Abstract
Immune surveillance by T helper type 1 (Th1) cells is critical for the host response to tumors and infection, but also contributes to autoimmunity and graft-versus-host disease (GvHD) after transplantation. The inhibitory molecule programmed death ligand-1 (PDL1) has been shown to anergize human Th1 cells, but other mechanisms of PDL1-mediated Th1 inhibition such as the conversion of Th1 cells to a regulatory phenotype have not been well characterized. We hypothesized that PDL1 may cause Th1 cells to manifest differentiation plasticity. Conventional T cells or irradiated K562 myeloid tumor cells overexpressing PDL1 converted TBET+ Th1 cells into FOXP3+ regulatory T cells (TREGS) in vivo, thereby preventing human-into-mouse xenogeneic GvHD (xGvHD). Either blocking PD1 expression on Th1 cells by siRNA targeting or abrogation of PD1 signaling by SHP1/2 pharmacologic inhibition stabilized Th1 cell differentiation during PDL1 challenge and restored the capacity of Th1 cells to mediate lethal xGVHD. PD1 signaling therefore induces human Th1 cells to manifest in vivo plasticity, resulting in a TREG phenotype that severely impairs cell-mediated immunity. Converting human Th1 cells to a regulatory phenotype with PD1 signaling provides a potential way to block GvHD after transplantation. Moreover, because this conversion can be prevented by blocking PD1 expression or pharmacologically inhibiting SHP1/2, this pathway provides a new therapeutic direction for enhancing T cell immunity to cancer and infection.
Introduction
CD4+ T-helper (Th) cells of the Th1 phenotype are critical for host protection against tumors(1) and infections(2) but must be tightly regulated to promote self-tolerance(3) and to limit alloimmunity during transplantation therapy(4). Th1 cells are amenable to regulation by multiple mechanisms, such as FAS-mediated antigen-induced cell death(5) and regulatory T (TREG) cell inhibition(3). One such mechanism of regulation is differentiation ‘plasticity’ among Th cell subsets, with functional subset inter-conversion depending on the cytokine micro-environment [reviewed in(6)]. Such plasticity, which has primarily been studied in murine T cells, is most prevalent for TREG and Th17 subsets: TREG cells can morph into Th17 cells upon IL-1 or IL-6 receptor signaling and subsequent STAT3 activation(7, 8), and Th17 cells can switch into a Th1 phenotype under the influence of IL-12 signaling and subsequent STAT4 activation(9).
In contrast, the Th1/Th2 subsets are relatively fixed in their differentiation status (6); nonetheless, counter-regulatory cytokines have long been known to promote Th1 to Th2 conversion(10) or Th2 to Th1 shifts(11) by activating specific transcription factors. Although STAT4 activation is required for initial Th1 polarization(12), STAT1 activation through IFN-α or IFN-γ receptor signaling appears to be the major contributor to Th1 cell stability through promotion of TBET transcription factor expression(13, 14).
Recently, the PD1/PDL1 pathway has emerged as a central player in immune regulation [reviewed in(15)]. Cancer cells that express PDL1 (also known as B7-H1) promote tumor progression through inhibition of PD1-expressing immune effectors(16); in addition, PDL1 modulates cell-mediated immunity in the infectious disease setting(17). Furthermore, allogeneic effector T cell responses are susceptible to PD1 pathway modulation, as evidenced in models of graft-versus-host disease (GvHD)(18) and graft rejection(19). However, there have been no reports in the literature to suggest that PD1/PDL1 interactions play a special role in the modulation of Th1 cell plasticity.
PD1/PDL1 interactions have been characterized previously to inhibit T cell receptor (TCR) signaling by recruiting the SHP-1 and SHP-2 (SHP1/2) phosphatases, which interfere with TCR signaling(20) and induce a TCR stop signal that limits T cell interactions with dendritic cells (DC)(21). Such immune modulation mechanisms are not specific for Th1 cells: For example, PD1 activation inhibits the suppressor function of TREG cells(22) and impairs monocyte immunity(23) in humans infected with hepatitis C. In addition to such direct mechanisms of T cell anergy, PDL1 also indirectly modulates T cells by inducing plasmacytoid DCs, which increase TREG cell numbers and result in TREG cell suppression of anti-tumor responses in a PDL1-dependent manner(24). This DC/TREG cell biology can be bi-directional, as human PDL1-expressing TREGS condition monocytes to express PDL1 and suppress cell-mediated immunity(25). Furthermore, PD1 activation of naïve T cells favors inducible TREG formation by promoting phosphatase and tensin homolog (PTEN) expression and limiting downstream mTOR activation(26). Taken together, this literature indicates that PDL1 modulates Th1 cells directly via anergy or indirectly through TREG cell induction, but there exists no evidence that PDL1 alters Th1 cell differentiation. In this report, using an in vivo model of human-into-mouse xenogeneic graft-versus-host disease (xGVHD), we have determined that PDL1 indeed directly modulates Th1 cell differentiation by promoting a tolerogenic TREG phenotype.
Results
Th1-mediated xenogeneic GVHD is abrogated by TREG cells or PDL1-expressing T cells
We previously found that human TREG cells prevented lethal, Th1 cell-mediated xGVHD through a PDL1-dependent mechanism(25). To directly evaluate the effect of PDL1 on Th1 cell biology, we constructed a lentivirus (LV) incorporating the full-length PDL1 cDNA. The vector, which encoded a fusion protein of TMPK for cell fate control(27) and CD19 for cell surface marking, is referred to as “CD19.TMPK/PDL1-LV” (Supplemental Fig. 1a; vector design). Transduction of purified human CD4+ T cells with a control-LV that expressed the fusion protein alone (CD19.TMPK-LV) or with CD19.TMPK/PDL1-LV yielded nearly 30% productive infection frequency by CD19/PDL1 flow cytometry analyses (Supplemental Fig. 1b left panels[representative data] and right panels[summary of n=7]). The functionalities of the TMPK cell fate control sequence was confirmed in vitro and in vivo by measuring the sensitivity of LV transduced T cells to AZT addition (Supplemental Fig. 2).
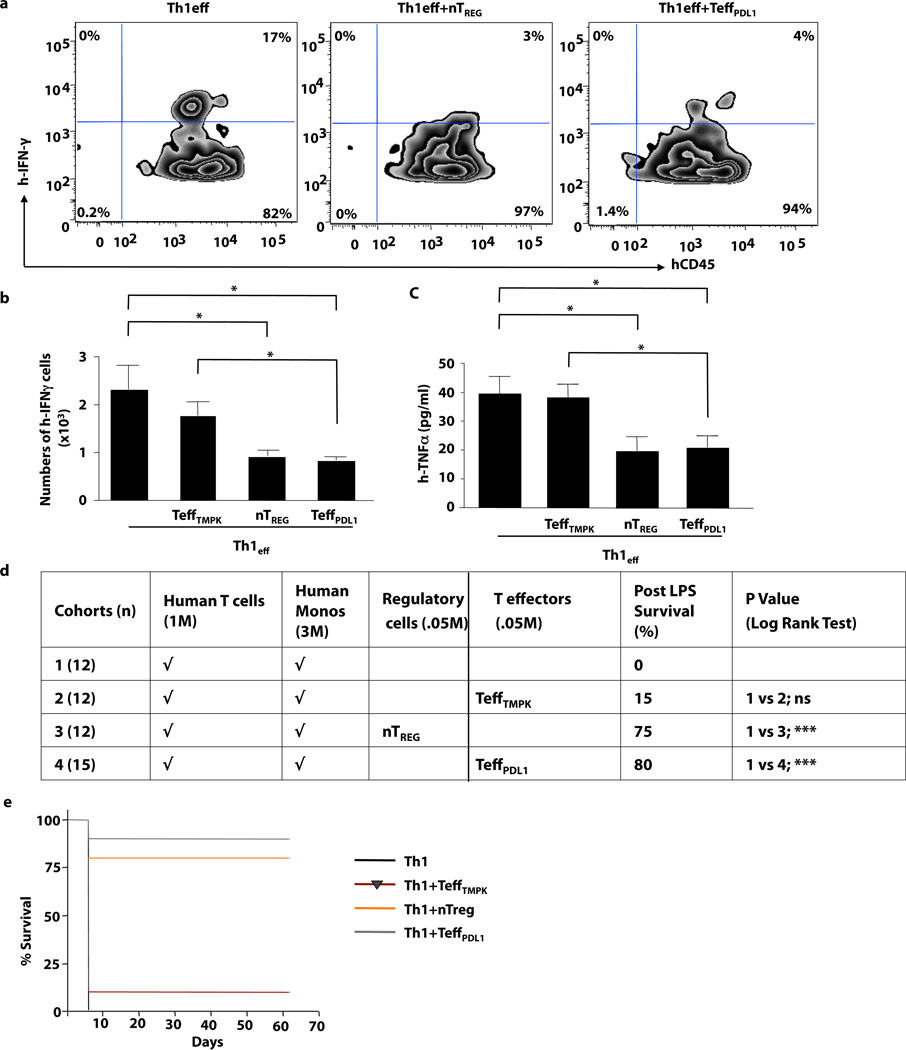
We next compared T cells transduced with CD19.TMPK-LV (TeffPDL1) with purified TREG cells for their capacity to prevent Th1 cell-mediated xGVHD. Th1-polarized human T cells (Th1) were adoptively transferred alone or with either control LV-transduced T cells (TeffTMPK), PDL1 LV-transduced T cells (TeffPDL1), or purified TREGS. Hosts were subsequently challenged with LPS to induce cytokine-mediated xGVHD(28). TREG cells and PDL1 LV-transduced T cells similarly inhibited post-transplant T cell IFN-γ production (Fig. 1a, [representative example] and 1b [summary of n=10 per cohort]), systemic TNF-α production [Fig. 1c], and lethality [Fig. 1d]). Recipients of Th1 cells in combination with TeffPDL1 cells or purified TREG cells were protected long-term from xGVHD through 65 days post-transplant (Fig. 1e). In vivo PDL1 expression on TeffPDL1 cells was stable in these long-term survivors (Supplemental Fig. 1c, d and e). Therefore, forced expression of PDL1 in conventional T cells mimicked TREG therapy for inhibition of Th1 cell-mediated xGVHD.
