Abstract
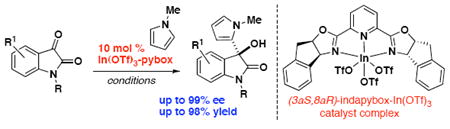
The indium(III)-catalyzed enantioselective and regioselective addition of pyrroles to isatins is described. The effects of metal and solvent on the reactivity and selectivity are compared and discussed, demonstrating that the indium(III)-indapybox complex provides the most effective catalyst. A case of divergent reactivity between pyrroles and indoles is presented.
Oxindoles are significant synthetic targets due to their biological activity and prevelance in various natural products and medicinal compounds.1 Recent reports highlight reactions of various nucleophiles that have been investigated for the synthesis of 3-hydroxy-3-oxindoles.2 Work from our group has previously demonstrated that both scandium(III) and indium(III) triflates catalyze the nucleophilic addition of indoles to isatin electrophiles to afford 3-hydroxy-2-oxindoles in high yields and high enantioselectivity.3 Here we report the first asymmetric catalytic method for pyrrole addition to isatins catalyzed by an indium(III)-pybox complex formed with a 2,6-bis[(3aS,8aR)-3a,8a-dihydro-8H-indeno[1,2-d]oxazolin-2-yl]pyridine ligand.4,5
Enantioselective catalysis with chiral indium complexes is gaining attention due to the catalytic activity and improved stability under atmospheric conditions that indium offers.6 Recent examples of catalysis with chiral indium(III) complexes have demonstrated stereoselective carbonyl ene, Mukaiyama-Michael, and Mukaiyama aldol reactions.7,8 While similar in reactivity to indoles, pyrroles present additional synthetic challenges due to issues of regioselectivity and oligomerization.9 Mixtures of C2 and C3 regioisomeric products are often observed with an inherent bias for the C2 regioisomer based on resonance stabilization.10 Methods for regio- and enantioselective pyrrole additions have been previously reported, often utilizing highly activated electrophiles and low temperatures.11
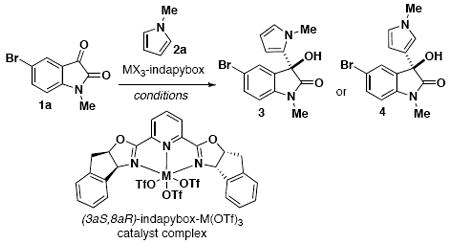
We initially screened various Lewis acidic metal complexes for activation of isatin 1a to optimize the enantioselective addition of N-methylpyrrole (Table 1). Using the scandium(III)-indapybox catalyst afforded the desired 3-hydroxy-2-oxindole product 3 with excellent enantioselectivity (93-98% ee) under various conditions (entries 1-5); however, a mixture of C2- and C3-substituted regioisomers 3 and 4 was consistently observed. Reactions were performed using an excess (2-3 equiv) of pyrrole nucleophile in order to overcome oligomerization and increase the rate of the reaction. Yttrium(III) triflate was also investigated and shown to proceed with good regioselectivity, but lower yield and enantioselectivity were observed (entries 6-9). Further investigations showed that the indium(III) triflate complex is also effective in promoting pyrrole additions with high enantioselectivity, up to 99% ee (entries 10-13).12 Indium(III) chloride showed very low reactivity in all cases (entries 14-17).
Table 1.
