Abstract
Background
Postmenopausal women with hormone receptor-positive early breast cancer have persistent, long-term risk of breast cancer recurrence and death. Therefore, trials evaluating endocrine therapies for this patient population require extended follow-up. We present an update of efficacy outcomes in the Breast International Group (BIG) 1-98 study at 8.1 years median follow-up.
Methods
BIG 1-98 is a randomized, phase III, double-blind trial of 8010 postmenopausal women with hormone receptor-positive early breast cancer that compares five years of tamoxifen or letrozole monotherapy or sequential treatment with two years of one of these agents followed by three years of the other. The primary efficacy endpoint is disease-free survival (DFS: events comprise invasive breast cancer relapse, second primaries [contralateral breast and non-breast], or death without prior cancer event), and secondary endpoints are overall survival (OS), distant recurrence-free interval (DRFI) and breast cancer-free interval (BCFI). The monotherapy comparison includes patients randomized to tamoxifen × 5 years (n=2459) or letrozole × 5 years (n=2463). In 2005, after significant DFS benefit was reported for letrozole as compared with tamoxifen, a protocol amendment facilitated the crossover to letrozole of patients who were still receiving tamoxifen alone; Cox models and Kaplan-Meier estimates with inverse probability of censoring weighting (IPCW) are used to account for selective crossover to letrozole of 619 patients in the tamoxifen arm. The comparison of sequential treatments to letrozole monotherapy includes patients enrolled in the four-arm option of the trial and randomized to letrozole × 5 years (n=1546), letrozole × 2 years followed by tamoxifen × 3 years (n=1540), or tamoxifen × 2 years followed by letrozole × 3 years (n=1548). All patients have completed study treatment; follow up is continuing for those enrolled in the four-arm option. BIG 1-98 is registered at clinicaltrials.gov NCT00004205.
Findings
At a median follow-up of 8.7 years from randomization (range 0–12.4), letrozole monotherapy is significantly better than tamoxifen, whether using IPCW or intention-to-treat (ITT) analysis [IPCW: DFS HR 0.82 (95% CI 0.74–0.92), OS HR 0.79 (0.69–0.900, DRFI HR 0.79 (0.68–0.92), BCFI HR 0.80 (0.70–0.92); ITT: DFS HR 0.86 (0.78–0.96), OS HR 0.87 (0.77–0.999), DRFI HR 0.86 (0.74–0.998), BCFI HR 0.86 (0.76–0.98)]. At a median follow-up of 8.0 years from randomization (range 0–11.2), there were no statistically significant differences in any of the four endpoints for either sequence compared with letrozole monotherapy. Eight-year ITT estimates [each with SE ≤ 1.1%] for letrozole monotherapy, letrozole followed by tamoxifen, and tamoxifen followed by letrozole were 78.6%, 77.8%, 77.3% for DFS; 87.5%, 87.7%, 85.9% for OS; 89.9%, 88.7%, 88.1% for DRFI; and 86.1%, 85.3%, 84.3% for BCFI.
Interpretation
For postmenopausal women with endocrine-responsive early breast cancer, a reduction in breast cancer recurrence and mortality is obtained by letrozole monotherapy when compared to tamoxifen. Sequential treatments involving tamoxifen and letrozole do not improve outcome compared with letrozole monotherapy, but may represent useful strategies considering individual patient’s risk of recurrence and treatment tolerability: more thromboembolic events, vaginal bleeding, hot flushes and night sweats with tamoxifen, while more vaginal dryness, bone fractures, osteoporosis, arthralgia/myalgia, and higher grade cardiac events with letrozole.
Funding
Novartis, United States National Cancer Institute, International Breast Cancer Study Group.
Keywords: aromatase inhibitor, letrozole, breast cancer, adjuvant therapy, endocrine therapy, tamoxifen
Introduction
Aromatase inhibitors are now part of standard treatment for most postmenopausal women with estrogen receptor (ER) and/or progesterone receptor (PgR) positive early invasive breast cancer.1 Generally these agents are given either alone or in sequence before or after tamoxifen. The BIG 1-98 trial’s enhanced design allowed the comparison of tamoxifen and letrozole monotherapies as well as the comparison of the sequential treatments with monotherapy,2–5 and included updates every two years of the primary analyses to monitor long-term safety and efficacy of the treatment strategies. This population has persistent, long-term risk of breast cancer recurrence requiring extended follow-up.6 The importance of extended follow-up of women in breast cancer adjuvant trials was discussed in a recent editorial.7 We present updated results at 8.1 years median follow-up (12 years since entry of the first patient) to provide a comparison of five years of monotherapy with letrozole versus tamoxifen and a direct comparisons of each sequential treatment with letrozole monotherapy.
