Abstract
The majority of patients with biliary tract carcinoma (cholangiocarcinoma and cancer of the gall bladder) present with advanced, irresectable tumours associated with poor prognosis. Death commonly occurs secondary to recurrent biliary obstruction and intra-biliary sepsis, rather than metastatic disease. The only current potentially curative treatment is surgical resection, but this remains possible in less than a third of patients.
The incidence and mortality rates associated with biliary tract carcinoma continue to rise, mandating the development of novel strategies for early detection, improved resection techniques and treatment of residual lesions. However, there remains limited data on the use of neo-adjuvant and adjuvant techniques and much of the literature to date concerns palliation of inoperable disease. Here, we review the current evidence base for surgical, neo-adjuvant and adjuvant management techniques in biliary tract carcinoma.
Keywords: Biliary Tract Carcinoma, Cholangiocarcinoma, Gall Bladder Carcinoma, Surgery, Neo-Adjuvant Therapy, Adjuvant Therapy
Introduction
Biliary tract carcinoma (BTC) comprises both cholangiocarcinoma and cancer of the gall bladder. Over 90% of BTCs are adenocarcinomas that arise from the epithelia of the biliary tract [1] and BTC is therefore often considered as one pathological entity. BTC has an incidence of 1–2 per 100,000 UK population [2, 3], although the UK and worldwide incidence (particularly of intra-hepatic BTC [4]), and mortality rates associated with BTC continue to rise [3–6].
Approximately 60% of BTC patients are male and in their 7th decade of life [7], as well as often being heavy smokers or diabetics [4]. Further known associations include primary sclerosing cholangitis (PSC), chronic or recurrent biliary infection, cirrhosis, hepatitis C, Caroli disease and hepatolithiasis.
BTC is characterized by the gradual infiltration of, and spread along, the biliary tract, often progressing locally for prolonged periods of time prior to diagnosis. The natural history of the disease is the gradual development of cholestasis, cholangitis and hepatic failure, and the majority of patients present late in the course of disease with signs of biliary obstruction and sepsis. Death commonly results from recurrent or refractory biliary obstruction and intra-biliary sepsis, rather than metastatic disease.
The increasing incidence and mortality rates associated with BTC mandate the development of techniques for early detection of lesions, improved resection and treatment of inoperable and residual disease following surgery. Here, a search strategy incorporating PubMed and Medline search engines and utilising the key words biliary tract carcinoma; cholangiocarcinoma; gall bladder carcinoma; management; surgery; chemotherapy; radiotherapy; photodynamic therapy; and radiofrequency ablation, was used to review the current evidence base for surgical, adjuvant and neo-adjuvant management techniques in BTC.
Surgical Management Strategies in BTC
Pre-Operative Staging
Surgery is currently the only potentially curative treatment for BTC, but is appropriate in only 13–55% of cases [4, 8–11]. Assessment of suitability for surgical resection relies upon accurate pre-operative staging to identify tumour origin and extent, involvement of vascular structures such as the portal vein and hepatic artery, and the presence or absence of extrahepatic metastases. However, insidious infiltration along bile ducts, associated with the small volume and multifocal nature of many tumours means that BTC is commonly understaged by cross-sectional imaging modalities.
Approximately 60–70% of BTC arise in the perihilar region (Klatskin tumours), involving the main extrahepatic bile duct and potentially extending to, and through, the bile duct bifurcation at the level of the liver [1]. 20–30% of tumours arise outside the liver from the common bile duct (CBD) beyond the cystic duct, sometimes extending to the ampulla, and often leading to strictures rather than compressive masses [1]. Such extrahepatic lesions generally present with painless jaundice although approximately 10% will present with signs of sepsis and cholangitis. Tumours arising from smaller bile ducts within the liver parenchyma often form a solitary mass that contribute to non-specific symptoms such as right upper quadrant discomfort, anorexia and nausea
The surgical staging of BTC remains problematic in that multiple descriptions of tumour staging exist (e.g. TNM and Bismuth staging) [12, 13], many of which are based upon differing clinical or surgical information, thus making comparison of trial data difficult. Traditional staging and assessment of resectability relies upon imaging of the abdomen and chest to exclude metastatic disease and should take place prior to procedures such as endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC) that can induce inflammation and render staging and surgery more complex [14]. Magnetic resonance imaging (MRI) with contrast angiography offers accurate and reliable information on biliary anatomy, local invasion and extent of ductal and vascular involvement; whereas, high resolution computed tomography (CT) provides high quality information on the extent of tumour spread outside the biliary system. Endoscopic ultrasound can give further information upon the depth of potential lesions and the identification of regional lymph nodes, as well as assisting in the biopsy of suspicious areas [15]. Malignant invasion of the portal bifurcation is of particular relevance and can be identified by PTC with some success [16, 17]. Positron-emission computed tomography (PET-CT) may increasingly become important in the determination of local and distant disease spread [18]: a recent study demonstrated that high tracer uptake was a significant prognostic factor for overall survival in patients with BTC, and may therefore be a useful adjunct in guiding treatment strategies [19]. However, the current use of PET-CT remains limited by the large proportion of BTC patients that present with cholangitis, which renders interpretation of results difficult.
Involvement of the hepatic artery or portal vein was previously considered a common contraindication to resection; however, many centres now advocate en bloc resection of vascular structures with vascular reconstruction [20]. Current contraindications to surgery include extension of the tumour to segmental bile ducts of both right and left liver lobes, and significant pre-existing lobar atrophy [11]. Up to one third of presenting patients are found to have lymph node, peritoneal or hepatic involvement upon radiological imaging [21]; however, staging laparoscopy may be crucial in assessment of disease extent, even in those patients assessed as resectable following extensive imaging review, as it can lead to identification of unresectability criteria in up to a third of patients [21, 22].
Pre-Operative Optimisation
Preoperative jaundice is an independent risk factor for poor post-operative outcome [23]; however, studies investigating the effects of pre-operative biliary drainage have shown that, although they are effective in reducing bilirubin levels [23], such techniques confer no significant effect upon post-operative morbidity and mortality rates [24]. Further, in patients with distal bile duct obstruction preoperative biliary drainage has been associated with increased rates of cholangitis and prolonged length of hospital stay [25]. However, drainage should still be considered in patients at high-risk of cholangitis and liver failure from unrelieved biliary obstruction [6, 14].
Patients with unresolving jaundice following drainage, and those scheduled to undergo significant hepatic resection with a predicted future liver remnant of less than 25%, may require ipsilateral portal vein embolisation or ligation to promote compensatory hypertrophy of the residual liver remnant, thus improving hepatic reserve and reducing post-operative liver dysfunction [26–28]. However, the precise indications, approaches and types of embolic material to be used for portal vein embolisation, as well as the most optimal method of assessing the function of the future liver remnant, vary between centres and there are no randomised controlled trials to guide management [29].
