Abstract
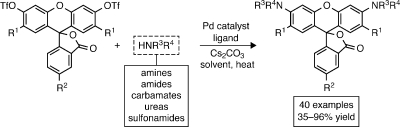
A unified, convenient, and efficient strategy for the preparation of rhodamines and N,N′-diacylated rhodamines has been developed. Fluorescein ditriflates were found to undergo palladium-catalyzed C–N cross-coupling with amines, amides, carbamates, and other nitrogen nucleophiles to provide direct access to known and novel rhodamine derivatives, including fluorescent dyes, quenchers, and latent fluorophores.
Rhodamine dyes and their fluorogenic derivatives enjoy widespread use as laser dyes, tracer agents, and biological probes.(1) This broad utility stems from the ability to modify the optical properties of the dye by appending different substituents on the rhodamine nitrogens. N-Alkyl rhodamines are valuable fluorescent dyes where the absorption and fluorescence emission can be tuned by altering the number and type of alkyl groups. Attachment of aryl functionalities yields strongly absorbing, nonfluorescent dyes, which can serve as quenchers for Förster resonance energy transfer (FRET) experiments.(2) Acylation of the rhodamine nitrogens locks the molecule in the nonfluorescent lactone form; such compounds serve as useful latent fluorescent compounds,(1) including fluorogenic enzyme substrates(3) and photoactivatable “caged” fluorophores.(4)
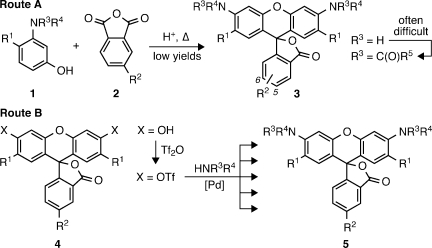
Despite the utility and flexibility of rhodamines, the established synthetic approach to this dye scaffold is archaic and difficult. Rhodamines are typically prepared through the acid-catalyzed condensation of an aminophenol (1) with a phthalic anhydride (2) to yield 3 (Scheme 1, Route A). Unfortunately, only a limited number of 3-aminophenols are compatible with this century-old route. Use of phthalic anhydrides bearing a substituent (R2 in 3) for bioconjugation yields products as intractable mixtures of 5- and 6-substituted regioisomers. Consequently, commercially available functionalized rhodamines are expensive and often sold as regioisomeric mixtures.(1c) Furthermore, derivatization of the already elusive rhodamines into fluorogenic derivatives via N-acylation is often inefficient, due primarily to the low nucleophilicity of the rhodamine nitrogens.3b,4c
Scheme 1. Strategies for Rhodamine Synthesis.
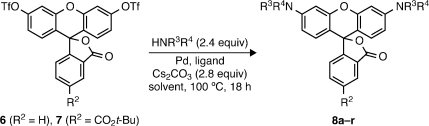
Given the difficulties with existing syntheses, we sought an alternative route wherein the C(aryl)–N bonds of rhodamines are formed at a late stage.(5) We envisoned using the Buchwald–Hartwig cross-coupling of nitrogen nucleophiles with fluorescein ditriflates (Scheme 1, Route B). Pd-catalyzed cross-coupling has emerged as a powerful method for C–N bond formation,(6) yet these amination reactions have seen little use in the preparation of xanthene dyes. Lippard and co-workers reported a single example of coupling pyrrolidine to a dibromofluoran.(7) Peng and Yang prepared a rhodol library via cross-coupling of a fluorescein monotriflate with various amines.(8) We recently used this strategy to construct a precursor for a photoactivatable “caged” rhodamine.(4c) These examples inspired us to further explore the C–N cross-coupling (Route B) as a general strategy for the direct conversion of fluorescein ditriflates to not only N-alkyl rhodamines but also N-aryl (5, HNR3R4 = aniline) and N-acyl derivatives (HNR3R4 = amide, carbamate, etc.). Most importantly, isomerically pure 5- and 6-substituted fluorescein dyes are readily synthesized on a multigram scale,(9) making this approach a convenient and divergent synthetic route to regioisomerically pure rhodamine dyes.
