Abstract
The role of tumour-stromal interactions in progression is generally well accepted but their role in initiation or treatment is less well understood. It is now generally agreed that rather than consisting solely of malignant cells, tumours consist of a complex dynamic mixture of cancer cells, host fibroblasts, endothelial cells, and immune cells that interact with each other and micro-environmental factors to drive tumour progression. We are particularly interested in stromal cells (for example fibroblasts) and stromal factors (for example fibronectin) as important players in tumour progression since they have also been implicated in drug resistance. Here we develop an integrated approach to understand the role of such stromal cells and factors in the growth and maintenance of tumours as well as their potential impact on treatment resistance, specifically in application to melanoma. Using a suite of experimental assays we show that melanoma cells can stimulate the recruitment of fibroblasts and activate them, resulting in melanoma cell growth by providing both structural (extra-cellular matrix proteins) and chemical support (growth factors). Motivated by these experimental results we construct a compartment model and use it to investigate the roles of both stromal activation and tumour aggressiveness in melanoma growth and progression. We utilise this model to investigate the role fibroblasts might play in melanoma treatment resistance and the clinically observed flare phenomena that is seen when a patient, who appears resistant to a targeted drug, is removed from that treatment. Our model makes the unexpected prediction that targeted therapies may actually hasten tumour progression once resistance has occurred. If confirmed experimentally, this provocative prediction may bring important new insights into how drug resistance could be managed clinically.
Introduction
Tumours are not composed entirely of cancer cells and instead consist of a dynamic mix of malignant cells, endothelial cells, fibroblasts and immune cells that all interact to drive tumour progression.1,2 Although the role of endothelial cells and the immune system in cancer development are now well established, considerably less is known about the putative function of host fibroblasts in this process. A role for fibroblasts in cancer progression has been long suspected based upon the observation that most solid tumours present as dense, palpable masses.3 The dense, fibrotic nature of solid tumours is thought to be a direct consequence of fibroblast infiltration and the subsequent deposition of extracellular matrix (ECM) proteins.3 The phenotype of the fibroblasts found within tumours (activated fibroblasts or cancer associated fibroblasts: CAFs) is very different to that of normal skin fibroblasts and closely resembles the phenotype of myofibroblasts. Myofibroblasts have a large, spindle like morphology, express contractile fibres of α-smooth muscle actin and exhibit unique cell matrix adhesion complexes (such as the fibronexus). Fibroblasts mediate their physiological effects upon wound healing, angiogenesis and tissue remodelling via the release of growth factors, proteases (such as the matrix metalloproteinases) and through the deposition of ECM proteins (such as laminin, tenascin and fibronectin).4,5
Melanoma arises from the malignant transformation of skin melanocytes and is the deadliest form of skin cancer. The prognosis for disseminated melanoma is dismal and is associated with median survival rates of 8.1 months, with only 2% of patients surviving for 5 years.6 Pathological examination of melanoma specimens shows the tumours to be infiltrated by fibroblasts, endothelial cells and inflammatory cells.3,7 A role for fibroblasts in the pathogenesis of melanoma is suggested by co-culture experiments demonstrating that fibroblasts (and fibroblast-derived factors) stimulate the growth of melanoma cell lines.8,9 In addition, melanoma progression is associated with a switch in cell-cell communication so that the emerging tumour cells down regulate their inhibitory interactions with skin keratinocytes and instead adhere to and interact with host fibroblasts.7
In epithelial tumours, activated fibroblasts are known to play multiple roles in tumour progression. In prostate cancer fibroblasts have been shown to potentially have a dual role, inhibiting progression at the PIN stage and promoting progression after breakout. This may in part be mediated by the growth promoting/inhibiting aspects of transforming growth factor β (TGFβ), a factor that fibroblasts are known to secrete.10,11 Recent studies have shown that fibroblasts are essential for the invasion of carcinoma cells with co-culture experiments showing that fibroblasts localise to the leading edge of the invasive front and generate tracks for the invading carcinoma cells follow.12,13 Studies from our group have also shown an important role for fibroblasts in the angiogenic response of carcinoma cells.14 In this instance the activated fibroblasts were shown to secrete vascular endothelial growth factor (VEGF) that directly mediated the recruitment and differentiation of endothelial cells.14
Although the role of fibroblasts in tumour progression is becoming better understood, their potential impact on drug resistance remains unclear. However, there is good evidence that the adhesion of cancer cells to ECM proteins contributes to therapeutic escape. This is reasonably well documented for multiple myeloma, where resistance is mediated by tumour cell adhesion to fibronectin and is termed cell adhesion mediated drug resistance (CAMDR).15,16 This form of resistance has also been described for lung cancer, where the adhesion of lung cancer cell lines onto fibronectin, laminin and collagen IV directly mediates resistance to the chemotherapy drugs cisplatin, adri-amycin and etoposide through the down regulation of apoptosis.17 Similar findings have also been reported for uveal melanoma cell lines adhering to laminin, collagen IV and fibronectin.18 For cutaneous melanoma it is known that plating cells onto fibronectin prevents anoikis and that knockdown of tenascin-C can sensitise melanoma cells to doxorubicin treatment.19,20
Our working hypothesis is that since fibroblasts secrete ECM proteins as well as growth factors they directly contribute to both tumour growth and drug resistance. In this paper we use an integrated mathematical and experimental approach to investigate our hypothesis for melanoma. Initially, we present experimental evidence that melanoma cells actively recruit and activate primary human skin fibroblasts and stimulate them to deposit ECM proteins that are known to be pro-survival for melanoma cells. We then use this evidence as motivation to construct a mathematical model in order to better understand the interactions between tumour cells and fibroblasts. By simulating the model for a range of parameters we are able to generate a spectrum of possible biological outcomes. The implications of these outcomes are then investigated in the context of cytotoxic chemotherapies. We present some experimental results that directly examine the impact of fibroblasts on treatment. Together these studies provide the beginnings of an integrated framework for improved combination therapy through the targeting of both the host and tumour cells.
Fibroblast interaction with cancer cells
Melanoma cells recruit primary skin fibroblasts
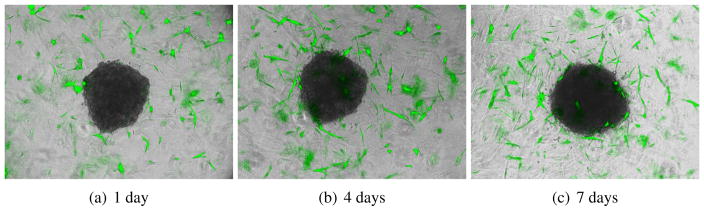
We began by evaluating whether melanoma cells would attract fibroblasts in a 3D co-culture model in which preformed melanoma spheroids were implanted into a collagen gel containing GFP-tagged fibroblasts. It was noted that after 4 days of culture, the fibroblasts started to migrate towards the melanoma spheroid, by 7 days some fibroblasts had started to infiltrate and grow into the spheroid (Figure 1).
Figure 1.
Melanoma cells recruit fibroblasts that then infiltrate the tumour. We implanted melanoma spheroids into a 3D collagen gel containing fibroblasts tagged with green fluorescent protein (green). After 4 days of culture, the fibroblasts are migrating towards the melanoma spheroid; by 7 days some fibroblasts start to infiltrate and grow into the spheroid.
