Abstract
Ischemic preconditioning (IPC) is a well-documented phenomenon. Short episodes of sublethal ischemia provide cardioprotective effects for subsequent longer duration ischemic events. Although the exact mechanism of IPC is not yet known, the chemical basis of IPC seems to involve preservation of ATP or collateral vascularization recruitment. In this case report, we present visual evidence of ischemic preconditioning using Heartscape Technologies 80 Lead ECG device. The 80 Lead ECG is described as a body surface mapping modality, converting its inputted 80 lead ECG data into a 3-Dimensional color coded map. The 80 lead ECG device can detect instantaneous ischemic changes. Different studies have been performed to show different clinical and biochemical aspects of IPC. However data regarding direct visual evidence of this phenomenon is lacking. The secondary objective of this study is to show the ability of 80 lead ECG to identify ST-segment elevation and depression during ischemic events. The utility of 80 Lead ECG body surface mapping is enormous when evaluating ischemic events.
Keywords: ischemic preconditioning, Cardioprotection, Electrocardiogram
Introduction
Ischemic preconditioning (IPC) is a phenomenon during which transient sub lethal episodes of ischemia before a prolonged ischemia/reperfusion injury provide cardioprotection for subsequent longer duration ischemic events.1 Hearts exposed to IPC have a better metabolic and ionic status during prolonged ischemia compared to naive hearts.2 Recent studies in surgical models of cardioplegic arrest and reperfusion have suggested that the preconditioned, arrested heart may have an increased tolerance to prolonged ischemia and improved functional recovery after reperfusion.3 The initial ischemic event activates numerous signal transduction pathways, which serve to maintain myocyte contractility and function.4 Experimentally, a reduction in myocardium infarct size can be induced by subjecting the heart to episodes of non-lethal myocardial ischaemia and reperfusion prior to the sustained coronary artery occlusion.5 In-hospital risk of adverse events, the risk of death, and non fatal myocardial infarction (MI) are reduced significantly in patients experiencing IPC. Failure of IPC production during percutaneous coronary intervention (PCI), serves as an independent predictor of future ischemic event.6 Different studies have been performed to show different clinical and biochemical aspects of IPC. However data regarding direct visual evidence of this phenomenon is lacking. Also, there are not many studies on the use of 80 lead ECG to evaluate ischemic events. In this case report, we present the visual evidence of IPC, using Heartscape Technologies 80 lead ECG device, a body surface mapping modality that converts 80 lead ECG data into a 3-D color coded map. The secondary objective of this study is to show the ability of 80 lead ECG to identify ST-segment elevation and depression during ischemic events.
Case Presentation
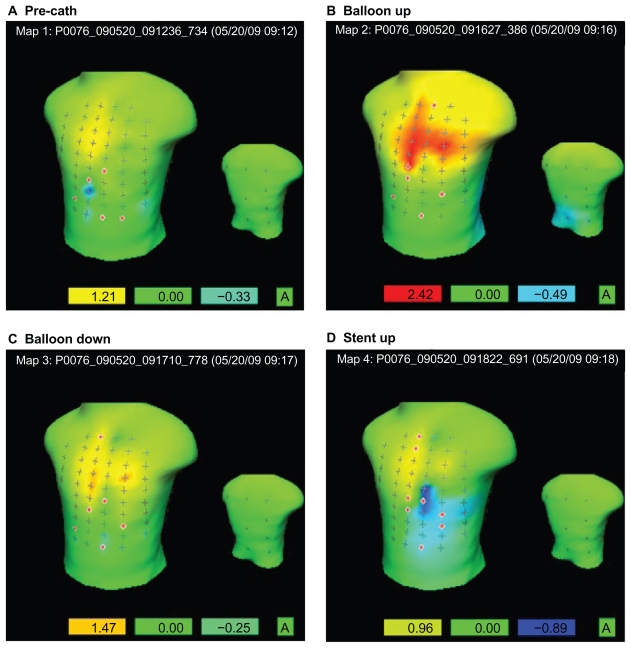