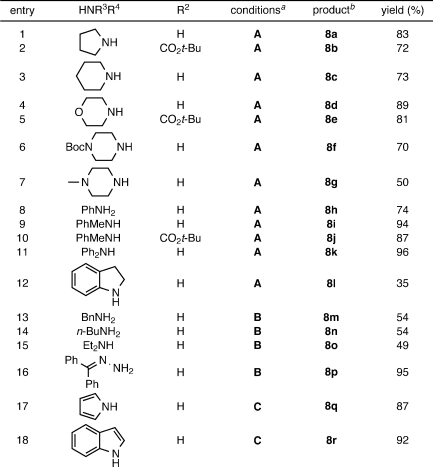
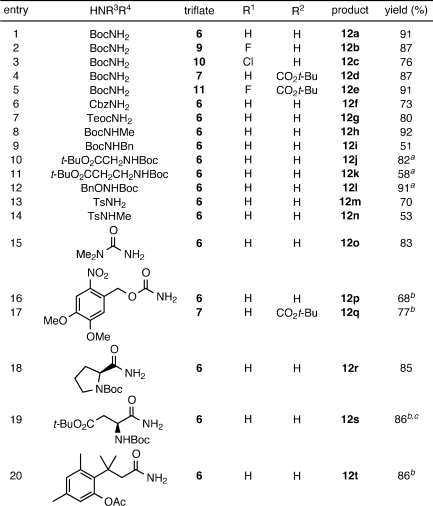
We initially investigated the ability of the cross-coupling to generate N-alkyl and N-aryl rhodamine dyes. Following preparation of several fluorescein ditriflates by straightforward reaction of known fluoresceins with Tf2O,(10) bis-amination of the parent analog 6 or the 5-carboxyfluorescein-derived 7 was explored with a variety of amines (Table 1). In all cases, the primary competitive side reaction was triflate hydrolysis to give fluorescein and/or rhodols. The undesired detriflation—most notable at low catalyst levels—was mitigated in a manner similar to that of Yang(8) by increasing loadings to 20 mol % Pd and 30 mol % ligand (10% and 15% per triflate, respectively). Well-established Buchwald–Hartwig conditions6,11 using Pd(OAc)2, BINAP, and Cs2CO3 in toluene at 100 °C (A) were effective in coupling 6 and 7 with cyclic amines (entries 1–7) and anilines (entries 8–12) to provide tetralkyl rhodamines 8a–g and aryl rhodamines(2)8h–l in good to excellent yields. Surprisingly, these conditions resulted in poor yields and low conversions for a number of secondary acyclic and primary aliphatic amines. The use of Pd2dba3 with the active biaryl ligand XPhos(12) in dioxane (B) expanded the scope to include these types of amines (entries 13–15) and provided convenient access to known dyes such as rhodamine B (8o). The cross-coupling was also tolerant of atypical, electronically diverse amination substrates, including benzophenone hydrazone(13) (entry 16) and, under Xantphos14,15 conditions (C), nitrogen heteroaromatics (entries 17–18).(16)
Table 1. Amination of Fluorescein Ditriflates.
A: 20 mol % Pd(OAc)2, 30 mol % BINAP, toluene. B: 10 mol % Pd2dba3, 30 mol % XPhos, dioxane. C: 10 mol % Pd2dba3, 30 mol % Xantphos, dioxane.
See Supporting Information for optical spectroscopy of rhodamine products.
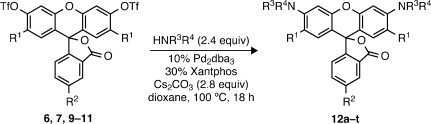
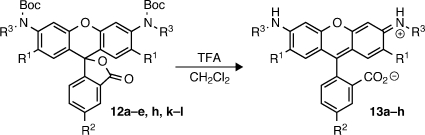
Carbamates, amides, and other N-acyl building blocks were of particular interest as substrates in the C–N cross-coupling of fluorescein ditriflates. In addition to providing efficient access to fluorogenic rhodamine derivatives, such compounds can also serve as surrogates for amines (e.g., ammonia) that are unsuitable for the direct amination process due to volatility, poor reactivity, or difficult product purification.(17) Moreover, using protecting-group-based carbamates (e.g., BocNH2) provides convenient access to “lactone-locked” forms of the dyes that are easier to purify and manipulate than the free rhodamines. To that end, a thorough investigation of the ditriflate amidation was undertaken (Table 2). In concurrence with the substantial precedent for palladium-catalyzed amidations, Pd2dba3/Xantphos conditions, with Cs2CO3 as base and dioxane as solvent at elevated temperature (80–100 °C), were found to be effective for nearly all substrates tested.6,18 Comparatively high catalyst loadings were once again necessary to minimize triflate hydrolysis. As illustrated in entries 1–7, Boc-, Cbz-, and Teoc-masked ammonia equivalents underwent smooth cross-coupling with 6, 7, and 9–11 to afford dicarbamates 12a–g in excellent yields (73–91%). These included rhodamine 110 (Rh110) derivatives with halide substituents on the xanthene core (R1 = F, Cl) and the crucial carboxyl handle on the bottom ring (R2 = CO2t-Bu). Gratifyingly, more hindered secondary carbamates18,19 were also effectively arylated under these conditions to provide several Boc-protected rhodamines bearing N-alkyl groups (entries 8–11), including those with ester functionalities (12j–k).
