Abstract
Background
Alzheimer's disease (AD) is a progressive neurodegenerative disorder. In AD, monocytes migrate across the blood-brain barrier and differentiate into microglia, are linked to inflammatory responses and display age-dependent decreases in telomere lengths.
Methods
Six monocyte-specific chemokines and the (telomere-associated) tumor suppressor proteins p53 and p21 were determined by multiplex immunoassay in plasma and monocyte extracts of patients with AD or mild cognitive impairment, and levels were compared between patients and controls (without cognitive impairment).
Results
CCL15 (macrophage inflammatory protein-1δ), CXCL9 (monokine-induced by interferon-γ) and p21 levels were decreased in monocytes of AD patients compared with controls.
Conclusion
The combination of monocytic CCL15 and p21 together with the Mini-Mental State Examination enables to differentiate AD patients from controls with high specificity and sensitivity.
Key Words: Alzheimer's disease; Blood parameters; Chemokines; Dementia markers; Differential diagnosis, Alzheimer's disease; Monocytes
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder morphologically characterized by β-amyloid (Aβ) plaque formation, tau pathology, neurodegeneration and inflammatory processes [1,2]. A probable diagnosis of AD is currently based on clinical evaluation, laboratory tests and brain imaging [3]. In order to support and simplify the diagnostic procedure, an intense search is underway for disease-specific biomarkers in the cerebrospinal fluid (CSF), blood plasma and blood cells. In the CSF, three biomarkers (Aβ42, total tau and phospho-tau-181) have been well established [4,5]. In blood, no specific biomarkers have been found despite intense research in proteins and genes of blood cells [6].
Monocytes are formed in the bone marrow and are continuously released into the blood [7]. They are involved in peripheral immune and inflammatory mechanisms, and in response to inflammatory stimuli monocytes are recruited to the AD brain [8,9,10]. Inflammatory processes are abundantly found in AD, resulting in impaired regulation of several cytokines and chemokines in the blood [11,12]. Chemokines are produced by a wide range of cell types, including monocytes, and have been found to be altered in AD. Reports on inflammatory cytokine and chemokine levels in the plasma and serum of AD patients vary significantly. For example, enhanced levels of interleukin-6, tumor necrosis factor-α or CCL15 [macrophage inflammatory protein (MIP)-1δ] have been found [13,14,15]. While other studies report decreased or unchanged protein levels [16,17], CXCL9 [monokine-induced by interferon γ (MIG)] is enhanced in the plasma of AD patients [18]. CCL2 [monocyte chemoattractant protein (MCP)-1] is also increased in serum and brain tissue, but it is reduced in peripheral blood mononuclear cells (PBMCs) [19,20,21]. It has been reported that monocytes produce CCL2 in response to Aβ [22,23]. Moreover, in AD patients microglia display replicative senescence earlier than in non-demented individuals, which may contribute to Aβ plaque formation [24,25]. AD patients also show shorter telomere lengths in monocytes compared with controls, and it is well known that a critical telomere length can trigger DNA damage, which is directly linked to the upregulation of tumor suppressor proteins p53 and p21 [26,27,28,29].
The aim of the present study was to investigate whether the levels of six selected specific monocyte chemokines/cytokines and the telomere-related tumor suppressor proteins p53 and p21 differ between patients with AD or mild cognitive impairment (MCI) and healthy subjects. We hypothesized that the combined statistical analysis of CCL15, p21 and the clinical Mini-Mental State Examination (MMSE) helps to differentiate AD patients from healthy controls with high sensitivity and specificity.
Patients and Methods
Patient Selection
Control (cognitively not impaired) subjects and patients suffering from AD or MCI were recruited from the Departments of Psychiatry in Innsbruck and Klagenfurt, Austria. All groups were assessed by the same diagnostic procedure. AD and MCI diagnoses were established by a structured routine process including clinical assessment, neuropsychological tests (MMSE) and neuroimaging (MRI) to exclude other brain pathologies [30]. MCI was diagnosed according to the criteria of Petersen et al. [31]. Probable AD was diagnosed according to National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria [32]. A general blood examination is part of the routine diagnostic procedure. All participants were scored using the geriatric depression scale (GDS). Exclusion criteria for healthy subjects, and MCI and AD patients included (1) another primary neurological or mental disorder (except depression, since it has been shown that depressive subjects without cognitive impairment were not different from controls in a selection of 16 plasma markers, and depressive patients with cognitive impairment did also not differ from cognitively impaired patients [15]), (2) any kind of metabolic decompensation or any sign of peripheral inflammation (e.g. rheumatic disease), (3) long-term alcohol or drug abuse or (4) any current, clinically significant cardiovascular disease. In order to test the accuracy of the laboratory examination, blood from 63 subjects routinely explored at the Memory Clinic (Innsbruck Medical University) was analyzed on a blind basis and compared with the clinical diagnosis. The study was approved by the Ethics Committee of Innsbruck Medical University.