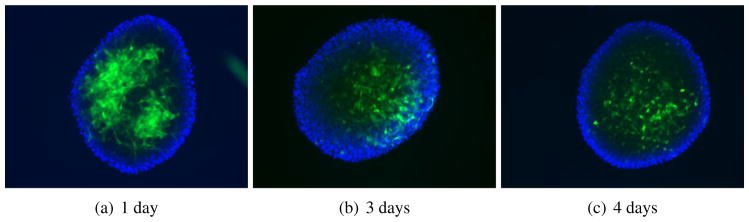
For our next experiment, we added GFP-fibroblasts on top of non-implanted melanoma spheroids and tracked the infiltration of the fibroblasts into the spheroid structure. After 24 hrs of addition, the GFP-fibroblasts were found to adhere to the melanoma spheroid surface in a focal manner, as time progressed, the fibroblasts grew into the spheroid and were found to be equally distributed throughout the spheroid by 96 hrs (Figure 2).
Figure 2.
GFP-tagged fibroblasts adhere to and infiltrate melanoma spheroids. 5000 fibroblasts were carefully pipetted on top of preformed melanoma spheroids on top of agar. Images show immunofluorescence microscopy of whole spheroids showing the infiltration of the fibroblasts (green).
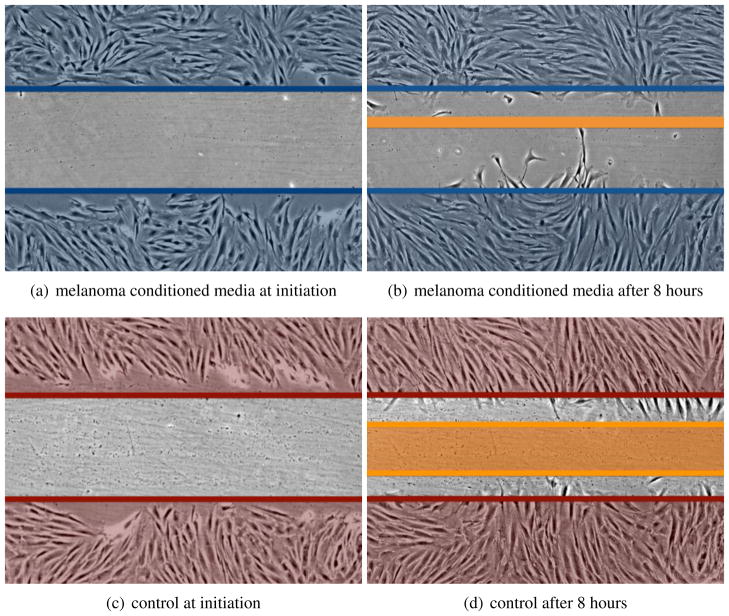
To determine whether the melanoma cells used soluble factors to recruit the fibroblasts we next performed a scratch experiment where fibroblasts were treated with either serum free media or serum free media conditioned for 48 hrs by 1205Lu melanoma cells. It was found that the melanoma conditioned media increased rate of wound closure of the fibroblasts (a 38% increase in wound closure and 3.4-fold more fibroblasts growing into the scratch in conditioned media treated cultures compared to controls) demonstrating the presence of melanoma derived motility factors (Figure 3).
Figure 3.
Conditioned media from melanoma cells increases the motility of fibroblasts. Human skin fibroblasts (FF2441) were grown to confluence before being subjected to a scratch wound in the presence of either serum free media, or serum free media conditioned for 48 hrs by 1205Lu melanoma cells. Representative images show the extent of wound closure at 8 hrs.
Melanoma cells increase fibroblast activation and matrix deposition
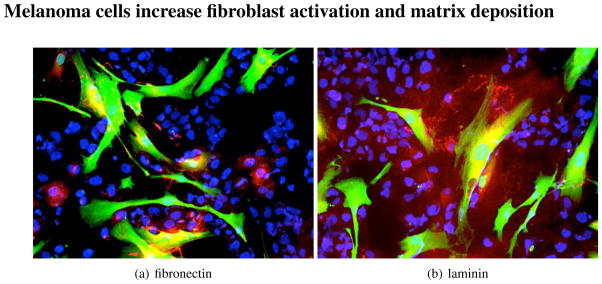
We next asked whether melanoma derived factors would activate the fibroblasts and whether this would in turn cause the fibroblasts to produce a pro-survival ECM. Co-culture of melanoma cells (WM1366 and WM793) with primary human skin fibroblasts led to an increase in expression of fibronectin, tenascin, laminin and collagen IV in the fibroblasts (Figure 4 and not shown). Similar results were also observed when melanoma cells and fibroblasts were co-cultured as spheroids (Figure 5). Interestingly, it was noted that the presence of the melanoma cells significantly up regulated the levels of ECM production in the fibroblasts, compared to spheroids composed of fibroblasts alone and that little ECM deposition was observed in spheroids of melanoma cells alone (Figure 6). The role of fibroblast derived stroma in providing structural support for the spheroid was supported by the observation that melanoma/fibroblast co-culture spheroids were much smaller, more compact and organised than the spheroid structures formed when melanoma cells alone were included.
Figure 4.
Melanoma cells (blue) activate fibroblasts (green), leading to increased ECM deposition (red). 2D monolayer cultures of WM793 melanoma cells (shown by blue 4′,6-diamidino-2-phenylindole (DAPI) staining) and GFP-tagged fibroblasts (green) after 48 hrs of co-culture. Slides are stained red for Figure 4(a) fibronectin or Figure 4(b) laminin.
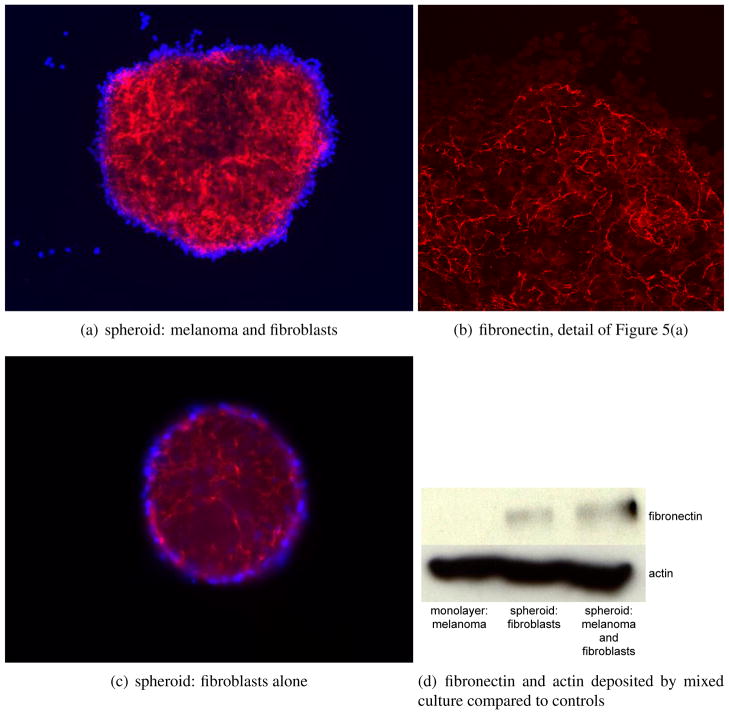
Figure 5.