Surgical Resection of BTC
The precise nature and extent of surgery depends upon tumour location and spread. Intrahepatic tumours necessitate segmental liver resection; whereas, extrahepatic tumours affecting the common bile duct (CBD) require resection of the liver hilum, biliary tree and lymphatics, with additional partial hepatic resection if the bile duct confluence is also affected [30]. Hilar tumours often require concurrent resection of an affected caudate lobe. [6] More distal biliary tract cancers can be managed by Whipple’s pancreaticoduodenectomy.
Short-Term Outcome
Although the safety of liver resection procedures has increased in recent years [31, 32], complications including bile leakage, intra-abdominal abscess and post-operative liver failure occur in up to 29% of patients [33], and are increased by the presence of significant pre-operative co-morbidities; pre-operative hyperbilirubinaemia, poor nutritional status and hypoalbuminaemia; and post-operative complications such as cholangitis [34, 35]. Thus, the necessity for aggressive procedures, to provide potentially curative outcomes, must be balanced by the fact that post-operative morbidity and mortality, including the risk of hepatic failure, increases with the size of resection [34, 36].
30-day mortality following resection of hilar BTC is 8.3–25%, with significant complications occurring in 50–65% [9, 11, 20, 34]. A multitude of past series have demonstrated that the most significant factor leading to increased peri-operative morbidity and mortality is the undertaking of extended resection or vascular resection, as compared to wide local excision [26, 37–39]. More recently, however, data suggests that all cause mortality between patients undergoing extended resection (including portal vein resection) and those without, may be similar [40], despite the fact that patients undergoing portal vein resection are likely to have more advanced tumours [20]. Neuhaus et al [36] demonstrated that perioperative mortality rates were comparable for radical and standard resections (13% vs. 10%) with the main cause of death in extended resections being hepatic insufficiency secondary to reduced volume of functioning parenchyma [36]. Surgeons at Nagoya university similarly operated upon 53 patients undergoing concomitant hepatic artery resection and reconstruction, with and without portal vein resection, to demonstrate a post-operative mortality rate of only 2% (1/53) [41].
Portal vein embolisation has been crucial in reducing rates of liver failure following extended hepatectomy from 20% to 6% [41] and has helped to expand the surgical indications for biliary tract cancer [13–15]
Long-Term Outcome
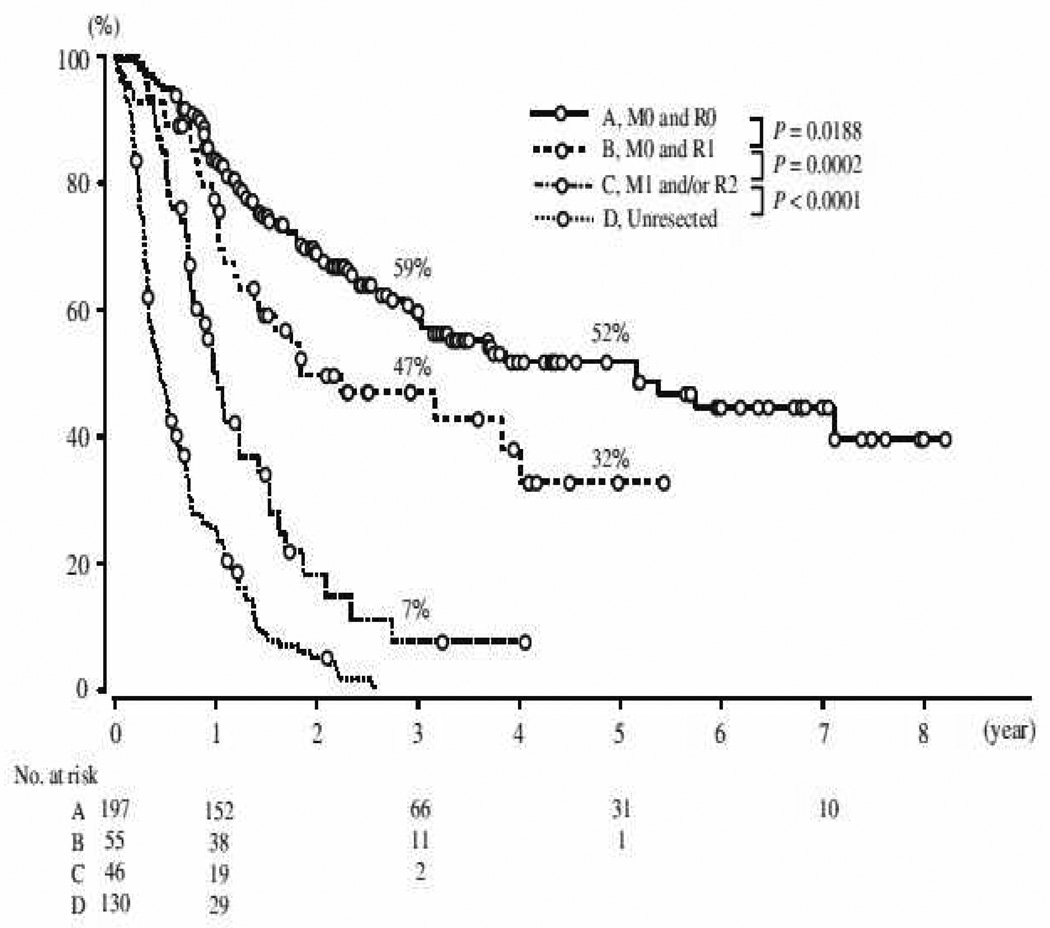
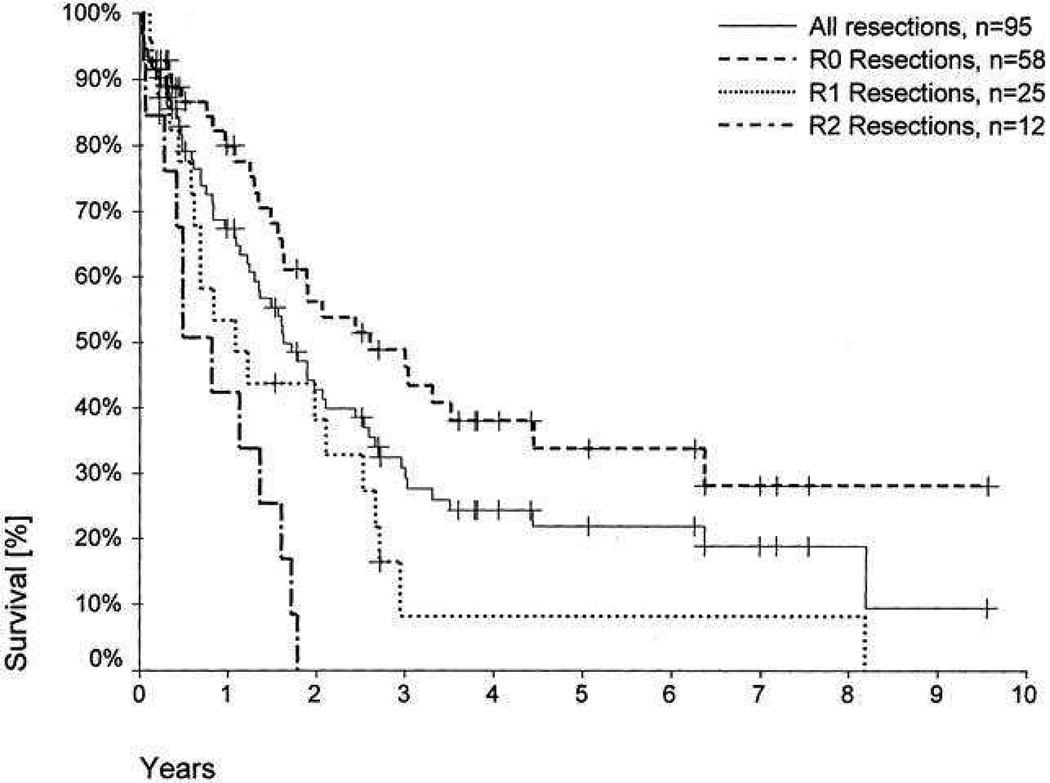