Table 2. Cross-Coupling of Fluorescein Ditriflates with Carbamates and Other Nitrogen Nucleophiles.
Reaction performed at 80 °C for 18 h.
Reaction performed at 80 °C for 2–3 h.
Product resulted exclusively from coupling at primary amide.
The reactivity of several other types of related nitrogen nucleophiles was also examined. The protected hydroxylamine tert-butyl benzyloxycarbamate was found to couple with 6 in excellent yield (entry 12, 91%). This result is significant as few reports exist detailing the C–N cross-coupling of hydroxylamines,(20) and 12l represents the first example involving a triflate. Primary and secondary sulfonamides were also viable substrates (entries 13–14), as was a urea(3b) (entry 15). Hence, the robustness of this reaction allows for the rapid preparation of lesser-known, yet potentially useful rhodamine derivatives (e.g., N,N′-disulfonyl rhodamines).(21)
As mentioned earlier, more elaborate N,N′-diacyl rhodamines possessing photolytically or enzymatically labile acyl moieties are themselves valuable as latent fluorophores. Rather than employ unreliable rhodamine acylations, we hoped to achieve the direct preparation of these fluorogenic molecules via the same ditriflate amidation strategy. We found the appropriately functionalized nucleophiles were well tolerated when coupled with fluorescein ditriflates (entries 16–20). A carbamate containing the photolabile ortho-nitroveratryloxycarbonyl (NVOC) cage was reacted with 6 and 7 to conveniently afford NVOC2-Rh110 (12p, entry 16, 68%) and the regioisomerically pure 5-tert-butoxycarbonyl analog 12q (entry 17, 77%).(4c) Primary amides of amino acids were also cross-coupled to 6 in excellent yields (entries 18–19). Rhodamine-linked amino acids like 12s have seen significant use as fluorogenic substrates for proteases.(3a) Finally, a rhodamine 110 substrate bearing the esterase-labile trimethyl lock(3b) moiety (12t) was easily prepared in high yield through coupling of the trimethyl lock amide with 6 (entry 20).
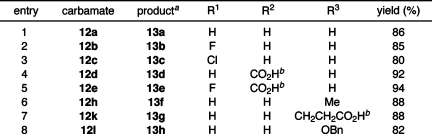
To further illustrate the ease of preparing rhodamine dyes via this strategy, the N,N′-di-Boc coupling products were deprotected (Table 3). Standard conditions (TFA/CH2Cl2, room temperature) cleanly removed the Boc groups and cleaved pendant tert-butyl esters, providing the free, deacylated rhodamines in excellent to nearly quantitative yields (80–94%). Several rhodamine 110 analogs were prepared in this expedient manner (entries 1–5), including the extremely useful—and expensive(22)—5-carboxy-Rh110 (13d, entry 4) and the 2′,7′-difluororhodamines 13b and 13e (entries 2 and 5) recently reported by Hell and co-workers.(23)N-Alkyl rhodamines (entries 6–7) were similarly prepared in a straightforward fashion. Entry 8 is notable because it represents the first preparation of an N-alkoxy rhodamine; exploration of the utility of this novel chemotype is ongoing.
Table 3. Deprotection of Boc-Protected Rhodamines.
See Supporting Information for optical spectroscopy of rhodamine products.
tert-Butyl esters also cleaved during Boc deprotection.