Melanoma cells can induce matrix production in fibroblasts. Mixing melanoma cells with fibroblasts increases the deposition and organisation of fibronectin when compared to growing fibroblasts on their own. Melanoma line is WM1366, fibroblasts are FF2441. Red staining is fibronectin, blue is DAPI. Figure 5(a) fibronectin produced by a fibroblast and melanoma cell spheroid. Figure 5(b) detail of fibronectin in the mixed spheroid (confocal). Figure 5(c) fibronectin produced by a fibroblast spheroid. Figure 5(d) levels of fibronectin and actin in 2D adherent cultures of melanoma cells alone, 3D cultures of melanoma alone and melanoma and fibroblast co-cultures. Melanoma cells alone produce no fibronectin.
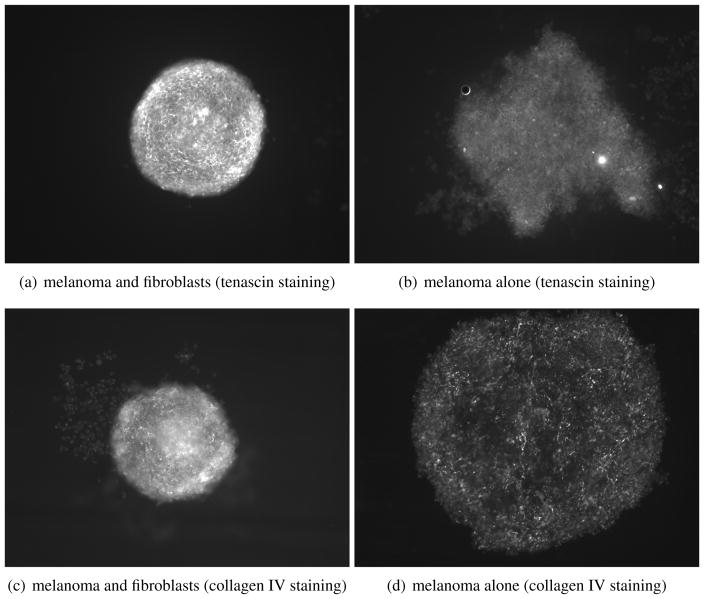
Figure 6.
Fibroblasts organise and give structure to tumours. The presence of fibroblasts increases the organisation and ECM deposition of sphere cultures compared to melanoma cell monoculture spheroids. Spheres were allowed to grow for 72 hrs before being fixed and stained for tenascin and collagen IV (also laminin, not shown).
In summary, from our experiments we have shown that melanoma cells can stimulate the recruitment of fibroblasts and activate them. In turn, these activated fibroblasts contribute to melanoma progression by providing both structural (ECM proteins) and chemical support (growth factors). Using the results obtained from our suite of experiments above we will construct our mathematical model and investigate the range of potential behaviours that it can produce and what implications this has for melanoma progression.
A model of tumour growth dynamics
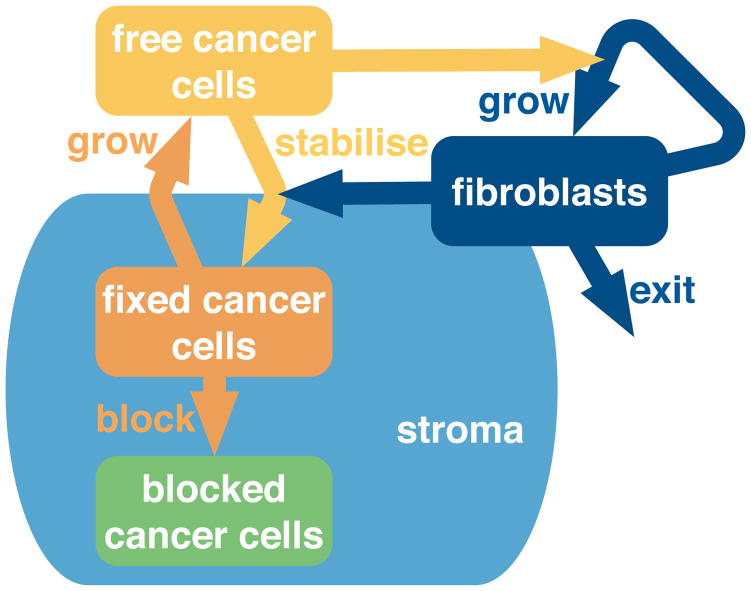
We choose a compartment model to describe the tumour. The two basic cells types in our model are cancer cells and fibroblasts. However, just two compartments is too coarse to show dynamic progression. We know that fibroblasts are drawn to cancer cells and become activated. For simplicity we assume that all of the fibroblasts shown explicitly are already activated; this gives a single fibroblast compartment. We divide the cancer cells population into three different compartments: free, fixed and blocked.
Free cancer cells are at the leading edge of the tumour mass and are the newest. They require stability, so they produce a signal to activate the fibroblasts. The fibroblasts produce matrix proteins which stabilise the cancer cells so they become fixed cancer cells. Fixed cancer cells can divide; their offspring are free cancer cells. As the fixed cancer cells divide, they become constrained in space. They have no room to divide, and so transition into the blocked cancer cell population. These blocked cancer cells are non-proliferative, in a state equivalent to quiescence. They could be necrotic, but we do not remove any of this population. We show a schematic representation of this compartment model in Figure 7. We show stroma in the schema; it is an implicit component of the model. The stroma represents matrix proteins and other stabilising components secreted by the fibroblasts. The fixed and blocked cells are stabilised by the stroma while the free cells lack this stability.
Figure 7.
Cancer cells and fibroblasts stimulate each other; the tumour progresses and grows. A compartment model describes the cell states and the interactions between them. Free cancer cells are new and unsupported by matrix. To obtain matrix, they attract fibroblasts, or stimulate them to divide. The fibroblasts produce matrix, which stabilises the free cancers cells: they become fixed. Fixed cancer cells can divide, but by doing so block themselves in physically – becoming blocked cancer cells. Stroma is implicit in the model; we show in the diagram that it is associated with the fixed and blocked cancer cells – but not with the free cancer cells. We allow fibroblasts to exit, which means they become inactive, or leave the tumour.
The compartment diagram shown in Figure 7 converts readily into a system of differential equations. We introduce variable names for each of the populations: free cancer cells – c, fixed cancer cells – s (indicating stabilised), blocked cancer cells – b and fibroblasts – f. Each interaction has a corresponding rate parameter: cancer cell division – θ, fibroblast division – φ, cancer cell stabilisation – σ, cancer cell self-blocking – β, fibroblast inactivation – δ. These parameters modify population interaction terms, producing our equation system:
Where two populations interact, a spatial constraint reduces the interaction rate. This dilutes the effect of a large population: a cell is stimulated by the proportions of cell types in its environment, not by the magnitude of them. For example, if there is one fibroblast surrounded by a million cancer cells, it produces much the same amount of fibronectin as if it were surrounded by a hundred cancer cells. So we scale the interaction terms by the total population size: n = c + s + b + f. We write the scaled population term with a bar over the symbol, so f̄= f/n, c̄ = c/n. This scaled variable is a proportion of the whole population, a percentage, so takes values between 0 and 1. This means it acts as a modifier to the base rate of the action, only reducing it. In our example, this says a fibroblast has a maximum fibronectin production rate, and if the population of stimulating cells is diluted by other cell types then the fibroblast will produce fibronectin more slowly.
