Abstract
Background/Aim
Mutations in MAPT cause frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17). Patients with the MAPT R406W mutation were reported to show phenotypic heterogeneity in different ethnic backgrounds. We here report the clinical and genetic characteristics of Japanese families with the R406W mutation.
Methods
We examined the clinical and neuroimaging features of 6 patients from three families with the R406W mutation. We determined the genotypes of intragenic MAPT single-nucleotide polymorphisms (SNPs) and the flanking microsatellite markers to search for a common founder.
Results
The initial symptom was memory loss with the average age at onset being 54 years. Anterograde amnesia with episodic memory impairment was the predominant phenotype. Behavioral and personality changes or parkinsonism is not a prominent feature. A brain MRI study revealed marked atrophy of the medial temporal lobe. Genetic analysis of SNPs and microsatellite markers revealed that the affected members of the three families share common genotypes.
Conclusion
The findings of the affected members in this study, which corroborate previously reported findings of European families, suggest that the R406W mutation may represent a phenotype of predominant anterograde amnesia in FTLD-17. Our genetic data suggest that a founder effect may account for some families with the R406W mutation.
Key Words: Amnestic syndrome, Familial dementia, Founder effect, FTDP-17, MAPT mutation
Introduction
Mutations in the microtubule-associated protein tau gene (MAPT) cause an inherited neurodegenerative disease, i.e. frontotemporal dementia (FTD) with parkinsonism linked to chromosome 17 (FTDP-17) [1]. More than 100 families with FTLD-17 have been described worldwide. It has been demonstrated that phenotypic presentations in patients with FTDP-17 vary considerably [2]. The phenotype of FTDP-17 can be generally divided into two subtypes: FTD- and parkinsonism-predominant phenotypes [3]. Memory impairment occurs less frequently as the primary presenting feature, and only in a few cases of FTDP-17, the phenotype of predominant memory disturbance is similar to that in Alzheimer's disease (AD) [4,5].
The R406W mutation in exon 13 of MAPT was previously described in different ethnic backgrounds [6,7,8,9,10,11,12]. The patients with the R406W mutation showed a relatively late onset age with an average of 56 years. In the families from Western countries, the patients were reported to exhibit a phenotype resembling AD with early memory impairment [8,9,10,12]. In a Dutch family, FTD-like symptoms such as personality changes in addition to initial memory impairment were reported [6]. Moreover, in a previous study, a Japanese patient with the R406W mutation with early disease onset (47 years) and rapidly progressive FTD-like psychiatric symptoms was reported [13]. These reports suggest that phenotypic variation may also be present in the families with the R406W mutation.
To further clarify the clinical characteristics of patients with the R406W mutation, we here report the clinical and neuroimaging features of three unrelated Japanese families with the R406W mutation. We also performed genetic analysis to determine the possibility of a common founder in these families.
Methods
Patients
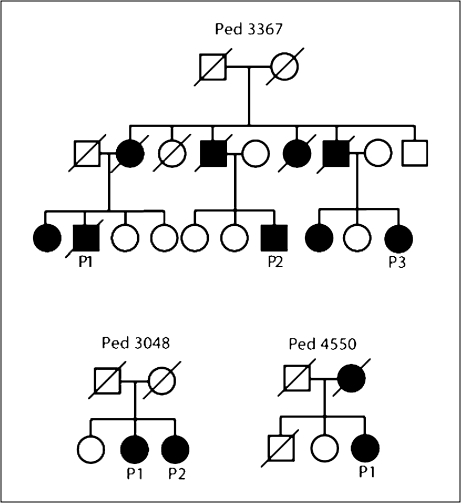
Mutational analysis of two pedigrees with the R406W mutation, Ped 3367 and Ped 3048, were previously described [11,14]. We found a new family (P4550) with the R406W mutation. In this family, the proband's mother died in her 60s with the clinical diagnosis of AD. The family trees are shown in figure 1. Although these families all reside in the Niigata Prefecture, Japan, family records revealed no shared surname and no geographical connections; thus, they are considered to be apparently unrelated. Six patients from three pedigrees were neurologically examined by board-certified neurologists. Six patients were examined by brain MRI and 3 by single photon emission computed tomography (SPECT). CSF samples were obtained from 2 patients; tau and phosphorylated tau (p181 tau) were quantified using a commercially available enzyme immunoassay kit (Innogenetics, Belgium), as previously described [15]. Written informed consent was obtained from all the patients or their caregivers. This study was approved by the Institutional Review Board of the Niigata University School of Medicine.