Methods
Study design
BIG 1-98 is a randomized, phase III, double-blind trial that recruited postmenopausal women with estrogen receptor and/or progesterone receptor positive early breast cancer.2–5 Initially, from 1998 to 2000, women were randomly assigned to receive monotherapy with letrozole (Femara®, Novartis) 2.5 mg orally daily or tamoxifen 20 mg orally daily for five years, and later, from 1999 to 2003, were randomly assigned to one of four arms: monotherapy with tamoxifen or letrozole for five years or sequential therapy comprising letrozole for two years followed by tamoxifen for three years, or tamoxifen for two years followed by letrozole for three years (Fig 1).8 Randomization was performed centrally at the International Breast Cancer Study Group (IBCSG) randomization center with the use of permuted blocks and was stratified according to the two- or four-arm randomization option, participating institution and chemotherapy use. The following procedure was used to assure concealment of the randomized assignment and blinding of patients, investigators, data managers and medical reviewers: Study drug was prepared centrally as double dummy packs containing both tamoxifen (active or placebo) and letrozole (active or placebo) tablets. Each pack included a six-month supply of study drug and was labeled with a study drug identification number. Study drug supplies were available at the local pharmacy of the participating site. When a patient was enrolled the IBCSG randomization center provided a study drug number corresponding to the randomized treatment – either active tamoxifen or active letrozole – and the associated pack was given to the patient. Resupply was carried out for subsequent six-monthly intervals using an interactive voice recognition system to transmit a study drug identification number available in the local pharmacy corresponding to the proper treatment to be received during the next six months. The trial was registered at clinicaltrials.gov: NCT00004205.
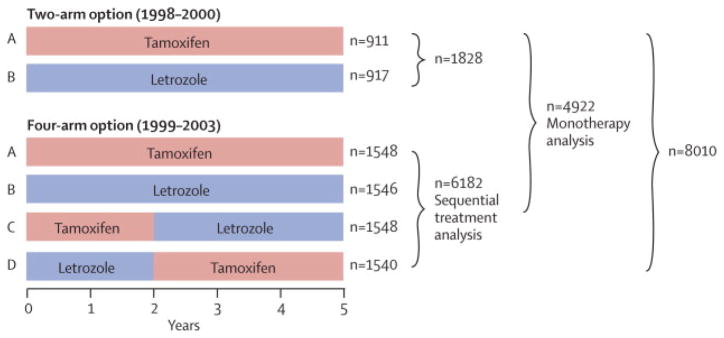
Figure 1.
BIG 1-98 Design: Of 8028 patients enrolled, 18 did not receive study treatment and withdrew consent for use of their data, leaving 8010 for the intent-to-treat (ITT) population (median follow-up 8.1 years, range 0–12.4). The monotherapy analysis includes 4922 patients randomly assigned to letrozole monotherapy or tamoxifen monotherapy either as part of the two-arm or four-arm randomization option (median follow-up 8.7 years, range 0–12.4). The sequential therapy analysis includes 6182 patients randomly assigned to one of four treatment groups as part of the four-arm randomization option (median follow-up 8.0 years, range 0–11.2).
Symptoms, side effects, and clinical examination findings were recorded at baseline, every 6 months for the first five years and yearly thereafter. All patients have completed study treatment and detailed safety results for adverse events that occurred during the five-year treatment period have been reported elsewhere.4,5 As previously reported, patients on tamoxifen experienced more thromboembolic events, vaginal bleeding, hot flushes, and night sweats. Patients on letrozole experienced more vaginal dryness, bone fractures, osteoporosis, arthralgia/myalgia, and higher grade cardiac events. It is important to note that these analyses present the incidence of AEs for one regimen (letrozole) compared to the other (tamoxifen), and it is possible that tamoxifen in particular may offer protection from cardiac or bone events. The incidences of the AEs occurring in the sequential arms generally show results similar to the monotherapies during the time the patient was on the individual agents (i.e., first two years or last three years). Comprehensive information on treatment interventions after five years was not systematically collected.
The primary study endpoint was disease-free survival (DFS), defined as the time from randomization to the first of the following events: invasive recurrence in local, regional, or distant sites; a new invasive cancer in the contralateral breast; any second (non-breast) primary cancer; or death without a prior cancer event. Other endpoints have been defined using STEEP criteria, and include overall survival (OS), invasive breast cancer-free interval (BCFI), and distant recurrence-free interval (DRFI).9 The trial primary analytic approach was intention-to-treat (ITT); if an event was not observed, then follow-up was censored at the date of last disease assessment.
The 2005 results,2 showing superiority of letrozole, led to the recommendation by the International Breast Cancer Study Group (IBCSG) Data and Safety Monitoring Committee (DSMC), and a decision by the BIG 1-98 Steering Committee, to inform patients randomly assigned tamoxifen monotherapy of their treatment to allow informed decisions about their future care. An amendment of the protocol in April 2005 provided letrozole therapy to any patient assigned to tamoxifen monotherapy who was disease-free, receiving or recently (within 6 months) stopped tamoxifen and wishing to cross over to letrozole (selective crossover). The three letrozole-containing treatment groups remained blinded.
The International Breast Cancer Study Group (IBCSG) is responsible for study design and coordination, data collection and management, medical review, data analysis, and reporting (including decision to publish). The IBCSG Statistical Center had unblinded access to the database, and the IBCSG Data Management Center had blinded access to the database. Ethics committees and relevant health authorities of each participating institution approved the study protocol. All patients gave written informed consent. The DSMC received safety data semiannually throughout the trial and reviewed predefined interim and the final efficacy analyses.
