Abstract
We tested whether dopamine receptor activation modulates the voltage-gated Na+ current of goldfish retinal ganglion cells, using a fast voltage-clamp amplifier, perforated-patch whole-cell mode, and a physiological extracellular Na+ concentration. As found in other cells, activators of D1-type dopamine receptors and of protein kinase A reduced the amplitude of current activated by depolarizations from resting potential, without altering the current kinetics or activation range. However, D1-type dopamine receptor activation also accelerated the rate of entry into inactivation during subthreshold depolarizations, and slowed the rate of recovery from inactivation after single, brief depolarizations. Our results provide the first evidence in any preparation that D1-type receptor activation can produce both of these latter effects.
Keywords: Na+ current, inactivation, dopamine, excitability, retina
INTRODUCTION
Signal processing and spike generation are modulated in several well-known neural circuits by dopamine. Although this involves various electrophysiological changes in some systems, one change that is commonly recruited to influence excitability is the reduction of voltage-gated Na+ current (INa). Recent studies have shown that this reduction usually entails activation of a cAMP-dependent protein kinase (PKA), and that PKA activation can reduce INa in different ways. In hippocampal neurons, Cantrell et al. (1999) reported that D1-type dopamine receptor and PKA activation reduce INa without changing the rates of fast or slow inactivation from closed or open states. In striatal neurons, Schiffman et al. (1998) showed that INa can be reduced by the PKA-phosphorylated form of the phosphatase inhibitor, DARPP-32. In prefrontal cortical neurons, Carr et al. (2003) found that PKA activation reduces INa by accelerating the entry of fast-inactivated Na+ channels into a slow-inactivated state.
The ability to reduce INa by different means suggests several possible functional consequences. For example, increasing the rate of slow inactivation should accelerate losses of excitability during sustained or repeated excitatory inputs, because slow inactivation accumulates during sustained and repeated depolarizations (Chandler and Meves 1970; Rudy 1975; Brismar 1977; Fleidervisch et al. 1996; Martina and Jonas 1997; Toib et al. 1998; Mickus et al. 1999; Ellerkmann et al. 2001). Unlike fast inactivation, where entry and recovery are both rapid, recovery from slow inactivation is slow, at least in part, and entry can be fast or slow (e.g., Rudy 1978, 1981). Does this imply that, in cells where entry into inactivation is slow, PKA activation could modulate spikes only after several spikes fired? This is of interest because some neurons transmit information with relatively few spikes (Lisman 1997; Usrey et al. 1998; Reinagel et al. 1999; Kara and Reid 2003), and one would wonder if PKA activation modulates INa in ways that affect these spikes. To investigate this possibility, we tested the effect of a D1-type dopamine receptor agonist and PKA activators on the inactivation of INa in retinal ganglion cells. These cells are well-suited for this purpose because D1-type dopamine receptor activation reduces spike generation in them even after blocking their voltage-gated Ca2+ current (Vaquero et al. 2001) and because they generate brief spike volleys in response to various inputs (Barlow et al. 1971; Sakuranaga et al. 1987; Berry et al. 1997; Balasubramanian and Berry 2002).
Parts of this study have been published previously in a meeting abstract (Hayashida and Ishida 2003).
MATERIALS and METHODS
The experiments described here were performed on retinal ganglion cells of adult goldfish (Carrasius auratus) because D1-type receptor activation inhibits spiking in these cells (Vaquero et al. 2001), anti-dopamine receptor antibodies bind to them (Mora-Ferrer et al. 1999), and dopaminergic cells synapse onto them (Yazulla and Zucker 1988). However, dopamine receptors are also found in bipolar cells that synapse onto ganglion cells (Hedden and Dowling 1978). Therefore, to ensure that effects were mediated by receptors in ganglion cells, all experiments were performed on isolated ganglion cells. Cells were isolated mechanically without exposure to enzymes (Hayashida et al. 2004), identified by methods described previously (Ishida and Cohen 1988), and used within 24 hours of plating. All animal care and experimental protocols were approved by the Animal Use and Care Administrative Advisory Committee of the University of California, Davis.