Plasma and Monocyte Collection
Monocytes were isolated as described recently in detail [33]. Briefly, blood (10 ml) was collected in EDTA tubes for normal routine clinical assessment and processed within 3 h. Plasma and PBMCs were isolated from whole blood by centrifugation (400 g, 30 min, room temperature) on a continuous-density Biocoll gradient (1.077 g/ml; Biochrom, Germany). Two thirds of the upper plasma phase and the interphase with the PBMCs, which is visible as a white stratum between the plasma phase and Biocoll, was carefully removed. Plasma was directly frozen at −80°C until use. PBMCs were washed in 50 ml of phosphate-buffered saline (PBS), centrifuged at 250 g for 6 min, and the pellet was dissolved in PBS with 1% bovine serum albumin. Monoyctes were isolated by negative magnetic isolation, as described by the manufacturer (Miltenyi Biotech, Germany). Briefly, PBMCs were incubated for 10 min on ice with a cocktail of various biotinylated antibodies (CD3, CD7, CD16, CD19, CD56, CD123 and CD235a). Then anti-biotin magnetic beads were added, and the mixture was incubated on ice for further 15 min, washed and the cells were applied to MACS MS columns (Miltenyi Biotech) on a strong magnet. The non-labelled monocytes were eluted and collected. Finally, cells were frozen at −80°C until analysis.
Measurement of Chemokines by SearchLight ELISA
The monocyte pellet was dissolved in 150 μl PBS with a protease inhibitor cocktail (Sigma, Austria), sonicated on ice (3 × 10 s, 125 W/cm2, 140 μm amplitude, 100%) and centrifuged (10 min, 14,000 g, 4°C). The supernatant of the monocyte extracts or plasma was used to detect CCL3 (MIP-1α), CCL4 (MIP-1β), CCL15 (MIP-1δ), CCL2 (MCP-1), CCL22 [MIP-1δ, macrophage-derived chemokine (MDC)] and CXCL9 (MIG) using the Thermo Scientific SearchLight Protein Array Technology (THP Medical Products, Austria), as described in our recent publication [15]. Briefly, 50 μl of calibrated protein standards or extracts were added to coated wells and incubated for 3 h. After washing, the biotinylated antibodies were added. After incubation for 30 min, the wells were washed again and incubated with streptavidin-horseradish peroxidase conjugate. After the final washing step, the SuperSignal chemiluminescent substrate was added. All incubation steps were carried out on a shaker at room temperature. The luminescent signal was detected using CCD imaging and a SearchLight Array Analyst system. The concentration of each sample was quantified by comparing the spot intensities to the corresponding standard curves calculated from the standard sample results using the SearchLight Array Analyst Software.
ELISA for p21 and p53 Protein
The p21 and p53 protein levels of monocytes and plasma were measured using commercial ELISA kits (p21 Waf1/Cip1 ELISA; Invitrogen, Austria; p53 pan ELISA; Roche, Austria). Detection of p21 and p53 was performed as described by the manufacturer. Briefly, 100 μl standard or extracts were added to coated wells and incubated for 2 h. After washing, the detection antibodies were added. After incubation for 1 h, the wells were washed again and incubated with a horseradish peroxidase conjugate for 30 min. After washing, stabilized chromogen substrate was added. After 30 min of incubation, the stop solution was added and absorbance was measured at 450 nm using a Zenyth 3100 ELISA reader.
Statistical Analysis of Individual Markers
The ability of the individual markers to discriminate between diagnostic groups was tested by analysis of variance (ANOVA). Markers for which a significant group effect had been detected by ANOVA were further determined by post hoc pairwise comparisons of groups using Fisher's least significant difference (LSD) method. No further adjustment for multiple testing was required as the number of diagnostic groups to be compared was three; in this case significance in the global F test and LSD testing (p ≤ 0.05) was sufficient to keep the family-wise error rate at 0.05.
Prediction of Group Membership by Combining Several Markers
Two different methods were used to predict a subject's group membership on the basis of biomarkers and MMSE, namely binary logistic regression and discriminant analysis. The first method allows classification into only two groups, whereas the latter enables classification into three (or more) groups.
Discrimination between Pairs of Groups (Healthy versus MCI/AD, Healthy/MCI versus AD). Logistic regression with forward stepwise variable selection was used to identify those variables which, in combination, best predicted group membership in a two-group setting. A second analysis with backward stepwise selection served as a control (producing identical results). The predicted probabilities obtained from logistic regression were entered into a receiver-operating characteristic (ROC) analysis. From this analysis, estimates of the sensitivity and specificity at various cutoff levels and of the area under the ROC curve (AUC) were derived.
Discrimination between All Three Groups. Linear discriminant analysis with forward stepwise variable selection and equal a priori probabilities for all three groups (healthy, MCI and AD) was used for this purpose. Stepwise selection of predictor variables was based on Wilk's λ and the corresponding F statistic, entering all variables with a value of p < 0.05 into the model. Predicted group membership was determined by means of Fisher's linear discriminant functions. Rates of correct classification and misclassification were estimated both directly and by means of cross-classification (bias-corrected version). In order to compare prediction by laboratory data only (1) and by combining laboratory parameters with MMSE (2), the discriminant analysis was run twice, once for the predictor set (1) and once for the combination set (2), testing for improved prediction by adding MMSE.