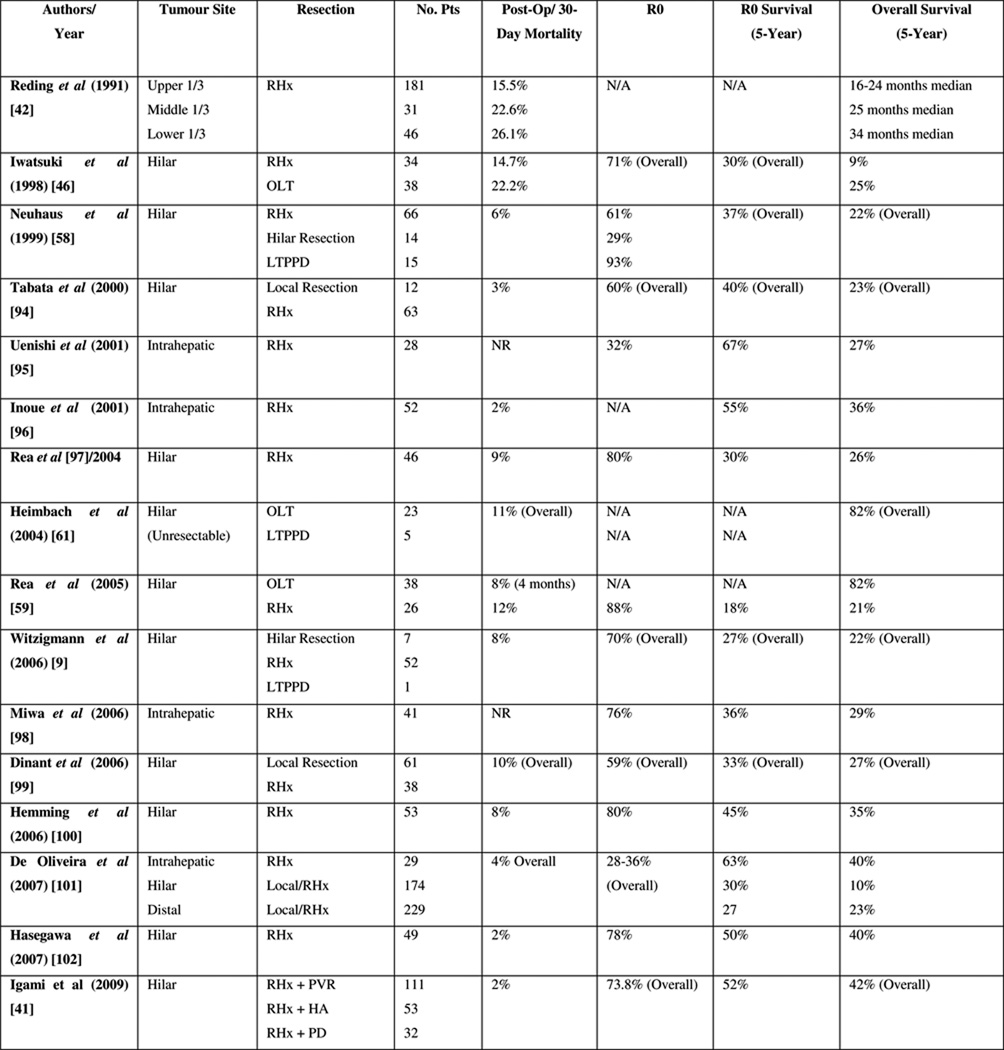
Perihilar BTC is associated with a five-year survival of 10–40% overall and 30–52% following R0 resection; whereas distal BTC carries a five-year survival rate of 23–50% overall and 27–62% in those undergoing R0 resection [6, 9, 42–46] (see Figures 1 & 2). After resection, 5-year survival rates for intrahepatic cholangiocarcinomas vary from 8% to 47% with the highest survival in patients with negative resection margins [35, 37, 47, 48]. Independent prognostic factors for survival include early stage, R0 resection, well-differentiated tumour grade, the absence of multiple hepatic tumours, regional node disease or macroscopic portal vein invasion, and small tumour size [4, 9–11, 20]. Regional lymph node metastases are common with hilar cholangiocarcinoma (up to 35% in a recent series), but there is no evidence that extended lymph node dissection improves survival [49]. However, even following successful resection, recurrence is common and can occur in up to 63% overall within the liver remnant within two years [4].
Figure 1.
Survival curves stratified according to residual tumour status (R) and M staging (TNM), following resection of hilar cholangiocarcinoma (2001–2008; Igami et al) [41]
Figure 2.
Patient survival, according to residual tumour status (R), following resection of hilar cholangiocarcinoma (1988–2000; Neuhaus et al [58])
Achieving microscopically negative resection margins is thus crucial to outcome, as R1 and R2 resections are associated with an overall five-year survival rate of 0% [9]. However, the capacity of BTC to invade and encase local, vital structures at presentation (such as the hepatic artery and portal vein), the limitations of staging techniques and the limited ability to recognise microscopic disease at operation means that difficulties remain in the precise planning and identification of the extent of tissue to resect. Thus, current surgical strategies provide R0 resection rates of only 60–78% [10, 11, 50–52].
Ongoing improvements in pre-operative optimisation and operative technique mean that the number of potential resections continues to increase (from 17% (1985–1994) vs. 69% (1995–2006) in one study [10]) and there is evidence to suggest that recent alterations in surgical approach, such as concomitant, radical, extended liver resection, have led to higher R0 resection rates, improved disease-specific and disease-free survival and decreased incidence of initial recurrence within the liver in patients with resectable BTC [10, 11, 20, 34, 40, 53, 54]. Thus, series since 2000 have reported median overall survival following surgical resection for BTC of 20–36 months overall and up to 65 months following R0 resection [4, 10, 34, 40].