Fig. 1.
Pedigrees of families with the MAPTR406W mutation. Circle = Female; square = male; slash through the symbol = deceased individual; black symbol = affected individual.
Molecular Genetic Analysis
Genomic DNA was extracted from peripheral blood leukocytes by a standard procedure. Mutational analysis was performed by sequencing both strands of all PCR-amplified coding exons and flanking intronic sequences of MAPT, APP, PSEN1, PSEN2, and PRGN, as previously reported [11]. The APOE genotype was determined by restriction enzyme digestion of PCR products using Hha1.
To determine whether the three families with the R406W mutation share a common founder, we determined the genotypes of six single-nucleotide polymorphisms (SNPs) in MAPT and the six flanking microsatellite markers on chromosome 17q21 in the affected individuals. Information on SNP genotypes can be retrieved from www.ncbi.nlm.nih.gov/projects/SNP or http://snp.ims.u-tokyo.ac.jp/. The microsatellite markers spanning ∼1.3 Mb containing the MAPT region include D17S930, D17S1861, D17S1804, D17S920, D17S693, and D17S1859. The SNP genotypes were determined by direct sequencing of the PCR products amplified using appropriate primer pairs. Allele sizes of the microsatellite markers were determined by PCR amplification using fluorescently labeled primers. All the primer sequences are available on request.
Results
Clinical Characteristics
The clinical characteristics of the 6 patients with the R406W mutation are summarized in table 1. The age at onset of the patients was 54 ± 7 years (mean ± SD; range: 46–68 years). These patients commonly showed insidious onset of memory disturbance such as repetition of questions and difficulties in remembering appointments and names. Anterograde amnesia including a decline in episodic memory was the primarily presenting feature in all patients, resulting in impairment in ability to work and perform daily activities. The Mini-Mental State Examination (MMSE) score at a mean disease duration of 10 years was 17 ± 6 (range: 9–25). Behavioral and personality changes were not noted by these patients or their caregivers in the early course of the disease. Mild personality changes and disinhibition, such as restlessness and garrulousness, were observed in 3 patients with a long duration of the disease (Ped 3367-P3; Ped 3048-P2, and Ped 4550-P1). Neurological examination of these patients revealed no motor signs of parkinsonism. The clinical diagnosis of early-onset AD was suspected in these families prior to genetic investigation.
Table 1.
Clinical characteristics of the patients with the MAPT R406W mutation
| Ped 3367-P1 | Ped 3367-P2 | Ped 3367-P3 | Ped 3048-P1 | Ped 3048-P2 | Ped4550-P1 | |
|---|---|---|---|---|---|---|
| Age at onset, years | 51 | 47 | 50 | 56 | 54 | 68 |
| Age at examination, years | 56 | 48 | 67 | 73 | 67 | 77 |
| Duration of disease, years | 5 | 1 | 17 | 17 | 13 | 9 |
| MMSE score | 25 | 22 | 9 | 14 | 17 | 16 |
| Dementia stage | CDR1 | CDR 0.5 | CDR 2 | CDR 2 | FAST 5 | CDR 2 |
| Initial symptom | memory loss | memory loss | memory loss | memory loss | memory loss | memory loss |
| Memory impairment | +++ | +++ | +++ | +++ | +++ | +++ |
| Personality changes | − | − | + | − | − | + |
| Disinhibition | − | − | + | − | + | + |
| Parkinsonism | − | − | − | − | − | − |
| CSF tau level, pg/ml | 282 | 116 | n/a | n/a | n/a | n/a |
| CSF p181 tau level, pg/ml | 80 | 74 | n/a | n/a | n/a | n/a |
| APOE typing | 3∗4 | 3∗4 | 2∗3 | 3∗3 | 3∗3 | 2∗3 |
CDR = Clinical dementia rating scale; FAST = functional assessment stage; n/a = data not available; grading of severity of clinical features: – = absent, + = mild, ++ = moderate, +++ = severe.
