decrease in E2SO4-to-E2 ratio in plasma in the fetuses infused icv with E2SO4 (Table 2). Plasma concentrations of both E2SO4 and E2 increased in the E2SO4-infused fetuses. We assume that some of the E2SO4-infused icv escapes into the fetal bloodstream, possibly through the arachnoid villi. The increase in plasma E2 could be that E2 is the product of the deconjugation reaction. If so, and assuming that the brain is an active site of deconjugation, a substantial fraction of the infused E2SO4 might enter the fetal blood as E2.
Our theoretical construct has been that conjugation and deconjugation of estrogens is a mechanism by which estrogen action in the brain is modified in the face of constant estrogen plasma concentrations or a mechanism by which estrogen action can be amplified or blunted in the face of increasing plasma estrogen concentrations at the end of gestation. All of the brain regions that we have included in this study express both ER-α and ER-β.4 In the hypothalamus, hippocampus, and brain stem, for example, there are increasing patterns of expression of ER-α in the latter half of gestation, suggesting an increased capacity for estrogen action in these tissues as the fetus matures and readies for birth. At the same time, the balance of sulfoconjugation and deconjugation can modify the sensitivity of the target tissue to the circulating estrogen. Nevertheless, it is not possible to calculate the true balance of deconjugation and conjugation based solely on mRNA ratios or protein abundance in Western blots. The Michaelis-Menten constant (Km) of each enzyme is in the low μmol/L range, as tested with estrone and estrone sulfate,29,30 and the actual rate of reaction for each enzyme could vary as a function of the actual plasma and cellular concentrations of substrate and as a function of posttranslational processing.31
The action of E2SO4 appears to overlap but not identical to the action of E2. Similar to the action of E2, E2SO4 reduces ER-α and FSH in pituitary. This is likely the result of the large increase in plasma E2 concentration and therefore a direct effect of E2. On the other hand, there was no stimulation of PGHS-2 gene expression in the fetal brain or pituitary. The increased PGHS-2 gene expression in response to E2 infusion is associated with increased neuronal activity throughout the fetal brain2 and (Powers and Wood, unpublished observations). It is possible that E2SO4 acts as a neurosteroid, interacting with GABAa and/or NMDA receptors, analogous to the action of pregnenolone sulfate.32–34 If so, the molecular response in the various brain regions is likely to be the result of estrogen action (after deconjugation) and neurosteroid activation or deactivation of pathways by the sulfo-conjugated steroid. In the case of PGHS-2, the summary action of E2SO4 is not likely to be activation of the same pathways that are activated by E2 alone.
The reduction in gene expression of POMC in the fetal pituitary and the gene expression of CRH in the hypothalamus were unexpected. The plasma hormone concentrations were consistent with an activation of the fetal HPA axis, with small but statistically significant increases in fetal plasma ACTH concentration and larger and quite variable but statistically significant increases in fetal plasma cortisol concentration. While this could theoretically be accounted for by an increased efficiency of POMC processing, we did not find any difference in the mRNA abundance of PC-1 in the fetal pituitary (Table 3), and there was no difference in the ACTH/POMC ratio in fetal plasma (Table 2). It is possible that the POMC and ACTH originate in neurointermediate lobe. Our method for the extraction of mRNA does not differentiate between anterior lobe corticotropes and neurointermediate lobe. If the E2SO4 stimulated a large increase in POMC and ACTH secretion from neurointermediate lobe, the resulting increase in fetal plasma cortisol might have reduced corticotrope POMC expression and hypothalamic CRH expression by negative feedback. Another possibility is that the POMC in fetal plasma originated in the neuroendocrine cells of the fetal lung. We have previously demonstrated that the lung synthesizes and secretes POMC, although we have not found evidence of processing of the pulmonary POMC to ACTH.35–38 Yet another possibility for interaction with the fetal HPA axis is the possible effect of the E2 or E2SO4 in plasma on the measured variables. For example, E2 increases adrenal sensitivity to ACTH in adult female rats while reducing CRH mRNA in the paraventricular nuclei.39 The increase in fetal plasma ACTH in this and in previous studies involving treatment of fetuses with E2 2,3,22 or E2SO4 9 suggests that the neuroendocrine response to E2 in the fetal sheep differs from that in the rat; it is possible that the increase in fetal plasma cortisol and the reduction in POMC and CRH mRNA are all downstream consequences of an E2 stimulation of adrenal sensitivity to ACTH.