Results
Patient Characteristics
The characteristics of the patients and the controls are presented in table 1. As expected, MMSE scores differed significantly between AD patients and controls, but not between MCI patients and controls. Eight controls, and 23 MCI and 25 AD patients were clinically diagnosed with mild depression (GDS score 10–19). None of the controls, but 12 MCI and 4 AD patients had a diagnosis of severe depression (GDS score 20–30). Healthy controls did not show a significant difference in GDS scores compared with AD patients, whereas MCI patients exhibited significantly higher GDS scores than AD patients or healthy controls (table 1).
Table 1.
Patient characteristics
| Groups | n | M/F | Age, years | MMSE | CDS |
|---|---|---|---|---|---|
| Patients included for biomarker search | |||||
| Control | 40 | 19/21 | 72.2 ±6.3 | 28.5 ±1.3 | 6.8 ± 4.4 |
| MCI | 67 | 23/44 | 73.8 ±8.0 | 26.9 ±2.0 | 11.5 ±7.8∗∗∗ |
| AD | 92 | 21/71 | 78.8 ±7.1∗∗∗ | 18.2 ±6.1∗∗∗ | 8.2 ± 5.3 |
| Patients included for the blinded study | |||||
| Control | 13 | 6/7 | 70.1 ±6.8 | 28.4 ±1.6 | 6.7 ±6.3 |
| MCI | 33 | 11/22 | 75.8 ±8.5∗ | 25.6 ±2.8∗∗ | 9.3 ±4.9 |
| AD | 17 | 8/9 | 79.7 ±6.6∗∗∗ | 20.0 ±4.0∗∗∗ | 8.6 ±6.1 |
Monocytes were isolated from healthy controls, and MCI and AD patients (means ± SD). n = Number of samples of male/female patients (M/F). Statistical analysis was performed by one-way ANOVA with a Fisher LSD post hoc test.
p < 0.05;
p < 0.01;
p < 0.001, vs. control; the other values were nonsignificant.
Plasma Levels
Chemokine plasma levels did not differ between controls and MCI patients (table 2). Plasma levels of CCL15 and CXCL9 were significantly elevated in AD patients compared with controls. Plasma levels of CCL4, CCL2 and CCL22 did not differ between patients and controls. CCL3, p53 and p21 levels in plasma were below the limit of quantitation of the assay (table 2).
Table 2.
Chemokines and cytokines in plasma and monocytes
| Chemokine | LOQ pg/well | Control | MCI | AD | |
|---|---|---|---|---|---|
| Plasma pg/ml | CCL15, MIP-1δ | 1.0 | 3,752 ±282 (43) | 4,283 ±179 (91) | 4,668±167(148)∗∗ |
| CXCL9, MIG | 0.45 | 1,096 ±168 (33) | 925 ±164 (36) | 2,591 ±428 (36)∗∗ | |
| CCL3,MIP-lα | 1.0 | <LOQ | <LOQ | <LOQ | |
| CCL4,MIP-1β | 0.24 | 108± 12 (11) | 102 ±15 (12) | 86± 11 (11) | |
| CCL2, MCP-1 | 0.24 | 362±21 (11) | 381 ±23 (12) | 430±48(11) | |
| CCL22, MDC |
0.12 |
308±52 (11) |
212±13 (12) |
245 ±32 (11) |
|
| p21 | 1.5 | <LOQ | <LOQ | <LOQ | |
| p53 | 15.0 | <LOQ | <LOQ | <LOQ | |
| Monocytes pg/mg | CCL15, MIP-1δ | 1.0 | 15.2 ±1.5 (40) | 9.2 ±0.9 (67)∗∗∗ | 9.9 ±0.8 (92)∗∗∗ |
| CXCL9, MIG | 0.45 | 89 ±15 (37) | 55 ±11 (63)∗ | 46 ±5 (85)∗∗ | |
| CCL3,MIP-lα | 1.0 | 57 ±38 (8) | 32 ± 6 (8) | 52 ±17 (8) | |
| CCL4,MIP-1β | 0.24 | 278 ±66 (8) | 210±46(8) | 472 ±212 (8) | |
| CCL2, MCP-1 | 0.24 | 341 ±56 (8) | 229 ±54 (8) | 562 ±190 (8) | |
| CCL22, MDC |
0.12 |
70 ±12 (8) |
124 ±30 (8) |
99 ±35 (8) |
|
| p21 | 1.5 | 87±17(34) | 80 ±13 (48) | 32 ±4 (68)∗∗∗ | |
| p53 | 15.0 | <LOQ | <LOQ | <LOQ | |
Plasma and monocyte chemokines, and tumor suppressors p53 and p21 were analyzed from controls, and AD and MCI patients using commercial ELISA and Searchlight multiplex ELISA (means ± SEM). Numbers of samples analyzed are given in parentheses. LOQ = Limit of quantitation.
p < 0.05;
p < 0.01;
p < 0.001, vs. control (one-way ANOVA with Fisher's LSD post hoc test).