Because an anti-D1-type dopamine receptor antiserum stains ganglion cell somata (Mora-Ferrer et al. 1999); cAMP levels in these somata can be elevated and lowered by a D1-type receptor agonist and antagonist, respectively (Vaquero et al. 2001); and the dendritic arbor, axon, and gap junctions of intact ganglion cells would prevent a space-clamp sufficient for the voltage-clamp measurements attempted here, recordings were made from isolated somata having no neurites or no more than a few short neurites. Because ganglion cells respond to D1-type dopamine receptor agonists in perforated-, but not ruptured-, patch mode (Vaquero et al. 2001), measurements were made only in perforated-patch mode (Horn and Marty 1988) using amphotericin B as the perforating agent (for details, see Hayashida et al. 2004). Currents and voltages were measured, with a discontinuous single-electrode voltage-clamp amplifier (npi electronic, SEC-05LX; Tamm, Germany), from cells in an extracellular solution that contained (in mM): 140 NaCl, 3.5 KCl, 3.4 MgCl2, 0.1 CaCl2, 10 d-glucose, 5 HEPES; pH adjusted to 7.4 with NaOH. The Na+ concentration was matched to cyprinid plasma levels (Houston and Madden 1968), and was not lowered to reduce series resistance errors, for two reasons. One was to avoid the possibility that lowering extracellular Na+ levels alters the rates of entry into slow-inactivation and recovery from fast-inactivation, as found in other cells (Townsend and Horn 1997; Kuo and Liao 2000). The second is that the membrane potentials measured during the currents reported here differed from the command potentials by less than a few mV [Figs. 1–3; see Hayashida et al. (2004) for details of the supercharging pulses, capacitance neutralization, feedback gain, switching frequency and other amplifier settings]. The lowered Ca2+ and elevated Mg2+ concentrations in this external solution blocked voltage-gated Ca2+ current (Vaquero et al. 2001) and thus precluded effects of Ca2+ influx on Na+ channel phosphorylation (Kondratyuk and Rossie 1997) and changes in whole-cell current due to Ca2+ current modulation (Liu and Lasater 1994).
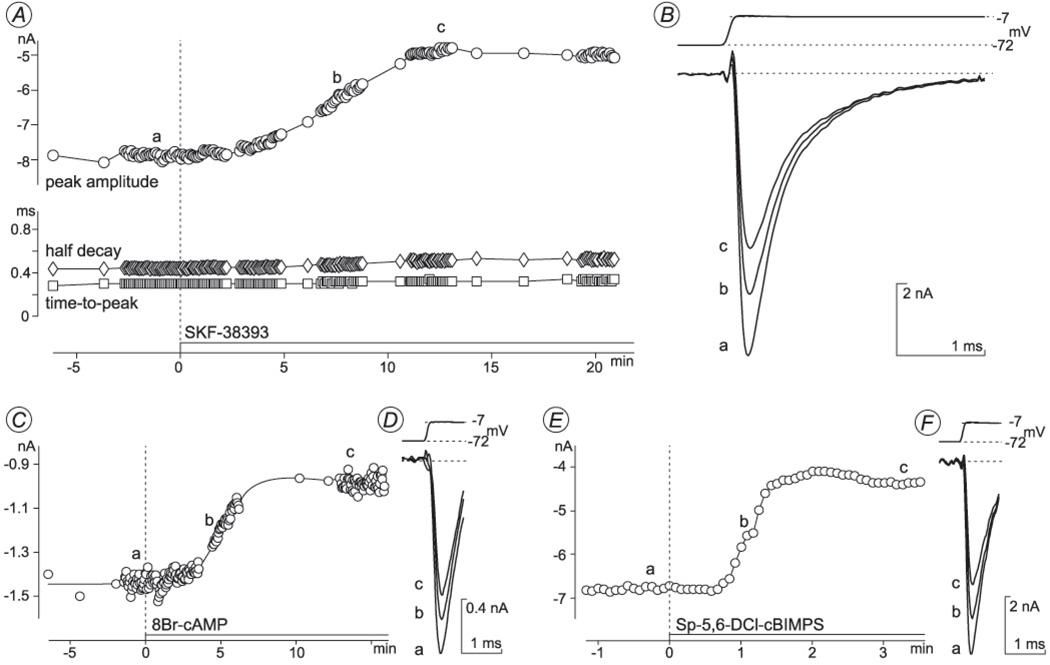
Fig. 1. Reduction of Na+ current (INa) amplitude by D1-type dopamine receptor related ligands.
A, B: SKF-38393 (28 µM). C, D: 8-bromo-cAMP (166 µM). E, F: Sp-5,6-DCl-cBIMPS (43 µM). INa was activated by 1–3-msec depolarizations from the holding potential (−72 mV) to a test potential of −7 mV. Jumps were repeated, at most, once per 5 sec. After the control INa amplitude remained stable for >1 min, ligands were applied. Circles plot peak INa amplitude during each test depolarization. Squares plot time from onset of test depolarization to INa peak. Diamonds plot time for INa to decline from peak to half the peak amplitude. Dashed vertical lines show time at which each ligand application began. In each pair of panels (A, B; C, D; E, F), the current traces labeled 𝖺, 𝖻, and 𝖼 were recorded at the times indicated by these letters in the corresponding amplitude plots. Leak and capacitive currents were subtracted off-line. Each pair of panels presents data from a different cell. Recordings were made with K+-free external and electrode solutions in A, B and with K+-containing solutions in C–F.