We conclude that the fetal brain contains the enzymatic capacity to interconvert sulfo-conjugated and unconjugated estrogens, and that the expression of the conjugating and deconjugating enzymes are altered as a function of fetal gestational age and proximity to the timing of birth. We also conclude that, while E2SO4 stimulates the fetal HPA axis, the response to E2SO4 is not identical to that of E2. The results suggest the possibility that the sulfo-conjugated steroid has other neuromodulatory activities, perhaps acting as a neurosteroid. The results also suggest that enzyme abundance and activity are important modulators of estrogen action in the fetal CNS.
Acknowledgments
We thank Drs Maureen Keller-Wood and Elaine Sumners for their advice and input in the design and execution of these experiments.
Footnotes
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
This work was supported by R01-HD057561 from NIH to CEW, by trainee support of RDC by T35-HL007930, and by support of JW by the University of Florida University Scholars Program.
Reference
- 1. Yu HK, Cabalum T, Jansen CA, Buster JE, Nathanielsz PW. Androstenedione, testosterone, and estradiol concentrations in fetal and maternal plasma in late pregnancy in the sheep. Endocrinology. 1983;113(6):2216–2220 [DOI] [PubMed] [Google Scholar]
- 2. Purinton SC, Wood CE. Oestrogen augments the fetal ovine hypothalamus-pituitary-adrenal axis in response to hypotension. J Physiol. 2002;544(pt 3):919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saoud CJ, Wood CE. Modulation of ovine fetal adrenocorticotropin secretion by androstenedione and 17beta-estradiol. Am J Physiol. 1997;272(4 pt 2):R1128–R1134 [DOI] [PubMed] [Google Scholar]
- 4. Schaub CE, Gersting JA, Keller-Wood M, Wood CE. Development of ER-alpha and ER-beta expression in the developing ovine brain and pituitary. Gene Expr Patterns. 2008;8(6):457–463 [DOI] [PubMed] [Google Scholar]
- 5. Mitchell BF, Lye SJ, Lukash L, Challis JRG. Androstenedione metabolism in the late gestation sheep fetus. Endocrinology. 1986;118(1):63–68 [DOI] [PubMed] [Google Scholar]
- 6. Pierrepoint CG, Anderson AB, Harvey G, Turnbull AC, Griffiths K. The conversion in vitro of C19-steroids to oestrogen sulphates by the sheep placenta. J Endocrinol. 1971;50(3):537–538 [DOI] [PubMed] [Google Scholar]
- 7. Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870 [DOI] [PubMed] [Google Scholar]
- 8. Payne AH, Lawrence CC, Foster DL, Jaffe RB. Intranuclear binding of 17b-estradiol and estrone in female ovine pituitaries following incubation with estrone sulfate. J Biol Chem. 1973;248:1598–1602 [PubMed] [Google Scholar]
- 9. Wood CE, Gridley KE, Keller-Wood M. Biological activity of 17beta-estradiol-3-sulfate in ovine fetal plasma and uptake in fetal brain. Endocrinology. 2003;144(2):599–604 [DOI] [PubMed] [Google Scholar]
- 10. Carnegie JA, Robertson HA. Conjugated and unconjugated estrogens in fetal and maternal fluids of the pregnant ewe: a possible role for estrone sulfate during early pregnancy. Biol Reprod. 1978;19(1):202–211 [DOI] [PubMed] [Google Scholar]
- 11. Tsang CPW. Changes in plasma levels of estrone sulfate and estrone in the pregnant ewe around parturition. Steroids. 1974;23(6):855–868 [DOI] [PubMed] [Google Scholar]
- 12. Hobkirk R. Steroid sulfotransferases and steroid sulfate sulfatases: characteristics and biological roles. Can J Biochem Cell Biol. 1985;63(11):1127–44 [DOI] [PubMed] [Google Scholar]
- 13. Purinton SC, Newman H, Castro MI, Wood CE. Ontogeny of estrogen sulfatase activity in ovine fetal hypothalamus, hippocampus, and brain stem. Am J Physiol. 1999;276(6):R1647–R1652 [DOI] [PubMed] [Google Scholar]
- 14. Purinton SC, Wood CE. Ovine fetal estrogen sulfotransferase in brain regions important for hypothalamus-pituitary-adrenal axis control. Neuroendocrinology. 2000;71(4):237–242 [DOI] [PubMed] [Google Scholar]
- 15. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283(2):R281–R283 [DOI] [PubMed] [Google Scholar]
- 16. Wood CE. Control of parturition in ruminants. J Reprod Fertil Suppl. 1999;54:115–126 [PubMed] [Google Scholar]