Figure 1. Th1 Cell-Mediated xGVHD in an LPS Model is Prevented by PDL1-expressing Effector T Cells.
Th1 cells were adoptively transferred to rag2−/−cγ−/− mice alone or in combination with control-LV transduced T cells, Treg cells, or PDL1-transduced T cells. (a) The percentage of IFN-γ secreting human T cells 5 days after adoptive transfer. (b) Absolute number of post-transplant splenic IFN-γ+ human T cells was quantified for each cohort. (c) Recipients were challenged with LPS and serum was harvested after 90 min and evaluated for TNF-α content. (d) Cohorts of host mice received the human cell inocula specified, followed by LPS administration at day 5 after transfer to induce lethal cytokine-mediated xGVHD (n, number per cohort: M, × 106). (e) Survival curve after xGVHD induction (* indicates p ≤ 0.05; *** indicates p ≤ 0.005).
Human Th1 Cells Show Rapid Plasticity In Vivo to a TREG Phenotype via PDL1
To confirm that PDL1 induced Th1 cell differentiation plasticity rather than clonal deletion, the fate of CFSE-labeled Th1 cells was monitored at 24 hours after transplant (representative flow data; Supplemental Fig. 3a, left panel). PDL1 LV-transduced T cells did not abrogate Th1 cell engraftment (Supplemental Fig. 3a, right panel), thereby ruling out a clonal deletion mechanism. PDL1 LV-transduced T cells down-regulated Th1 cell IFN-γ production (Supplemental Fig. 3b) and increased the absolute number of Th1 cells that expressed FOXP3 but did not secrete IFN-γ (Supplemental Fig. 3c). As such, Th1 cells showed differentiation flexibility towards a regulatory phenotype through interactions with PDL1.
PDL1-mediated plasticity occurs in ex vivo generated purified Th1 cells and in vivo-derived pathogenic Th1 cells
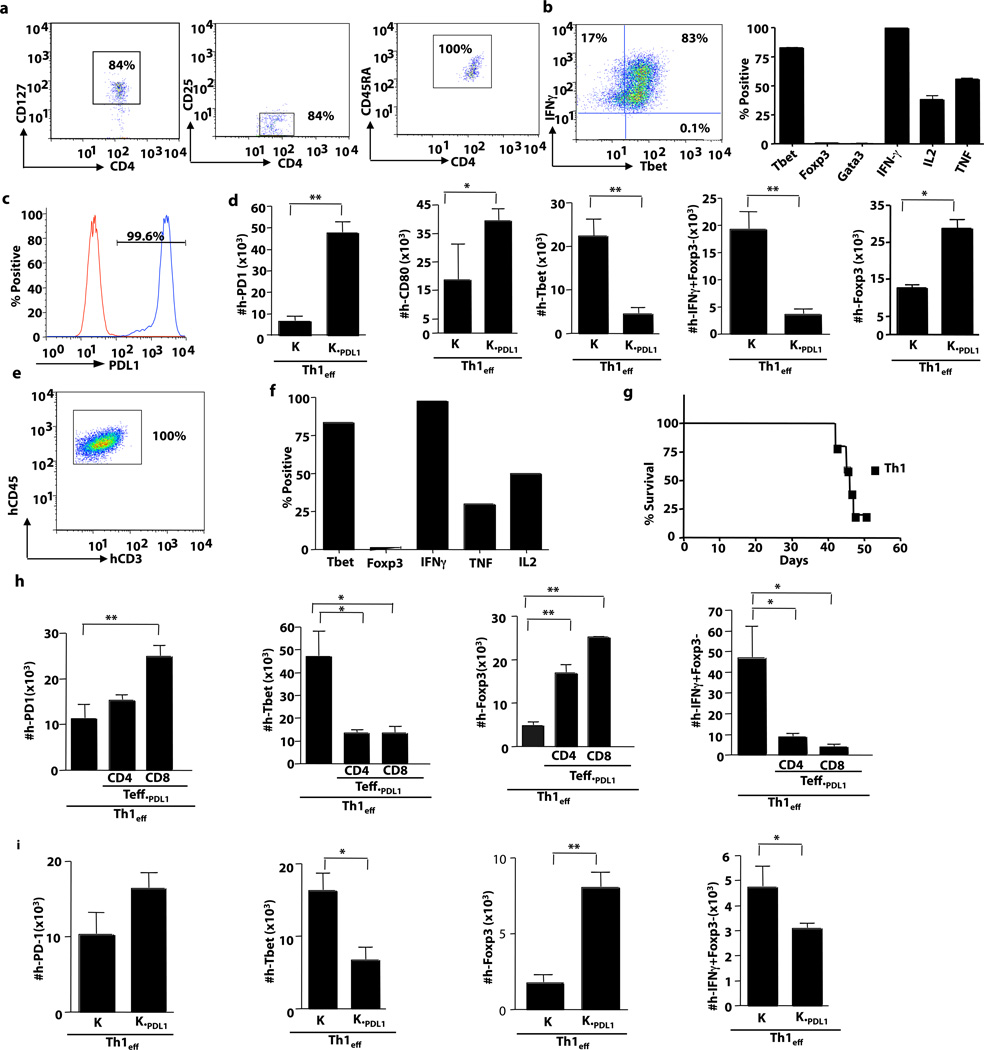
Highly purified human Th1 cells were generated ex vivo using flow sorting to enrich for naïve CD4+ T cells at culture initiation (Fig. 2a) and expansion under Th1 polarizing conditions for an extended, 12-day culture interval (Fig. 2b). Such highly polarized Th1 cells were injected in vivo along with irradiated K562 cells that were transduced with PDL1 (K562PDL1) (Fig. 2c). Post adoptive transfer, K562PDL1 treated human Th1 cells had increased expression of the PDL1 binding partners PD1 and CD80 (Fig.2d, left panels), B7H4 and HVEM Supplemental Fig. 4a), with a concomitant reduction in TBET, IFN-γ (Fig. 2d, middle panels) IL2 and TNFα (Supplemental Fig. 4b, right panels). Deviation from the Th1 phenotype correlated with an increase in FOXP3 expression (Fig.2d, right panel; Supplemental Fig. 4b, middle and left panels).
Figure 2. PDL-1 modulation occurs in highly purified, ex vivo generated Th1 cells and in vivo-derived pathogenic human Th1 cells.
(a) Naïve CD4+ T cells were purified by flow sorting based on their CD127+, CD25−, and CD45RA+ status. Sorted populations were expanded for 12 days in the presence of anti-CD3, -CD28 coated beads in media containing IL-2, an antibody to IL4, and rhIL12. (b) At day 12, Th1 cells were harvested and restimulated with PMA and ionomycin and subjected to intra-cellular flow cytometry. (right panel) Representative flow plot showing percentage of Th1 cells that were IFN-γ+ TBET+. (left panel) Characterization of Th1 cells based on transcription factor profile and cytokine secretion. (c) Representative histogram of PDL1 expression on the transduced K562 tumor cell line (blue);control K562 cell line (red). (d) Expanded Th1 cells were adoptively transferred into NSG mice alone or in combination with K562 cells alone (K) or K562 cells that expressed PDL1 (KPDL1). At day 5 after adoptive transfer, splenocytes were harvested and absolute numbers cells that expressed hPD1 hCD80, hTBET, hFOXP3 and hIFN-γ were quantified. (e) Human Th1 cells were adoptively transferred into NSG mice and flow cytometry was performed at day 50 after transplant to detect human Th1 cell engraftment. (f) Engrafted human Th1 cells were restimulated ex vivo and characterized for transcription factor and cytokine expression. (g) Survival curve for human Th1 cell transfer into NSG hosts. (h) The in vivo-derived, pathogenic human Th1 cells were harvested at day 50 after transplant and transferred to secondary murine recipients either alone or in the presence of CD4+ T cells expressing PDL1 or CD8+ T cells expressing PDL1. At day 5 after adoptive transfer, splenocytes were harvested and absolute numbers of cells expressing hPD1, hTBET, hFOXP3 and hIFN-γ were quantified by flow cytometry. (i) Additional experiments were performed where in vivo-derived pathogenic human Th1 cells were co-injected with irradiated K562 cells (K) or irradiated K562 cells expressing PDL1 (KPDL1). Splenocytes were harvested at day 5 and absolute numbers of cells expressing hPD1, hTBET, hFOXP3 and hIFN-γ were quantified (* indicates p ≤ 0.05, ** indicates p ≤ 0.005).