Many of the interactions consist of transitions between states; in this case the total cancer population is conserved. This is reflected in the algebraic form: if we add all the cancer cell equations, only the cell division term remains – d(c +s +b)/dt = θs. The fibroblast population is not conserved, since there is a death term (labelled exit in Figure 7). This term models fibroblasts becoming inactive, or leaving the tumour.
Tumour growth is affected by fibroblasts
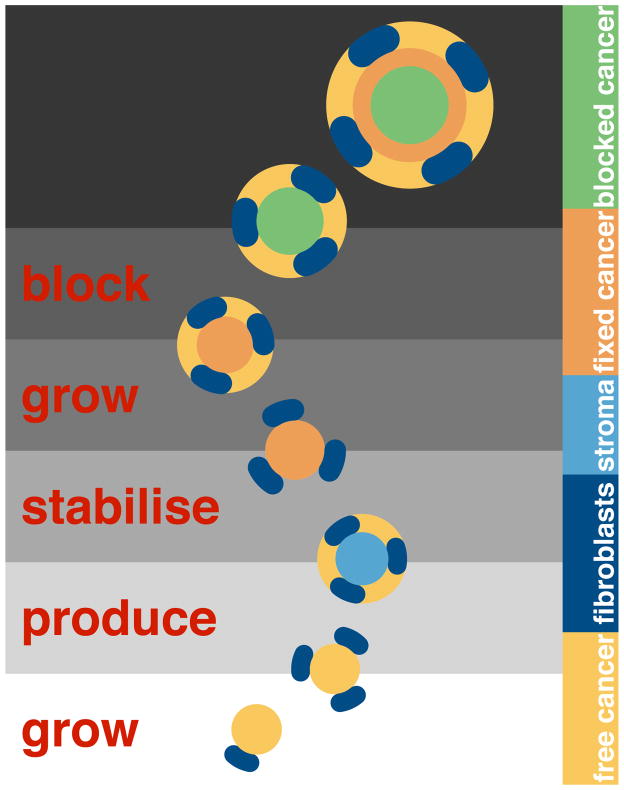
To explain the model construction, we consider the model interactions as a sequence of steps. At the start, there are only free cancer cells and a few fibroblasts. The cancer cells stimulate the fibroblasts, so they increase in number. Due to stimulation, the fibroblasts also produce stroma. In the fourth step, the free cancer cells migrate onto the stroma and stabilise. These fixed cancer cells divide and produce new free cancer cells. As a result of division, the fixed cells block themselves in spatially, so move to the blocked compartment. We show this sequence in Figure 8.
Figure 8.
Each interaction is a step in tumour growth. Here we show the first few steps of the dynamic model. This discrete breakdown is artificial, but demonstrates the assumptions at work. The population of each compartment grows. The initial conditions are simple: a pool of inactive fibroblasts, and a few free cancer cells. Time progresses vertically with the initial state of the system at the bottom of the figure. At the start, there are only free cancer cells (yellow) and a few fibroblasts (dark blue). The cancer cells stimulate the fibroblasts to increase in number. Then the fibroblasts produce stroma (light blue). In the fourth step, the free cancer cells migrate onto the stroma and stabilise (orange). These fixed cancer cells divide and produce new free cancer cells. In the process of division, the fixed cells block themselves in, and so become blocked (green).
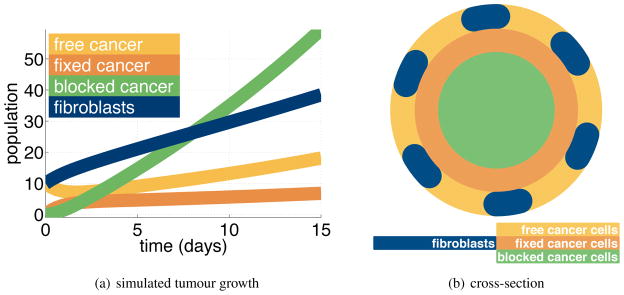
Having constructed our tumour growth model we can investigate tumour growth dynamics. We solve the system of equations, Eq. (1), numerically, using the Matlab solver ode15s. A typical solution is given in Figure 9(a). The simulation produces growth in all populations with the strongest growth occurring in the blocked cancer cell population. In the long term, all the populations grow exponentially at the same rate.
Figure 9.
Figure 9(a)The simulation yields tumour growth. Most of the population is blocked cancer cells. We see a smaller number of the other populations: fibroblasts, free cancer cells, fixed cancer cells. In the long term, all the populations increase exponentially at the same rate. Figure 9(b)The simulation represents a layered tumour with a quiescent core. Here we show a cross section.
After some time, the tumour approximates a layered sphere, with a quiescent or blocked core. Surrounding this core is a layer of cancer cells which are fixed – stabilised by stroma produced by the fibroblasts. On the outside is a shell of new cancer cells, free of any secreted stromal support. Both the fixed and blocked cancer populations are mixtures of cancer cells, fibroblasts and secreted stroma. Figure 9(b) is a cross section of this structure.
Tumours have a spectrum of malignancy in terms of both proliferative and invasive ability; we believe that different cancer cell lines also have different capacities for recruiting and stimulating fibroblasts. Specifically, we expect that more aggressive cancers stimulate fibroblasts more than less aggressive cancers. In our model, the parameter φ is the amount the cancer cells stimulate the fibroblasts. We set φ in our model (Eq. (1)) to 0.7 to simulate a less aggressive cancer, less able to recruit fibroblasts. To model a more aggressive cancer, we set φ = 1. Fibroblasts can also vary in the rate they produce stroma. Stroma is the supportive tissue of epithelial organ or tumour, consisting of connective tissues and blood vessels. In the model this is quantified as the parameter σ For normal fibroblasts, σ is low at 0.2 whereas activated fibroblasts produce much more stroma: σ= 1.