Optimization for pyrrole addition[a]
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | metal salt | solvent | t (°C) | time (h) | ratio 3:4[b] | yield (%)[c] | % ee (major)[d] |
| 1 | Sc(OTf)3 | DCM | rt | 24 | 77:23 | 90 | 98 |
| 2 | Sc(OTf)3 | DCM | 4 | 24 | 70:30 | 99 | 93 |
| 3 | Sc(OTf)3 | DCM | −20 | 48 | 77:23 | 89 | 97 |
| 4 | Sc(OTf)3 | MeCN | −20 | 36 | 78:22 | 91 | 98 |
| 5 | Sc(OTf)3 | MeCN | 4 | 36 | 80:20 | 17 | 98 |
| 6 | Y(OTf)3 | DCM | 4 | 120 | nd | <5 | nd |
| 7 | Y(OTf)3 | MeCN | 4 | 72 | 80:20 | 45 | 63 |
| 8 | Y(OTf)3 | DCM | −20 | 168 | 86:14 | 21 | 32 |
| 9 | Y(OTf)3 | MeCN | −20 | 168 | 93:7 | 24 | 60 |
| 10 | In(OTf)3 | DCM | −20 | 60 | 69:31 | 78 | 99 |
| 11[e] | In(OTf)3 | MeCN | −20 | 48 | 98:2 | 98 | 97 |
| 12 | In(OTf)3 | tol | 4 | 48 | 70:30 | 74 | 96 |
| 13 | In(OTf)3 | tol | −20 | 48 | 71:29 | 32 | 67 |
| 14 | InCl3 | DCM | −20 | 168 | nd | <5 | nd |
| 15 | InCl3 | MeCN | −20 | 168 | nd | <5 | nd |
| 16 | InCl3 | DCM | rt | 168 | nd | 10 | 14 |
| 17 | InCl3 | MeCN | rt | 168 | nd | <5 | nd |
All reactions performed under argon with 10 mol % catalyst loading, using 2-3 equiv of pyrrole, with activated 4 Å MS.
Based on 1H NMR analysis of unpurified product; regioisomers are separable by column chromatography.
Represents total yield isolated for both isomers after separation.
Determined by HPLC analysis of unpurified sample with AD-H stationary phase; minor regioisomer is ≥95% ee
Reported as an average of 2 or more reactions.
Varying the temperature and solvent with indium(III) triflate catalyst allowed us to identify optimal conditions for the single addition of N-methylpyrrole with 98:2 regioselectivity and 99% ee (entry 11). Generally, oligomerization was minimized and conversion increased by using acetonitrile at lower temperatures. Although previous reports have shown that Lewis acids can catalyze the condensation of isatin and pyrroles for the synthesis of 3,3-dipyrrolyloxindoles,13 the addition of a second pyrrole was not observed here using these optimized conditions. Formation of such diaryloxindole structures could still be induced under slightly modified conditions in other cases (see Scheme 2). Through this optimization process, it was established that 1) regioselectivity is influenced by both metal and solvent effects; 2) oligomerization can be mitigated by using acetonitrile solvent at lower reaction temperatures; and 3) high enantioselectivity is maintained with the In(OTf)3-pybox complex under a variety of conditions.
Scheme 2.

Conditions controlling product formation
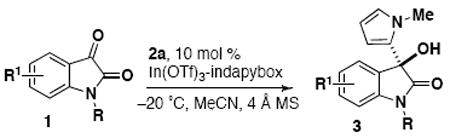
Proceeding with the optimized indium(III) conditions, the scope of isatins was investigated (Table 2). First, a series of various N-alkylated isatins was investigated and shown to maintain high enantioselectivity and regioselectivity. Substitution with methoxy and various halogen groups affords consistently high yields, regioselectivity (≥90:10) and enantioselectivity (94-99% ee). While the 5-chloro N-methylisatin proceeded with high yield and selectivity (entry 2), shifting the chloro substituent to the 4-position had a detrimental effect on the reactivity and only the isatin starting material was recovered (entry 3).
Table 2.
Scope of isatins with N-methylpyrroles[a]
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | R | R1 | time (d) | product | ratio 3:4[b] | yield of 3 (%)[c] | ee (%)[d] |
| 1 | CH3 | 5-Br | 3 | 3a | 98:2 | 98 | 97 |
| 2 | CH3 | 5-Cl | 3 | 3b | 98:2 | 95 | 94 |
| 3 | CH3 | 4-Cl | 5 | 3c | - | no rxn | - |
| 4 | CH3 | 5-F | 3 | 3d | 98:2 | 94 | 97 |
| 5 | CH3 | H | 4 | 3e | >99:1 | 83 | 98 |
| 6 | CH3 | OCH3 | 4 | 3f | >99:1 | 82 | 99 |
| 7 | C3H3 | 5-Br | 4 | 3g | 95:5 | 83 | 98 |
| 8 | C3H3 | 5-F | 3 | 3h | 90:10 | 80 | 99 |
| 9 | C3H3 | OCH3 | 4 | 3i | 99:1 | 91 | >99 |
| 10 | PMB | 5-Br | 3 | 3j | 94:6 | 88 | >99 |
| 11 | PMB | 5-F | 4 | 3k | 93:7 | 84 | >99 |
All reactions performed under argon using 2-3 equiv of pyrrole.