Role of Funding Source
Novartis (Basel, Switzerland), the manufacturer of letrozole, distributed the study drugs, provided financial support, and imposed no restrictions on the investigators with respect to trial data. The manuscript was prepared by the authors, who had full access to the data and made final decisions on content. The Steering Committee (including a minority representation from Novartis; Webappendix Section 1) reviewed the manuscript and offered changes. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Statistical Analysis
Two analytic populations are presented, the monotherapy analysis population and the sequential treatment analysis population (Fig 1). The monotherapy population includes 4922 patients randomized in the 2-arm or 4-arm option to receive either tamoxifen for five years or letrozole for five years. The sequential treatment population includes the 6182 patients randomized in the 4-arm option (Fig 1). The statistical design has been previously described,8 and a CONSORT diagram is available in the Webappendix Fig A1.
The selective crossover to letrozole of the patients in the tamoxifen monotherapy group, after release of the primary trial results in 2005, complicates its comparison with other treatment groups in updated analyses. Among the 2459 patients assigned tamoxifen monotherapy, 619 (25.2%) selectively crossed over to receive letrozole prior to a disease-free survival event, mostly between three and five years from the start of therapy. Evidence from large, phase III studies has shown that patients who switched to an aromatase inhibitor after 2 to 3 years of tamoxifen had a survival benefit compared with patients who continued on tamoxifen for 5 years.10 Therefore, the 25.2% of patients in the BIG 1-98 tamoxifen group who selectively crossed over to letrozole actually received a treatment known to be superior to tamoxifen alone. Consequently, updated ITT analyses involving tamoxifen monotherapy are likely to produce attenuated (biased) estimates of the magnitude of treatment effect. To better estimate the magnitude of the letrozole treatment effect relative to tamoxifen monotherapy had there been no selective crossover, we used inverse probability censoring weighted (IPCW) Cox models to estimate hazard ratios (HR) and 95% confidence intervals (CI).11 IPCW modeling artificially creates a scenario of informative missing data by first censoring the follow-up of each woman at the time she crossed over, and then restoring the lost follow-up by applying weighting to the follow-up experience of women with similar characteristics who remain on tamoxifen. IPCW analyses provide valid estimates, assuming no unmeasured confounders of an endpoint and selective crossover. IPCW Kaplan-Meier estimates of time to event distributions were calculated. HRs, 95% CIs and Wald chi-square p-values using unweighted Cox models to implement the ITT approach are also reported for completeness. Models for the monotherapy population are stratified by randomization option (2- or 4-arm) and chemotherapy use; models for the sequential treatment population are stratified by chemotherapy use.
Results
Updated DFS events