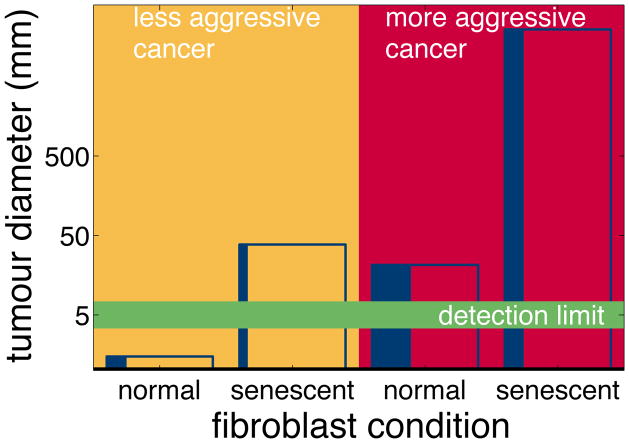
We simulate the four possible scenarios produced by the permutations of cancer aggression and fibroblast senescence. In the first scenario, less aggressive cancer cells together with normal fibroblasts grow very slowly. The tumour is so small, even after two years, that it is too small to be detected by a clinician. In the second scenario, less aggressive cancer cells do not stimulate fibroblast division so there are relatively few fibroblasts present. However, these fibroblasts are activated and so produce stroma rapidly. A substantial tumour grows, in comparison to the first scenario. In the third scenario, aggressive cancer cells stimulate fibroblast production, so the tumour has a high proportion of fibroblasts. However, these normal fibroblasts produce less stroma; cancer cells stabilise slowly, so the tumour grows fairly slowly too. In this case, the aggressive cancer has produced a smaller tumour than the previous scenario of a less aggressive cancer paired with activated fibroblasts. The fourth scenario is aggressive cancers cells paired with activated fibroblasts: the tumour grows rapidly. We show these results in Figure 10.
Figure 10.
Fibroblast condition is as important as cancer type. In each column we show the results of a single simulation of two years of tumour growth. The height of each column shows the total tumour diameter. The width of the vertical blue band shows the proportion of fibroblasts within each tumour. We vary the aggressiveness of the cancer cells, and the condition of the fibroblasts. In the first column, less aggressive cancer cells together with normal fibroblasts grow very slowly. The tumour is so small, even after two years, that it is too small to be detected by a clinician. In the second column, less aggressive cancer cells do not stimulate fibroblast division so there are relatively few fibroblasts present. However, these fibroblasts are activated and so produce stroma rapidly. A substantial tumour grows, in comparison to the first scenario. In the third column, aggressive cancer cells stimulate fibroblast production, so the tumour has a high proportion of fibroblasts. However, these normal fibroblasts produce less stroma; cancer cells stabilise slowly, so the tumour grows fairly slowly too. In this case, the aggressive cancer has produced a smaller tumour than the previous scenario of a less aggressive cancer paired with activated fibroblasts. The fourth column is aggressive cancers cells paired with activated fibroblasts: the tumour grows rapidly.
Discussion
To date, the role of fibroblasts in tumour initiation and progression has been best studied in epithelial tumours (prostate, breast, esophagus, squamous cell and colon carcinoma).4,12–14 Here, the epithelial tumour cells recruit and activate fibroblasts and the fibroblasts in turn fulfil important biological functions that are often lacking in the cancer cells, such as growth factor secretion and matrix remodelling.12–14 Relatively little is known about the interaction of melanoma cells with fibroblasts and is mostly limited to the observation that melanoma cells bind to fibroblasts via N-cadherin and that melanoma cells activate fibroblasts leading to the secretion of growth factors.21,22 In an initial series of motivating biological studies, we show that melanoma cells actively recruit fibroblasts and stimulate them to secrete matrix. In addition, we observe that the fibroblasts infiltrate into the growing melanoma spheroids, and that this increases the level of structural support. These observations recapitulate those made by pathologists showing that melanomas specimens often show signs of fibroplasia, and may possess desmoplastic characteristics (the presence of fibroblasts, fibrocytes and clearly visible fibres of extracellular matrix).23 It is likely that melanoma cells stimulate the fibroblasts through soluble factors such as transforming growth factor-β and through direct cell-cell interaction. Our experiments support this hypothesis and demonstrate that both co-culture of melanoma cells with fibroblasts and the addition of conditioned media from melanoma cells can increase matrix production in fibroblasts and stimulate their motility.
Using these experimental results as primary motivation for a mathematical model of cancer growth that includes fibroblasts as a key player, we developed a compartmental model consisting of four differential equations. The four equations describe the temporal dynamics of four variables, three of which are classified as subsets of cancer and the fourth is the fibroblasts themselves. The sub-compartmentalisation of cancer into free, fixed and blocked is one of the novel aspects of this model and is in part motivated by the dual roles, of growth promotion and stabilisation, that fibroblasts play in cancer development.
The blocked population represents tumour cells that are effectively trapped in the inner core of the tumour, bound together by stromal components that serve to stabilise them. This quiescent population may be the most difficult to reach therapeutically, for reasons we will discuss in the following section. The fixed population is one that has just transitioned from being free as a result of direct interactions with the fibroblasts, which provided stroma for structural stabilisation. The free population are the cancer cells which have not yet been supplied with stroma by the fibroblasts.
The model makes three important predictions.
Fibroblast recruitment and division plays an important role in tumour growth and organisation, providing both growth promoting and structural support.
Fibroblast activation has an even more significant impact on tumour growth as it leads to rapidly growing tumours that consist of a large blocked cancer population.
In reality, predictions 1 and 2 often occur in combination leading to fatal scale cancers in a very short time frame.
Perhaps, most importantly, is that these tumours consist of large populations of blocked, quiescent tumour cells, since this blocked population will be the most difficult to reach therapeutically. In the next section we investigate this issue by considering the role of tumour-fibroblast interactions in treatment resistance.
Fibroblasts aid resistance to treatment
The role of fibroblasts in tumour progression is becoming better understood, however, their potential impact on drug resistance remains unclear. Fibroblasts have been shown to enhance drug resistance in multiple myeloma via cell adhesion to fibronectin.15,16 Similar findings have also been reported for uveal melanoma cell lines adhering to laminin, collagen IV and fibronectin.18 For cutaneous melanoma it is known that plating cells onto fibronectin prevents anoikis and that knockdown of tenascin-C can sensitise melanoma cells to doxorubicin treatment.19,20 Although we have not considered it in the present study, it is also likely that other host cell types may also protect the cancer cells from drug-induced apoptosis. Recent work has shown that plating either lung or breast carcinoma cells onto monolayers of astrocytes conveys protection from chemotherapy-induced apoptosis through the upregulation of GSTA5, BCL2L1 and TWIST1.24 Here we present new results that directly validate the role of fibroblasts in melanoma drug resistance and use these results to motivate a theoretical investigation of different treatment strategies.