Neuhaus et al [36] compared standard right and left hepatectomy with more radical resection (trisegmentectomy with or without portal vein resection (PVR)) in 133 patients with BTC and reported an improved five-year survival rate (23% and 18% vs. 72% and 52% respectively). Further studies have similarly demonstrated that within cohorts of patients undergoing R0 resection, operative methods of further extending the resection margins (into the bile duct branches and liver, caudate lobe, and vascular structures) may be the only independent predictors of long-term survival [11]. However, deciding exactly how much tissue to resect and how radically to operate remains challenging. Ebata et al [20] illustrated that approximately a third of all resected portal veins were found to be free from tumour and that, although the presence of microscopic portal vein invasion did not influence long-term outcome, the presence of macroscopic portal vein invasion did. Moreover, microscopic invasion of the portal vein can be clinically misdiagnosed, due to dense fibrosis occurring adjacent to the portal vein.
Complete tumour resection appears to offer the best opportunity for long-term survival [4, 9] and improved adjuvant and palliative management techniques should not alter the concept of an aggressive resectional approach [9]. Hepatectomy with bile duct resection and portal vein resection and anastomosis, where necessary, should now be considered as minimal standard surgical treatment for BTC, as it is associated with improved survival and decreased hepatic recurrence [10] and is now being performed in up to 30% of patients [20].
Liver Transplantation
The concept of liver transplantation for BTC, although attractive as it obviates requirements for negative resection margins, was originally criticised because of the high recurrence rate and poor prognosis associated with the technique during early studies [55–57]. More recently, however, patients with unresectable perihilar or intrahepatic BTC, in the absence of extrahepatic disease, have been successfully treated by orthotopic liver transplantation after en bloc excision of the liver, bile ducts and hilar lymph nodes. The concomitant use of neoadjuvant radiation and operative staging has allowed many centres to develop working protocols for liver transplantation in BTC and to achieve histologically negative margins in up to 93% [58] and five-year survival rates of up to 80% in patients with unresectable disease [59–61].
Rea et al [59] published an 11-year experience of orthotopic liver transplantation, preceded by neaoadjuvant chemoradiation, in 38 patients with operatively-confirmed stage I and II hilar BTC. All patients were deemed unresectable or had BTC on a background of PSC and the study was the first to incorporate a contemporary, comparative resection group. One-, three-, and five-year patient survival rates were 92%, 82%, and 82% after transplantation (82%, 48%, and 21% after resection (P = 0.022)) and there were significantly fewer recurrences in the transplant patients (13% vs. 27%).
However, inherent difficulties remain upon comparison of unresectable, transplanted and resectable, resected patients, particularly as all transplanted patients in this series underwent neo-adjuvant therapy, were younger and had node-negative disease removed by R0 operation [62]. However, the resultant survival and recurrence figures remain impressive given the poor outcomes associated with intrahepatic or perihilar BTC on a background of PSC [63], and the fact that recurrence rates of up to 76% can occur at either the site of anastomosis or intrahepatically, even in those patients with R0 resection margins following radical surgery [36, 58].
The model for end-stage liver disease (MELD) criteria was subsequently proposed [64] with exceptions made for liver transplant candidates with malignant strictures, or hilar BTC of less than 3cm, in the absence of regional lymph node and peritoneal involvement following neoadjuvant therapy, confirmed by operative staging. Studies to date have also highlighted the importance of neoadjuvant therapy as a crucial determinant in achieving optimal outcomes [59]. Therefore, although the number of potential procedures is likely to be limited by a lack of available donors, orthotopic liver transplantation remains an option for a selected minority of patients, particularly in the context of unresectable disease.
Neo-Adjuvant Management Strategies in BTC
Chemotherapy and chemoradiation
The ABC-02 study has defined the standard of care for advanced biliary tract cancer. The median overall survival (OS) was 11.7 months for a combination of Cisplatin and Gemcitabine and 8.1 months for Gemcitabine alone [65]. Improved progression free survival (PFS) and disease control rates were also demonstrated. Until the publication of these data, non-randomised phase II studies provided the evidence base for treatment of BTC. A systematic review in 2005 identified 13 studies which used gemcitabine alone or in combination with other agents [66]. Three of these studies used a Cisplatin (Cis) and Gemcitabine (Gem) regimen that demonstrated median survivals of 4.6, 6.5 and 10.4 months. A Japanese trial of 83 patients conducted using the same treatment regimens as ABC-02 [67] documented a median OS of 11.2 months in the CisGem arm and 7.7 months in the Gem arm, consistent with ABC-02. The response rate in these lead studies is between 18–26% and would restrict the benefit of a neoadjuvant strategy. Neoadjuvant programmes, such as those in colorectal or breast cancer, are investigating the added benefit of biological agents in addition to standard therapy to improve the response rate as neoadjuvant programmes commonly require response rates of greater than 50% to be sustainable. The French BINGO trial randomised 101 patients to receive gemcitabine and oxaliplatin with or without cetuximab [68]. They reported 4 month PFS rates of 50% in the GEMOX arm and 61% in the GEMOX with cetuximab arm. Mature data will determine the validity of this approach. Neoadjuvant chemoradiation data is limited to a single study. de Aretxabala et al [69] treated 18 gallbladder cancer patients, with invasion deeper than the muscular layer, to five day continuous infusions of neoadjuvant 5-Fluorouracil (5-FU) (at days one and 28) and radiotherapy (total dose 4500 cGy). Following chemoradiotherapy, 15 patients suffered haematological problems and five patients were excluded from surgical treatment. The resulting 13 patients that underwent surgery had no obvious survival benefit compared to those receiving no neoadjuvant chemoradiotherapy. These limited data suggest that neoadjuvant chemoradiation may be of use in highly selected patients and that it may be more effective in cholangiocarcinoma than gallbladder cancer, but there is a need for good quality, randomised trials.
Radiotherapy
Radiotherapy can be applied to BTC via combinations of external beam, intraluminal brachytherapy (often administered via drainage tubes) and radioactive spheres [70]. However, intrahepatic BTC remains difficult to treat via brachytherapy as the catheter and radioactive source cannot easily traverse the hilum; although transcutaneous, image-guided approaches are possible.
Studies to date have made comparison with historical controls and demonstrate contrasting effects of neo-adjuvant treatment in BTC. McMasters et al [71] described a case series of nine patients with extrahepatic cholangiocarcinoma that had previously been considered unresectable, who were treated with external beam radiotherapy. All nine were downstaged sufficiently to be able to undergo surgical resection following radiotherapy, resulting in a 100% negative resection margin rate as compared to only 54% in 31 patients that did not undergo pre-operative treatment.