At this protocol-specified update 12 years since trial commencement, there were 2074 DFS events and 1284 deaths, compared with 1569 and 923 at the 10-year update, among all 8010 patients with median follow-up time of 8.1 years. The additional 505 DFS events, which were mostly observed between 5 and 11 years from randomization, comprised 279 (55%) breast cancer recurrences, 106 (21%) second non-breast malignancies, 101 (20%) deaths without prior cancer event, and 19 (4%) events that could not be reliably classified. 74% (5936 of 8010) of patients were reported at their most recent follow-up to be alive and without a DFS event. Lost to follow-up rates were low and similar across treatment groups: 5.3% (260 of 4922) for the monotherapy analysis and 3.3% (206 of 6182) for the sequential treatment analyses.
Updated Monotherapy Analysis
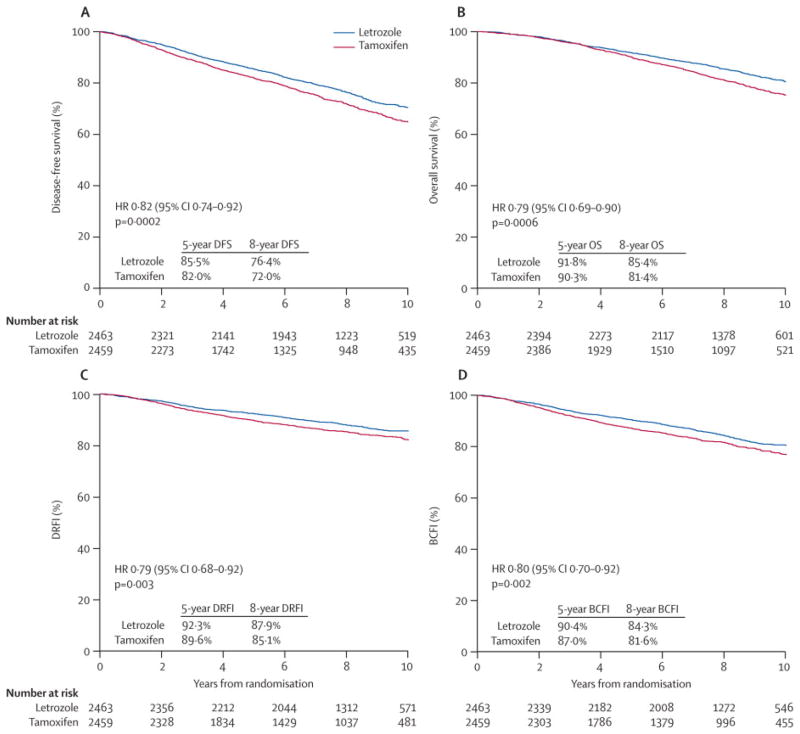
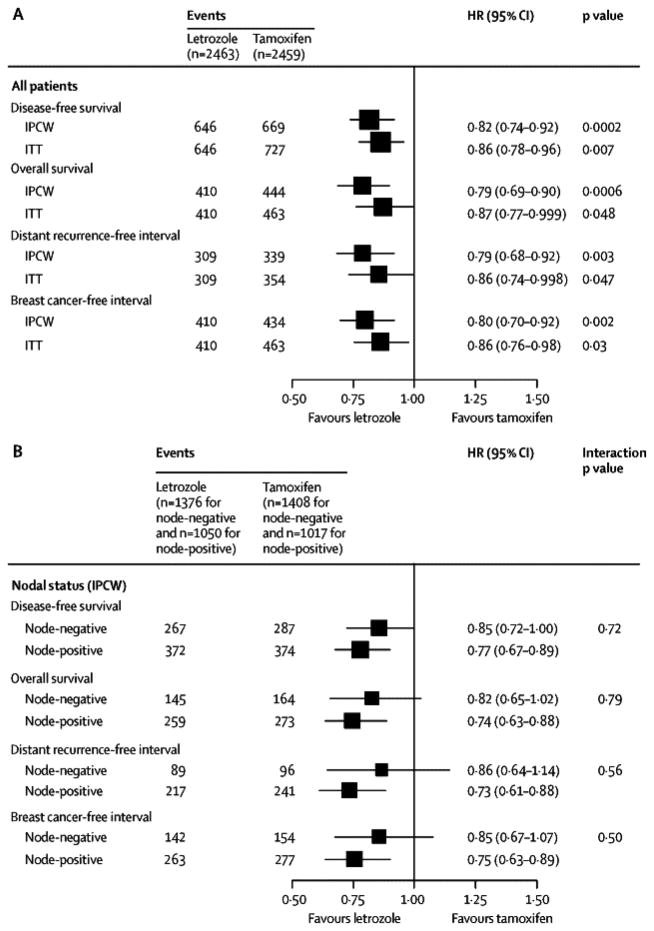
The monotherapy analysis cohort of 4922 patients randomized to receive five years of tamoxifen or letrozole included 42% (2067 of 4922) with node-positive disease, 38% (1859 of 4922) with primary tumor size greater than 2cm, 46% (2274 of 4922) who received mastectomy, and 25% (1232 of 4922) who received adjuvant or neo-adjuvant chemotherapy (41% of the node-positive subgroup (851 of 2067) and 14% of the node-negative subgroup (376 of 2784)). The median age at randomization was 61 years (range, 38 to 90 years). The median follow-up for the updated analysis, which includes patients assigned to letrozole or tamoxifen monotherapy either as part of the two-arm or four-arm randomization option, was 8.7 years. The IPCW Cox models showed a reduction in the hazard of a DFS event with letrozole (HR: 0.82, 95% CI 0.74 to 0.92; Fig. 2A) and a reduction in the hazard of death (HR: 0.79, 95% CI 0.69 to 0.90; Fig. 2B) compared to tamoxifen. There was a reduction in the hazard of a distant recurrence event with letrozole (HR: 0.79, 95% CI 0.68 to 0.92; Fig. 2C) and a reduction in the hazard of a breast cancer event (HR: 0.80, 95% CI 0.70 to 0.92; Fig. 2D). The relative treatment effects expressed as hazard ratios were homogeneous across node-positive and node-negative subgroups (treatment-by-nodal status interaction p-values = 0.72, 0.79, 0.56, 0.50 for DFS, OS, DRFI and BCFI respectively; Fig. 3).
Figure 2.
Monotherapy Analysis: Inverse probability of censoring weighted (IPCW) Kaplan-Meier estimates of (A) disease-free survival (DFS), (B) overall survival (OS), (C) distant recurrence-free interval (DRFI), and (D) breast cancer-free interval. The median follow-up time is 8.7 years.
Figure 3.
Monotherapy Analysis: Hazard ratios and 95% CI comparing tamoxifen versus letrozole for four endpoints, estimated using inverse probability of censoring weighted (IPCW) Cox models overall and by nodal status, and estimated using unweighted Cox models to implement the ITT approach. The models were stratified by randomization option and chemotherapy use. The size of the boxes is inversely proportional to the standard error of the hazard ratio.
The comparison of letrozole and tamoxifen monotherapies according to the ITT analysis are also shown in Fig. 3. The ITT analysis estimated hazard ratios of smaller magnitude than the IPCW analysis, nonetheless, also demonstrated statistically significant improvements in DFS, OS, DRFI and BCFI with letrozole compared to tamoxifen (each p≤0.05). Sites of first DFS event are summarized in the Webappendix Section 2, Table A1.