We further assessed the effect of PDL1 on human Th1 cells that were demonstrated to be pathogenic in vivo on the basis of their causation of xenogeneic GVHD. Such pathogenic human Th1 cells (Fig. 2e) caused lethality in a subset of murine hosts (Fig. 2g) and upon T cell harvest at day 50 after transplant for secondary transfer experiments, were found to express an effector phenotype (Fig. 2f). Similar to in vitro polarized Th1 cells, in vivo-derived human Th1 cells with demonstrable pathogenicity were susceptible to PDL1-mediated plasticity. Such in vivo-derived human Th1 cells were similarly converted to a regulatory phenotype whether the PDL1 was delivered by CD4+ T cells or CD8+ T cells as PDL1 vehicle (Fig. 2h, Supplemental Fig. 4c and 4d); furthermore, PDL1-expressing K562 cells also converted the in vivo-derived human Th1 cells (Fig. 2i, Supplemental Fig. 4e and 4f).
We next characterized the phenotype of the PDL1-expressing CD4+ and CD8+ T cells, and evaluated whether Th1 cell exposure might modulate the PDL1-expressing T cells (reverse signaling). PDL1 transduction increased T cell expression of BTLA ligands (Supplemental Fig. 5a, left panels), galectin 9 (Supplemental Fig. 5a, middle panel), and CD80 (Supplemental Fig. 5a, right panel) and reduced T cell expression of TBET (Supplemental Fig. 5b, left panel) and IFN-γ (Supplemental Fig. 5b, right panel); FOXP3 expression was not altered by PDL1 transduction (Supplemental Fig. 5b, middle panel). PDL1-transduced T cells that were exposed in vivo to Th1 effectors had decreased expression of BTLA ligands, galectin 9, and CD80 (Supplemental Fig. 5c) and maintained low expression of TBET and FOXP3 with no significant alteration in IFN-γ production (Supplemental Fig. 5d). In vitro co-culture of PDL1-expressing T cells with Th1 cells did not significantly alter expression of any of these molecules on the PDL1-expressing T cells (Supplemental Fig. 5e; Supplemental Fig. 5f). In sum, these in vivo data may suggest that reverse signaling occurred in our system.
PDL1-Mediated Th1 to TREG Conversion is Durable In Vivo
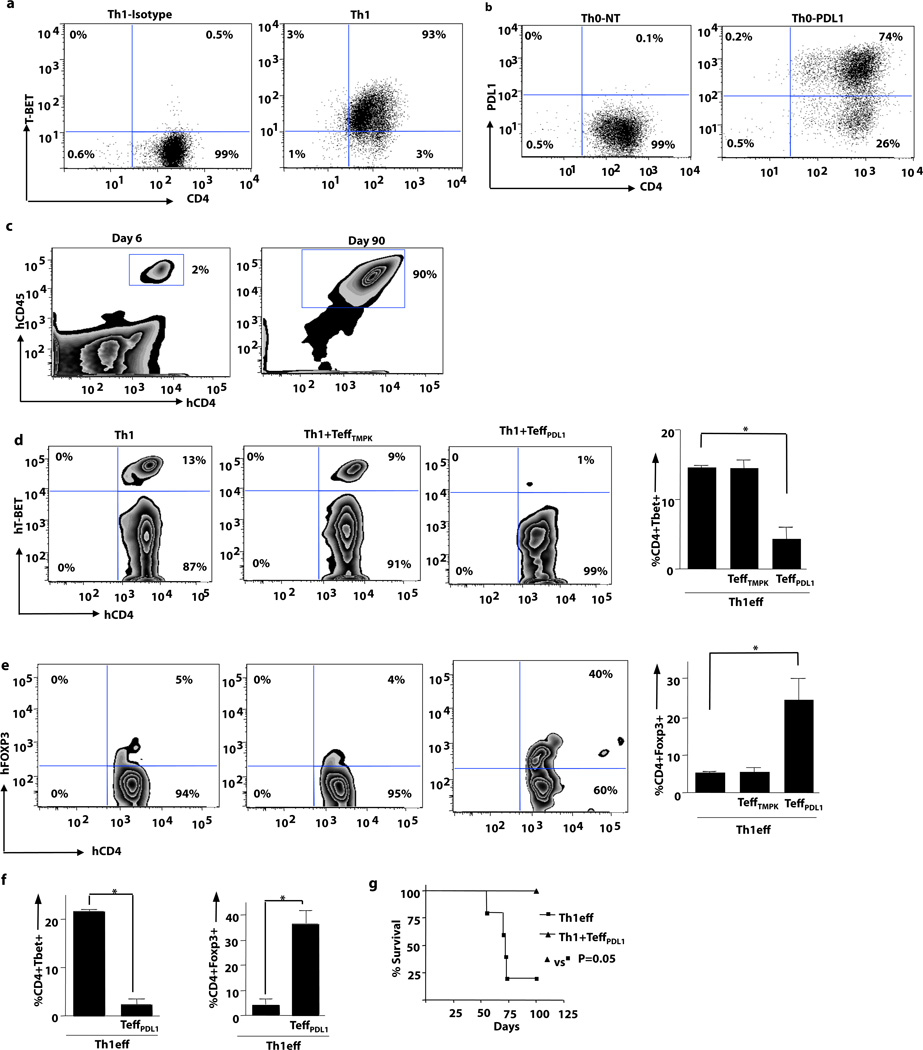
We then evaluated the long-term stability of the Th1 cell phenotype in vivo during xGVHD. Th1 cells, 93% of which expressed TBET (Fig. 3a; representative flow data), were adoptively transferred into murine recipients either alone or with PDL1-expressing T cells (Fig. 3b; representative flow data). Human Th1 cells engrafted and persisted long-term (Fig. 3c, left and right panel; days 6 and 90 after transplant, respectively). At day 6 after transplant, Th1 cells preferentially expressed TBET (Fig. 3d, left panel) rather than FOXP3 (Fig. 3e, left panel). A similar transcription factor profile was observed in recipients of Th1 cells plus T cells expressing CD19.TMPK alone (Fig. 3d, middle panel; Fig. 3e, middle panel). In contrast, recipients of Th1 cells plus T cells expressing CD19.TMPK/PDL1 had reduced TBET+ T cells (Fig. 3d, right panel) and increased FOXP3+ T cells (Fig. 3e, right panel). This shift in Th1 cell transcription factor expression was observed consistently (Fig. 3d summary plot; Fig. 3e summary plot). At day 90 after transplant, T cells from the minority of Th1 cell recipients that did not undergo lethal xGVHD maintained TBET expression without FOXP3 expression (Fig. 3f,). In contrast, recipients of Th1 cells plus PDL1-expressing T cells expressed abundant FOXP3 and dramatically reduced TBET levels (Fig. 3f). Importantly, this conversion of Th1 cells into a regulatory phenotype was associated with complete prevention of xGVHD lethality (Fig. 3g).
Figure 3. Th1 Cells Mediate Lethal xGVHD: Abrogation by PDL1- expressing Effector T Cells.
(a) Human Th1 cells were expanded ex vivo. Prior to adoptive transfer, Th1 cells were evaluated by intracellular flow cytometry for transcription factor expression and cytokine content (b) Prior to adoptive transfer, transduced human T cells were evaluated for PDL1 expression (non-transduced cells; PDL1-transduced cells). (c) NSG mice were injected with Th1 cells (1 × 106) alone or with PDL1-transduced T cells (5 × 104). Human T cell splenic engraftment for recipients of Th1 cells plus PDL1-transduced cells is shown at day 6 and day 90 after transfer. (d) TBET expression by intracellular flow cytometry was monitored in cohorts that received Th1 cells alone, Th1 cells plus control LV-transduced T cells, or Th1 cells plus PDL1-LV transduced T cells. Right panel shows pooled data for TBET expression for each cohort. (e) FOXP3 expression by i.c. flow cytometry was monitored at day 6 after transplant in cohorts that received Th1 cells alone, Th1 cells plus control LV-transduced T cells, or Th1 cells plus PDL1-LV transduced T cells. Right panel shows pooled data for FOXP3 expression for each cohort. (f) TBET and FOXP3 expression at day 90 after transplant in recipients of Th1 cells or Th1 cells plus PDL1-expressing T cells. (g) Survival curve for recipients of Th1 cells alone or Th1 cells plus PDL1-expressing T cells. (* indicates p ≤ 0.05, ** indicates p ≤ 0.005).