Fig. 3. SKF-38393 modulates rates of entry into and recovery from inactivation.
The recording electrode solution contained (in mM): 110 K-d-gluconic acid, 15 KCl, 15 NaOH, 2.6 MgCl2, 0.34 CaCl2, 1 EGTA, 10 HEPES; pH adjusted to 7.4 with methanesulfonic acid (MSA). This solution was designed to measure INa in the presence of physiological levels of intracellular Na+ and K+ (Stys et al. 1997), and to avoid the possibility that INa kinetics were altered by ions used to replace K+ (Schauf and Bullock 1978). To ensure that the effects observed were due to changes in INa rather than changes in K+ current, K+-free solutions were also used. The K+-free extracellular solution contained (in mM): 110 NaCl, 3 CsCl, 30 tetraethylammonium-Cl, 2.4 MgCl2, 0.1 CaCl2, 10 d-glucose, 5 HEPES; pH adjusted to 7.4 with CsOH. The K+-free recording electrode solution contained (in mM): 140 CsOH, 15 NaCl, 2.6 MgCl2, 0.34 CaCl2, 1 EGTA, 10 HEPES; pH was adjusted to 7.4 with MSA. We noticed no significant difference between the effects on INa obtained with K+-containing and K+-free solutions, and therefore pooled the data obtained with these solutions in this report. The osmolality of the extracellular and recording electrode solutions were 280 mOsmo/kg and 260 mOsmo/kg, respectively. The extracellular solution was grounded via an agar bridge, and recordings were made at room temperature (23 °C). Pharmacological agents were either bath-applied or superfused over cells with a 𝖴-tube, and to avoid any chance that effects measured in one cell were contaminated by effects lingering from prior applications, test agents were introduced only once into a given dish. Chemicals were obtained from Sigma (St. Louis, MO), with the following exceptions: CaCl2 (BDH Laboratory Supplies, Poole, England); CsCl and CsOH (ICN Biomedical, Aurora, OH); 8-bromo-cAMP, dimethylsulfoxide, tetraethylammonium-Cl, and tetrodotoxin (Calbiochem, La Jolla, CA); Pluronic F-127 (Molecular Probes, Eugene, OR); and Sp-5,6-DCl-cBIMPS (5,6-Dichloro-1-β-D-ribofuranosylbenzimidazole-3’,5’-cyclic monophosphorothioate, Sp-isomer; Biolog Life Science Institute, Bremen, Germany).
The output signals from the amplifier were analog-filtered (5–20 kHz, 2-pole Bessel) and digitally sampled (10–50 kHz). To reduce noise, the recorded signals were digitally filtered off-line (4 kHz, 8-pole Bessel). In some instances, linear leakage and membrane capacitive currents were subtracted off-line by using scaled, averaged current traces elicited by small voltage jumps. The membrane potentials reported here were corrected for liquid junction potentials that arose from differences between the extracellular and recording electrode solutions. pCLAMP software (v. 8.1.01, Axon Instruments; Union City, CA) was used for voltage protocol generation and data acquisition. SigmaPlot (version 5.0.5; SPSS, Chicago, IL) and Matlab (version 6.5.1.199709 Release13; The MathWorks, Natick, MA) were used for data analyses.
RESULTS
Voltage-gated Na+ current (INa) was measured in retinal ganglion cell somata to examine two possibilities. The first was whether SKF-38393 (a D1-type dopamine receptor agonist), 8-bromo-cAMP (a membrane-permeable cAMP analog), and Sp-5,6-DCl-cBIMPS (a membrane-permeable PKA activator) alter the amplitude and kinetics of INa activated by brief depolarizations. The second was whether these ligands alter the accumulation of INa inactivation, i.e. the entry into and/or recovery from inactivation. In both sets of experiments, the holding potential was set to −72 mV to mimic normal resting potential (Vaquero et al. 2001), and because studies of other preparations found that PKA effects on INa are attenuated at more negative voltages (Schubert et al. 1989; Gershon et al. 1992; Li et al. 1993; Cantrell et al. 1999; Carr et al. 2003).