Updated Sequential Treatment Analysis
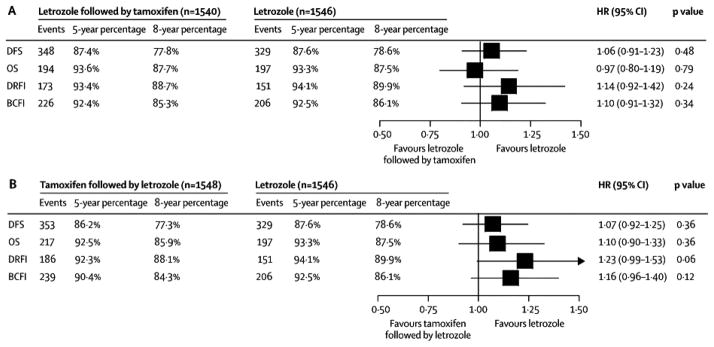
The 6182 patients randomized during the 4-arm option comprise the sequential treatment analysis population (Fig. 1). This population included 41% (2517 of 6182) patients with node-positive disease, 36% (2201 of 6182) with primary tumor size greater than 2cm, 39% (2397 of 6182) who received mastectomy, and 26% (1586 of 6182) who received adjuvant or neo-adjuvant chemotherapy. The median age at randomization was 61 years (range, 38 to 89 years). The median follow-up for the sequential treatment analyses was 8.0 years. The sequential treatments of tamoxifen followed by letrozole and letrozole followed by tamoxifen did not significantly reduce the risk of a DFS event compared with letrozole monotherapy (tamoxifen→letrozole vs. letrozole HR: 1.07, 95% CI 0.92 to 1.25; letrozole→tamoxifen vs. letrozole HR: 1.06, 95% CI 0.91 to 1.23; Fig. 4). The hazard ratio for the comparison of the two sequential arms (letrozole→tamoxifen vs. tamoxifen→letrozole) with respect to DFS is 0.99, 95% CI 0.85 to 1.14. The estimated eight-year DFS percentages for the three letrozole-containing regimens of the sequential treatment analysis population were 78.6%, 77.8% and 77.3%, for the letrozole monotherapy, sequential letrozole followed by tamoxifen, and sequential tamoxifen followed by letrozole groups, respectively (Fig. 4; Webappendix Section 2, Fig A2). There were no statistically significant differences in OS, DRFI, or BCFI for either sequence compared with letrozole monotherapy (Fig. 4). With 151 to 353 events available per treatment group depending on endpoint (Fig. 4), the results are underpowered to demonstrate statistical equivalence. Sites of first DFS event are summarized in the Webappendix Section 2, Table A2 and Kaplan-Meier estimates of the 5-year and 8-year percentages of the four endpoints (with standard errors) are summarized in Table A3.
Figure 4.
Sequential Treatment Analysis: Hazard ratios and 95% CI comparing (A) tamoxifen followed by letrozole versus letrozole monotherapy, (B) letrozole followed by tamoxifen versus letrozole monotherapy, for the four endpoints using Cox models stratified for chemotherapy use. All analyses are ITT, as the letrozole-containing regimens remained blinded, and comparisons with tamoxifen alone are not shown. The size of the boxes is inversely proportional to the standard error of the hazard ratio. Five- and eight-year estimates of endpoints are calculated using the Kaplan-Meier method, and the standard error (SE) of each estimate is ≤ 1.1%. The median follow-up time is 8.0 years.
Discussion
Although it has been 12 years since the BIG 1-98 trial opened for accrual, disease-free survival events continue to occur in large numbers in this population of postmenopausal women with endocrine-responsive early breast cancer. The trial protocol specified that updates of the primary analyses would be performed every two years in recognition of the prolonged, persistent hazard of breast cancer recurrence in this population.6 At this update, 2074 DFS events were observed among all 8010 patients, compared with 1569 at the protocol-specified update two years ago, a 32% increase in number of events. Statistically, these additional events during follow-up improve the precision of the treatment effect estimate for the secondary endpoints of OS, DRFI and BCFI (Table 1), and the ability to examine relative efficacy in subgroups, e.g. node-positive vs. node-negative, where the timing of the events differs. Long-term follow-up of the BIG 1-98 trial population continues, which will enable analysis of changes in patterns of events over time including treatment-by-time interaction and carry-over effect.
Table 1.
Monotherapy analysis: estimates of relative treatment benefit of letrozole versus tamoxifen monotherapy according to follow-up time.
| ITT | IPCW | ||||
|---|---|---|---|---|---|
| Endpoint and Analysis Dataset* | Median Follow-up | Events | HR (95% CI) | P-value | HR (95% CI) |
| Disease-free survival | |||||
| 8-year [3] | 4.25y | 770 | 0.82 (0.71, 0.95) | 0.007 | n/a |
| 10-year [5] | 6.3y | 1074 | 0.88 (0.78, 0.99) | 0.03 | 0.83 (0.74, 0.94) |
| 12-year | 8.7y | 1373 | 0.86 (0.78, 0.96) | 0.007 | 0.82 (0.74, 0.92) |
| Overall survival | |||||
| 8-year [3] | 4.25y | 405 | 0.91 (0.75, 1.11) | 0.35 | n/a |
| 10-year [5] | 6.3y | 646 | 0.87 (0.75, 1.02) | 0.08 | 0.82 (0.70, 0.95) |
| 12-year | 8.7y | 873 | 0.87 (0.77, 0.999) | 0.048 | 0.79 (0.69, 0.90) |
| Distant recurrence-free interval | |||||
| 8-year [3] | 4.25y | 427 | 0.81 (0.67, 0.98) | 0.03 | n/a |
| 10-year [5] | 6.3y | 555 | 0.85 (0.72, 1.00) | 0.05 | 0.80 (0.67, 0.94) |
| 12-year | 8.7y | 663 | 0.86 (0.74, 0.998) | 0.047 | 0.79 (0.68, 0.92) |
| Breast cancer-free interval | |||||
| 8-year [3] | 4.25y | 522 | 0.78 (0.65, 0.92) | 0.004 | n/a |
| 10-year [5] | 6.3y | 697 | 0.86 (0.74, 0.99) | 0.04 | 0.81 (0.70, 0.94) |
| 12-year | 8.7y | 873 | 0.86 (0.76, 0.98) | 0.030 | 0.80 (0.70, 0.92) |
At the 8-year, 10-year, and 12-year datasets (time from randomization of first patient), the percentages of total person-years of follow-up for the ITT tamoxifen randomized group that accumulated after selective crossover to letrozole were 0%, 7% (1040 of 14361) and 13% (2324 of 18208), respectively.