Th1 Cell Differentiation Plasticity Requires an Intact PD1 Receptor
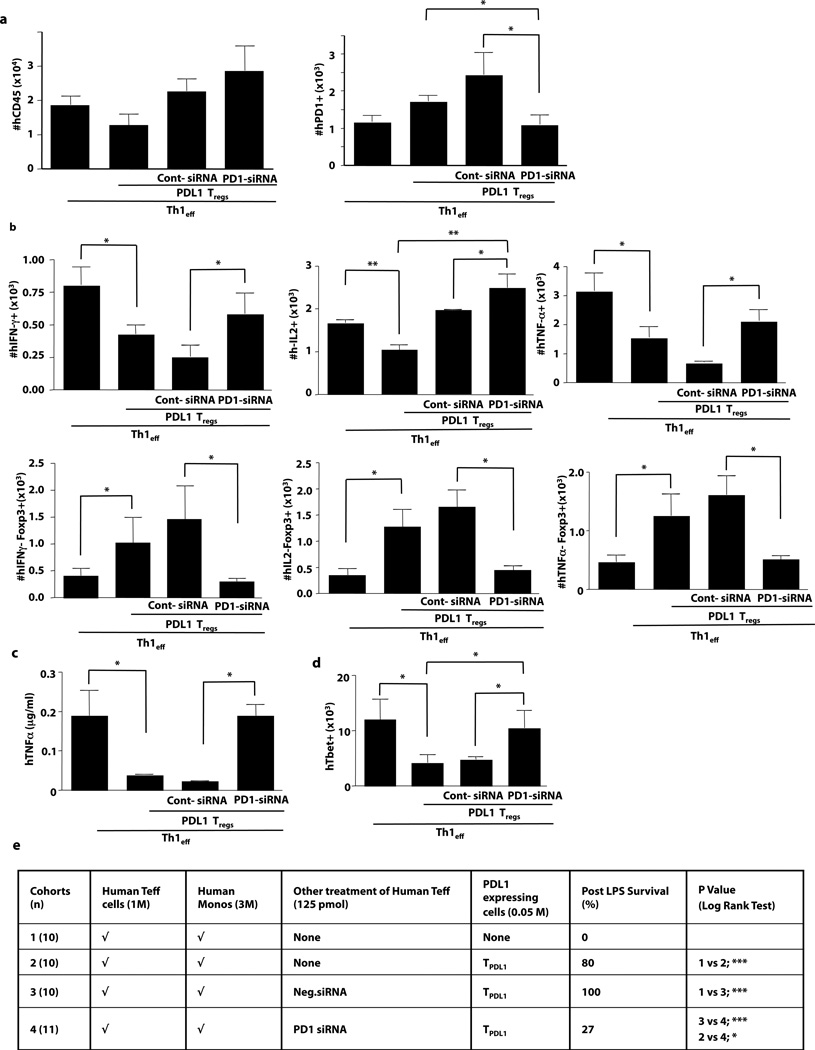
PDL1 can modulate T cell function through binding to PD1 or CD80 (B7.1)(29). To determine whether PD1 was operational in our system, in vivo experiments were performed using Th1 cells knocked down for PD1 receptor expression via an siRNA approach (Th1.PD1kd). Importantly, Th1.PD1kd cells engrafted similarly to control Th1 cells (Fig. 4a, left panel). Consistent with our previous finding that PDL1 up-regulated PD1 expression(25), Th1 cells had increased PD1 levels in vivo when co-administered with PDL1-expressing T cells; however, engrafted Th1.PD1kd cells did not up-regulate PD1 (Fig. 4a, right panel), thereby confirming in vivo PD1 knockdown. Co-administration of PDL1-expressing T cells reduced Th1 cell-mediated in vivo effector function, as indicated by reduced numbers of FOXP3-negative T cells that were IFN-γ positive, IL-2 positive, and TNF-α-positive (Fig. 4b top panels). In contrast, engrafted Th1.PD1kd cells were relatively resistant to the down-regulatory effect of PDL1-expressing T cells. Furthermore, whereas transfer of PDL1-expressing T cells increased the number of Th1 cells that expressed FOXP3 in the absence of effector cytokine secretion (Fig. 4b; bottom panels), Th1.PD1kd cells did not express this regulatory phenotype in vivo. Following adoptive transfer of PDL1-expressing T cells, Th1.PD1kd cells maintained TBET expression and were fully capable of initiating a systemic TNF-α response that was associated with lethal xGVHD (Fig. 4c, 4d, and 4e). In sum, these data indicate that PDL1 mediated an in vivo conversion of human Th1 cells towards a TREG phenotype by a mechanism involving the PD1 receptor.
Figure 4. PDL1-mediated Abrogation of Th1 Effector Cell Function Requires Intact PD1.
NSG host mice received, as indicated, a combination of human Th1 cells (either unmodified, control siRNA-treated, or PD1 siRNA-treated) and PDL1-transduced T cells. (a) At day 6, absolute numbers of human T cells (left panel) and PD1-expressing T cells (right panel) were enumerated in the spleen. (b) Splenocytes were stimulated (PMA/ionomycin) and the number of FOXP3-negative T cells expressing IFN-γ, IL-2 and TNF-α were quantified; conversely, the number of FOXP3-positive T cells deficient in production of IFN-γ, IL-2, and TNF-α were quantified. (c) Recipients were challenged with LPS and serum was harvested 90 min later for measurement of TNF-α. (d) Absolute numbers of engrafted human T cells expressing TBET were quantified. (e) Cohorts of host mice received the human cell inocula specified, followed by LPS administration on day 5 after transfer for assessment of lethal cytokine-mediated xGVHD (n, number per cohort). Data panels represent n=10 replicates per cohort (* indicates p ≤ 0.05, ** indicates p ≤ 0.005).
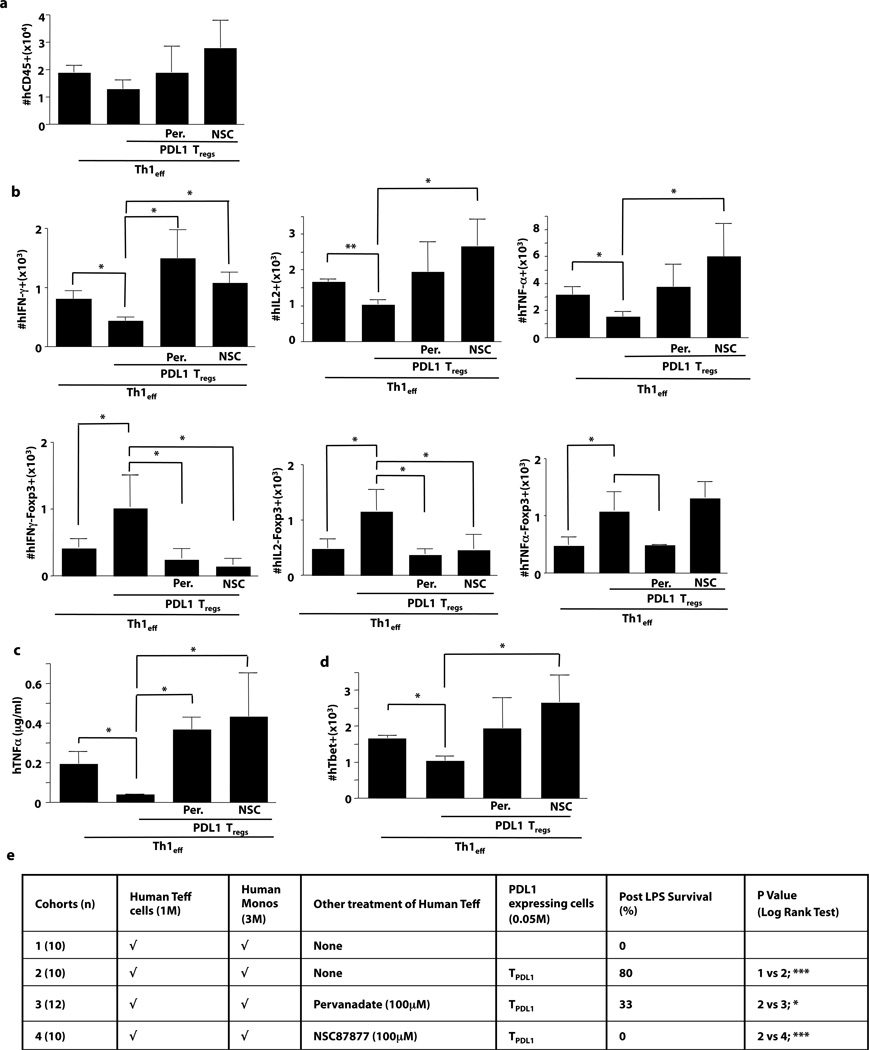
Th1 to TREG Conversion Involves SHP1/SHP2 Signaling
Because PD1 receptor signaling occurs via SHP1/2(20, 30), we hypothesized that ex vivo pharmacologic inhibition of this pathway using pervanadate(20) or NSC87877(31, 32) might generate Th1 cells (Th1.SHP1/2in) resistant to the tolerizing effect of PDL1. SHP1/2 inhibition did not influence Th1 cell engraftment (Fig. 5a). Remarkably, Th1.SHP1/2in cells maintained their effector phenotype in vivo when co-administered with PDL1-expressing T cells, as indicated by preserved or increased expression of IFN-γ, IL-2, and TNF-α (Fig. 5b, top panels) in combination with a reduction of PDL1-mediated up-regulation of FOXP3 (Fig. 5b, bottom panels). Finally, even in the presence of PDL1-expressing T cells, Th1.SHP1/2in cells promoted systemic TNF-α production, had preserved or increased TBET expression, and were potent mediators of lethal xGVHD (Fig. 5c, 5d, and 5e). Therefore, blockade of PD1 receptor or inhibition of down-stream SHP1/2 signaling prevented PDL1-mediated Th1 cell differentiation plasticity.