Monocyte Tissue Levels
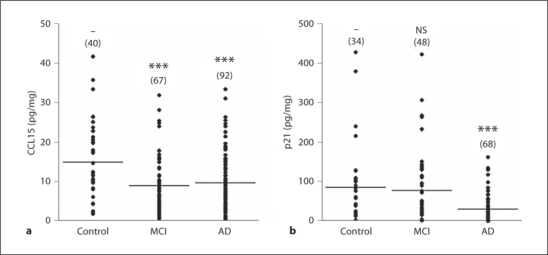
In monocytes, CCL15 (fig. 1) and CXCL9 levels were significantly lower in MCI patients than in healthy subjects (table 2). In AD patients, CCL15 (fig. 1), CXCL9 and p21 (fig. 1) levels were significantly decreased compared with controls (table 2). Monocytic CCL4 and CCL2 displayed a tendency to an increase in AD patients. Monocytic CCL3 and CCL22 levels of AD and MCI patients did not differ from those of healthy controls. The protein levels of p53 in monocytes were below the limit of quantitation of the assay (table 2).
Fig. 1.
Scatter plots of CCL15 (a) and p21 (b) levels in monocytes of controls, MCI and AD patients. Lines indicate means. Sample numbers are given in parentheses. ∗∗∗ p < 0.001, vs. control; NS = Nonsignificant. ANOVA followed by post hoc pairwise group comparisons using Fisher's LSD method.
Discrimination between Diagnostic Groups by Logistic Regression and ROC Analysis
A summary of the results of logistic regression and ROC analyses is given in table 3. Using monocytic biomarkers only, discrimination between controls and cognitively impaired subjects (either MCI or AD) was only modest. ROC analysis yielded an AUC of 0.748 in combination with a sensitivity of 69.6% and a specificity of 72.7%. Discrimination between subjects with and without AD (control/MCI) was even poorer, with a sensitivity of 61.7% and a specificity of 61.4% (AUC = 0.679). Considering cognitively impaired subjects only, discrimination between MCI and AD was also poor (sensitivity 63.2%, specificity 64.6%, AUC 0.693, data not shown). Discrimination between controls and MCI/AD patients substantially improved when MMSE was added to the set of predictors: sensitivity of 80.7% and specificity of 84.7% (AUC = 0.912). Discrimination between patients with AD and those without AD was even better, with sensitivity and specificity reaching values >90%. However, for clinical applications, the classification into two groups given in this subsection probably has only limited value. A classification of the subjects into three diagnostic groups is presented in the next subsection.
Table 3.
Results of logistic regression and ROC analyses
| Model | Logistic regression |
ROC analysisa |
|||||
|---|---|---|---|---|---|---|---|
| significant predictors | model information |
AUC | sensitivity | specificity | |||
| χ2 | d.f. | p value | |||||
| Prediction by laboratory data only | |||||||
| Control vs. MCI/AD | CCL15 | 18.8 | 1 | <0.0001 | 0.748 | 69.6% | 72.7% |
| Control/MCI vs. AD | p21 | 16.5 | 1 | <0.0001 | 0.679 | 61.7% | 61.4% |
| Prediction by laboratory data and MMSE | |||||||
| Control vs. MCI/AD | CCL15, MMSE | 70.1 | 2 | <0.0001 | 0.912 | 80.7% | 84.8% |
| Control/MCI vs. AD | p21,MMSE | 157.6 | 2 | <0.0001 | 0.987 | 94.9% | 93.2% |
The cutoff value was chosen to maximize the sum of sensitivity and specificity.
Prediction of Diagnostic Group Membership by Discriminant Analysis
Linear discriminant analysis with stepwise variable selection and equal a priori probabilities for all three groups (healthy, MCI and AD) led us to include monocytic CCL15 (step 1: Wilk's λ = 0.851; d.f. = 1, 2, 145; F = 11.2; p < 0.001) and monocytic p21 (step 2: Wilk's λ = 0.784; d.f. = 2, 2, 145; F = 9.25; p < 0.001) in the model as significant predictors, whereas CXCL9 was not entered as it did not significantly improve group prediction. When MMSE was added as a predictor, diagnostic accuracy increased significantly (Wilk's λ = 0.346; d.f. = 3, 2, 144; F = 33.1; p < 0.001). Both CCL15 and p21 remained in the model as significant predictors. A summary of the classification results is shown in table 4. Sensitivity for the identification of MCI cases was high (81.8%). Sensitivity for the identification of AD cases was somewhat lower (72.1%), but most of the misclassified AD cases were categorized as MCI (23.7%); only 3 cases (5.1%) were erroneously classified as healthy. The specificity of the prediction model was only modest (57.6% correctly identified healthy controls), but all misclassified healthy controls were categorized as MCI, none as AD. Working with Fisher's linear discriminant functions (f0, f1, f2), the analysis yielded a formula allowing the prediction of diagnostic group membership on the basis of a patient's CCL15, p21 and MMSE values (table 4).
Table 4.
Classification of results of discriminant analysis
| Diagnosis | Predicted group membership |
||
|---|---|---|---|
| healthy | MCI | AD | |
| Healthy (n = 33) | 19 (57.6%) | 14 (42.4%) | 0 (0.0%) |
| MCI (n = 55) | 10 (18.2%) | 45(81.8%) | 0 (0.0%) |
| AD (n = 59) | 3 (5.1%) | 14 (23.7%) | 42 (71.2%) |
| Goodness of prediction (bias correction by means of cross-classification) |
|||
|---|---|---|---|
| correct | slightly misclassified (one category off)a | completely misclassifiedb | |
| Healthy (n = 33) | 57.6% (54.5%) | 42.4% (45.5%) | 0% (0%) |
| MCI (n = 55) | 81.8% (78.2%) | 18.2% (21.8%) | 0% (0%) |
| AD (n = 59) | 71.2% (71.2%) | 23.7% (23.7%) | 5.1% (5.1%) |
| Total (n= 147) | 72.1% (70.1%) | 25.9% (27.9%) | 2.0% (2.0%) |
Classification formulae:
Healthy f0 = 0.01449·ρ21 + 0.314·CCL15 + 1.815·MMSE − 30.174;
MCI fl = 0.01352·ρ21 + 0.171·CCL15 + 1.697·MMSE − 25.060;
AD f2 = 0.00506·ρ21 + 0.201·CCL15 + 1.153·MMSE − 12.653.