However, Gonzalez Gonzalez et al [72] prescribed combinations of pre- and post-operative external beam radiotherapy and brachytherapy to 71 patients with resectable proximal bile duct cancer. Preoperative radiotherapy had no impact upon one, three or five year survival rates, although they did find that recurrence within the surgical scar did not occur in patients undergoing preoperative treatment, as compared to 15% of those in which no preoperative radiotherapy was given.
Photodynamic Therapy
Photodynamic therapy (PDT) results in localized tissue necrosis or apoptosis, via the application of either visible or near-infrared light (usually from a low-power, red laser) after prior administration of a photosensitizing agent. Haematoporphyrin derivatives, which are excited by light of a specific wavelength, are most commonly used as photo-sensitising agents, and can be activated by either endoscopic or percutaneous application of a light source. The phototoxicity lasts for 4–6 weeks in decreasing intensity [73]; however, since non-ionising light is used, PDT does not carry the cumulative toxicity associated with radiotherapy. Thus, once a PDT-treated area has healed, it can be treated again as necessary. Interestingly, photosensitising agents such as Photofrin®, Foscan®, LS11, MACE and 5-aminolevulinic acid (ALA) have shown preferential accumulation in malignant biliary tissue, as compared to normal bile duct tissue [74, 75]. In theory, local ablation of spreading tumour or dysplastic epithelium prior to surgery may help to improve R0 resection rates and increase survival, but neo-adjuvant studies are lacking to date.
Berr et al [76] gave Photofrin® PDT to a single patient with hilar BTC, to illustrate tumour necrosis within the bile duct to a depth of 4–5mm, resulting in local control for 18 months. Wiedmann et al [77] subsequently described a case series of seven patients with advanced proximal bile duct cancer that were treated with neoadjuvant Photofrin® PDT. PDT resulted in R0 resections being achieved in all patients, with tumour recurrence occurring in only two patients, at six and nine months post-surgery. No functionally relevant stricture formation was observed at the biliary-enteric anastomoses (median follow up 15 months), viable tumour cells were absent from the inner 4 mm layer of the surgical specimens and one-year recurrence-free survival was 83%. Median survival was 11.2 months, although all the patients eventually progressed locally or systemically.
The limited information to date suggests that neoadjuvant PDT of localized BTC has the potential to reduce the rates of positive resection margins and local disease recurrence, but requires further study.
Adjuvant Management Strategies in BTC
The rate of local recurrence of BTC remains high and improvements in survival would occur if methods were available for reducing the rate of loco-regional recurrence. Currently there is no established treatment protocol for those patients who undergo attempted curative resection for BTC but in whom negative histological margins are not achieved. Perineural invasion has been shown to be an independent prognostic factor in BTC [78] and may be a helpful indicator in selecting patients for adjuvant therapy. Adjuvant studies in BTC to date have incorporated small numbers, with minimal or no controls, and with heterogeneous cohorts of resected and non-resected patients.
Radiotherapy
One of the major challenges to the efficacy of radiotherapy in BTC is the deep-seated nature of the tumour, combined with the presence of multiple superficial and radio-sensitive structures in the treatment path. Intra-operative radiotherapy allows radiation to be applied closely to the target focus, whilst excluding more radio-sensitive tissues, and may help to minimise the risk of complications occurring secondary to higher radiation doses and enlarged radiation fields.
Certainly in peri-hilar BTC, some studies appear to demonstrate significantly improved loco-regional control rates [79] and survival rates [79–81] in patients undergoing various combinations of intra- and postoperative radiotherapy. Limited experiences have been described but adjuvant radiotherapy may also increase survival in extrahepatic BTC, although loco-regional recurrence remains the commonest cause of treatment failure [82]. Similarly, adjuvant radiotherapy appears to prolong local control rates [83] and survival [82–84] in patients with advanced gallbladder cancer.
However, there are a number of contrasting studies appearing to demonstrate that radiotherapy may not prolong survival [85], and may in fact contribute to significant side-effects and decreased quality of life [72, 81]. Complications may occur in up to 88% of patients treated with radiation [81], with abdominal pain and cholangitis occurring in almost half the patient population and representing the commonest side-effects of treatment [81]. Brachytherapy, which is usually associated with reduced complications due to the precise placement of radiation sources and the subsequent localised effect, has also been associated with significant morbidity with one study reporting retrograde bile leaks in almost a quarter of patients [81]. Further, patients receiving a total radiation dose above 55 Gy may even have a reduced survival [72].
A selection bias may be inherent in many reports to date as they tend to compare medically-fit patients with resectable, favourable tumours undergoing radiotherapy, with control groups, often composed of patients with unresectable tumours, metastatic disease or poor performance status. Pitt et al [86] attempted to circumvent this problem by stratifying 31 resected and 19 operatively-palliated patients, all of whom were confirmed to have perihilar BTC with no metastases, a patent portal vein and a good quality of life to receive combination radiation therapy (n=23) or not (n=27). Among the resected patients, radiation had no effect upon mean, median or actuarial survival, with similar results seen in the palliated patients.
Gwak et al [87] administered post-operative, external beam radiotherapy (45–54 Gy) to 31 patients with extrahepatic biliary tract cancer and compared them to 47 patients undergoing resection alone. Twenty patients undergoing adjuvant radiation had microscopically-positive resection margins; whereas, 27 in the no radiation group did. There was a trend towards improved 5-year overall survival in radiation patients (21% radiation vs. 11.6% no radiation), although these patients were younger (57 years vs. 65 years). However, patients with positive resection margins who received adjuvant radiation therapy had significantly higher median disease-free survival (21 months vs. 10 months’ p = 0.042). Further, adjuvant radiation patients also had decreased local treatment failure (61.7% vs. 35.6%; p = 0.02). In conclusion, although some studies have demonstrated the usefulness of adjuvant radiotherapy, large, prospective, randomised studies are limited and convincing evidence for radiation therapy as a standard adjuvant treatment for BTC is lacking. There is no evidence to support adjuvant radiotherapy in patients with margin-negative resection margins [86].
Chemo- and Chemoradiotherapy
Most trials evaluating the efficacy of chemotherapuetic agents in BTC have been small, single centre trials combining all types of BTC. Because of the poor response rates with single agent therapy, many centres have begun to combine chemo- and radiotherapy in an attempt to improve efficacy.
Kim et al [88] recently followed resection in 72 extrahepatic BTC patients (47 with negative margins and 25 with positive margins) with post-operative external beam radiotherapy (40Gy) and concomitant boluses of 5-FU (500mg/m²), and monthly maintenance therapy 5-FU (500mg/m²) for the first year. The five-year survival rates were 36% following R0 resection, 35% following R1 resection and 0% following R2 resection. Nakeeb et al [89] similarly reported a series of 140 biliary malignancies over a twelve-year period to show that a regimen of confocal radiation, 5-FU and gemcitabine, employed more frequently since 1998, resulted in better survival (p<0.05) than a less sophisticated regimen used in the early 1990s.