Figure 5. PDL1 Induction of Th1 Cell Plasticity Requires Intact SHP1/2 Signaling.
NSG host mice received, as indicated, a combination of human Th1 cells (either unmodified, pervanadate-treated [Per], or NSC87877-treated [NSC]) and PDL1-transduced T cells. (a) At day 6, absolute numbers of human T cells engrafted in the spleen were enumerated. (b) Splenocytes were stimulated (PMA/ionomycin) and the number of FOXP3-negative T cells expressing IFN-γ, IL-2, or TNF-α were quantified; conversely, the number of FOXP3-positive T cells deficient in production of IFN-γ, IL-2, and TNF-α were quantified. (c) Recipients were challenged with LPS and serum was harvested 120 min later for measurement of TNF-γ. (d) Absolute numbers of engrafted human T cells expressing TBET were quantified. (e) Cohorts of host mice received the human cell inocula specified, followed by LPS administration on day 5 after transfer for assessment of lethal cytokine-mediated xGVHD (n, number per cohort). Data panels represent n=10 replicates per cohort (* indicates p ≤ 0.05, ** indicates p ≤ 0.005).
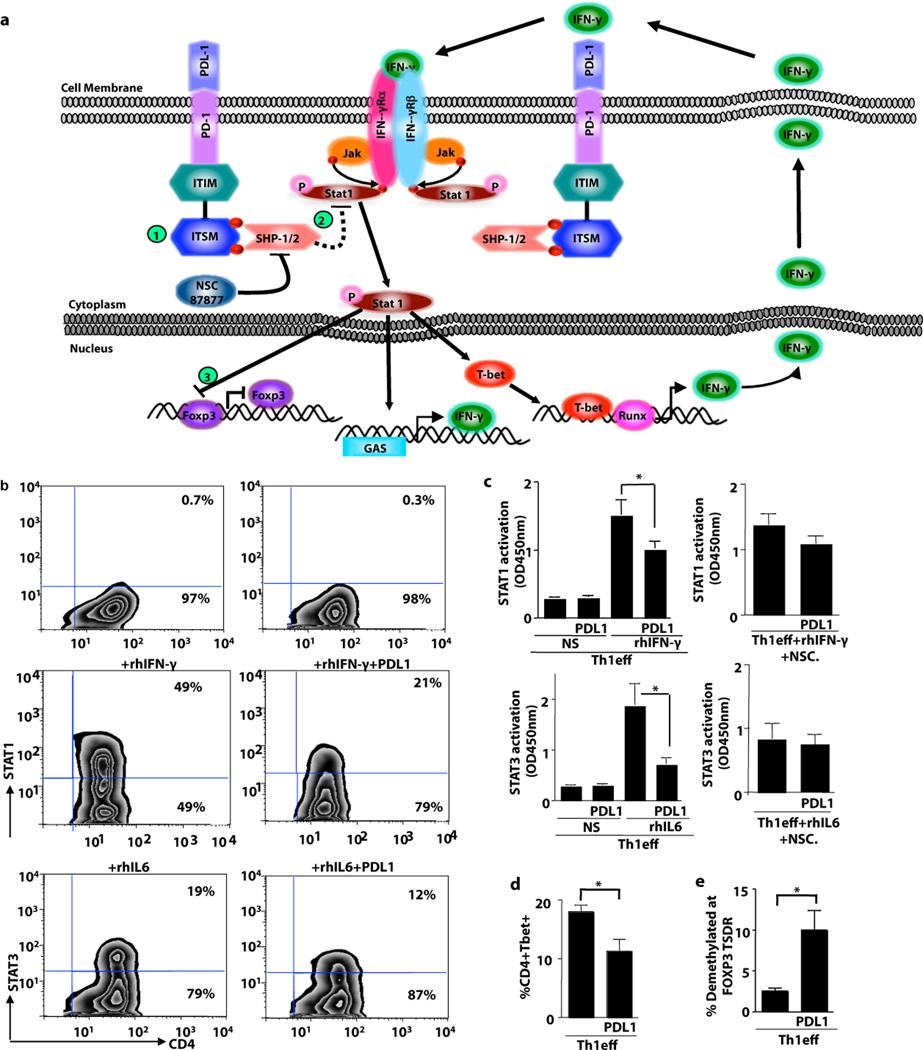
PD1 Signaling Reduces Th1 Cell STAT1 Activation
The question remained: how does PD1 activation and subsequent SHP1/2 signaling induce Th1 cell plasticity? Using a conditional knockout model, it was previously shown that SHP2 inactivates STAT molecules: Specifically, lack of SHP2 increased STAT3 phosphorylation, thereby resulting in constitutive T cell activation and inflammatory disease(33). Previous studies have not evaluated whether STAT1, which is critical for maintaining the Th1 phenotype and serves to negatively regulate FOXP3 expression in murine T cells (41), might be similarly down-regulated by SHP1/2 activation. Given this information, we generated a working model (Fig. 6a) whereby SHP1/2 activation might reduce STAT1 phosphorylation, thereby promoting instability of Th1 cell polarity and propagation of FOXP3 expression. To initiate testing of this hypothesis, Th1 cells were re-stimulated in the presence of the STAT1 activating cytokine IFN-γ either alone or in combination with PDL1-conjugated beads. As anticipated, IFN-γ stimulation increased STAT1 phosphorylation (Fig. 6b; Supplemental Fig. 6a, left panel). Consistent with our hypothesis, in the presence of PDL1, Th1 cells had reduced IFN-γ mediated STAT1 phosphorylation (Fig. 6b, representative data; Supplemental Fig. 6a left panel). Further experiments were performed to evaluate whether PDL-1 mediated Th1 cell plasticity might be mimicked by other known immunosuppressive molecules. Decreases in Th1 cell STAT1 phosphorylation were observed not only with PDL1 exposure, but also with addition of TGF-β or purified TREG cells (Supplemental Fig. 6a left panel); Th1 cell co-culture did not modulate STAT1 phosphorylation within the PDL1-transduced T cells. Furthermore, Th1 nuclear STAT1 phosphorylation upon IFN-γ stimulation was also decreased in the presence of PDL1 (Fig. 6c, top left panel). This reduction in nuclear STAT1 phosphorylation was abrogated both by SHP1/2 pharmacologic inhibition (Fig. 6c, top right panel) and siRNA-mediated knock-down of PD1 (Supplemental Fig. 7d). Th1 cell STAT4 phosphorylation was reduced after PDL1 exposure (Supplemental Fig. 6b, left panel). Finally, PDL1-transduced T cells were relatively deficient in STAT4 phosphorylation (Supplemental Fig. 6b, middle panel); after co-culture with Th1 cells, STAT4 phosphorylation in PDL1 expanded cells was not significantly altered by Th1 cell co-culture (Supplemental Fig. 6b, right panel).
Figure 6. PDL1 Induction of Th1 Cell Plasticity Associates with STAT1 Inactivation.