Abbreviations: HR=hazard ratio; CI=confidence interval; ITT=intention-to-treat; IPCW=inverse probability of censoring weighted; n/a=not applicable;
The comparison of the monotherapy treatments of BIG 1-98 in this updated analysis continues to clearly demonstrate the superiority of letrozole over tamoxifen for these patients. In addition, with more than 500 DFS events observed among the 2784 patients with node-negative disease, the updated data more strongly support that, on average, letrozole is beneficial in both the node-positive and the node-negative subgroups. We have presented the results using IPCW analysis methods, because, compared with the ITT approach, which is known to be biased in this case, IPCW provides better estimates of the magnitude of the true treatment effect that would have been observed had there been no selective crossover.12 It is noteworthy that the ITT analysis of the monotherapy population--which ignores the trial’s provision of letrozole for a portion of the five years of therapy to one-quarter of the patients assigned tamoxifen--also supports letrozole as the better single-agent endocrine treatment, showing statistically significant improvements in DFS, OS, DRFI and BCFI (each p≤0.05). However as summarized in Table 1, the hazard ratio for OS estimated using ITT is unchanged as compared with the previous report two years ago, but has narrower confidence interval and a p-value that is now below the threshold of 0.05.
In the sequential treatment analyses, neither sequence--tamoxifen followed by letrozole nor letrozole followed by tamoxifen--showed superiority over letrozole monotherapy. As the study was not designed to test equivalence, statistical equivalence cannot be demonstrated. While letrozole followed by tamoxifen appeared to provide similar DFS and OS compared with letrozole monotherapy (upper 95% confidence intervals below 1.25), letrozole monotherapy tended to be superior to tamoxifen followed by letrozole, especially for control of distant recurrence among patients at higher risk of early relapse. Therefore overall risk and tradeoffs with respect to side effects and other burdens will influence the preferred choice of treatment.
Whether clinical and pathological features can identify patient groups for whom it is more or less important that a five year program include only or some aromatase inhibitor therapy has been described for early relapse,13 and more recently for five-year outcome.14 In the recent report by Viale et al,14 we simulated the clinical approach to treatment decision-making using a synthesized assessment of risk based on multiple factors. A composite measure of prognostic risk was calculated for each patient from a Cox proportional hazards model using factors based on the 2007 St. Gallen Consensus15 (i.e., number of involved lymph nodes, tumor grade, tumor size, and presence of peritumoral vascular invasion as determined by local pathology; and ER, PgR, Ki-67, and HER2 status as determined by central pathology review), plus age. The nonparametric Subpopulation Treatment Effect Pattern Plot (STEPP)16 shows the estimates of five-year DFS for subpopulations across the continuum of risk without regard to treatment (Webappendix Fig. A3.A) and separately by treatment (Fig. A3.B).14 As shown in Webappendix Figure A3.B and in Viale et al,14 it appears that all four treatments had similar five-year DFS for patients at lowest risk (left end of the x-axis), the three letrozole-containing treatments had similar five-year DFS for intermediate risk (middle), while letrozole for five years had better outcome for those at highest risk. The recent meta analysis by Amir et al17 on toxicity of adjuvant endocrine therapy for postmenopausal patients concludes that, compared with ‘upfront’ aromatase inhibitor therapy administered for five years, switching from tamoxifen to an aromatase inhibitor may offer the best balance between efficacy and toxicity. Their analysis does not include information on the sequential treatment arms from BIG 1-98, and in particular does not consider the unique evidence provided by BIG 1-98 on the sequential use of ‘upfront’ letrozole for two years followed by tamoxifen for five years, available in the BIG 1-98 Collaborative Group’s article and appendix.4 ‘Upfront’ letrozole might be reasonable for patients at high risk for early relapse, but sequential regimens may be useful strategies for others considering treatment tolerability.