Activated fibroblasts prevents apoptosis in melanoma cell lines following cis-platin treatment
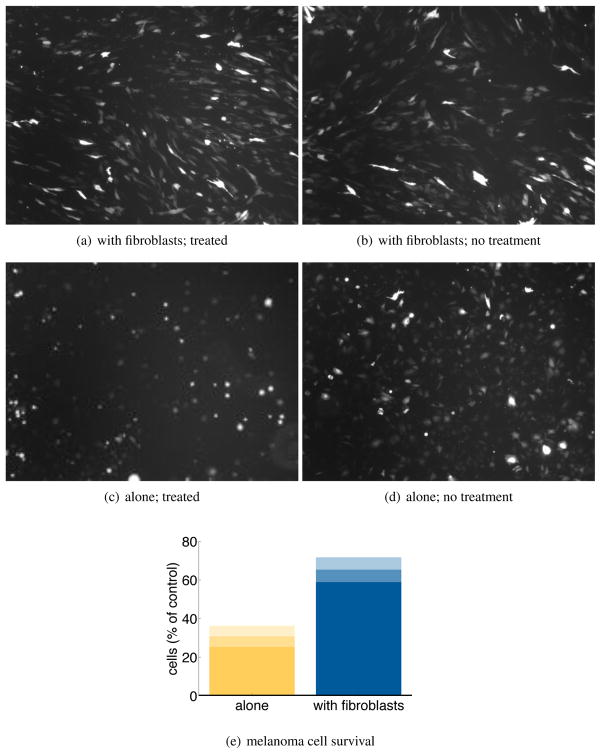
To experimentally test the role of fibroblasts in melanoma drug resistance, GFP-tagged 1205Lu melanoma cells were either grown on regular tissue culture plastic or on top of an activated fibroblast monolayer and then treated with cisplatin for 72 hrs (20 μM). It was noted that treating the melanoma cells grown on top of cell culture plastic reduced the melanoma cell number to 30.8% of that of vehicle (0) treated cultures (Figure 11). In contrast, melanoma cells grown on top of the fibroblast monolayer had a significant (*P > 0.05) survival advantage, with cisplatin treatment found to reduce the number of cells to 65.3% of control values (Figure 11).
Figure 11.
Activated fibroblasts increase the survival of melanoma cells under therapeutic drug treatment. Figure 11(a)-Figure 11(d) Representative microscope pictures showing GFP-tagged 1205Lu melanoma cells plated on either a fibroblast monolayer or normal tissue culture plastic and treated with either vehicle (0) or cisplatin (20 μM, 4 hours). Assessment is made 72 hours after the addition of cisplatin. Figure 11(e) Melanoma survival as a percentage of the vehicle treated control, with the mean bounded by standard deviation. Data is from 3 independent experiments.
These experimental results clearly show that the plating melanoma cells onto a fibroblast monolayer reduces the level of cell death observed following treatment with cisplatin. Although these results do not specify the underlying biological mechanism, we believe there are likely to be multiple mechanisms through which this fibroblast induced drug resistance can be mediated. For example, ECM-integrin engagement, paracrine growth factor secretion, and through direct homotypic cell-cell adhesion between melanoma cells and fibroblasts. Regardless of the precise mechanism it is clear that fibroblasts contribute to melanoma drug resistance, these results and those of the previous section, support our hypothesis that fibroblasts secrete ECM proteins as well as growth factors that directly contribute to both tumour growth and drug resistance.
Flare response to treatment is caused by fibroblast pool
The treatment of disseminated melanoma has been revolutionized by the discovery that ≈50% of all melanomas harbor activating mutations in the serine/threonine kinase BRAF .25–27 A recent phase III clinical trial of the BRAF inhibitor vemurafenib (formerly PLX4032), showed significant responses in ≈50% of patients whose melanomas harbored an activating BRAF mutation (Progression Free Survival: 7 months). On the basis of the phase II findings and the expectation of an increased rate of overall survival, vemurafenib was recently FDA-approved for the treatment of un-resectable BRAF V600E mutant melanoma.28,29 However, even these approaches suffer from the emergence of drug resistance and patients relapse within approximately seven months. As relapse occurs patients are removed from the targeted therapy and often a rapid regrowth of the tumour occurs,30 a so called “flare” response, that reflects both the rapidly growing tumour’s enhanced metabolism and an increase in the time to progression or death following cessation of drug.31 Thus far, the flare response has been best characterized in non-small cell lung cancer patients on EGFR inhibitor therapy, where it is likely to have important implications for future clinical trial design.31 Using the model we developed in the previous section we now investigate the impact of targeted treatments and what implications they might have for the “flare” response.
A targeted therapy will only kill the tumour population, not the fibroblasts. The blocked cells are therapeutically the most difficult to treat being quiescent, surrounded by other cells and bound up with matrix proteins. Therefore, we assume that all of the free and fixed populations and a large proportion of the blocked population are killed by the therapy. However, once the free and fixed populations are removed, the remaining blocked cells are no longer blocked, becoming fixed, so able to divide again.
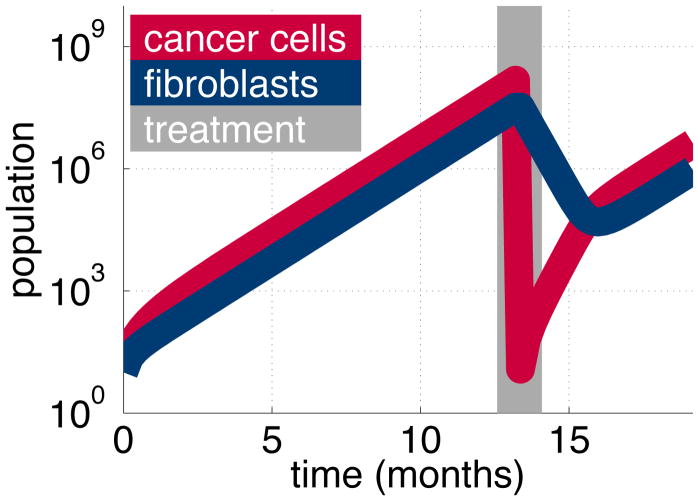
We simulate therapy with our model. We grow a mixed tumour of cancer cells and fibroblasts then apply treatment for a short period: just 1 week. The cancer cell population regrows rapidly after the targeted treatment. This accelerated growth is caused by the large fibroblast population that remains post treatment. Its production of stroma is taken up by the new, free cancer cells. These cells become fixed, and start dividing. We see a return to the standard growth rate once the cancer cell population stabilises to the original ratio it had with the fibroblast population (Figure 12). The rapid regrowth of the cancer cells represents a flare response, driven by the large pool of fibroblasts that are untouched by the targeted treatment.
Figure 12.
After treatment (gray), the large fibroblast population (blue) causes the cancer cell population (red) to regrow rapidly. We simulate a mixed tumour of cancer cells and fibroblasts. Targeted therapy reduces the cancer cell population but not the fibroblasts. After treatment, the large pool of fibroblasts means that the cancer population regrows much more rapidly than before.
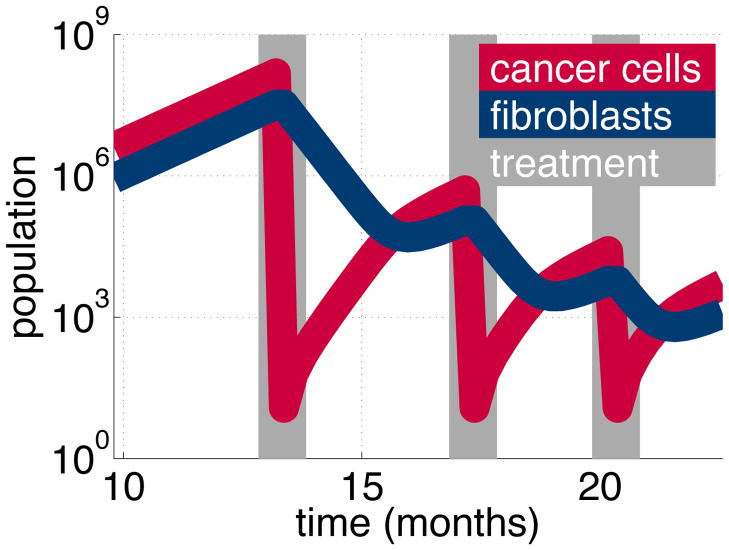
Multiple treatments reduce flare
The flare response is driven by the pool of fibroblasts. This pool declines naturally in the absence of cancer stimulation, but over a longer time scale. We wish to allow the fibroblast pool to decline further, and so we apply further treatments. In our model, it is simple to repeat treatment at the ideal time, when the cancer cells start stimulating a regrowth in the fibroblast pool. We repeat this strategy and eventually the fibroblast population returns to normal levels. With a small fibroblast pool, the tumour regrows slowly and flare is avoided (Figure 13). Our result depends on the fibroblast homeostasis assumption in the model. This ensures the fibroblast population returns to normal levels in the absence of cancer cell stimulation. These results suggests a novel approach to dealing with the flare response.