(a) Working model for Th1 cell plasticity: (1) PDL1/PD1 interactions recruit SHP1/2; (2) SHP1/2 inactivates STAT1, thereby abrogating IFN-mediated maintenance of TBET; (3) STAT1 inhibits FOXP3. (b) To investigate aspects of this model, Th1 cells were restimulated in the presence of IFN-γ or IL-6 for 30 min prior to phosphoflow cytometry. Representative examples shown are Th1 cells without additional cytokine addition either alone or with PDL1-coated beads; Th1 cells plus a STAT1 activating cytokine (IFN-γ) alone or with PDL1-coated beads; and Th1 cells plus a STAT3 activating cytokine (IL-6) alone or with PDL1-coated beads. (c) Nuclear lysates of Th1 cells were obtained (n=5 donors) and subjected to an ELISA-based STAT activation assay. STAT1 activation was evaluated in the presence of PDL1 (top left panel) or PDL1 plus the SHP1/2 inhibitor NSC87877 (top right panel); STAT3 activation was evaluated in the presence of PDL1 (bottom left panel) or PDL1 plus NSC87877 (bottom right panel). (d) Th1 cells were evaluated for TBET expression in the presence or absence of PDL1. (e) Th1 cells were evaluated for demethylation status of the FOXP3 TSDR locus. (For each panel: * indicates p ≤ 0.05, ** indicates p ≤ 0.005, *** indicates p ≤ 0.0005).
As anticipated based on the prior literature(33), the inhibitory effect of SHP1/2 activation on STAT molecules was not limited to STAT1: That is, using IL-6 to activate STAT3, we found that PDL1 also inactivated this pathway in Th1 cells (Fig. 6b, representative data). IL-6 driven nuclear STAT3 phosphorylation was also decreased in Th1 cells by PDL1 expression (Fig. 6c, bottom right panel). In this model system, PD1 activation and subsequent reduction in STAT activation was associated with a reduction in Th1 cell expression of TBET and IFN-γ (Fig. 6e; Supplemental Fig. 6d). Interestingly, Th1 cell exposure to PDL1-coated beads increased the demethylation of the TSDR locus of the FOXP3 gene (Fig. 6f); this effect appeared to occur independently of TGF-β signaling because there was no alteration of p-Smad expression in Th1 cells (Supplemental Fig. 6e). Addition of IL-10, TGF-β, or purified TREG cells to Th1 cells inhibited STAT1 and STAT4 phosphorylation and reduced TBET and IFN-γ expression in a manner similar to PDL1 exposure (Supplemental Fig. 6a,b and d), did not alter expression of the PDL1 binding partners PD1 or CD80 (Supplemental Fig. 6c), and did not alter TSDR demethylation (0.2% of Th1 cells demethylated at FOXP3 TSDR). In sum these data indicate that PDL1 mediated Th1 cell plasticity are somewhat unique in relation to other immunological factors.
Discussion
CD4+ T cells of the Th1 cytokine phenotype, which are essential to cell-mediated immunity against cancer and infectious disease, can be rendered ineffective by multiple down-regulatory mechanisms including antigen-induced cell death(5), anergy due to cytokines such as TGF-β(34) or due to surface molecules such as CTLA-4(35) or PDL1(20, 21), suppression by TREG cells(3), or conversion to a Th2 phenotype(10). Here, we demonstrate that bona fide human Th1 cells are susceptible to conversion into a TREG phenotype. These observations extend an understanding of the mechanisms whereby PDL1 limits T cell function and illustrate the abundant in vivo differentiation plasticity of human Th1 cells.
This new finding that human Th1 cells can rapidly morph into TREG cells in vivo under the influence of PDL1 provides further evidence that functional T cell subset inter-conversion plays an important role in immune regulation. Because the Th1 cells that we evaluated nearly uniformly expressed TBET and IFN-γ and were relatively devoid of FOXP3 expression, it is unlikely that the rapid, PDL1-induced alteration in Th1 cell phenotype that we observed was due to an outgrowth of ‘contaminating’ TREG cells in the Th1 cell inoculum. Of note, PDL1 modulated both ex vivo generated Th1 cells and human Th1 cells that were demonstrated to be pathogenic on the basis of their induction of xenogeneic GVHD. T-helper cell functional plasticity was initially described in seminal work relating to Th1/Th2 cross-regulation(10, 11). This Th1/Th2 paradigm has been updated and expanded recently, as it has been demonstrated that Th1 plus Th2 regulation can occur at the single cell level via transcription factor co-expression: Th2 cells under the influence of interferon and IL-12 signaling can gain TBET expression while preserving GATA-3 expression(36). Another well-described example of CD4+ T cell subset plasticity involves TREG cell susceptibility towards Th17 differentiation via the reprogramming effect of the STAT3 activating cytokines IL-1 and IL-6(8). Importantly, such TREG to Th17 subset in vivo conversion can be alleviated by blocking IL-6, thereby increasing TREG cells and reducing Th1 and Th17 cell-mediated murine GVHD(37). In still yet another example, non-pathogenic Th17 cells convert in vivo into Th1 cells with subsequent induction of autoimmune diabetes(38, 39); IL-12 induced activation of STAT4 in Th17 cells represents an important mechanism for Th17 conversion to IFN-γ secreting, TBET expressing Th1 cells(40).
Our new observations extend an understanding of CD4+ T cell plasticity in two new important directions. First, Th1 cells have never been identified as a subset susceptible to reprogramming to a TREG phenotype; therefore, Th1 cells are both amenable to regulation by TREG cells(3) and as described here, can indeed become TREG cells under the influence of PDL1. This finding stands somewhat in contrast to observations from a recent study, which found that murine Th1 cells had limited capacity for conversion to a TREG phenotype; indeed, Th1 cells caused a reduction in TREG cell numbers in vivo(41). And second, previous studies have exclusively focused on differentiation plasticity mediated by cytokine-mediated STAT pathway activation through phosphorylation. Distinct from this prior literature, we have identified an example of plasticity due to an inhibitory co-stimulatory molecule, PDL1, which reduces Th1 cell STAT activation through the phosphatase function of the SHP1/2 signaling pathway that lies downstream to the PD1 receptor. These results are consistent with the critical dependence of Th1 cells on appropriate STAT activation for phenotype maintenance(12, 42) and suggest that the observed Th1-to-TREG cell plasticity may occur by a ‘default’ pathway (STAT inactivation) rather than through PDL1 induction of any potential TREG promoting factor.
In addition to extending an understanding of CD4+ T cell differentiation plasticity, the current results enhance knowledge pertaining to the primacy of PDL1/PD1 interactions in T cell regulation. That is, previous to this report, PD1 suppression of T cell function has been primarily attributed to anergy via SHP1/2 inhibition of TCR activation(20) or to tolerance achieved through blocking of TCR stop signals required for T cell interaction with antigen-expressing dendritic cells(21). Distinct from these prior reports, we found that PDL1/PD1 interactions caused the in vivo conversion of effector Th1 cells into TREG cells; in our model, this form of T cell differentiation plasticity was tolerizing, as evidenced by the inability of an otherwise lethal inocula of Th1 cells to mediate xenogeneic GVHD. As such, PD1-mediated T cell tolerance can be attained by inhibition of T cell activation, or as we have shown, by altering T cell differentiation. Based on these findings, it is now possible to propose that the increased frequency of TREG cells in the tumor microenvironment may relate not only to an indirect mechanism of TREG recruitment by CCL22 elaborated in response to inflammation from infiltrating anti-tumor Th1 cells(43) but also from a direct mechanism of tumor cell PDL1-mediated conversion of Th1 cells to a TREG phenotype.
The current findings may also point to new clinical translational approaches. First, we found that pre-treatment of adoptively-transferred Th1 cells with anti-PD1 siRNA or SHP1/2 pharmacologic inhibitors successfully abrogated PDL1-mediated conversion to a TREG phenotype and fully restored Th1 cell capacity to mediate lethal xGVHD. Of note, anti-PD1 monoclonal antibodies are being evaluated in early-stage clinical trials and have shown promising anti-tumor effects (reviewed in(44)); given our results, it will be of interest to determine whether recipients of anti-PD1 therapies have preservation of a Th1 cytokine phenotype. And, it is possible that adoptive transfer of Th1 cells in combination with an anti-PD1 strategy might represent an approach to preserve the in vivo phenotype of Th1 cell therapy. Alternatively, our data indicate that it may be possible to use SHP1/2 inhibitors, which are currently being evaluated in pre-clinical models(44), for the protection of adoptively transferred Th1 cells against TREG cell conversion. It is important to note that our experiments utilized either T cells or immortalized K562 tumor cells to deliver the tolerizing PDL1 signal and that it will thus be essential to determine whether a similar biology occurs in the tumor microenvironment.