The present study examined five years of adjuvant endocrine therapy. Patients continue to relapse after such therapy. Other trials have since demonstrated the value of extended aromatase inhibitor therapy after five years of tamoxifen adjuvant therapy.18,19 The ongoing Study of Letrozole Extension (SOLE) trial20 investigates this concept in more detail and adds the evaluation of intermittent letrozole therapy as extended adjuvant therapy based on promising results from pre-clinical models.21,22
This update of BIG 1-98 at 8.1 years median follow-up reinforces the evidence that letrozole monotherapy is superior to tamoxifen in controlling breast cancer recurrence and improving survival for postmenopausal women with endocrine-responsive early breast cancer. Use of a sequence may be reasonable for patients at low to intermediate risk of relapse, those for whom starting or continuing letrozole is contraindicated, or in cases where five years of letrozole may not be available. Trials investigating endocrine treatments in hormone receptor-positive populations require an investment in long-term follow-up to ensure reliable choices for patient care.
Lancet Oncology Panel (requested by LO)
Systematic review
This paper presents the updated results of a practice-changing clinical trial, BIG 1-98, that was opened for accrual in 1998. The BIG 1-98 trial was designed to compare five years of tamoxifen with five years of the aromatase inhibitor letrozole, and to compare the strategy of the sequential treatments with the monotherapy approach. These comparisons are presented in this report at 8.1 years median follow-up (12 years after the first patient was entered). During the past 12 years, evolving data from other trials and the selective crossover of one quarter of the tamoxifen-treated patients to a more effective treatment in this trial after the trial’s first report in 2005, led to the adaptation of analysis plans to present the most accurate and clinically-useful long-term results to the oncology community.
At the time this trial opened, the role of aromatase inhibitors for use “upfront” in early breast cancer was being tested in only one other trial, the ATAC trial, and no results of such therapy for this indication were available for early breast cancer. BIG 1-98 is the only trial to evaluate the sequence of letrozole for 2 years followed by tamoxifen for 3 years. The first results from ATAC were reported in 2002, and reports of trials investigating the switching to aromatase inhibitors after 2–3 years of tamoxifen began appearing in 2003 and 2004. Thus, when BIG 1-98 was designed in 1998, one could not have predicted the highly statistically and clinically significant improvement in disease-free survival achieved with letrozole compared with tamoxifen, nor the sequence of tamoxifen followed by an aromatase inhibitor compared with tamoxifen.
Interpretation
Letrozole for five years is the best treatment option, on average, for postmenopausal women with hormone receptor-positive early breast cancer. Based on the totality of the evidence from the aromatase inhibitor trials, aromatase inhibitor therapy is recommended to be a part of adjuvant treatment for these women. This update of BIG 1-98 demonstrates the benefit of letrozole for 5 years compared with tamoxifen for 5 years for all endpoints, including overall survival, whether the selective crossover is appropriately accounted for using the IPCW analysis, or whether the intent-to-treat (as randomized) analysis is used. Neither of the sequences of letrozole and tamoxifen is better than letrozole alone, but they may represent useful strategies that can be considered based on the patient’s risk of recurrence, preferences, and treatment tolerability.
Supplementary Material
Acknowledgments
Support: The BIG 1-98 trial was financed by Novartis and coordinated by IBCSG. Other support for the IBCSG: Swedish Cancer Society, The Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group, Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research (SAKK), United States National Cancer Institute Grant CA-75362, Cancer Research Switzerland/Oncosuisse, and the Foundation for Clinical Cancer Research of Eastern Switzerland (OSKK).
Footnotes
Members of the BIG 1-98 Collaborative Group are listed in Webappendix Section 1, available with the full text of this article at [journal website].
Author Contributions
Meredith M. Regan. Data analysis and interpretation, manuscript writing, final approval of manuscript.
Patrick Neven. Data interpretation, acquisition of data, manuscript writing/revising, final approval of manuscript
Anita Giobbie-Hurder. Data analysis and interpretation, manuscript writing/revising, final approval of manuscript.
Aron Goldhirsch. Trial design, data interpretation, manuscript writing/revising, final approval of manuscript
Bent Ejlertsen. Data interpretation, acquisition of data, manuscript writing/revising, final approval of manuscript
Louis Mauriac. Trial design, data interpretation, acquisition of data, manuscript writing/revising, final approval of manuscript
John F. Forbes Trial design, data interpretation, acquisition of data, manuscript writing/revising, final approval of manuscript
Ian Smith. Trial design, data interpretation, manuscript writing/revising, final approval of manuscript
István Láng. Acquisition of data, manuscript writing/revising, final approval of manuscript
Andrew Wardley. Acquisition of data, manuscript writing/revising, final approval of manuscript
Manuela Rabaglio. Medical review of data, data interpretation, manuscript writing/revising, final approval of manuscript.
Karen N. Price. Trial design, data interpretation, manuscript writing, final approval of manuscript.
Richard D. Gelber. Trial design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Alan S. Coates. Trial design, data interpretation, manuscript writing/revising, final approval of manuscript.
Beat Thürlimann. Trial design, data interpretation, acquisition of data, manuscript writing, final approval of manuscript.
Conflicts of Interest
Consultant or Advisory Role: Andrew Wardley, Novartis; Bent Ejlertsen, Pfizer; Stock Ownership: Beat Thürlimann, Novartis; Honoraria: John F. Forbes, AstraZeneca; Andrew Wardley, Novartis; Aron Goldhirsch, Novartis, Pfizer; Beat Thürlimann, AstraZeneca, Novartis; Research Funding: Richard D. Gelber, Novartis; Bent Ejlertsen; Novartis; Travel grants: Alan Coates, Roche and Takeda
No medical writer or editor was involved in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Meredith M. Regan, International Breast Cancer Study Group (IBCSG) Statistical Center, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Boston, MA, USA. Harvard Medical School and Harvard School of Public Health, Boston, MA, USA.