The Mayo clinic reported a five-year survival rate of 64% in 21 completely resected gallbladder cancer patients undergoing external beam radiation (median dose 54Gy) and concurrent 5-FU, a result superior to historical controls from their centre [90]. Similarly, a group from Duke University [91] described their experience of 22 patients with resected, non-metastatic gallbladder cancer who underwent external beam radiotherapy alone (n=4) or radiotherapy with concurrent 5-FU (n=18). In this series, patients with a microscopically positive margin following resection had similar outcomes to patients with a negative margin. Although these studies incorporate small numbers, they appear to support the use of radical resection followed by chemoradiotherapy in BTC.
Interestingly, a combined questionnaire from the IHPBA (International Hepatopancreaticobiliary Association), AHPBA (American Hepatopancreaticobiliary Association) and the American College of Surgeons Oncology Group in 2001/2 (returned from 331 authorities in 39 countries), revealed that adjuvant chemoradiotherapy is currently sparingly employed in Europe (29%) with many more centres in the Americas (71%) and Asia/Pacific regions (55%) utilising this modality [89]. The ongoing UK BILCAP randomised controlled trial, investigating the role of adjuvant chemotherapy with capecitabine (an oral 5-fluorouracil analogue), following surgical resection of BTC, is attempting to further explore these possibilities.
Photodynamic Therapy
Nanashima et al [92] assessed the efficacy of adjuvant PDT in eight patients that underwent surgical resection for BTC. Residual tumour cells were microscopically detected in the hepatic duct stump in six patients; one patient developed bile duct occlusion due to recurrence and in one patient biliary stenosis developed secondary to remnant tumor. Via cholangioscopy, the group observed marked destruction of both the tumour and ductal epithelium on the first day after PDT. In patients undergoing PDT of the stump, four showed no tumour recurrence for 17, 12, 12 and six months, respectively. In the two patients with biliary occlusion due to tumour regrowth, resolution of biliary stenosis was noted on day 7.
Adjuvant PDT may therefore be helpful in the treatment of BTC, particularly as the use of intra-operative frozen section is inaccurate and initial reports of negative surgical margins may in fact be found to be R1 on haematoxylin and eosin staining. One option may therefore be to leave a stent, or an external drain, in place across a high risk biliary anastomosis and offer PDT once definitive histology is reported as R1.
Radiofrequency Ablation
RFA may represent another expanding technology capable of providing a significant adjuvant or neo-adjuvant contribution to the treatment of BTC, although data remains limited. Zgodinski et al [93] have described the only case report of RFA to date, in an obese patient with multiple significant co-morbidities and an incidental solitary, primary intrahepatic BTC who remained disease free for 24 months after laparoscopic-guided RFA.
Conclusion
Despite significant advances in the optimisation and surgical management of patients with BTC, the only chance for long-term survival remains surgical resection with negative pathological margins or liver transplantation. Unfortunately, this remains possible in only a minority of selected patients and both neo-adjuvant and adjuvant techniques currently provide only limited success in improving survival of those patients with advanced disease or positive margins following surgery. The development of novel strategies and treatment techniques are crucial. However, the shortage of randomized controlled trials in liver surgery is compounded by the low feasibility of conducting an adequately powered RCT in liver surgery, using outcomes such as mortality or specific complications, due to the large study sample sizes that would be required. Further, conclusions of underpowered RCTs should be interpreted with caution. To achieve improved outcomes, better understanding of tumour biology and the influence of various hormones and systemic factors may be necessary.
Figure 3.
R0 resection rates, early mortality and five-year survival rates following surgical resection.
Acknowledgements
JS drafted the manuscript and receives support from the ‘No Surrender Charitable Trust’ as the inaugural recipient of the ‘Jason Boas Fellowship’.
All authors edited the manuscript. All authors read and approved the final manuscript.
The work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health's National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme.
Glossary
- OLT
Orthotopic Liver Transplantation
- LTPPD
Liver Transplantation + Partial pancreaticoduodenectomy
- RHx
Radical Hepatic Resection
- PVR
Portal Vein Resection
- HA
Hepatic Artery Resection
- PD
Pancreaticoduodenectomy
- NR
Not Reported
Footnotes
The authors declare that they have no competing interests.
References
- 1.Nakeeb A, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463–473. doi: 10.1097/00000658-199610000-00005. discussion 473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 3.Taylor-Robinson SD, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut. 2001;48(6):816–820. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endo I, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 5.Khan SA, et al. Cholangiocarcinoma and its management. Gut. 2007;56(12):1755–1756. doi: 10.1136/gut.2007.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan SA, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):VI1–VI9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West J, et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer. 2006;94(11):1751–1758. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215(1):31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witzigmann H, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006;244(2):230–239. doi: 10.1097/01.sla.0000217639.10331.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito F, et al. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008;248(2):273–279. doi: 10.1097/SLA.0b013e31817f2bfd. [DOI] [PubMed] [Google Scholar]
- 11.Jarnagin WR, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–517. doi: 10.1097/00000658-200110000-00010. discussion 517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blechacz BR, Sanchez W, Gores GJ. A conceptual proposal for staging ductal cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25(3):238–239. doi: 10.1097/MOG.0b013e3283292383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matull WR, Khan SA, Pereirai SP. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2007;99(5):407. doi: 10.1093/jnci/djk068. author reply 407-8. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CD, et al. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 2004;9(1):43–57. doi: 10.1634/theoncologist.9-1-43. [DOI] [PubMed] [Google Scholar]
- 15.Gleeson FC, et al. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67(3):438–443. doi: 10.1016/j.gie.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Williamson BW, Blumgart LH, McKellar NJ. Management of tumors of the liver. Combined use of arteriography and venography in the assessment of resectability, especially in hilar tumors. Am J Surg. 1980;139(2):210–215. doi: 10.1016/0002-9610(80)90256-1. [DOI] [PubMed] [Google Scholar]
- 17.Nishio H, et al. Value of percutaneous transhepatic portography before hepatectomy for hilar cholangiocarcinoma. Br J Surg. 1999;86(11):1415–1421. doi: 10.1046/j.1365-2168.1999.01270.x. [DOI] [PubMed] [Google Scholar]