Detailed neuropsychological tests were performed on 1 patient (Ped 3367-P1) at the age of 56 years, who was alert and cooperative on examination. His speech output was not disturbed, and there were no features of echolalia, verbal stereotype, or perseverations. The MMSE score was 25/30. Assessment using the Wechsler Adult Intelligence Scale III yielded a verbal IQ of 97, a performance IQ of 90, and a full IQ of 93. In the Wechsler Memory Scale-Revised test, the patient showed low index scores in the delayed recall subtest (score: <50) and general memory subtest (score: 65), whereas his index score in the attention/concentration subtest (score: 98) was normal. His frontal assessment battery score [16] was 16/18. The clinical assessment of attention consisting of the visual cancellation task, memory updating test, paced auditory serial addition test, position Stroop test, and continuous performance test [17] yielded normal results.
Molecular Genetic Analysis
Mutational analysis of APP, PSEN1, PSEN2, MAPT, and PGRN revealed that 6 patients from three pedigrees carry a R406W mutation in MAPT. To examine whether these families share a common founder haplotype, we determined the genotypes of intragenic MAPT SNPs and the flanking microsatellite markers. All the affected individuals from the three pedigrees with the R406W mutation shared common genotypes of intragenic MAPT SNPs (A-C-C-C-A-delT) and the flanking microsatellite markers (105-105-238-100-95-183 at D17S930-D17S1861-D17S1804-D17S920-D17S693-D17S1859; table 2). The estimated frequency of the disease-associated haplotype constructed by MAPT SNPs and the microsatellite markers (105-105-238-A-C-C-C-A-T-delT-100-95-183) in control subjects from the same population was 3.3% (n = 60). All the subjects were homozygous for the H1 haplotype, as previously reported [18].
Table 2.
Shared genotypes of MAPT SNPs (in bold) and microsatellite markers in three R406W families
| Microsatellite marker/SNP | Distance from MAPTa/location in MAPT | Ped3367 P1 | Ped 3367 P2 | Ped 3367 P3 | Ped 3048 P1 | Ped 4550 P1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D17S930 | 1,147 | 105 | 105 | 105 | 105 | 103 | 105 | 105 | 105 | 105 | 105 |
| D17S1861 | 1,063 | 107 | 105 | 103 | 105 | 105 | 105 | 105 | 105 | 103 | 105 |
| D17S1804 | 855 | 238 | 238 | 238 | 238 | 238 | 238 | 238 | 238 | 236 | 238 |
| rs2 42557 | intron 1 | G | A | G | A | G | A | G | A | G | A |
| rs63750222 | exon 4A | C | C | C | C | C | C | C | C | C | C |
| rs2258689 | exon 6 | T | C | T | C | T | C | T | C | C | C |
| rs2435211 | intron 6 | T | C | T | C | C | C | T | C | C | C |
| rs2435200 | intron 9 | G | A | G | A | G | A | G | A | A | A |
| pR406W | exon 12 | C | T | C | T | C | T | C | T | C | T |
| rs5820605 | exon 14 | T | − | T | − | − | − | T | − | − | − |
| D17S920 | 423 | 100 | 100 | 100 | 100 | 102 | 100 | 102 | 100 | 102 | 100 |
| D17S693 | 575 | 99 | 95 | 95 | 95 | 99 | 95 | 99 | 95 | 95 | 95 |
| D17S1859 | 1,425 | 181 | 183 | 177 | 183 | 177 | 183 | 181 | 183 | 177 | 183 |
Distance from MAPT (in kb) was calculated on the basis of the latest human assembly GRCh37.p2 (www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/index.shtml).