A 60 year old Caucasian male with complaints of chest pain and a positive stress test, underwent PCI and possible angiogram. The patient consented to be enrolled in this study, and the written consent was obtained from the patient. The patient was connected to the 80 Lead ECG device according to company instructions. The angiogram revealed 90% blockage on proximal LAD coronary artery. A 2.5 mm × 18 mm Xience stent was inserted. The use of the 80-lead ECG did not interfere with coronary artery visualization during PCI. An 80 lead ECG was recorded pre-catheterization, 30 seconds after balloon inflation, immediately after balloon deflation, and 30 seconds after stent deployment. The duration of balloon inflation, balloon deflation, and stent deployment was equal (2 minutes each). No medications were given in between the first balloon inflation and the stent deployment. After the procedure, the 80 lead ECGs were reviewed and analyzed by cardiologists. Interpreting the results of the body surface map requires some training. A green area represents an isoelectric state or no deflection from the isoelectric line (the isoelectric line indicates no voltage difference between the T and P wave in the subsequent beat). A red colored area indicates a positive deflection or deflection above the isoelectric line and a blue area indicates a negative deflection or deflection below the isoelectric line. Figure 1 represents the ST0 filter map which is a variation of the ST isopotential map that measures the voltage at the beginning of the ST segment or J point. This specialized ST0 filter map displays only the significant ST-segment deflections and normalizes any small deflections that are likely artifacts or normal variations. The thresholds for abnormal ST elevation or depression vary depending on the distance from the surface lead to the myocardium. Values exceeding threshold are indicated as ±1.5 mm in the anterior, septal and lateral walls, ±1.0 mm in the inferior wall, and ±0.5 mm in the posterior wall. The left box below each torso map indicates the maximum positive deflection in the respective recording and the right box below each map indicates the value of the maximum negative deflection. In the pre-catheterization setting, there is no significant positive or negative deflection from baseline, as seen in Figure 1A. This can be appreciated both numerically by the values in the boxes (none exceeding threshold values) and visually by the absence of significant red or blue areas. When the balloon was inflated (Fig. 1B), there is marked significant positive elevation of 2.42 mm, also indicated by the bright red colored area in the upper anterior wall, indicating significant ischemia. When the balloon was deflated, there is no significant elevation as indicated by the 1.47 mm deviation from baseline and negative deflection of −0.25 mm, seen in Figure 1C. When the stent was deployed (Fig. 1D), there was a marked decrease in the positive deflection from baseline as compared to when the balloon was inflated. In fact, the maximum recorded baseline deflection when the stent was deployed was 0.96 mm, a value below significant threshold deviation. The 12 lead ECG at every phase was in accordance with the data of 80 lead ECG. This can be evidence that the sublethal ischemic insult provided by the balloon inflation conditioned the myocardium to a subsequent ischemic event, in this case, the deployment of a stent.
Figure 1.
80 lead st0 filter map recordings during PCI. (A) In the pre-catheterization setting, there is no significant positive or negative deflection from baseline. (B) When the balloon was inflated, there is marked significant positive elevation of 2.42 mm, also indicated by the bright red colored area in the upper anterior wall, indicating significant ischemia. (C) When the balloon was deflated, there is no significant elevation as indicated by the 1.47 mm deviation from baseline and negative deflection of −0.25 mm. (D) When the stent was deployed, there was a marked decrease in the positive deflection from baseline as compared to when the balloon was inflated.