Patrick Neven, Department of Medical Oncology, University Hospital Gasthuisberg, Catholic University of Leuven, Belgium.
Anita Giobbie-Hurder, IBCSG Statistical Center, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Boston, MA, USA.
Aron Goldhirsch, European Institute of Oncology, Milan, Italy, and Swiss Center for Breast Health, Sant’Anna Clinics, Lugano-Sorengo, Switzerland.
Bent Ejlertsen, Danish Breast Cancer Cooperative Group (DBCG), Rigshospitalet, Copenhagen, Denmark.
Louis Mauriac, Fédération Nationale des Centres de Lutte Contre le Cancer, Institut Bergonié, Bordeaux, France.
John F. Forbes, Australian New Zealand Breast Cancer Trials Group, University of Newcastle, Calvary Mater Newcastle, Newcastle, New South Wales, Australia.
Ian Smith, The Royal Marsden Hospital, London, Royal Marsden NHS Trust, Surrey, England, United Kingdom.
István Láng, National Institute of Oncology, Budapest, Hungary.
Andrew Wardley, Christie Hospital NHS Trust, South Manchester University Hospital Trust, Manchester, England, United Kingdom.
Manuela Rabaglio, IBCSG Coordinating Center and Inselspital, Bern, Switzerland.
Karen N. Price, IBCSG Statistical Center and Frontier Science and Technology Research Foundation, Boston, MA, USA.
Richard D. Gelber, IBCSG Statistical Center, Dana-Farber Cancer Institute, Harvard School of Public Health and Frontier Science and Technology Research Foundation, Boston, MA.
Alan S. Coates, International Breast Cancer Study Group and University of Sydney, Sydney, Australia.
Beat Thürlimann, Breast Center, Kantonsspital, St. Gallen and Swiss Group for Clinical Cancer Research (SAKK), Switzerland.
References
- 1.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology Clinical Practice Guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BIG 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 3.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486–92. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 4.BIG 1-98 Collaborative Group. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–76. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colleoni M, Giobbie-Hurder A, Regan MM, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011;29:1117–24. doi: 10.1200/JCO.2010.31.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–46. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 7.Pritchard KI, Sousa B. Long-term follow-up of women in trials of adjuvant therapy for breast cancer: Is it still important? J Clin Oncol. 2011;29:1651–2. doi: 10.1200/JCO.2010.34.2766. [DOI] [PubMed] [Google Scholar]
- 8.Giobbie-Hurder A, Price KN, Gelber RG. Design, conduct, and analyses of Breast International Group (BIG) 1-98: a randomized, double-blind, phase-III study comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Clinical Trials. 2009;6:272–87. doi: 10.1177/1740774509105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–32. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 10.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–18. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 11.Regan MM, Price KN, Giobbie-Hurder A, Thürlimann B, Gelber RD. Interpreting Breast International Group (BIG) 1-98: A randomized, double-blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Breast Cancer Res. 2011;13:209. doi: 10.1186/bcr2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein DM, Schoenfeld DA. Correcting for discretionary treatment crossover in an analysis of survival in the Breast International Group BIG 1-98 Trial by using the inverse probability of censoring weighted method. J Clin Oncol. 2011;29:1093–5. doi: 10.1200/JCO.2010.33.9374. [DOI] [PubMed] [Google Scholar]
- 13.Mauriac L, Keshaviah A, Debled M, et al. Predictors of early relapse in postmenopausal women with hormone receptor-positive breast cancer in the BIG 1-98 trial. Ann Oncol. 2007;18:859–67. doi: 10.1093/annonc/mdm001. [DOI] [PubMed] [Google Scholar]
- 14.Viale G, Regan MM, Dell’Orto P, et al. Which patients benefit most from adjuvant aromatase inhibitors? Results using a composite measure of prognostic risk in the BIG 1-98 randomized trial. Ann Oncol. doi: 10.1093/annonc/mdq738. E-published 18 Feb 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–44. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 16.Lazar AA, Cole BF, Bonetti M, Gelber RD. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: Subpopulation Treatment Effect Pattern Plot. J Clin Oncol. 2010;28:4539–44. doi: 10.1200/JCO.2009.27.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: A systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1–11. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 18.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 19.Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965–71. doi: 10.1200/JCO.2007.14.0228. [DOI] [PubMed] [Google Scholar]
- 20.Phase III randomized study of continuous letrozole versus intermittent letrozole in postmenopausal women with hormone receptor-positive, node-positive, early-stage breast cancer after completion of 4 to 6 years of prior adjuvant endocrine therapy. [accessed 2May2011]; http://www.cancer.gov/clinicaltrials/IBCSG-35-07.
- 21.Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26:3073–82. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 22.Sabnis GJ, Macedo LF, Goloubeva O, Schayowitz A, Brodie AM. Stopping treatment can reverse acquired resistance to letrozole. Cancer Res. 2008;68:4518–24. doi: 10.1158/0008-5472.CAN-07-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.