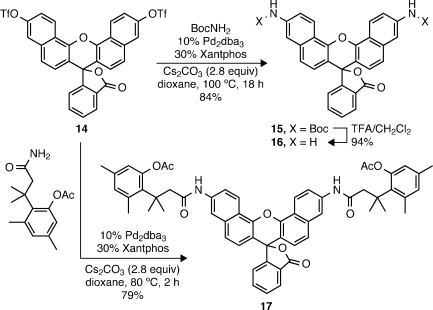
Finally, we explored the utility of the cross-coupling strategy for other dye scaffolds. As shown in Scheme 2, coupling of naphthofluorescein ditriflate (14) with tert-butyl carbamate followed by deprotection with TFA provided naphthorhodamine 16 in excellent yield (81%, two steps).(24) The chemistry proved flexible enough to allow the preparation of the novel naphthorhodamine derivative 17 bearing the esterase-labile trimethyl lock. Thus, the facile coupling chemistry could prove useful for generating a variety of novel nitrogenous dyes from phenolic precursors, as also evidenced by recent work on the coumarin system by Ting and co-workers.(25)
Scheme 2. Cross-Coupling Route to Naphthorhodamines.
In summary, we have developed a general, efficient, and unified strategy for the synthesis of rhodamines and N,N′-diacylated rhodamines. Fluorescein ditriflates, which are easily prepared from readily available, regioisomerically pure fluoresceins, were found to undergo palladium-catalyzed C–N cross-coupling with amines, amides, carbamates, and related nucleophiles. Amination with alkyl and aryl amines allowed for convenient synthesis of fluorophores and FRET quencher dyes. Where the synthesis of rhodamine dyes by direct amination was impractical, protecting-group-based carbamates were effectively employed. Appropriately functionalized carbamates and amides were also coupled with ditriflates to efficiently assemble rhodamine-based latent fluorophores, including fluorogenic enzyme substrates and photoactivatable dyes. This process constitutes a divergent strategy for the rapid synthesis of many types of rhodamines and will enable the fine-tuning of optical and chemical properties for specific applications.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute. We thank S. M. Sternson, L. M. Wysocki, and P. H. Lee (Janelia Farm Research Campus) for contributive discussions.
Supporting Information Available
Experimental procedures and spectral data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- a Haugland R. P.; Spence M. T. Z.; Johnson I. D.; Basey A.. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 10th ed.; Molecular Probes: Eugene, OR, 2005. [Google Scholar]; b Lavis L. D.; Raines R. T. ACS Chem. Biol. 2008, 3, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Beija M.; Afonso C. A. M.; Martinho J. M. G. Chem. Soc. Rev. 2009, 38, 2410. [DOI] [PubMed] [Google Scholar]
- a Ioffe I. S.; Zal′manovich M. Z. Zh. Obshch. Khim. 1962, 32, 1480. [Google Scholar]; b Haugland R. P.; Singer V. L.; Yue S. L. U.S. Patent 6,399,392 B1, June 4, 2002.
- a Hug H.; Los M.; Hirt W.; Debatin K.-M. Biochemistry 1999, 38, 13906. [DOI] [PubMed] [Google Scholar]; b Lavis L. D.; Chao T.-Y.; Raines R. T. ACS Chem. Biol. 2006, 1, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Mitchison T. J.; Sawin K. E.; Theriot J. A.; Gee K.; Mallavarapu A. Methods Enzymol. 1998, 291, 63. [DOI] [PubMed] [Google Scholar]; b Puliti D.; Warther D.; Orange C.; Specht A.; Goeldner M. Bioorg. Med. Chem. 2011, 19, 1023. [DOI] [PubMed] [Google Scholar]; c Wysocki L. W.; Grimm J. B.; Tkachuk A. N.; Brown T. A.; Betzig E.; Lavis L. D.. Angew. Chem., Int. Ed.2011, 50, 11206. [DOI] [PMC free article] [PubMed]
- High-temperature nucleophilic substitution of dihalofluorans with amines has been used previously but is limited in scope. See ref (2) and: Song X.; Johnson A.; Foley J. J. Am. Chem. Soc. 2008, 130, 17652. [DOI] [PubMed] [Google Scholar]
- Reviews: ; a Hartwig J. F. In Modern Arene Chemistry; Astruc D., Ed.; Wiley-VCH: Weinheim, 2002; p 107. [Google Scholar]; b Muci A. R.; Buchwald S. L. Top. Curr. Chem. 2002, 219, 131. [Google Scholar]; c Jiang L.; Buchwald S. L. In Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; de Meijere A., Diederich F., Eds.; Wiley-VCH: Weinheim, 2004; p 699. [Google Scholar]
- Woodroofe C. C.; Lim M. H.; Bu W.; Lippard S. J. Tetrahedron 2005, 61, 3097. [Google Scholar]
- Peng T.; Yang D. Org. Lett. 2010, 12, 496. [DOI] [PubMed] [Google Scholar]
- a Coons A. H.; Kaplan M. H. J. Exp. Med. 1950, 91, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sun W.-C.; Gee K. R.; Klaubert D. H.; Haugland R. P. J. Org. Chem. 1997, 62, 6469. [Google Scholar]; c Rossi F. M.; Kao J. P. Bioconjugate Chem. 1997, 8, 495. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for experimental details.