Neuroimaging Findings
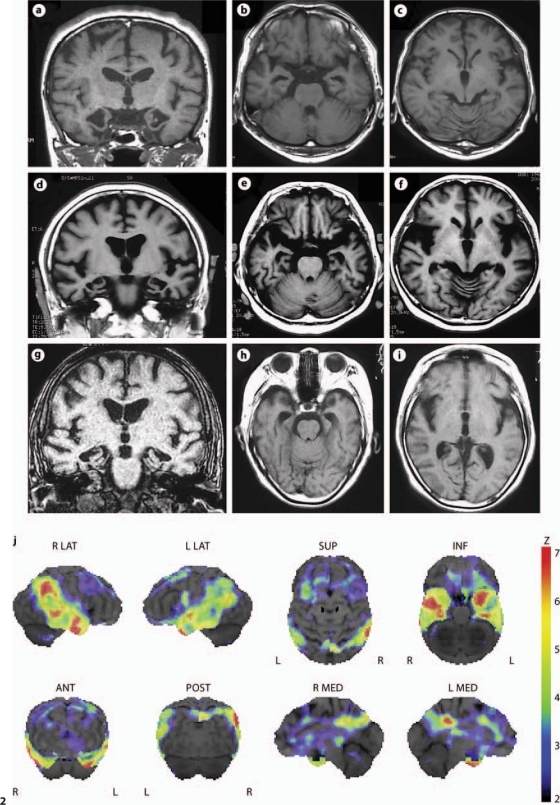
The brain structure was evaluated by MRI in 6 patients. All the affected subjects showed a similar structural change of severe atrophy mainly involving the medial temporal lobe (fig. 2a–i). Mild cortical atrophy may be observed without apparent hemisphere predominance. Parenchymal signal changes were absent.
Fig. 2.
Representative T1-weighted MR images of 3 patients: Ped 3367-P1 at the age of 56 years (a–c); Ped 3367-P3 at the age of 67 years (d–f), and Ped 4550-P1 at the age of 77 years (g–i). Coronal images revealed marked atrophy of the medial temporal lobe including the hippocampus and parahypocampal gyrus (a, d, e). On axial images, an enlarged inferior horn of the lateral ventricles was observed (b, e, h). Atrophic change in the frontal lobe is less noticeable (c, f, i). j Decreased rCBF by 123I-IMP SPECT with 3D-SSP in Ped 3367-P3 at the age of 67 years. After global normalization to the mean blood flow for the entire brain, rCBF in the patient was compared with that in normal controls by the Z test. Color-coding represents the statistical significance (Z score) of the decrease in rCBF. Decreases in rCBF were observed in the bilateral temporal lobes as well as in the cingulate gyrus of anterior (ANT) and posterior (POST) portions. L = Left; R = right; INF = inferior; LAT = lateral; MED = medial; SUP = superior.
We investigated regional cerebral blood flow (rCBF) patterns in 3 patients by SPECT. The patients in the early stage of the disease did not show a prominent decrease in rCBF. In the patient with a long disease course of 17 years at the age of 67 years (Ped 3367-P3), the decreased rCBF in the bilateral temporal lobes and the cingulate gyrus of the anterior and posterior portions was visualized by N-isopropyl-p-[123I]iodoamphetamine (123I-IMP) SPECT using three-dimensional stereotactic surface projection (3D-SSP; fig. 2j) [19].
CSF Analysis
We were able to determine the total and phosphorylated p181 tau levels in the CSF of 2 patients (Ped 3367-P1 and Ped 3367-P2; table 1). The average total tau level in the patients with the R406W mutation is slightly higher than that in our control subjects (157 ± 65 pg/ml, n = 24) and lower than that in patients with AD (555 ± 248 pg/ml, n = 31) [15]. p181 tau was higher than that in the controls (29 ± 10 pg/ml, n = 21) and comparable to that in patients with AD (86 ± 39 pg/ml, n = 31).