Determined upon 1H NMR analysis of unpurified product.
Isolated yield of major product. Most yields reported as an average of two or more reactions.
Determined by HPLC analysis on chiral stationary phase.
The intolerance of the 4-chloro group suggests that the 4-position is close enough to the electrophilic carbon center to prevent nucleophilic addition due to steric or electronic interference with either the pyrrole nucleophile or the metal-ligand complex. Our previous studies have shown that the 4-chloro group of isatin 1c is still well-tolerated for the enantioselective addition of indoles to isatins with chiral Sc(OTf)3 catalysts,3 which would indicate that the interaction is specific to the case of the pyrrole. Here we have performed a direct comparison for the addition of N-methylpyrrole and N-methylindole with 4-chloro-N-methylisatin (Scheme 1). We have observed that the source of metal does not influence the reaction because the indole reaction proceeds with both scandium(III) and indium(III) conditions, and the pyrrole addition does not proceed with either metal. When the indole and pyrrole nucleophiles are both added to a reaction, the indole addition still proceeds to completion in less than one day. The success of these indole reactions (e.g. 5b and 5c) confirms that the effect is not based on an interaction of the metal-ligand complex with the 4-chloro substituent, but rather that the distinction in reactivity is attributed solely to the nucleophilic species (i.e. pyrrole vs indole).
Scheme 1.
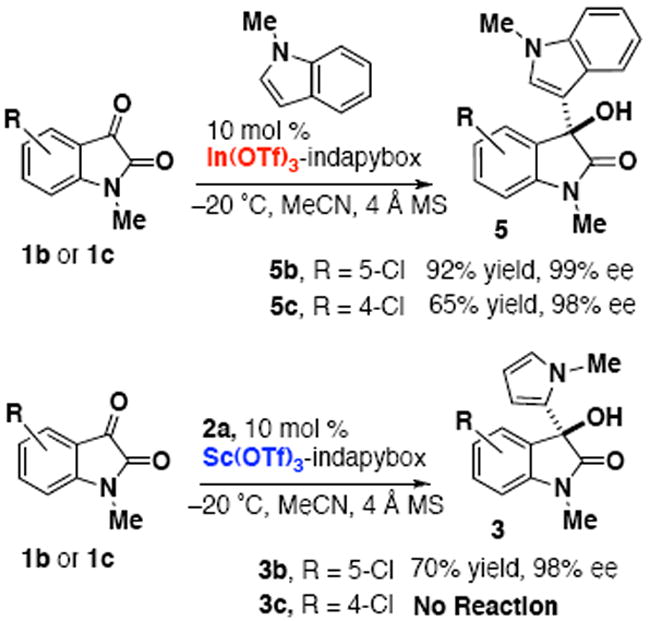
Divergent reactivity of indoles and pyrroles
Further investigation of scope shows that NH isatins also afford products with excellent enantioselectivity and regioselectivity (Table 3). It was noted that reactions containing NH isatins demonstrated a greater sensitivity to air and moisture because the reduced reactivity allowed time for moisture to diminish the reactivity of hygroscopic indium(III) complex. Using more rigorous air-free techniques or adding catalytic NaSbF6 was effective to promote the reaction and maintain high enantioselectivity (entries 4-6).7b,14,15
Table 3.