- Åhman J.; Buchwald S. L. Tetrahedron Lett. 1997, 38, 6363. [Google Scholar]
- a Huang X.; Anderson K. W.; Zim D.; Jiang L.; Klapars A.; Buchwald S. L. J. Am. Chem. Soc. 2003, 125, 6653. [DOI] [PubMed] [Google Scholar]; b Surry D. S.; Buchwald S. L. Angew. Chem., Int. Ed. 2008, 47, 6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wagaw S.; Yang B. H.; Buchwald S. L. J. Am. Chem. Soc. 1999, 121, 10251. [Google Scholar]; b Chen A.; Gee K.; Kang H.-C. U.S. Patent Appl. US 2010/0291547 A1, Nov. 18, 2010.
- Old D. W.; Harris M. C.; Buchwald S. L. Org. Lett. 2000, 2, 1403. [DOI] [PubMed] [Google Scholar]
- Xantphos was found to be optimal for heteroaromatics; other ligands (e.g., BINAP) were effective, albeit with diminished yield.
- Interestingly, bis(heteroaryl)xanthenes 8q and 8r are colorless and nonfluorescent in solution and in the solid state, suggesting they (like N-acyl derivatives) exist primarily in the lactone form.
- Bhagwanth S.; Waterson A. G.; Adjabeng G. M.; Hornberger K. R. J. Org. Chem. 2009, 74, 4634 and references cited therein. [DOI] [PubMed] [Google Scholar]
- Yin J.; Buchwald S. L. Org. Lett. 2000, 2, 1101. [DOI] [PubMed] [Google Scholar]
- Hicks J. D.; Hyde A. M.; Cuezva A. M.; Buchwald S. L. J. Am. Chem. Soc. 2009, 131, 16720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jones K. L.; Porzelle A.; Hall A.; Woodrow M. D.; Tomkinson N. C. O. Org. Lett. 2008, 10, 797. [DOI] [PubMed] [Google Scholar]; b Porzelle A.; Woodrow M. D.; Tomkinson N. C. O. Org. Lett. 2009, 11, 233. [DOI] [PubMed] [Google Scholar]
- Shibata A.; Furukawa K.; Abe H.; Tsuneda S.; Ito Y. Bioorg. Med. Chem. Lett. 2008, 18, 2246. [DOI] [PubMed] [Google Scholar]
- Price for single regioisomer (5-carboxy-Rh110): $59 000/g (AAT Bioquest).
- Mitronova G. Y.; Belov V. N.; Bossi M. L.; Wurm C. A.; Meyer L.; Medda R.; Moneron G.; Bretschneider S.; Eggeling C.; Jakobs S.; Hell S. W. Chem.—Eur. J. 2010, 16, 4477. [DOI] [PubMed] [Google Scholar]
- a Lee L. G.; Berry G. M.; Chen C.-H. Cytometry 1989, 10, 151. [DOI] [PubMed] [Google Scholar]; b Furukawa K.; Abe H.; Wang J.; Uda M.; Koshino H.; Tsuneda S.; Ito Y. Org. Biomol. Chem. 2009, 7, 671. [DOI] [PubMed] [Google Scholar]
- Jin X.; Uttamapinant C.; Ting A. Y. ChemBioChem 2011, 12, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.