- 18.Corvera CU, et al. 18F–fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206(1):57–65. doi: 10.1016/j.jamcollsurg.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa H, et al. Prognostic importance of standardized uptake value on F-18 fluorodeoxyglucose-positron emission tomography in biliary tract carcinoma. J Surg Oncol. 2009 doi: 10.1002/jso.21356. [DOI] [PubMed] [Google Scholar]
- 20.Ebata T, et al. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238(5):720–727. doi: 10.1097/01.sla.0000094437.68038.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goere D, et al. Utility of staging laparoscopy in subsets of biliary cancers : laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc. 2006;20(5):721–725. doi: 10.1007/s00464-005-0583-x. [DOI] [PubMed] [Google Scholar]
- 22.Corvera CU, Weber SM, Jarnagin WR. Role of laparoscopy in the evaluation of biliary tract cancer. Surg Oncol Clin N Am. 2002;11(4):877–891. doi: 10.1016/s1055-3207(02)00033-9. [DOI] [PubMed] [Google Scholar]
- 23.Grandadam S, et al. Role of Preoperative Optimization of the Liver for Resection in Patients with Hilar Cholangiocarcinoma Type III. Ann Surg Oncol. doi: 10.1245/s10434-010-1168-z. [DOI] [PubMed] [Google Scholar]
- 24.Figueras J, et al. Changing strategies in diagnosis and management of hilar cholangiocarcinoma. Liver Transpl. 2000;6(6):786–794. doi: 10.1053/jlts.2000.18507. [DOI] [PubMed] [Google Scholar]
- 25.van der Gaag NA, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 362(2):129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 26.Nimura Y, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7(2):155–162. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- 27.Hemming AW, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237(5):686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. discussion 691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdalla EK, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137(6):675–680. doi: 10.1001/archsurg.137.6.675. discussion 680-1. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama Y, et al. Recent advances in the treatment of hilar cholangiocarcinoma: portal vein embolization. J Hepatobiliary Pancreat Surg. 2007;14(5):447–454. doi: 10.1007/s00534-006-1193-2. [DOI] [PubMed] [Google Scholar]
- 30.Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):189–199. doi: 10.1055/s-2004-828895. [DOI] [PubMed] [Google Scholar]
- 31.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 32.Khan SA, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37(6):806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 33.van den Broek MA, et al. Feasibility of randomized controlled trials in liver surgery using surgery-related mortality or morbidity as endpoint. Br J Surg. 2009;96(9):1005–1014. doi: 10.1002/bjs.6663. [DOI] [PubMed] [Google Scholar]
- 34.Gerhards MF, et al. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma--a single center experience. Surgery. 2000;127(4):395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]
- 35.Su CH, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223(4):384–394. doi: 10.1097/00000658-199604000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuhaus P, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388(3):194–200. doi: 10.1007/s00423-003-0383-5. [DOI] [PubMed] [Google Scholar]
- 37.Nakeeb A, et al. Improved survival in resected biliary malignancies. Surgery. 2002;132(4):555–563. doi: 10.1067/msy.2002.127555. discission 563-4. [DOI] [PubMed] [Google Scholar]
- 38.Hadjis NS, et al. Outcome of radical surgery in hilar cholangiocarcinoma. Surgery. 1990;107(6):597–604. [PubMed] [Google Scholar]
- 39.Pichlmayr R, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg. 1996;224(5):628–638. doi: 10.1097/00000658-199611000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kondo S, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240(1):95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Igami T, et al. Surgical treatment of hilar cholangiocarcinoma in the “new era”: the Nagoya University experience. J Hepatobiliary Pancreat Surg. 2009 doi: 10.1007/s00534-009-0209-0. [DOI] [PubMed] [Google Scholar]
- 42.Reding R, et al. Surgical management of 552 carcinomas of the extrahepatic bile ducts (gallbladder and periampullary tumors excluded). Results of the French Surgical Association Survey. Ann Surg. 1991;213(3):236–241. doi: 10.1097/00000658-199103000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aljiffry M, et al. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208(1):134–147. doi: 10.1016/j.jamcollsurg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the extrahepatic bile ducts. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70(6):1498–1501. doi: 10.1002/1097-0142(19920915)70:6<1498::aid-cncr2820700609>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 45.Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70(6):1493–1497. doi: 10.1002/1097-0142(19920915)70:6<1493::aid-cncr2820700608>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 46.Iwatsuki S, et al. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187(4):358–364. doi: 10.1016/s1072-7515(98)00207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron JL, et al. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg. 1990;159(1):91–97. doi: 10.1016/s0002-9610(05)80612-9. discussion 97-8. [DOI] [PubMed] [Google Scholar]
- 48.Nimura Y, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14(4):535–543. doi: 10.1007/BF01658686. discussion 544. [DOI] [PubMed] [Google Scholar]
- 49.Kitagawa Y, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233(3):385–392. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford) 2005;7(4):259–262. doi: 10.1080/13651820500373010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hidalgo E, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur J Surg Oncol. 2008;34(7):787–794. doi: 10.1016/j.ejso.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Silva MA, et al. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol. 2005;31(5):533–539. doi: 10.1016/j.ejso.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 53.Killeen RP, et al. Achievable outcomes in the management of proximal cholangiocarcinoma: an update prepared using “evidence-based practice” techniques. Abdom Imaging. 2008;33(1):54–57. doi: 10.1007/s00261-007-9312-3. [DOI] [PubMed] [Google Scholar]
- 54.Lee SG, et al. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7(2):135–141. doi: 10.1007/s005340050167. [DOI] [PubMed] [Google Scholar]
- 55.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69(8):1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 56.Robles R, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239(2):265–271. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alessiani M, et al. Assessment of five-year experience with abdominal organ cluster transplantation. J Am Coll Surg. 1995;180(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 58.Neuhaus P, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230(6):808–818. doi: 10.1097/00000658-199912000-00010. discussion 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rea DJ, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242(3):451–458. doi: 10.1097/01.sla.0000179678.13285.fa. discussion 458-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rea DJ, et al. Transplantation for cholangiocarcinoma: when and for whom? Surg Oncol Clin N Am. 2009;18(2):325–337. doi: 10.1016/j.soc.2008.12.008. ix. [DOI] [PubMed] [Google Scholar]
- 61.Heimbach JK, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24(2):201–207. doi: 10.1055/s-2004-828896. [DOI] [PubMed] [Google Scholar]