Discussion
It is widely accepted that IPC provides protection to the myocardium. It has been proposed that pre-infarct angina provides the sublethal insult which protects the myocardium from the subsequent infarct with respect to the size of infarct, contractile dysfunction, arrhythmia generation and prognosis.6 IPC can be induced for myocardial protection prior to planned cases such as coronary artery bypass graft surgery (CABG).7 Monitoring and comparing the ischemic changes instantaneously during such procedures can provide significant information regarding regional myocardial injury or prognosis. Currently, the only device that allows visualization of ischemic changes is Heartscape Technologies 80 Lead ECG device. In contrast to the 12 leads of data and the limited anterior or front view of the heart from a traditional EKG, an 80 lead EKG utilizes 80 leads placed on both the front and back of the patient to analyze a 360-degree spatial view of the heart. This system on the computer console displays a recognizable waveform from each single electrode position. The deviation of ST segments or the conduction patterns can be readily appreciated then by single lead representations or by rhythm strips from each and every one of the 80 electrode positions. In addition, the system will configure any measurement of interest (ST segment at J-point, ST segment 60 ms, QRS deviations) as a color-coded precordial reconstitution of the measurement showing the location of elevations or depressions, as color-coded hot spots or cold spots, located specifically in their lead positions relative to a human torso graphic. With this graphic in the actual device, as you point with a mouse towards any single spot, a hot spot or a cold spot, the waveform that measurement is originating from is displayed instantaneously. Being first explained by Murry et al,8 IPC has been extremely investigated. In the study of Warren et al it was shown that IPC could be induced in 80% of elective PCI cases. In this study, it was also shown that, the frequency of post-procedural cardiac enzyme elevation, was significantly lower in patients experiencing IPC. In addition, the risk of death and non-fatal MI at 1 year follow-up were reduced by 85%, and 55% respectively, in patients who manifested IPC.9 Recent studies on surgical models of cardioplegic arrest and reperfusion have suggested that, preconditioned arrested heart may have an increased tolerance to prolonged ischemia and improved functional recovery after reperfusion.10 Mitochondrial adenosine triphosphate-dependent potassium channel has been suggested as a “final common pathway”,11–13 and numerous agonists are believed to mediate their respective preconditioning effect via this mechanism. 14 To study IPC on humans, PCI model of coronary occlusion (brief myocardial ischemia) has been extensively used, whereas the use of ST segment deviation (in contrast to myocardial necrosis) has served as a surrogate marker of ischemic injury.6 To our best knowledge, despite multiple studies on clinical aspects and molecular pathways of IPC, there has been no investigation to focus on the direct visualization of IPC during PCI.
Conclusion
In this study, we show that the 80 lead ECG device, can detect instantaneous ischemic changes. The utility of 80 lead ECG body surface mapping is enormous, when evaluating ischemic events.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Hoole SP, Heck PM, Sharples L, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–7. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010;24:225–34. doi: 10.1007/s10557-010-6236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikonomidis JS, Weisel RD, Mickle DA. Ischemic preconditioning: cardioprotection for cardiac surgery. J Card Surg. 1994;9:526–31. doi: 10.1111/jocs.1994.9.3s.526. [DOI] [PubMed] [Google Scholar]
- 4.Ali ZA, Callaghan CJ, Lim E, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116:I98–105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008 Aug 1;79(3):377–86. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 6.Miura T. Myocardial Response to Ischemic Preconditioning: Is it a Novel Predictor of Prognosis? JACC. 2003;42:1004–5. doi: 10.1016/s0735-1097(03)00908-2. [DOI] [PubMed] [Google Scholar]
- 7.Tyagi P, Tayal G. Ischemic Preconditioning of Myocardium. Acta Pharmacol Sin. 2002:865–70. [PubMed] [Google Scholar]
- 8.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 9.Laskey WK, Beach D. Frequency and clinical significance of ischemic preconditioning during percutaneous coronary intervention. J Am Coll Cardiol. 2003;42:998–1003. doi: 10.1016/s0735-1097(03)00909-4. [DOI] [PubMed] [Google Scholar]
- 10.Ikonomidis JS, Weisel RD, Mickle DA. Ischemic preconditioning: cardioprotection for cardiac surgery. J Card Surg. 1994;9:526–31. doi: 10.1111/jocs.1994.9.3s.526. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annu Rev Physiol. 2000;62:79–109. doi: 10.1146/annurev.physiol.62.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Bryan L, Clement JP, 4th, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–45. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Sato T, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–9. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 14.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications. Part 2. Circulation. 2001;104:3158–67. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]