Rule: Classify as control if f0 > fl and f0 > f2; classify as MCI, if fl > f0 and fl > f2; classify as AD, if f2 > f0 and f2 > fl.
Actual group and predicted group in adjacent categories, e.g. actual group = healthy, predicted group = MCI.
Actual group and predicted group are completely different, e.g. actual group = healthy, predicted group = AD.
Blinded Laboratory Analysis
In order to test the accuracy of the prediction model based on laboratory biomarkers and MMSE, 63 patients routinely examined at the Memory Clinic were analyzed. Based on the formula, our ‘laboratory diagnosis’, including CCL15, p21 and MMSE, classified 60 subjects as cognitively impaired, while only 3 subjects were categorized as (cognitively not impaired) controls (table 5). In contrast, according to the clinical diagnosis, only 50 subjects were classified as cognitively impaired, while 13 were defined as controls without cognitive impairment (tables 1, 5). Eleven subjects were categorized as AD cases according to the blinded laboratory analysis, whereas the clinical examination identified 17 cases with AD (table 1). The classification results are detailed in tables 1 and 5. Overall, in 63.8% of the subjects (43 of 63), classification did not differ between both methods; slight deviations (healthy subjects classified as MCI, MCI patients classified as AD patients) occurred in 36.2% of the cases. There were no completely misclassified cases (healthy subjects classified as AD or vice versa).
Table 5.
Diagnosis from blinded samples using the formulae obtained by discriminant analysis
| Clinical diagnosis | Predicted diagnosis based on p21, MIP-1δ and MMSE using the formulae |
||
|---|---|---|---|
| healthy (n = 3) | MCI (n = 49) | AD(n=11) | |
| Healthy (n= 13) | 1 (7.7%) | 12 (92.3%) | 0 (0.0%) |
| MCI (n = 33) | 2 (6.1%) | 31 (93.9%) | 0 (0.0%) |
| AD (n = 17) | 0 (0.0%) | 6 (35.3%) | 11 (64.7%) |
Blood from subjects routinely examined at the Memory Clinic was analyzed under ‘blinded’ conditions using the three statistical formulae (see Results) including p21, CCL15 and MMSE. These data were subsequently compared with the clinical diagnosis.
Discussion
Our findings show significantly decreased plasma levels for two monocyte-specific chemokines (CCL15 and CXCL9) and a reduction in the tumor suppressor p21 in AD patients compared to healthy subjects. When combining these two laboratory biomarkers with a clinical marker (MMSE), AD patients can be differentiated from healthy controls and MCI with high sensitivity and specificity.
Monocytes and AD
There is strong evidence that monocytes play a role in neuroinflammatory processes seen in AD. Monocytes migrate across a compromised blood-brain barrier and express chemokine receptors to guide immune cells to inflammatory sites [9,10,34,35,36]. Monocytic cell adhesion molecules are altered in AD patients [33,37]. Further, the expression of chemokine receptors and cytokines was found to be increased in PBMCs of AD patients [38]. Thus, potentially pathogenic mechanisms in AD, e.g. immune cell activation, may correlate with impaired regulation of cytokines, chemokines and cell adhesion molecules seen in the disease.
Chemokines in Monocytes and Plasma
This study provides additional evidence that chemokines are specifically affected in AD. The plasma levels of two of six chemokines, i.e. CCL15 and CXCL9, were increased. Both chemokines play a major role in the recruitment of immune cells to sites of injury or infection. CCL15 belongs to a subgroup of CC chemokines (containing six cysteine residues) and is expressed in monocytes after exposure to inflammatory stimuli [39,40]. It has been shown that CCL15 plasma levels are reduced (d score −1.6) in AD patients compared to controls [17]. The present study found reduced levels of CCL15 in monocytes and higher plasma levels in patients suffering from AD, which is in agreement with our previous study [15]. CXCL9 has chemotactic functions and plays a role in inflammatory response modulation. It has been shown that CXCL9 plasma levels are higher in AD patients [18], which is consistent with our present observation. Most importantly, here we show that CXCL9 levels were significantly lower in monocytes of AD patients than in controls. Thus, it seems likely that reduced levels of CCL15 and CXCL9 in monocytes directly reflect an enhanced release of these cytokines into the blood, which fully correlates with our findings of enhanced plasma levels. However, it cannot be ruled out that the unchanged chemokines have a potential role in AD. CCL3 acts as a chemoattractant for a variety of cells and is produced and released by monocytes after stimulation with Aβ [23]. CCL4 has been isolated from monocytes, has chemotactic activity and showed no difference between the brains of AD patients and controls [21,41]. CCL2 plays a role in the migration of monocytes to sites of injury and infection, is produced in microglia and can be stimulated by Aβ [22,42]. Overall, it has been reported that CCL2 is upregulated in the brain, CSF, serum and plasma of AD patients [20,21,43,44]. However, CCL2 plasma levels were decreased in severe AD [45]. In CSF and serum, CCL2 did not differ between control, MCI and AD groups [46,47]. Even more controversially, CCL2 levels increased with age regardless of AD [48]. CCL22 and its receptor CCR4 play a role in homeostatic and inflammatory processes. The high heterogeneity between different studies may be ascribed to methodological differences, but also to the stage of disease, and the age and the overall health status of the patients.