Scope of NH isatins with N-methylpyrrole
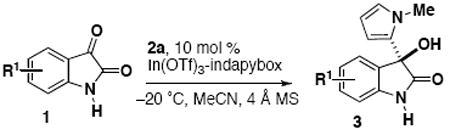 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | time (d) | product | ratio 3:4[a] | yield of 3 (%)[b] | ee (%)[c] |
| 1 | 5-F | 3 | 3l | >99:1 | 86 | >99 |
| 2 | 5-Cl | 3 | 3m | >99:1 | 96 | 98 |
| 3 | 5-OCF3 | 4 | 3n | >99:1 | 87 | >99 |
| 4[d] | H | 3 | 3o | >99:1 | 45 | 95 |
| 5[d] | 5-Br | 3 | 3p | >99:1 | 63 | 99 |
| 6 | 5-OMe | 4 | 3q | >99:1 | 65 | 96 |
Determined using 1H NMR spectroscopy for analysis of unpurified product.
Isolated yield of major product.
Determined by HPLC analysis on chiral stationary phase of unpurified sample.
Reactions performed with 10 mol % NaSbF6.
The addition of NaSbF6 may facilitate the removal of triflate ions and generate a more cationic catalyst complex that would exhibit increased catalytic activity. This outcome is supported by 19F NMR studies for both the In(OTf)3-pybox and Sc(OTf)3-pybox catalyst complexes. In both CD2Cl2 and CD3CN, the 19F NMR spectra show a single peak at −79 ppm for the M(OTf)3-pybox complex, indicating that the triflate ligands are partially or totally dissociated for the metal-ligand complex. The uncoordinated triflate anions are typically observed near −78 ppm, while the coordinated triflate ligand are known to be shifted downfield.16 The coordinating ability of acetonitrile also assists in displacing weakly coordinating triflate ligands. It should be noted that the lack of a signal for the bound triflate cannot be perceived as an indication that all triflate ligands are uncoordinated; bound triflate may not be present in solution in sufficient quantities to be visible by NMR spectroscopy. The Sc(OTf)3 salt alone is insoluble in CD2Cl2 and the In(OTf)3 salt alone is insoluble in both CD2Cl2 and CD3CN.
Next, the scope of pyrrole substrates for nucleophilic addition to isatins was investigated (Table 4). For all cases, enantioselectivity remains high (98-99% ee), but different reactivity was observed based on pyrrole substitution.9 The NH pyrrole (2b) reacts readily to form regioisomer 3r in high 98:2 regioselectivity, but this pyrrole also polymerizes quickly under the reaction conditions to afford only a 60% yield (entry 2). In order to maintain suitable yields with the NH pyrrole substrate, four equivalents of nucleophile were typically employed. The addition of N-benzylpyrrole (2c) also proceeded with a lower conversion (entry 3). The reaction with 2,4-dimethylpyrrole (2d) proceeds quickly and product 3t was observed by ESI-MS; however, product 3t was not stable to silica gel and degraded under purification conditions (entry 4). The reaction with 2-ethylpyrrole (2e) also proceeds quickly; full consumption of starting material is observed in less than one day, affording 50% of the single addition product 3u (entry 5). For the more reactive pyrroles 2d and 2e,9c the product selectivity can be shifted to favor the diaryloxindole product (6t and 6u) by using excess pyrrole (see scheme 2).
Table 4.
Scope of pyrroles with 5-chloro-N-methylisatin
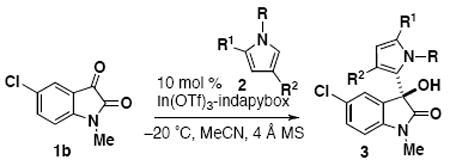 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | R | R1 | R2 | time (d) | product | ratio 3:4[a] | yield of 3 (%) | ee (%)[b] |
| 1 | CH3 | H | H | 3 | 3b | 98:2 | 98 | 97 |
| 2[c] | H | H | H | 2 | 3r | >99:1 | 60 | 99 |
| 3 | Bn | H | H | 2 | 3s | >99:1 | 45 | 99 |
| 4[d] | H | CH3 | CH3 | 4 | 3t | >99:1 | nd[e] | n/a |
| 5[d] | H | Et | H | 1 | 3u | nd | 50[f] | >99 |
Determined upon 1H NMR analysis of unpurified reaction.
Determined by HPLC analysis with chiral stationary phase.
Performed using 4 equiv of pyrrole.