Discussion
The three families with the MAPT R406W mutation presented in this study showed relatively uniform clinical characteristics as follows: (1) presenile insidious onset of memory loss, (2) predominant early clinical features of anterograde amnesia, (3) relatively spare behavioral and personality changes or parkinsonism, (4) slow progression of disease, and (5) prominent atrophy of the medial temporal lobe involving the hippocampus. These clinical characteristics were similarly observed in Western families with the R406W mutation [8,9,10,12], suggesting that the predominant symptom of memory impairment with slowly progressive disease may be a cardinal clinical presentation of the R406W mutation irrespective of the ethnic background. However, it should be noted that different clinical features suggestive of FTD were also observed in a few patients with the R406W mutation [6,13].
The phenotype of prominent memory disturbance was occasionally noted in patients with MAPT mutations in introns 10 and 16 and P301L [4,5,20]. Similar to our patients, these patients were misdiagnosed as having AD prior to genetic or pathological analysis. Prominent early memory impairment is characteristic of AD and is an exclusion criterion for the diagnosis of FTD [21]. It remains unclear how some MAPT mutations lead to the phenotype of memory disturbance reminiscent of AD. In the case of R406W families, this phenotype may be explained by the prominent involvement of the hippocampus, which is considered the pathological substrate for memory impairment. Indeed, in the R406W patients in this study severe atrophy was noted in the hippocampus on MR images (fig. 2). This notion is supported by previous neuropathological studies showing that neuronal loss and gliosis are observed together with tau-positive neurofibrillary tangles and neuronal threads in the medial temporal lobe including the hippocampi [6,7,12,13,22].
Genetic analysis of the intragenic MAPT SNPs and the flanking microsatellite markers demonstrated that the three families with the R406W mutation in this study shared the same genotypes. This finding raises the intriguing notion that they may share extended chromosomal segments around the tau locus and have a common founder among their ancestors even though they are apparently unrelated pedigrees. This notion is supported by the observation that the frequency of the disease-associated haplotype in the general population is as low as 3.3%. The similarity of the phenotype observed in our families may be explained by the possibility that these families may represent a single extended R406W pedigree. In contrast to our findings, three families from Western Europe have been reported to carry different haplotypes among families [8], arguing against the founder effect; thus, the R406W mutation in these families seems to arise independently. Taken together, although the founder effect of the R406W mutation is responsible for the occurrence in some families, the same mutation may also occur independently in different kindreds.
In conclusion, findings on our and previously described cases suggest that the R406W mutation occurs pan-ethnically and may represent a peculiar phenotype of predominant anterograde amnesia of FTDP-17 in addition to two well-known core phenotypes of FTDP-17: the FTD- and parkinsonism-predominant phenotypes. Because anterograde amnesia is the prototypical presentation of AD, using solely the clinical diagnostic criteria in the families with the R406W mutation failed to properly identify the pathological substrate of their disease. A similar AD-like phenotype exhibiting anterograde amnesia was reported in a family with a mutation (c.154delA) in PGRN [23], another causative gene for FTLD. On the other hand, some PSEN1 mutations responsible for early-onset familial AD may cause the FTD-like phenotype [24,25]. Comprehensive mutational analysis of genes including PSEN1, PSEN2, APP, MAPT, and PGRN would be necessary for familial dementia with a broad spectrum of overlapping clinical phenotypes.
Acknowledgements
This study was supported by KAKENHI (09152381 and 08098982) from MEXT/JSPS, Japan, the Takeda Science Foundation, and the Mitsui Life Social Welfare Foundation. The authors thank Dr. Atushi Sato for the assistance in performing the neuropsychological tests.