p21 and p53 in Monocytes and Plasma
We assessed monocyte and plasma levels of tumor suppressor proteins p53 and p21, since we discovered a reduced telomere length in monocytes of AD patients (data not shown). Both proteins play a critical role in cell death and aging, and are directly related to telomere dysfunction [27,28,29]. The tumor suppressor p21 is a direct target of p53 and mediates the p53-dependent cell cycle arrest in response to DNA damage and subsequent DNA repair [27,49,50]. In the brain, p53 plays an important role in regulating amyloid-precursor protein (APP) [51], and APP protects neuronal cells against apoptosis by controlling p53 activation at the posttranslational level [52]. Further, it has been reported that p53 inhibition prevented microglial neurotoxicity [53]. In AD blood lymphocytes, the p53 protein and the dysfunction of the G1/S checkpoint have been considered as potential biomarkers [54]. Furthermore, it has been shown that p21-activated kinase interacts with APP and is markedly reduced in AD [55]. Thus, in the present study, we measured both tumor suppressor proteins in plasma and monocytes, but could not detect p53. However, to our knowledge, our data show for the first time that p21 is significantly reduced in monocytes of AD patients, which was not detectable in plasma. It seems likely that p21 is associated with telomere length reductions in monocytes but not secreted into the blood. The reduced p21 monocytic levels in AD patients could be a direct mechanism of the disease pathology or directly related to the telomere-shortening process.
MMSE as a Clinical Marker in Combination with Blood Markers
Our data show that the combination of two cytokines and the p21 suppressor protein alone enables neither satisfactory differentiation between cognitively impaired patients and healthy controls, nor a useful distinction of patients with AD from those without AD. However, when adding MMSE to the set of predictor variables, differentiation between the diagnostic groups was markedly improved. Notably, both MMSE and biomarkers (CCL15 and p21) were required to obtain a useful prediction. MMSE alone was also not sufficient. Our discriminant analysis based on CCL15, p21 and MMSE yielded a high sensitivity of 81.8% for the identification of MCI patients and a somewhat lower, but still fairly satisfactory, sensitivity of 71.2% for the correct detection of AD cases (particularly as almost all misclassified AD patients were categorized as MCI). Specificity levels were somewhat less satisfactory. Our allocation method based on biomarkers and MMSE tended to classify subjects as MCI, even if they were diagnosed as cognitively healthy by the clinician. However, the clinical diagnosis may not always be correct. Sometimes subjects with minor cognitive impairments are diagnosed as healthy by the clinician, whereas laboratory markers have the potential to identify AD-associated alterations at a very early stage.
The MMSE is a brief 30-point questionnaire that is used to screen for cognitive impairment and to estimate the severity of cognitive impairment at a given point in time. The MMSE total score may differentiate between normal age-associated memory symptoms and MCI (a possible prodromal state of AD) and also separate MCI from mild AD. As the MMSE was initially also used by the clinicians to diagnose AD, it seems to be a circle evidence to include MMSE in this type of diagnostic algorithm at first glance. We are fully aware of the clinical problems of an AD diagnosis, especially since the AD diagnosis is not confirmed by neuropathology. However, the aim of the blinded study was to receive the MMSE from the clinicians and to perform the blood tests, and to apply the blinded algorithm to separate AD/MCI/controls. Based on this algorithm, it was possible to include the ‘blinded MMSE value’ obtained by clinicians and combine it with the two blood biomarkers. This way, the inclusion of MMSE was a first circle evidence marker to establish the algorithm, which could not have been possible without any histopathology. In order to test the efficiency of the ‘laboratory diagnosis’, we tested 63 subjects on a blinded basis and compared the findings with the clinical diagnosis. Indeed, the diagnosis was consistent in 43 of 63 cases (63.8%). Moreover, in none of the cases was a subject with a clinical diagnosis of AD classified as cognitively healthy or vice versa. We were thus able to distinguish AD patients from controls with high sensitivity and specificity.
Limitations of the Study
This study, however, has some limitations. First, the number of subjects analyzed was small, although the findings were statistically significant. Due to the limited amount of samples, we could not perform measurements for all cytokines. Second, this was a cross-sectional study. Therefore, it cannot be determined whether the changes in chemokine levels cause the disease or are the results of brain pathology and inflammation in AD. Further research confirming these findings in large, prospective studies is needed. Current research efforts aim to identify individual markers or combinations of markers to facilitate objective diagnosis and treatment of AD. Changes in CCL15 and p21 levels are not proposed as a stand-alone test or screening tool, but they may be interesting biomarker candidates in a multiple-biomarker strategy. Further studies applying CCL15 and p21 together with multiple-biomarker strategies as well as other clinical markers may further improve diagnostic accuracy for early AD, MCI and other types of dementia (e.g. frontotemporal lobe dementia), too.
