Abstract
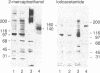
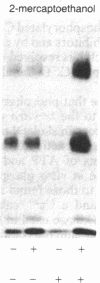
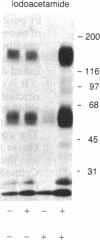
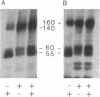
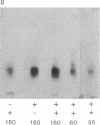
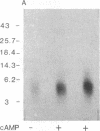
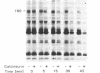
The phosphorylation and dephosphorylation of the dihydropyridine-sensitive Ca2+ channel was studied in transverse-tubule membranes isolated from rabbit skeletal muscle. Exposure of these membranes to either the cAMP-dependent protein kinase or a Ca2+/calmodulin-dependent protein kinase resulted in a rapid phosphorylation of a protein with properties similar to the major component of the skeletal muscle Ca2+ channel. The molecular mass of the phosphoprotein was 140 or 160 kDa, depending on the electrophoretic conditions. The stoichiometry of the phosphorylation was calculated to be 0.4-1.0 mol of phosphate per mol of protein. Neither the rate nor the extent of phosphorylation was affected by dihydropyridines. Limited proteolytic digestion of the protein that had been phosphorylated by either or both protein kinases yielded a single phosphopeptide of approximately equal to 5.4 kDa. The Ca2+-dependent phosphatase calcineurin dephosphorylated the membrane-bound Ca2+ channel that had been previously phosphorylated by either protein kinase. The results suggest that the major component of the dihydropyridine-sensitive Ca2+ channel from skeletal muscle can be effectively phosphorylated and dephosphorylated in its native state by cAMP- and Ca2+-dependent processes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Borsotto M., Barhanin J., Fosset M., Lazdunski M. The 1,4-dihydropyridine receptor associated with the skeletal muscle voltage-dependent Ca2+ channel. Purification and subunit composition. J Biol Chem. 1985 Nov 15;260(26):14255–14263. [PubMed] [Google Scholar]
- Borsotto M., Barhanin J., Norman R. I., Lazdunski M. Purification of the dihydropyridine receptor of the voltage-dependent Ca2+ channel from skeletal muscle transverse tubules using (+) [3H]PN 200-110. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1357–1366. doi: 10.1016/0006-291x(84)91241-5. [DOI] [PubMed] [Google Scholar]
- Borsotto M., Norman R. I., Fosset M., Lazdunski M. Solubilization of the nitrendipine receptor from skeletal muscle transverse tubule membranes. Interactions with specific inhibitors of the voltage-dependent Ca2+ channel. Eur J Biochem. 1984 Aug 1;142(3):449–455. doi: 10.1111/j.1432-1033.1984.tb08307.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium-transporting systems of plasma membranes, with special attention to their regulation. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:543–549. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cognard C., Romey G., Galizzi J. P., Fosset M., Lazdunski M. Dihydropyridine-sensitive Ca2+ channels in mammalian skeletal muscle cells in culture: electrophysiological properties and interactions with Ca2+ channel activator (Bay K8644) and inhibitor (PN 200-110). Proc Natl Acad Sci U S A. 1986 Mar;83(5):1518–1522. doi: 10.1073/pnas.83.5.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2528–2532. doi: 10.1073/pnas.82.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984 May 8;23(10):2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Galizzi J. P., Borsotto M., Barhanin J., Fosset M., Lazdunski M. Characterization and photoaffinity labeling of receptor sites for the Ca2+ channel inhibitors d-cis-diltiazem, (+/-)-bepridil, desmethoxyverapamil, and (+)-PN 200-110 in skeletal muscle transverse tubule membranes. J Biol Chem. 1986 Jan 25;261(3):1393–1397. [PubMed] [Google Scholar]