- 17. Gersting JA, Schaub CE, Wood CE. Development of prostaglandin endoperoxide synthase expression in the ovine fetal central nervous system and pituitary. Gene Expr Patterns. 2009;9(8):603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller-Wood M, Powers MJ, Gersting JA, Ali N, Wood CE. Genomic analysis of neuroendocrine development of fetal brain-pituitary-adrenal axis in late gestation. Physiol Genomics. 2006;24(3):218–224 [DOI] [PubMed] [Google Scholar]
- 19. Schaub CE, Keller-Wood M, Wood CE. Blockade of estrogen receptors decreases CNS and pituitary prostaglandin synthase expression in fetal sheep. Neuroendocrinology. 2008;87(2):121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wood CE, Cudd TA, Kane C, Engelke K. Fetal ACTH and blood pressure responses to thromboxane mimetic U46619. Am J Physiol. 1993;265(4 pt 2):R858–R862 [DOI] [PubMed] [Google Scholar]
- 21. Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1178–R1184 [DOI] [PubMed] [Google Scholar]
- 22. Wood CE, Giroux D. Central nervous system prostaglandin endoperoxide synthase-1 and -2 responses to oestradiol and cerebral hypoperfusion in late-gestation fetal sheep. J Physiol. 2003;549(pt 2):573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wood CE. Cerebral hypoperfusion increases estrogen receptor abundance in the ovine fetal brain and pituitary. Neuroendocrinology. 2008;87(4):216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winer BJ. Statistical Principles in Experimental Design.2nd ed. 1971;New York, NY: McGraw-Hill, [Google Scholar]
- 25. Saoud CJ, Wood CE. Developmental changes and molecular weight of immunoreactive glucocorticoid receptor protein in the ovine fetal hypothalamus and pituitary. Biochem Biophys Res Commun. 1996;229(3):916–921 [DOI] [PubMed] [Google Scholar]
- 26. Zar JH. Biostatistical Analysis.2nd ed. 1984;Englewood Cliffs, NJ: Prentice-Hall, [Google Scholar]
- 27. Roselli CE, Stormshak F. Prenatal programming of sexual partner preference: the ram model. J Neuroendocrinol. 2009;21(4):359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gong H, Jarzynka MJ, Cole TJ, et al. Glucocorticoids antagonize estrogens by glucocorticoid receptor-mediated activation of estrogen sulfotransferase. Cancer Res. 2008;68(18):7386–7393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prost O, Adessi GL. Estrone and dehydroepiandrosterone sulfatase activities in normal and pathological human endometrium biopsies. J Clin Endocrinol Metab. 1983l;56(4):653–661 [DOI] [PubMed] [Google Scholar]
- 30. Suzuki T, Hirato K, Yanaihara T, et al. Purification and properties of steroid sulfatase from human placenta. Endocrinol Jpn. 1992;39(1):93–101 [DOI] [PubMed] [Google Scholar]
- 31. Frese MA, Schulz S, Dierks T. Arylsulfatase G, a novel lysosomal sulfatase. J Biol Chem. 200825;283(17):11388–11395 [DOI] [PubMed] [Google Scholar]
- 32. Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning of the story. Int Rev Neurobiol. 2001;46:1–32 [DOI] [PubMed] [Google Scholar]
- 33. Petrovic M, Sedlacek M, Cais O, Horak M, Chodounska H, Vyklicky L., Jr Pregnenolone sulfate modulation of N-methyl-D-aspartate receptors is phosphorylation dependent. Neuroscience. 2009;160(3):616–628 [DOI] [PubMed] [Google Scholar]
- 34. Schumacher M, Liere P, Akwa Y, et al. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int. 2008;52(4-5):522–540 [DOI] [PubMed] [Google Scholar]
- 35. Cudd TA, Castro MI, Wood CE. Content, in vivo release, and bioactivity of fetal pulmonary immunoreactive adrenocorticotropin. Am J Physiol. 1993;265(4 pt 1):E667–E672 [DOI] [PubMed] [Google Scholar]
- 36. Cudd TA, Wood CE. Secretion and clearance of immunoreactive ACTH by fetal lung. Am J Physiol. 1995;268(5 pt 1):E845–E848 [DOI] [PubMed] [Google Scholar]
- 37. Wood CE, Barkoe D, The A, et al. Fetal pulmonary immunoreactive adrenocorticotropin: molecular weight and cellular localization. Regul Pept. 1998;73(3):191–196 [DOI] [PubMed] [Google Scholar]
- 38. Ali NS, Keller-Wood M, Wood CE. Ontogenetic changes in the extra-pituitary expression of pro-opiomelanocortin in the developing ovine fetus. Peptides. 2005;26(2):301–306 [DOI] [PubMed] [Google Scholar]
- 39. Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab. 2007;292(4):E1173–E1182 [DOI] [PubMed] [Google Scholar]