Second, we found that PDL1-mediated erosion of an otherwise stable Th1 phenotype was associated with lower levels of STAT activation, thereby implying that PDL1/PD1 interactions may operate in part by reducing a tonic level of STAT activation required for protection of the Th1 state. In clinical trials of adoptive T cell therapy, IL-2 is the cytokine most commonly administered in combination with T cell infusion(45). Our data would suggest that IFN-α or IL-12, which activate the Th1-dedicated pathways STAT1 and STAT4, respectively, might also be evaluated in combination with adoptive T cell therapy to preserve the Th1 state in vivo; of note, in animal models, T cells engineered to secrete IL-12 represent particularly potent anti-tumor effectors(46). And finally, we found that T cells genetically engineered to express PDL1 prevented lethal xenogeneic GVHD in a similar manner as purified TREG cells. These data suggest that the treatment of GVHD, prevention of solid organ graft rejection, or modulation of autoimmunity might be harnessed through a PDL1 gene transfer approach as an alternative to ongoing TREG cell therapy strategies(47). PDL1-modulation of Th1 cells inhibited xGVHD in two distinct in vivo models; nonetheless, it is not known how the current results may translate to GVHD as it occurs in the clinic setting. Of note, multiple cellular vehicles for the PDL1 gene transfer (namely, CD4+ and CD8+ T cells and K562 myeloid tumor cells) were capable of modulating Th1 cells, thereby indicating the flexibility of such a PDL1-mediated therapy.
Th1 cells, which are critical for anti-tumor and anti-infection immunity, are subjected to numerous previously described counter-regulatory mechanisms, and as described here, appear to also face the challenge of PDL1-mediated conversion to a TREG phenotype. One can envision two directions for clinical translational efforts based on this new understanding. First, adoptive T cell therapy clinical trials might be performed to evaluate whether Th1 cell function can be preserved in vivo through anti-PD1 reagents, SHP1/2 inhibitors, or modulation of specific STAT pathways. And second, cellular vehicles engineered for PDL1 expression might be evaluated as a surrogate for regulatory T cell therapy of autoimmunity or GVHD.
Materials and Methods
Mice
Various immune-deficient murine hosts were used, depending upon availability. Female RAG2−/−cγ−/− mice were obtained from Taconic and used at 8–12 weeks of age. Experiments were performed according to a protocol approved by the NCI Animal Care and Use Committee. Mice were housed in a sterile facility and received sterile water and pellets. Mice were injected with 0.1ml clodronate containing liposomes (Encapsula Nanoscience) for macrophage depletion and given low-dose irradiation (350 cGy). Female NOD/SCID mice were obtained from Jackson Laboratory and conditioned with low dose radiation (450 cGy). NODLtSz-scidIL2Rγnull mice (NSG) were obtained from Jackson Laboratory; NSG hosts did not undergo conditioning prior to human cell transfer.
Antibodies and reagents
X-VIVO 20 media was obtained from BioWhitaker and AB serum was from Gem Cell. CD4 microbeads were from Miltenyi Biotec. Anti-CD3, anti-CD28 and PDL1-Fc chimera coated tosyl-activated magnetic beads were manufactured. Rapamycin was from Wyeth (Rapamune®). Recombinant human (rh) IL-2 was from PeproTech and rhIFN-α-2b was from Schering Plough. Recombinant PDL1-Fc chimera was from R&D systems. All other antibodies (unless otherwise stated) were provided by BD Biosciences; anti-human FOXP3 PE was from Biolegend. Luminex kits for detection of IFN-γ and TNF-α were from Bio-Rad (Hercules, CA). 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester [5(6)-CFDA, SE; CFSE] was from Invitrogen. Zidovudine (AZT) was from Glaxosmithkline.
Vector design and construction
pDY.cPPT.EF-1α.EGFP.WPRE.SIN was modified from pHR’.cPPT.EF-1α.EGFP.WPRE.SIN by deletion of non-essential elements of the original transfer vector backbone and LTR sequences. Human codon-optimized, human PD-L1 cDNA was synthesized by Genscript and subcloned into pDY.cPPT.EF-1α.CD19ΔTMPK.WPRE.SIN (control-LV) to yield pDY.cPPT.EF-1α.CD19ΔTMPK.IRES.PDL1.WPRE.SIN (PDL1-LV). Fidelity of all vectors was validated by bidirectional sequencing. LVs pseudotyped with vesicular stomatitis virus-glycoprotein (VSV-g) were generated as described previously(48). In addition, we incorporated a fourth plasmid, pAdVAntage™ vector from Promega to increase LV titers. Virus supernatants were harvested 48 hours and 72 hours after transfection, filtered using 0.45-µm filter, and concentrated at 50,000 × g for 2 hours using an Optima L-100 XP Ultracentrifuge.LV pellets were re-suspended in serum-free X-VIVO 20 media. Typical functional titers of concentrated LVs were in the range of 5×108 to 1×109 IU/mL.
Human T cell transduction with Lentivirus
Normal donor peripheral blood cells were collected by apheresis on an IRB-approved protocol. Total lymphocytes were isolated by elutriation(49). Total CD4+ T cells were then enriched by CD4 microbeads according to manufacturer instructions. CD4+ T cells were co-stimulated at a 3 bead:1 T cell ratio in media containing IL-2 (20 IU/ml) and rapamycin (1µM). At day 3 of culture, cells were washed and replated at a concentation of 2 × 105 cells/ml in media containing IL-2 and lentiviral supernatant at an MOI of 20.
Flow cytometry
T cells were washed with PBS supplemented with 0.1% BSA and 0.01% azide, and stained using anti-: CD4 APC (clone: RPA-T4), CD19 PE (clone: H1B19), and PDL1 FITC (clone: M1H1). For assessment of cell death, T cells were stained for surface markers (CD4 FITC, CD19 PE and PDL1 PE-cy7), resuspended in Annexin V buffer, and stained with AV APC and 7AAD according to manufacturer’s instructions. For in vivo monitoring of human T cells, splenocytes were stained with CD45 FITC (clone: H130), CD3 Pecy5 (clone: H1T3a), PDL1 Pe-cy7, PD1 APC (clone: M1H4), CD80 PE (clone: L307.4), and CD19 APC-cy7 (clone: SJ25C1). For intra-cellular (IC) flow cytometry, fixation and permeabilization buffer was utilized (eBioscience). IC flow cytometry was performed with combinations of CD45 FITC, CD3 Pecy5, PDL1 Pecy7, TBET APC (clone: 4B10; eBioscience), FOXP3 PB (clone 249D; eBioscience), and CD19 APC-cy7; other reagents for IC flow cytometry included IL-2 FITC (clone: MQ1-17H12), IFN-γ PE (clone: 45.B3), CD3 Pecy5, PDL1 Pecy7, TNF-α APC (clone: MAB11; eBioscience), FOXP3 PB, CD45 APC-cy7 or CD45 FITC, active Caspase-3 PE, CD3 Pecy5, PDL1 Pecy7, PD1 APC, FOXP3 PB, and CD19 APC-cy7.
STAT phosphorylation assays
Flow cytometry to assess phosphorylation status of STAT molecules was performed using BD phosphoflow kit containing BD lyse/fix buffer and perm buffer III; STAT1 A488 (clone 4a) and STAT3 A647 (clone49; p-stat3) antibodies were utilized. Briefly, cells were incubated for 15 min with IFN-γ, fixed for 15 min at 37°C with phospho-lyse buffer, and then fixed in perm buffer III (30 min, 4°). Cells were washed with FACS buffer and stained for STAT and surface markers, incubated at room temperature (20 min), and analyzed with FACSCalibur® and CellQuest® software (BD). Nuclear lysates of stimulated T cells were tested for activated nuclear STAT1 and STAT3 by using the TransAM DNA binding ELISA assay obtained from Active Motif.
Protein determination by western blot analysis
Protein lysates were obtained from T cell cultures that were subjected to SiRNA transduction. Lysates were run on 10–20% SDS-PAGE gels and transferred onto nitrocellulose membrane. Membranes were blocked with 5% milk in TBST buffer (20mmol/L Tris HCl, 500 mmol/L NaCl, and 0.01% Tweeen 20) and incubated overnight at 4°C with primary antibodies (Ab) in TBST containing either 5% milk or BSA. Immunoreactivity was detected by sequential incubation with HRP-conjugated secondary Ab and enzymatic chemiluminescence (Cell Signaling Technology). Primary Abs utilized were from Cell Signaling and included anti-: SHP1, SHP2, Smad2, pSmad2, and beta-actin.
siRNA knockdown of PD1 and SHP1/2
siRNA oligonucleotides for PD1 (P1, P2, P3, and P4), SHP1, SHP2 and AllStar Negative control siRNA were purchased from Qiagen (Valencia, CA). Transfection of siRNA was performed according to manufacturer’s instructions (Amaxa). Transfected cells were co-stimulated as previously described and harvested for real time PCR, protein, and functional assays at day 3 post-transfection. PD1 knockdown was measured by flow cytometry and SHP1/2 knockdown was measured by western blotting. All in vivo experiments were performed with P4 siRNA, which had the most efficient PD1 knockdown. The cells were further flow sorted for >99% purity of PD1kd population and then adoptively transferred into mice.