- Galizzi J. P., Fosset M., Lazdunski M. Properties of receptors for the Ca2+-channel blocker verapamil in transverse-tubule membranes of skeletal muscle. Stereospecificity, effect of Ca2+ and other inorganic cations, evidence for two categories of sites and effect of nucleoside triphosphates. Eur J Biochem. 1984 Oct 15;144(2):211–215. doi: 10.1111/j.1432-1033.1984.tb08451.x. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R. Assay for calcium channels. Methods Enzymol. 1985;109:513–550. doi: 10.1016/0076-6879(85)09112-1. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R., Boschek C. B. Purification of the putative calcium channel from skeletal muscle with the aid of [3H]-nimodipine binding. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(1):1–11. doi: 10.1007/BF00498821. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Horne P., Triggle D. J., Venter J. C. Nitrendipine and isoproterenol induce phosphorylation of a 42,000 dalton protein that co-migrates with the affinity labeled calcium channel regulatory subunit. Biochem Biophys Res Commun. 1984 Jun 29;121(3):890–898. doi: 10.1016/0006-291x(84)90761-7. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazoglou T., Schackmann R. W., Fosset M., Shapiro B. M. Calcium channel antagonists inhibit the acrosome reaction and bind to plasma membranes of sea urchin sperm. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1460–1464. doi: 10.1073/pnas.82.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., McGuinness T., Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J Neurosci. 1983 Apr;3(4):818–831. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Krinks M. H., Manalan A. S., Cohen P., Stewart A. A. Isolation and characterization of bovine brain calcineurin: a calmodulin-stimulated protein phosphatase. Methods Enzymol. 1983;102:227–244. doi: 10.1016/s0076-6879(83)02024-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazurek N., Schindler H., Schürholz T., Pecht I. The cromolyn binding protein constitutes the Ca2+ channel of basophils opening upon immunological stimulus. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6841–6845. doi: 10.1073/pnas.81.21.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness T. L., Lai Y., Greengard P., Woodgett J. R., Cohen P. A multifunctional calmodulin-dependent protein kinase. Similarities between skeletal muscle glycogen synthase kinase and a brain synapsin I kinase. FEBS Lett. 1983 Nov 14;163(2):329–334. doi: 10.1016/0014-5793(83)80846-1. [DOI] [PubMed] [Google Scholar]
- Miller R. J., Freedman S. B. Are dihydropyridine binding sites voltage sensitive calcium channels? Life Sci. 1984 Mar 26;34(13):1205–1221. doi: 10.1016/0024-3205(84)90543-5. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Perry S. V. Calmodulin and myosin light-chain kinase of rabbit fast skeletal muscle. Biochem J. 1979 Apr 1;179(1):89–97. doi: 10.1042/bj1790089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Schmid A., Renaud J. F., Lazdunski M. Short term and long term effects of beta-adrenergic effectors and cyclic AMP on nitrendipine-sensitive voltage-dependent Ca2+ channels of skeletal muscle. J Biol Chem. 1985 Oct 25;260(24):13041–13046. [PubMed] [Google Scholar]
- Sperelakis N. Hormonal and neurotransmitter regulation of Ca++ influx through voltage-dependent slow channels in cardiac muscle membrane. Membr Biochem. 1984;5(2):131–166. doi: 10.3109/09687688409150275. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 5. Purification and properties of a Ca2+/calmodulin-dependent protein phosphatase (2B) from rabbit skeletal muscle. Eur J Biochem. 1983 May 2;132(2):289–295. doi: 10.1111/j.1432-1033.1983.tb07361.x. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Davison M. T., Cohen P. The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification, subunit structure and substrate specificity. Eur J Biochem. 1983 Nov 15;136(3):481–487. doi: 10.1111/j.1432-1033.1983.tb07766.x. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Sakuma M., Yagi K. Calmodulins from muscles of marine invertebrates, scallop and sea anemone. J Biochem. 1980 May;87(5):1313–1320. doi: 10.1093/oxfordjournals.jbchem.a132869. [DOI] [PubMed] [Google Scholar]