- 62.Callery MP. Transplantation for cholangiocarcinoma: Advance or supply-demand dilemma? Gastroenterology. 2006;130(7):2242–2244. doi: 10.1053/j.gastro.2006.03.056. discussion 2244. [DOI] [PubMed] [Google Scholar]
- 63.Rosen CB, et al. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991;213(1):21–25. doi: 10.1097/00000658-199101000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wiesner R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 65.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 66.Dingle BH, Rumble RB, Brouwers MC. The role of gemcitabine in the treatment of cholangiocarcinoma and gallbladder cancer: a systematic review. Can J Gastroenterol. 2005;19(12):711–716. doi: 10.1155/2005/565479. [DOI] [PubMed] [Google Scholar]
- 67.Okusaka T, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 103(4):469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tournigand C, Malka D, Desrame J. [45th Congress of the American Society of Clinical Oncology (ASCO) Orlando, May 30th–June 2nd 2009] J Chir (Paris) 2009;146(3):311–315. doi: 10.1016/j.jchir.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 69.de Aretxabala X, et al. Preoperative chemoradiotherapy in the treatment of gallbladder cancer. Am Surg. 1999;65(3):241–246. [PubMed] [Google Scholar]
- 70.Saito H, et al. Radiation therapy and photodynamic therapy for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15(1):63–68. doi: 10.1007/s00534-007-1281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMasters KM, et al. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174(6):605–608. doi: 10.1016/s0002-9610(97)00203-1. discussion 608-9. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez Gonzalez D, et al. Role of radiotherapy, in particular intraluminal brachytherapy, in the treatment of proximal bile duct carcinoma. Ann Oncol. 1999;10(Suppl 4):215–220. [PubMed] [Google Scholar]
- 73.Zoepf T. Photodynamic therapy of cholangiocarcinoma. HPB (Oxford) 2008;10(3):161–163. doi: 10.1080/13651820801992625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pahernik SA, et al. Distribution and pharmacokinetics of Photofrin in human bile duct cancer. J Photochem Photobiol B. 1998;47(1):58–62. doi: 10.1016/s1011-1344(98)00203-6. [DOI] [PubMed] [Google Scholar]
- 75.Kiesslich T, et al. Comparative characterization of the efficiency and cellular pharmacokinetics of Foscan- and Foslip-based photodynamic treatment in human biliary tract cancer cell lines. Photochem Photobiol Sci. 2007;6(6):619–627. doi: 10.1039/b617659c. [DOI] [PubMed] [Google Scholar]
- 76.Berr F, et al. Neoadjuvant photodynamic therapy before curative resection of proximal bile duct carcinoma. J Hepatol. 2000;32(2):352–357. doi: 10.1016/s0168-8278(00)80083-5. [DOI] [PubMed] [Google Scholar]
- 77.Wiedmann M, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer. 2003;97(11):2783–2790. doi: 10.1002/cncr.11401. [DOI] [PubMed] [Google Scholar]
- 78.Shirai K, et al. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2008;32(11):2395–2402. doi: 10.1007/s00268-008-9726-2. [DOI] [PubMed] [Google Scholar]
- 79.Todoroki T, et al. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys. 2000;46(3):581–587. doi: 10.1016/s0360-3016(99)00472-1. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez Gonzalez D, et al. Results of radiation therapy in carcinoma of the proximal bile duct (Klatskin tumor) Semin Liver Dis. 1990;10(2):131–141. doi: 10.1055/s-2008-1040466. [DOI] [PubMed] [Google Scholar]
- 81.Gerhards MF, et al. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27(2):173–179. doi: 10.1007/s00268-002-6434-1. [DOI] [PubMed] [Google Scholar]
- 82.Fields JN, Emami B. Carcinoma of the extrahepatic biliary system--results of primary and adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 1987;13(3):331–338. doi: 10.1016/0360-3016(87)90006-x. [DOI] [PubMed] [Google Scholar]
- 83.Todoroki T, et al. Benefits of combining radiotherapy with aggressive resection for stage IV gallbladder cancer. Hepatogastroenterology. 1999;46(27):1585–1591. [PubMed] [Google Scholar]
- 84.Todoroki T, et al. Resection combined with intraoperative radiation therapy (IORT) for stage IV (TNM) gallbladder carcinoma. World J Surg. 1991;15(3):357–366. doi: 10.1007/BF01658729. [DOI] [PubMed] [Google Scholar]
- 85.Stein DE, et al. Positive microscopic margins alter outcome in lymph node-negative cholangiocarcinoma when resection is combined with adjuvant radiotherapy. Am J Clin Oncol. 2005;28(1):21–23. doi: 10.1097/01.coc.0000139017.90599.f5. [DOI] [PubMed] [Google Scholar]
- 86.Pitt HA, et al. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221(6):788–797. doi: 10.1097/00000658-199506000-00017. discussion 797-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gwak HK, et al. Extrahepatic Bile Duct Cancers: Surgery Alone Versus Surgery plus Postoperative Radiation Therapy. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Kim S, et al. Role of postoperative radiotherapy in the management of extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2002;54(2):414–419. doi: 10.1016/s0360-3016(02)02952-8. [DOI] [PubMed] [Google Scholar]
- 89.Nakeeb A, Pitt HA. Radiation therapy, chemotherapy and chemoradiation in hilar cholangiocarcinoma. HPB (Oxford) 2005;7(4):278–282. doi: 10.1080/13651820500373028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kresl JJ, et al. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys. 2002;52(1):167–175. doi: 10.1016/s0360-3016(01)01764-3. [DOI] [PubMed] [Google Scholar]
- 91.Czito BG, et al. Adjuvant external-beam radiotherapy with concurrent chemotherapy after resection of primary gallbladder carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys. 2005;62(4):1030–1034. doi: 10.1016/j.ijrobp.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 92.Nanashima A, et al. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: a preliminary study. J Gastroenterol. 2004;39(11):1095–1001. doi: 10.1007/s00535-004-1449-z. [DOI] [PubMed] [Google Scholar]
- 93.Zgodzinski W, Espat NJ. Radiofrequency ablation for incidentally identified primary intrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11(33):5239–5240. doi: 10.3748/wjg.v11.i33.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tabata M, et al. Surgical treatment for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7(2):148–154. doi: 10.1007/s005340050169. [DOI] [PubMed] [Google Scholar]
- 95.Uenishi T, et al. Histologic factors affecting prognosis following hepatectomy for intrahepatic cholangiocarcinoma. World J Surg. 2001;25(7):865–869. doi: 10.1007/s00268-001-0042-3. [DOI] [PubMed] [Google Scholar]
- 96.Inoue K, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery. 2000;127(5):498–505. doi: 10.1067/msy.2000.104673. [DOI] [PubMed] [Google Scholar]
- 97.Rea DJ, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139(5):514–523. doi: 10.1001/archsurg.139.5.514. discussion 523-5. [DOI] [PubMed] [Google Scholar]
- 98.Miwa S, et al. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol. 2006;41(9):893–900. doi: 10.1007/s00535-006-1877-z. [DOI] [PubMed] [Google Scholar]
- 99.Dinant S, et al. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor) Ann Surg Oncol. 2006;13(6):872–880. doi: 10.1245/ASO.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 100.Hemming AW, et al. Portal vein resection for hilar cholangiocarcinoma. Am Surg. 2006;72(7):599–604. discussion 604-5. [PubMed] [Google Scholar]
- 101.DeOliveira ML, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hasegawa S, et al. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. 2007;31(6):1256–1263. doi: 10.1007/s00268-007-9001-y. [DOI] [PubMed] [Google Scholar]