Conclusion
Levels of CCL15, CXCL9 and p21 in monocytes were decreased in AD patients compared with healthy controls. Consequently, the combination of CCL15 and p21 with the MMSE enables differentiation of cognitively impaired subjects, specifically between AD patients and controls, with high specificity and sensitivity.
Disclosure Statement
There were no biomedical financial interests or potential conflicts of interest.
Acknowledgements
This study was supported by the Austrian Science Fund (L429-B05). T.H. was supported by the European Community (Moodinflame – Early Diagnosis, Treatment and Prevention of Mood Disorders Targeting the Activated Inflammatory Response System; FP7-Health-2007 Project No. 222963). We thank Ursula Kirzenberger-Winkler for her excellent technical assistance.
References
- 1.Hauw JJ, Seilhean D, Piette F, Uchihara T, Duyckaerts C. Alzheimer's disease lesions: from morphology to cell biology. Bull Acad Natl Med. 1996;180:1687–1700. [PubMed] [Google Scholar]
- 2.Burns A, Byrne EJ, Maurer K. Alzheimer's disease. Lancet. 2002;360:163–165. doi: 10.1016/S0140-6736(02)09420-5. [DOI] [PubMed] [Google Scholar]
- 3.Fradinger EA, Bitan G. En route to early diagnosis of Alzheimer's disease – are we there yet? Trends Biotechnol. 2005;23:531–533. doi: 10.1016/j.tibtech.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Blennow K. CSF biomarkers for mild cognitive impairment. J Intern Med. 2004;256:224–234. doi: 10.1111/j.1365-2796.2004.01368.x. [DOI] [PubMed] [Google Scholar]
- 5.Blennow K. CSF biomarkers for Alzheimer's disease: use in early diagnosis and evaluation of drug treatment. Expert Rev Mol Diagn. 2005;5:661–672. doi: 10.1586/14737159.5.5.661. [DOI] [PubMed] [Google Scholar]
- 6.Humpel C. Identifying and validating biomarkers for Alzheimer's disease. Trends Biotechnol. 2010;29:26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkman A. The origin and fate of the monocyte. Ser Haematol. 1970;3:62–92. [PubMed] [Google Scholar]
- 8.Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 9.Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer's disease brain and damage the blood-brain barrier. Eur J Clin Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 10.Malm TM, Koistinaho M, Pärepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to amyloid-β deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 12.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 13.Angelopoulos P, Agouridaki H, Vaiopoulos H, Siskou E, Doutsou K, Costa V, Baloyiannis SI. Cytokines in Alzheimer's disease and vascular dementia. Int J Neurosci. 2008;118:1659–1672. doi: 10.1080/00207450701392068. [DOI] [PubMed] [Google Scholar]
- 14.Bermejo P, Martín-Aragón S, Benedí J, Susín C, Felici E, Gil P, Ribera JM, Villar AM. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer's disease. Immunol Lett. 2008;117:198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Marksteiner J, Kemmler G, Weiss EM, Knaus G, Ullrich C, Mechtcheriakov S, Oberbauer H, Auffinger S, Hinterhölzl J, Hinterhuber H, Humpel C. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2011;32:539–540. doi: 10.1016/j.neurobiolaging.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Duijn CM, Hofman A, Nagelkerken L. Serum levels of interleukin-6 are not elevated in patients with Alzheimer's disease. Neurosci Lett. 1990;108:350–354. doi: 10.1016/0304-3940(90)90666-w. [DOI] [PubMed] [Google Scholar]
- 17.Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 18.Lee KS, Chung JH, Lee KH, Shin MJ, Oh BH, Hong CH. Bioplex analysis of plasma cytokines in Alzheimer's disease and mild cognitive impairment. Immunol Lett. 2008;121:105–109. doi: 10.1016/j.imlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Iarlori C, Gambi D, Gambi F, Lucci I, Feliciani C, Salvatore M, Reale M. Expression and production of two selected beta-chemokines in peripheral blood mononuclear cells from patients with Alzheimer's disease. Exp Gerontol. 2005;40:605–611. doi: 10.1016/j.exger.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corrà B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer's disease. Neurobiol Aging. 2006;12:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer's disease. Brain Pathol. 2009;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishizuka K, Kimura T, Igata-yi R, Katsuragi S, Takamatsu J, Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin Neurosci. 1997;51:135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 23.Fiala M, Zhang L, Gan X, Sherry B, Taub D, Graves MC, Hama S, Way D, Weinand M, Witte M, Lorton D, Kuo YM, Roher AE. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood-brain barrier model. Mol Med. 1998;4:480–489. [PMC free article] [PubMed] [Google Scholar]
- 24.Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007;10:61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- 25.Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, De Vivo I. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses' health study. PLoS One. 2008;3:1590. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 27.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 28.Vaziri H, Benchimol S. From telomere loss to p53 induction and activation of a DNA-damage pathway at senescence: the telomere loss/DNA damage model of cell aging. Exp Gerontol. 1996;31:295–301. doi: 10.1016/0531-5565(95)02025-x. [DOI] [PubMed] [Google Scholar]