Performed using 1.1 equiv of pyrrole.
Isolated 95% of the 3,3-diaryl product 6t with 5 equiv of pyrrole.
Isolated 52% of the 3,3-diaryl product 6u using 5 equiv of pyrrole.
For pyrroles substituted in the 2-position (e.g. 2d and 2e), reaction conditions can control selective formation of either the 3-hydroxy-2-oxindole, such as 3u, or the 3,3-dipyrrolyloxindole product, such as 6u. Similar 3,3-diaryl-oxindole structures have shown biological activity and thus provide targets of interest.17 Product selectivity for either 3u or 6u is achieved by controling the equivalents of pyrrole nucleophile and temperature (Scheme 2). When only one equivalent of pyrrole 2e is utilized, the reaction affords the 3-hydroxy-2-oxindole product 3u as the major product. However, the diaryloxindole product 6u can be isolated in 52% yield using three equivalents of pyrrole 2e. Likewise, the diaryloxindole product 6t, derived from pyrrole 2d, can be isolated in 95% yield when 5 equivalents of pyrrole are utilized.
In conclusion, we have developed the first enantioselective addition of pyrroles to isatins. The indium(III)-pybox catalyst provides sufficient catalytic activation of isatins, while also controlling the regio- and enantioselectivity for the addition. It was also observed that interactions with a 4-chloro substituent hinder the pyrrole addition in this system, leading to a case of divergent reactivity for indoles and pyrroles.
Supplementary Material
Acknowledgments
We acknowledge the University of California, Davis, and the donors of the American Chemical Society Petroleum Research Fund, and NIH/NIGMS (P41-GM0089153) for support of this research. A.K.F is recipient of a 3M Non-tenured faculty grant and E.G.G. acknowledges the United States Department of Education for a GAANN fellowship. Special thanks to Ngon Tran (UC Davis) for solving the X-ray structure of 3b.
Footnotes
Supporting Information Available: Experimental procedures and spectral data for all compounds; X-ray crystal structure coordinates and files in CIF format for 3b. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For several examples, see: Kagata T, Saito S, Shigemori H, Ohsaki A, Kubota H, Kobayashi J. J Nat Prod. 2006;69:1517. doi: 10.1021/np0602968.. Tokunaga T, Hume WE, Nagamine J, Kawamura T, Taiji M, Nagata R. Bioorg Med Chem Lett. 2005;15:1789. doi: 10.1016/j.bmcl.2005.02.042.. Koguchi Y, Kohno J, Nishio M, Takahashi K, Okuda T, Ohnuki T, Komatsubara S. J Antibiot. 2000;53:105. doi: 10.7164/antibiotics.53.105.. Zhang HP, Kamano Y, Ichihara Y, Kizu H, Komiyama K, Itokawa H, Pettit GR. Tetrahedron. 1995;51:5523.
- 2.For selected examples and reviews, see: Yong SR, Ung AT, Pyne SG, Skelton BW, White AH. Tetrahedron. 2007;63:5579.. Itoh T, Ishikawa H, Hayashi Y. Org Lett. 2009;11:3854. doi: 10.1021/ol901432a.. Badillo JJ, Hanhan NV, Franz AK. Curr Opin Drug Disc. 2010;13:758.. Zhou F, Liu Y-L, Zhou J. Adv Synth Catal. 2010;352:1381.. Zheng K, Yin C, Liu X, Lin L, Feng X. Angew Chem Int Ed. 2011;50:2573. doi: 10.1002/anie.201007145.. Liu L, Zhang S-L, Xue F, Lou G-S, Zhang H-Y, Ma S-C, Duan W-H, Wang W. Chem --Eur J. 2011;17:7791. doi: 10.1002/chem.201101025.