Xenogeneic GVHD model
Human effector CD4+Th1 (Th1) cells were generated by T cell culture for 6 days with co-stimulation and expansion in rhIL2 (20 IU/ml), anti-IL-4 (100 ng/ml), rhIFN-α-2b (1 × 106 IU/ml), and rapamycin (1µM)(28). On day 6 of culture, Th1 cells were harvested and injected i.v. by retro-orbital injection(50) into immune-deficient murine hosts; Th1 cell dose was 1 ×106 cells/recipient. Specific cohorts additionally received ex vivo generated TREG cells (generated from CD4+CD127− cells; dose of 0.5 × 104 cells/recipient); other cohorts received non-polarized CD4+ T cells transduced with control-LV or PDL1-LV (dose of 0.5 × 104 or 1 × 106 cells/recipient, as indicated in figure legend). Mice were challenged with LPS at day 5 post-transplant as indicated. In vivo experiments were also performed where Th1 cells were generated ex vivo using flow sorted CD4+CD127+CD25−CD45RA+ T cells as the culture input population and using a longer, 12-day Th1 cell expansion and polarization interval. In the natural history model of x-GVHD, LPS challenge was not performed; additional in vivo experiments were performed using human Th1 cells harvested at day 50 post-transplant of the natural history x-GVHD model. AZT was administered by i.p. injection (twice per day; dose of 50 mg/kg/day). The myeloid tumor cell line K562 (unmodified or PDL1 transduced) were used after irradiation (10,000 rads).
DNA methylation analysis
Th1 cells were either co-cultured for 3 days with PDL1-coated beads or isolated post-BMT from PDL1-treated or untreated mice. Genomic DNA was harvested and quantification of the degree of methylation at the FOXP3-TSDR locus was detected by real time PCR method (performed by Epiontis).
Statistical Analysis
Flow cytometry and cytokine data were analyzed using student’s 2-tailed t tests. Comparison values of p ≤ 0.05 were considered statistically significant. LPS lethality data was analyzed using log rank test.
Supplementary Material
Acknowledgements
We would like to thank Hong Kong for her technical expertise.
Funding sources: Supported by the Intramural Research and by P01AI080192 and JDRF Collaborative Centre for Cell Therapy.
Footnotes
Author Contributions: S.A. designed and performed research, performed analysis and wrote the manuscript. C.W.M., J.C.M.W., F.W., A.H., V.K., J.E.F, P.R.M., T.C.F. performed research, J.L.R, B.L.L, C.H.J, J.A.M and D.H.F designed research and wrote the manuscript. Competing Interests: J.R. has an advisory relationship with Bristol Meyer Squibb. The other authors declare that they have no competing interests. D.F. and J. M. are authors on a patent relating to TMPK cell fate. J.R. is an author on a patent related to the use of PD-L1 expressing aAPCs to treat autoimmune disease.
References and Notes
- 1.Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Mueller-Berghaus J, Kirkwood JM, Kwok WW, Storkus WJ. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland SM. Interferon gamma, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunol Res. 2007;38:342–346. doi: 10.1007/s12026-007-0045-8. [DOI] [PubMed] [Google Scholar]
- 3.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 142:914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariotti J, Foley J, Ryan K, Buxhoeveden N, Kapoor V, Amarnath S, Fowler DH. Graft rejection as a Th1-type process amenable to regulation by donor Th2-type cells through an interleukin-4/STAT6 pathway. Blood. 2008;112:4765–4775. doi: 10.1182/blood-2008-05-154278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsdell F, Seaman MS, Miller RE, Picha KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 6.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 8.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991;147:2713–2716. [PubMed] [Google Scholar]
- 11.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 13.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O'Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 15.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 17.Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 19.Lee I, Wang L, Wells AD, Ye Q, Han R, Dorf ME, Kuziel WA, Rollins BJ, Chen L, Hancock WW. Blocking the monocyte chemoattractant protein-1/CCR2 chemokine pathway induces permanent survival of islet allografts through a programmed death-1 ligand-1-dependent mechanism. J Immunol. 2003;171:6929–6935. doi: 10.4049/jimmunol.171.12.6929. [DOI] [PubMed] [Google Scholar]
- 20.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 21.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma CJ, Ni L, Zhang Y, Zhang CL, Wu XY, Atia AN, Thayer P, Moorman JP, Yao ZQ. PD-1 negatively regulates interleukin-12 expression by limiting STAT-1 phosphorylation in monocytes/macrophages duringchronic hepatitis C virus infection. Immunology. 2010 doi: 10.1111/j.1365-2567.2010.03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amarnath S, Costanzo CM, Mariotti J, Ullman JL, Telford WG, Kapoor V, Riley JL, Levine BL, June CH, Fong T, Warner NL, Fowler DH. Regulatory T cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS Biol. 8:e1000302. doi: 10.1371/journal.pbio.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T, Neschadim A, Konrad M, Fowler DH, Lavie A, Medin JA. Engineered human tmpk/AZT as a novel enzyme/prodrug axis for suicide gene therapy. Mol Ther. 2007;15:962–970. doi: 10.1038/mt.sj.6300122. [DOI] [PubMed] [Google Scholar]
- 28.Amarnath S, Flomerfelt FA, Costanzo CM, Foley JE, Mariotti J, Konecki DM, Gangopadhyay A, Eckhaus M, Wong S, Levine BL, June CH, Fowler DH. Rapamycin generates anti-apoptotic human Th1/Tc1 cells via autophagy for induction of xenogeneic GVHD. Autophagy. 6 doi: 10.4161/auto.6.4.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70:562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- 32.Song M, Park JE, Park SG, Lee do H, Choi HK, Park BC, Ryu SE, Kim JH, Cho S. NSC-87877, inhibitor of SHP-1/2 PTPs, inhibits dual-specificity phosphatase 26 (DUSP26) Biochem Biophys Res Commun. 2009;381:491–495. doi: 10.1016/j.bbrc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 33.Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, Miyata T, Itoh S, Narimatsu M, Maeda H, Fukada T, Itoh M, Okano H, Hibi M, Hirano T. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- 34.Li XF, Takiuchi H, Zou JP, Katagiri T, Yamamoto N, Nagata T, Ono S, Fujiwara H, Hamaoka T. Transforming growth factor-beta (TGF-beta)-mediated immunosuppression in the tumor-bearing state: enhanced production of TGF-beta and a progressive increase in TGF-beta susceptibility of anti-tumor CD4+ T cell function. Jpn J Cancer Res. 1993;84:315–325. doi: 10.1111/j.1349-7006.1993.tb02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 36.Hegazy AN, Peine M, Helmstetter C, Panse I, Frohlich A, Bergthaler A, Flatz L, Pinschewer DD, Radbruch A, Lohning M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 32:116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, Drobyski WR. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz EG, Mariani L, Radbruch A, Hofer T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity. 2009;30:673–683. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay JY, Menetrier-Caux C. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 44.Kline J, Gajewski TF. Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer. Curr Opin Investig Drugs. 11:1354–1359. [PubMed] [Google Scholar]
- 45.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor Regression in Patients With Metastatic Synovial Cell Sarcoma and Melanoma Using Genetically Engineered Lymphocytes Reactive With NY-ESO-1. J Clin Oncol. 29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, Morgan RA, Gattinoni L, Rosenberg SA, Trinchieri G, Restifo NP. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshimitsu M, Sato T, Tao K, Walia JS, Rasaiah VI, Sleep GT, Murray GJ, Poeppl AG, Underwood J, West L, Brady RO, Medin JA. Bioluminescent imaging of a marking transgene and correction of Fabry mice by neonatal injection of recombinant lentiviral vectors. Proc Natl Acad Sci U S A. 2004;101:16909–16914. doi: 10.1073/pnas.0407572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrahamsen TG, Carter CS, Read EJ, Rubin M, Goetzman HG, Lizzio EF, Lee YL, Hanson M, Pizzo PA, Hoffman T. Stimulatory effect of counterflow centrifugal elutriation in large-scale separation of peripheral blood monocytes can be reversed by storing the cells at 37 degrees C. J Clin Apher. 1991;6:48–53. doi: 10.1002/jca.2920060110. [DOI] [PubMed] [Google Scholar]
- 50.Nervi B, Rettig MP, Ritchey JK, Wang HL, Bauer G, Walker J, Bonyhadi ML, Berenson RJ, Prior JL, Piwnica-Worms D, Nolta JA, DiPersio JF. Factors affecting human T cell engraftment, trafficking, and associated xenogeneic graft-vs-host disease in NOD/SCID beta2mnull mice. Exp Hematol. 2007;35:1823–1838. doi: 10.1016/j.exphem.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.