- 29.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol. 2005;40:634–642. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 32.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 33.Hochstrasser T, Weiss E, Marksteiner J, Humpel C. Soluble cell adhesion molecules in monocytes of Alzheimer's disease and mild cognitive impairment. Exp Gerontol. 2010;45:70–74. doi: 10.1016/j.exger.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floris S, Ruuls SR, Wierinckx A, van der Pol SM, Döpp E, van der Meide PH, Dijkstra CD, De Vries HE. Interferon-beta directly influences monocyte infiltration into the central nervous system. J Neuroimmunol. 2002;127:69–79. doi: 10.1016/s0165-5728(02)00098-x. [DOI] [PubMed] [Google Scholar]
- 35.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malm TM, Magga J, Kuh GF, Vatanen T, Koistinaho M, Koistinaho J. Minocycline reduces engraftment and activation of bone marrow-derived cells but sustains their phagocytic activity in a mouse model of Alzheimer's disease. Glia. 2008;56:1767–1779. doi: 10.1002/glia.20726. [DOI] [PubMed] [Google Scholar]
- 37.Ciaramella A, Bizzoni F, Salani F, Vanni D, Spalletta G, Sanarico N, Vendetti S, Caltagirone C, Bossù P. Increased pro-inflammatory response by dendritic cells from patients with Alzheimer's disease. J Alzheimers Dis. 2010;19:559–572. doi: 10.3233/JAD-2010-1257. [DOI] [PubMed] [Google Scholar]
- 38.Reale M, Iarlori C, Feliciani C, Gambi D. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer's disease. J Alzheimers Dis. 2008;14:147–159. doi: 10.3233/jad-2008-14203. [DOI] [PubMed] [Google Scholar]
- 39.Youn BS, Zhang SM, Lee EK, et al. Molecular cloning of leukotactin-1: a novel human β-chemokine, a chemoattractant for neutrophils, monocytes, and lymphocytes, and a potent agonist at CC chemokine receptors 1 and 3. J Immunol. 1997;159:5201–5205. [PubMed] [Google Scholar]
- 40.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Guan E, Wang J, Norcross MA. Identification of human macrophage inflammatory proteins 1α and 1β as a native secreted heterodimer. J Biol Chem. 2001;276:12404–12409. doi: 10.1074/jbc.M006327200. [DOI] [PubMed] [Google Scholar]
- 42.El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer's disease. Neurobiol Aging. 1998;19:81–84. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 43.Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2003;16:136–144. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- 44.Fenoglio C, Galimberti D, Lovati C, Guidi I, Gatti A, Fogliarino S, Tiriticco M, Mariani C, Forloni G, Pettenati C, Baron P, Conti G, Bresolin N, Scarpini E. MCP-1 in Alzheimer's disease patients: A-2518G polymorphism and serum levels. Neurobiol Aging. 2004;25:1169–1173. doi: 10.1016/j.neurobiolaging.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, Guidi I, Blankenstein MA, Bresolin N, Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 46.Galimberti D, Schoonenboom N, Scarpini E, Scheltens P, Dutch-Italian Alzheimer Research Group chemokines in serum and cerebrospinal fluid of Alzheimer's disease patients. Ann Neurol. 2003;53:547–548. doi: 10.1002/ana.10531. [DOI] [PubMed] [Google Scholar]
- 47.Mattsson N, Tabatabaei S, Johansson P, Hansson O, Andreasson U, Månsson JE, Johansson JO, Olsson B, Wallin A, Svensson J, Blennow K, Zetterberg H. Cerebrospinal fluid microglial markers in Alzheimer's disease: elevated chitotriosidase activity but lack of diagnostic utility. Neuromolecular Med. 2011;13:151–159. doi: 10.1007/s12017-011-8147-9. [DOI] [PubMed] [Google Scholar]
- 48.Blasko I, Lederer W, Oberbauer H, Walch T, Kemmler G, Hinterhuber H, Marksteiner J, Humpel C. Measurement of thirteen biological markers in CSF of patients with Alzheimer's disease and other dementias. Dement Geriatr Cogn Disord. 2006;21:9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- 49.Harper JW, Adami GR, Wie N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 50.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 51.Cuesta A, Zambrano A, Royo M, Pascual A. The tumour suppressor p53 regulates the expression of amyloid precursor protein (APP) Biochem J. 2009;15:643–650. doi: 10.1042/BJ20081793. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Yang D, Wyss-Coray T, Yan J, Gan L, Sun Y, Mucke L. Wild-type but not Alzheimer-mutant amyloid precursor protein confers resistance against p53-mediated apoptosis. Proc Natl Acad Sci USA. 1999;96:7547–7552. doi: 10.1073/pnas.96.13.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davenport CM, Sevastou IG, Hooper C, Pocock JM. Inhibiting p53 pathways in microglia attenuates microglial-evoked neurotoxicity following exposure to Alzheimer peptides. J Neurochem. 2010;112:552–563. doi: 10.1111/j.1471-4159.2009.06485.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhou X, Jia J. P53-mediated G(1)/S checkpoint dysfunction in lymphocytes from Alzheimer's disease patients. Neurosci Lett. 2010;468:320–325. doi: 10.1016/j.neulet.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 55.Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]