References
- 1.Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D'Amato CJ, Gilman S. Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Ann Neurol. 1997;41:706–715. doi: 10.1002/ana.410410606. [DOI] [PubMed] [Google Scholar]
- 2.Reed LA, Wszolek ZK, Hutton M. Phenotypic correlations in FTDP-17. Neurobiol Aging. 2001;22:89–107. doi: 10.1016/s0197-4580(00)00202-5. [DOI] [PubMed] [Google Scholar]
- 3.Wszolek ZK, Tsuboi Y, Farrer M, Uitti RJ, Hutton ML. Hereditary tauopathies and parkinsonism. Adv Neurol. 2003;91:153–163. [PubMed] [Google Scholar]
- 4.Mirra SS, Murrell JR, Gearing M, Spillantini MG, Goedert M, Crowther RA, Levey AI, Jones R, Green J, Shoffner JM, Wainer BH, Schmidt ML, Trojanowski JQ, Ghetti B. Tau pathology in a family with dementia and a P301L mutation in tau. J Neuropathol Exp Neurol. 1999;8:335–345. doi: 10.1097/00005072-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Doran M, du Plessis DG, Ghadiali EJ, Mann DMA, Pickering-Brown S, Larner AJ. Familial early-onset dementia with tau intron 10 + 16 mutation with clinical features similar to those of Alzheimer disease. Arch Neurol. 2007;64:1535–1539. doi: 10.1001/archneur.64.10.1535. [DOI] [PubMed] [Google Scholar]
- 6.van Swieten JC, Stevens M, Rosso SM, Rizzu P, Joosse M, de Koning I, Kamphorst W, Ravid R, Spillantini MG, Niermeijer MF, Heutink P. Phenotypic variation in hereditary frontotemporal dementia with tau mutations. Ann Neurol. 1999;46:617–626. doi: 10.1002/1531-8249(199910)46:4<617::aid-ana10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Reed LA, Grabowski TJ, Schmidt ML, Morris JC, Goate A, Solodkin A, Van Hoesen GW, Schelper RL, Talbot CJ, Wragg MA, Trojanowski JQ. Autosomal dominant dementia with widespread neurofibrillary tangles. Ann Neurol. 1997;42:564–572. doi: 10.1002/ana.410420406. [DOI] [PubMed] [Google Scholar]
- 8.Rademakers R, Dermaut B, Peeters K, Cruts M, Heutink P, Goate A, Van Broeckhoven C. Tau (MAPT) mutation Arg406Trp presenting clinically with Alzheimer disease does not share a common founder in Western Europe. Hum Mutat. 2003;22:409–411. doi: 10.1002/humu.10269. [DOI] [PubMed] [Google Scholar]
- 9.Passant U, Ostojic J, Fabre SF, Gustafson L, Lannfelt L, Larsson E-M, Nilsson K, Rosen I. Familial presenile dementia with bitemporal atrophy. Dement Geriatr Cogn Disord. 2004;17:287–292. doi: 10.1159/000077156. [DOI] [PubMed] [Google Scholar]
- 10.Ostojic J, Elfgren C, Passant U, Nilsson K, Gustafson L, Lannfelt L, Fabre SF. The tau R406W mutation causes progressive presenile dementia with bitemporal atrophy. Dement Geriatr Cogn Disord. 2004;17:298–301. doi: 10.1159/000077158. [DOI] [PubMed] [Google Scholar]
- 11.Ikeuchi T, Kaneko H, Miyashita A, Nozaki H, Kasuga K, Tsukie T, Tsuchiya M, Imamura T, Ishizu H, Aoki K, Ishikawa A, Onodera O, Kuwano R, Nishizawa M. Mutational analysis in early-onset familial dementia in the Japanese population. Dement Geriatr Cogn Disord. 2008;26:43–49. doi: 10.1159/000141483. [DOI] [PubMed] [Google Scholar]
- 12.Lindquist SG, Holm IE, Schwartz M, Law I, Stokholm J, Batbayli M, Waldemar G, Nielsen JE. Alzheimer disease-like clinical phenotype in a family with FTDP-17 caused by a MAPT R406W mutation. Eur J Neurol. 2008;15:377–385. doi: 10.1111/j.1468-1331.2008.02069.x. [DOI] [PubMed] [Google Scholar]