Figure 13.
Repeating treatment (gray) controls the cancer population (red) while the fibroblast population (blue) gradually reduces. We simulate a mixed tumour of cancer cells and fibroblasts. Targeted therapy reduces the cancer cell population but not the fibroblasts. However, without stimulation, the fibroblast population declines naturally. Repeating treatment controls the cancer cell population, allowing the fibroblast population to revert to normal levels. After several treatments, the tumour regrows slowly, without flare.
Discussion
Aside from providing direct structural support for the growing tumour we expect the fibroblast-derived extracellular matrix to have direct affects upon the behaviour of the tumour cells. Experimental studies have shown that plating cancer cells onto purified ECM, such as laminin, collagen IV, fibronectin and tenascin directly regulate tumour survival through the engagement of anti-apoptotic signalling loops mediated through integrins.17 Integrins are the primary means by which cells sense their local environment and integrin activation triggers signalling through a number of pro-survival pathways including focal adhesion kinase (FAK), integrin-linked kinase (ILK), MAPK/ERK and PI3K/AKT/mTOR. Of these, the MAPK/ERK and PI3K/AKT/mTORsignalling pathways are known to be critical in transducing pro-survival effects following chemotherapeutic insult.32 Our experimental studies clearly show that the plating of melanoma cells onto a fibroblast monolayer reduces the level of cell death observed following treatment with cisplatin.
The theoretical model clearly implicates the cancer cell interactions with the fibroblasts as a key driver of therapeutic escape. Although it remains neutral about the biological mechanism underlying these effects since there are likely to be multiple mechanisms through which this effect can be mediated including ECM-integrin engagement, paracrine growth factor secretion and through direct homotypic cell-cell adhesion between melanoma cells and fibroblasts. Previous work from our group has shown that melanoma cells adhere strongly to fibroblasts through N-cadherin.21,33 This homotypic cell-cell adhesion contributes to melanoma survival by increasing levels of pro-survival AKT signalling in melanoma cells.21
Whilst the experimental results focused on the fibroblasts mediated resistance in response to a standard chemotherapeutic the theoretical model investigated the potential impact on a targeted therapeutic. With the key assumption that the targeted treatment would not affect the fibroblast population. This led to the surprising conclusion that the fibroblast population maybe facilitating the clinically observed flare response. Another novel hypothesis that the model makes concerns the timing of multiple targeted treatments: by ensuring that each treatment coincides with the observed growth in the fibroblast population, we were able to significantly reduce the flare response. However, we must be cautious about drawing conclusions from these model driven hypothesis as so far none of them have been tested experimentally.
Conclusions
In this paper we investigated the role that fibroblasts play in tumour growth, organisation and treatment resistance. Using a combined experimental and theoretical approach we converged upon several key predictions:
Fibroblast recruitment and division plays an important role in tumour growth and organisation, providing both growth promoting and structural support;
Fibroblast activation has an even more significant impact on tumour growth as it leads to rapidly growing tumours that consist of a large blocked cancer population;
Fibroblast recruitment and activation often occur in combination leading to fatal scale cancers in a very short time frame;
Fibroblasts contribute to melanoma drug resistance;
Fibroblasts may facilitate the clinically observed flare response;
Targeted therapies may actually hasten tumour progression, if stopped, once resistance has occurred;
Multiple treatments strategically spaced may reduce the flare response.
It is important to recognise that many of these predictions come from a theoretical model that represents a generic tumour that has not been parameterised from a specific experiment. However, if specific parameters were to be obtained the dynamics of the model would remain the same but be less or more exaggerated. Ultimately, the model depends on the synergistic relationship between tumour and stroma which may be stronger in some cancers than others, but we believe this relationship can fundamentally affect both tumour growth and treatment and should be an important focus for cancer research.
Materials and methods
Cell culture
The melanoma cells lines WM793, 1205Lu and WM1366 are grown as described in.34 Primary skin fibroblasts are grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% foetal bovine serum, these are infected with a lentiviral vector for GFP and fluorescence-activated cell sorting (FACS) sorted to ensure that 100% of the cells were GFP expressing.
Spheroid formation
Collagen implanted spheroids are prepared using the liquid overlay method described in35 from 5000 cells in total. Co-culture spheroids are prepared from a 50:50 mix of melanoma cells (WM1366 or 1205Lu) and primary human skin fibroblasts (FF2441). Fibroblast infiltration experiments are performed by adding 2500 GFP-tagged FF2441 cells onto WM1366 spheroids cultured on agar. Spheres are harvested after 24, 48, 72 and 96 hours, fixed, made permeable and stained with 4′,6-diamidino-2-phenylindole (DAPI) then examined under an upright inverted microscope.
Immunofluorescence staining
For the adherent cultures, a 50:50 mix of melanoma cells and fibroblasts are plated onto coverslips and grown for 72 hours before being fixed and made permeable as described in.36 Then they are imaged with a Leica confocal microscope at 40 times magnification. In other studies, co-culture spheroids consisting of melanoma cells and fibroblasts are fixed with formaldehyde for 1 hr, made permeable with 0.2% v/v Triton X-100 and stained using the antibodies listed below for 1 hour at 37C. The primary antibody for fibronectin is from BD Pharmingen, the antibody for tenascin is from Research Diagnostics, the antibody for laminin was from Oncogene and the antibody to collagen IV is from Oncogene. Anti-mouse and anti-rabbit secondary antibodies are from Invitrogen.
Scratch wound assay
FF2441 fibroblasts are grown to 100% confluence on 6 well plates in the presence of either serum-free DMEM or DMEM conditioned for 48 hours by 1205Lu melanoma cells. Cultures are then scratched with a p200 pipette tip at time zero and representative pictures are taken after 8 hours to show motility.
Western blotting
Proteins are extracted and blotted for as described in.36 Approximately 100 spheroids are harvested for each protein extraction. The antibody to fibronectin is from BD Pharmingen and the antibody to actin was from Sigma.