- 3.Hanhan NV, Sahin AH, Chang TW, Fettinger JC, Franz AK. Angew Chem Int Ed. 2010;49:744. doi: 10.1002/anie.200904393. [DOI] [PubMed] [Google Scholar]
- 4.(a) Evans DA, F KR, Song H, Scheidt KA, Xu R. J Am Chem Soc. 2007;129:10029. doi: 10.1021/ja072976i. [DOI] [PubMed] [Google Scholar]; (b) Hargaden GC, Guiry PJ. Chem Rev. 2009;109:2505. doi: 10.1021/cr800400z. [DOI] [PubMed] [Google Scholar]
- 5.In this study, the (S,R)-indapybox ligand was synthesized using a modified procedure, see supporting information and ref: Desimoni G, Faita G, Guala M, Pratelli C. Tetrahedron: Asymmetry. 2002;13:1651.
- 6.For a recent review of indium in organic synthesis, see: Yadav JS, Antony A, George J, Subba Reddy BV. Eur J Org Chem. 2010;2010(4):591.
- 7.(a) Zhao J-F, Tsui H-Y, Wu P-J, Lu J, Loh T-P. J Am Chem Soc. 2008;130:16492. doi: 10.1021/ja807501a. [DOI] [PubMed] [Google Scholar]; (b) Zhao J-F, Tan B-H, Loh T-P. Chem Sci. 2011;2:349. [Google Scholar]; (c) Chua S-S, Alni A, Chan L-TJ, Yamane M, Loh T-P. Tetrahedron. 2011;67:5079. [Google Scholar]
- 8.(a) Hayashi R, Cook GR. Org Lett. 2007;9:1311. doi: 10.1021/ol070235g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Giera DS, Schneider C. Org Lett. 2010;12:4884. doi: 10.1021/ol102023z. [DOI] [PubMed] [Google Scholar]
- 9.For information on pyrrole polymer formation, see: Can M, Özaslan H, Ildak Ö, Pekmez NÖ, Yildiz A. Polymer. 2004;45:7011.. For information on pyrrole regioisomers, see: Voldhardt P, Schore N. Organic Chemistry: Structure and Function. W. H. Freeman and Co.; New York: 2011. . For relative pyrrole reactivities, see: Nigst TA, Westermaier M, Ofial AR, Mayr H. Eur J Org Chem. 2008:2369.
- 10.Yadav JS, Reddy BVS, Abraham S, Sabitha G. Tetrahedron Lett. 2002;43:1565. [Google Scholar]
- 11.For recent examples of stereoselective pyrrole additions with other metals, see: Huang Y, Suzuki S, Shiro M, Shibata N. Org Lett. 2010;12:1136. doi: 10.1021/ol100171z.. Singh PK, Singh VKS. Org Lett. 2010;12:80. doi: 10.1021/ol902360b.
- 12.The regiochemistry and absolute configuration was assigned and confirmed based on X-ray crystallography (anomalous dispersion method) of 3b (see supporting information)
- 13.Yadav JS, SubbaReddy BV, Gayathri KU, Meraj S, Prasad AR. Synthesis. 2006:4121. [Google Scholar]
- 14.(a) Zhao J-F, Tjan T-BW, Loh T-P. Tetrahedron Lett. 2010;51:5649. [Google Scholar]; (b) Zhao J-F, Tjan T-BW, Tan B-H, Loh T-P. Org Lett. 2009;11:5714. doi: 10.1021/ol902507x. [DOI] [PubMed] [Google Scholar]
- 15.While investigating various additives, it was discovered that the use and efficient activation of 4 Å molecular sieves was critical for optimal catalyst performance, affording faster and more efficient conversion. Further investigation into the role of molecular sieves in this reaction is required. See ref: Posner GH, Dai H, Bull DS, Lee J, Eydoux F, Ishihara Y, Welsh W, Pryor N, Petr S. J Org Chem. 1996;61:671. doi: 10.1021/jo9515900.
- 16.Britovsek GJP, England J, Spitzmesser SK, White AJP, Williams DJ. Dalton Trans. 2005;945 doi: 10.1039/b414813d. [DOI] [PubMed] [Google Scholar]
- 17.Paira P, Hazra A, Kumar S, Paira R, Sahu KB, Naskar S, Saha P, Mondal S, Maity A, Banerjee S, Mondal NB. Bioorg Med Chem Lett. 2005;15:1789–1792. doi: 10.1016/j.bmcl.2009.06.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