- 13.Saito Y, Geyer A, Sasaki R, Kuzuhara S, Nanba E, Miyasaka T, Suzuki K, Murayama S. Early-onset, rapidly progressive familial tauopathy with R406W mutation. Neurology. 2002;58:811–813. doi: 10.1212/wnl.58.5.811. [DOI] [PubMed] [Google Scholar]
- 14.Kitade Y, Sato A, Imamura T. Two sibling patients with Alzheimer's disease associated with presenile onset and very slowly progressive clinical course (in Japanese) Jpn J Neuropsychol. 2006;22:260–268. [Google Scholar]
- 15.Kasuga K, Tokutake T, Ishikawa A, Uchiyama T, Tokuda T, Onodera O, Nishizawa M, Ikeuchi T. Differential levels of α-synuclein, β-amyloid42 and tau in CSF between patients with dementia with Lewy bodies and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2010;81:608–610. doi: 10.1136/jnnp.2009.197483. [DOI] [PubMed] [Google Scholar]
- 16.Dubois B, Litvan I. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 17.Kato M. The development and standardization of clinical assessment for attention (CAT) and clinical assessment for spontaneity (CAS) (in Japanese) Higher Brain Funct Res. 2006;26:310–319. [Google Scholar]
- 18.Evans W, Fung HC, Steele J, Eerola J, Tienari P, Pittman A, Silva R, Myers A, Vrieze FW, Singleton A, Hardy J. The tau H2 haplotype is almost exclusively Caucasian in origin. Neurosci Lett. 2004;369:183–185. doi: 10.1016/j.neulet.2004.05.119. [DOI] [PubMed] [Google Scholar]
- 19.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–1248. [PubMed] [Google Scholar]
- 20.Larner AJ. Mutation negative ‘early-onset familial Alzheimer disease’: consider screening for tau gene mutation. Alzheimer Dis Assoc Disord. 2008;22:194–195. doi: 10.1097/WAD.0b013e3181664ea4. [DOI] [PubMed] [Google Scholar]
- 21.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 22.Mott RT, Dickson DW, Trojanowski JQ, Zhukareva V, Lee VM, Forman M, van Deerlin V, Ervin JF, Wang DS, Schmechel DE, Hulette CM. Neuropathologic, biochemical, and molecular characterization of the frontotemporal dementias. J Neuropathol Exp Neurol. 2005;64:420–428. doi: 10.1093/jnen/64.5.420. [DOI] [PubMed] [Google Scholar]
- 23.Kelley BJ, Haidar W, Boeve BF, Baker M, Shiung M, Knopman DS, Rademakers R, Hutton M, Adamson J, Kuntz KM, Dickson DW, Parisi JE, Smith GE, Petersen RC. Alzheimer disease-like phenotype associated with the c.154delA mutation in progranulin. Arch Neurol. 2010;67:171–177. doi: 10.1001/archneurol.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dermaut B, Kumar-Singh S, Engelborghs S, Theuns J, Rademakers R, Saerens J, Pickut BA, Peeters K, Van Den Broeck M, Vennekens K, Claes S, Cruts M, Cras P, Martin JJ, Van Broeckhoven C, De Deyn PP. A novel presenilin 1 mutation associated with Pick's disease but not β-amyloid plaques. Ann Neurol. 2004;55:617–626. doi: 10.1002/ana.20083. [DOI] [PubMed] [Google Scholar]
- 25.Mendez MF, McMurtray A. Frontotemporal dementia-like phenotypes associated with presenilin-1 mutations. Am J Alzheimers Dis Other Dement. 2006;21:281–286. doi: 10.1177/1533317506290448. [DOI] [PMC free article] [PubMed] [Google Scholar]