Fibroblast mediated melanoma cell survival
Human fibroblasts are grown to confluence on 24 well plates and treated with transforming growth factor β for 48 hours (10mg/ml). 50,000 GFP-tagged human melanoma cells (1205Lu) are added to the fibroblast cultures and allowed to adhere overnight before being treated with either vehicle or cisplatin (4 hours; 20 μM) (Sigma). Co-cultures are then allowed to grow for a further 72 hours before being fixed and the GFP-tagged cells counted. Four high powered fields, at 40 times magnification, are counted for each treatment. In control experiments, GFP-tagged melanoma cells are plated directly onto 24 well cell culture plates that are not coated with fibroblasts. These are treated with either vehicle or cisplatin.
Acknowledgments
This work is paid for by the The Physical Microenvironment in Cancer Biology and Therapy (1U54CA143970-01) award. The award is made under the Physical Sciences-Oncology Program in the National Cancer Institute. This institute is part of the National Institutes of Health.
Footnotes
Supporting Information Available
This material is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Smalley KSM, Lioni M, Herlyn M. Targeting the stromal fibroblasts: a novel approach to melanoma therapy. Expert Review of Anticancer Therapy. 2005;5:1069–1078. doi: 10.1586/14737140.5.6.1069. [DOI] [PubMed] [Google Scholar]
- 2.Bissell M, Radisky D. Putting tumours in context. Nature Reviews Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. The Lancet Oncology. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 4.Cunha G, Hayward S, Wang Y, Ricke W. Role of the stromal microenvironment in carcinogenesis of the prostate. International Journal of Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 5.Elenbaas B, Weinberg R. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Experimental Cell Research. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 6.Balch C, Gershenwald J, Soong S, Thompson J, Atkins M, Byrd D, Buzaid A, Cochran A, Coit D, Ding S. Final version of 2009 AJCC melanoma staging and classification. Journal of Clinical Oncology. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haass N, Smalley K, Li L, Herlyn M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Research. 2005;18:150–159. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 8.Cornil I, Theodorescu D, Man S, Herlyn M, Jambrosic J, Kerbel R. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proceedings of the National Academy of Sciences. 1991;88:6028–6032. doi: 10.1073/pnas.88.14.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satyamoorthy K, Li G, Vaidya B, Patel D, Herlyn M. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and -catenin pathways. Cancer Research. 2001;61:7318–7324. [PubMed] [Google Scholar]
- 10.Basanta D, Strand DW, Lukner RB, Franco OE, Cliffel DE, Ayala GE, Hayward SW, Anderson ARA. The role of transforming growth factor-beta-mediated tumor-stroma interactions in prostate cancer progression: an integrative approach. Cancer Res. 2009;69:7111–20. doi: 10.1158/0008-5472.CAN-08-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, 2nd, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–81. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Wever O, Nguyen Q, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, Mareel M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. The FASEB Journal. 2004;18:1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 13.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall J, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGT-Pases in leading and following cells. Nature Cell Biology. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 14.Noma K, Smalley K, Lioni M, Naomoto Y, Tanaka N, El-Deiry W, King A, Nakagawa H, Herlyn M. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–1993. doi: 10.1053/j.gastro.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazlehurst LA, Argilagos RF, Emmons M, Boulware D, Beam CA, Sullivan DM, Dalton WS. Cell adhesion to fibronectin (CAM-DR) influences acquired mitoxantrone resistance in U937 cells. Cancer Res. 2006;66:2338–45. doi: 10.1158/0008-5472.CAN-05-3256. [DOI] [PubMed] [Google Scholar]
- 16.Shain KH, Dalton WS. Environmental-mediated drug resistance: a target for multiple myeloma therapy. Expert Rev Hematol. 2009;2:649–62. doi: 10.1586/ehm.09.55. [DOI] [PubMed] [Google Scholar]
- 17.Sethi T, Rintoul R, Moore S, MacKinnon A, Salter D, Choo C, Chilvers E, Drans-field I, Donnelly S, Strieter R. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nature Medicine. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 18.Bérubé M, Talbot M, Collin C, Paquet-Bouchard C, Germain L, Guérin SL, Petitclerc E. Role of the extracellular matrix proteins in the resistance of SP6. 5 uveal melanoma cells toward cisplatin. Int J Oncology. 2005;26:405–413. [PubMed] [Google Scholar]
- 19.Boisvert-Adamo K, Aplin A. B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene. 2006;25:4848–4856. doi: 10.1038/sj.onc.1209493. [DOI] [PubMed] [Google Scholar]
- 20.Fukunaga-Kalabis M, Martinez G, Nguyen T, Kim D, Santiago-Walker A, Roesch A, Herlyn M. Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene. 2010;29:6115–6124. doi: 10.1038/onc.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–25. [PubMed] [Google Scholar]
- 22.Li G, Satyamoorthy K, Meier F, Berking C, Bogenrieder T, Herlyn M. Function and regulation of melanoma-stromal fibroblast interactions: when seeds meet soil. Oncogene. 2003;22:3162–71. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- 23.Nakhleh RE, Wick MR, Rocamora A, Swanson PE, Dehner LP. Morphologic diversity in malignant melanomas. Am J Clin Pathol. 1990;93:731–40. doi: 10.1093/ajcp/93.6.731. [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, Maya M, He J, Kim SW, Weihua Z, Balasubramanian K, Fan D, Mills GB, Hung MC, Fidler IJ. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia (New York, NY) 2011;13:286. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bollag G, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smalley KSM. PLX-4032, a small-molecule B-Raf inhibitor for the potential treatment of malignant melanoma. Curr Opin Investig Drugs. 2010;11:699–706. [PubMed] [Google Scholar]
- 27.Tsai J, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riely G, Kris M, Zhao B, Akhurst T, Milton D, Moore E, Tyson L, Pao W, Rizvi N, Schwartz L, Miller V. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clinical Cancer Research. 2007;13:5150. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 31.Chaft J, Oxnard G, Sima C, Kris M, Miller V, Riely G. Disease Flare after Tyrosine Kinase Inhibitor Discontinuation in Patients with EGFR-Mutant Lung Cancer and Acquired Resistance to Erlotinib or Gefitinib: Implications for Clinical Trial Design. Clinical Cancer Research. 2011;17:6298–6303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraus AC, Ferber I, Bachmann SO, Specht H, Wimmel A, Gross MW, Schlegel J, Suske G, Schuermann M. In vitro chemo- and radio-resistance in small cell lung cancer correlates with cell adhesion and constitutive activation of AKT and MAP kinase pathways. Oncogene. 2002;21:8683–95. doi: 10.1038/sj.onc.1205939. [DOI] [PubMed] [Google Scholar]
- 33.Smalley KSM, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol. 2005;166:1541–54. doi: 10.1016/S0002-9440(10)62370-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paraiso KH, Fedorenko IV, Cantini L, Munko A, Hall M, Sondak V, Messina J, Flaherty K, Smalley K. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. British journal of cancer. 2010;102:1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smalley KSM, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Molecular Cancer Therapeutics. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 36.Smalley KSM, Contractor R, Haass NK, Lee JT, Nathanson KL, Medina C, Flaherty KT, Herlyn M. Ki67 expression levels are a better marker of reduced melanoma growth following MEK inhibitor treatment than phospho-ERK levels. British Journal of Cancer. 2007;96:445–449. doi: 10.1038/sj.bjc.6603596. [DOI] [PMC free article] [PubMed] [Google Scholar]