SKF-38393 reduced whole-cell INa amplitude, without altering the activation range or current kinetics. Figure 1A shows the amplitude and kinetics of INa activated by depolarizations to −7 mV before and during application of 28 µM SKF-38393. Currents were activated, at most, once per 5 sec to allow the current to recover fully from inactivation induced by the test pulses (see control values in Fig. 1). By around 10 min after the agonist application began, the amplitude of the INa peak fell by 38% (from 7.9 nA to 4.9 nA) and remained at this level for 10 min thereafter (i.e., for the remainder of this recording). SKF-38393 (12–30 µM) produced similar results in all of the cells tested, reducing the peak INa amplitude by 24 ± 3 % (mean ± SEM, n = 13) within the first 7–10 minutes of agonist application. During the same time, the voltage-dependence of activation (Fig. 2B, C), the time required for INa to reach peak amplitude (“time-to-peak”; Fig. 2D), and the time to decay to half-peak amplitude (“half decay”; Fig. 2E), did not change by statistically significant amounts.
Fig. 2. Reduction of INa amplitude without change in activation or fast-inactivation.
A: The middle and lower family of traces plot the INa recorded 2–3 min before and 14–17 min after beginning of application of 14 µM SKF-38393, respectively. The upper family of traces plots the membrane potential recorded during these currents. The holding potential was −72 mV. Test potentials were incremented from −67 to +3 mV, in 5-mV steps. Recordings were made with K+-free external and electrode solutions. Each voltage command was run twice in this cell, and every trace shown is the average of the data pairs recorded. Leak and capacitive currents were subtracted off-line. B–E: current measurements before (black) and after (red) SKF-38393 application, plotted against test potential. B: amplitude of current peaks in A. The lines are the best fits to third order Boltzmann functions multiplied by the Goldman-Hodgkin-Katz (GHK) current equation. C: Activation curve (INa amplitude after correction for the instantaneous current rectification calculated by the GHK current equation). The lines are the best fits to third order Boltzmann functions. D: Time from onset of test depolarization to INa peak. Lines, single exponential fits. E: Time for INa to decline from peak to half-peak amplitude. Lines, single exponential fits. C–E plot mean ± 1 S.E.M. of data from 5 cells.
As shown by the currents marked 𝖻 and 𝖼 in Fig. 1B, SKF-38393 reduced the INa amplitude throughout the duration of each test depolarization, i.e., it reduced the current before, during, and after the peak. The INa amplitude measured 2 msec after the onset of the depolarization pulse (after the current decayed to around 10% of the peak amplitude) was reduced by 35±6 % (n=4) of the control. (Thus, when the peak of the INa measured in SKF-38393 is normalized to that in the control, there is no significant difference between the current amplitudes measured at this 2-msec time point, as noted below in regard to Fig. 3.) We did not routinely attempt to quantify reductions of the small amounts of INa that flow at later times during longer test depolarizations because the voltage-clamp current noise obscured its amplitude.
8-bromo-cAMP and Sp-5,6-DCl-cBIMPS also reduced INa amplitude (Fig. 1C–F), without altering the current time-to-peak or half decay time (data not shown). Like SKF-38393, neither agent suppressed INa completely; 8-bromo-cAMP (170–350 µM) and Sp-5,6-DCl-cBIMPS (40 µM) reduced the peak INa by 31±2 % (n=3) and 43±4 % (n=3), respectively, of the control amplitude. The time course of the response to 8-bromo-cAMP was as slow as that to SKF-38393 (Fig. 1C). The response to cBIMPS was faster, but still required 1–2 min to reach steady-state (Fig. 1E).
To test the effect of modulation on the availability of INa during subthreshold depolarizations, we modified the voltages used to study gradual entry into inactivation in other cells (Chandler and Meves 1970; Rudy 1975; Brismar 1977; Martina and Jonas 1997) so that our protocols incorporated measured values of resting potential, the voltage that ganglion cells depolarize to before generating a spike, the voltage that ganglion cells repolarize to between spikes, and the voltage that activates the maximum Na+ conductance in these cells (see Hidaka and Ishida 1998; Vaquero et al. 2001; Hayashida et al. 2004). Thus, entry into inactivation was assessed by depolarizing cells from a holding potential of −72 mV to a conditioning potential of −52 mV for different amounts of time, and testing INa by a depolarization to −7 mV. After each test depolarization, cells were returned to the holding potential for 15 sec, before starting the next episode of depolarization. At these intervals, the INa amplitudes activated without conditioning depolarizations were constant (data not shown), and the loss of INa during one conditioning depolarization was unlikely to contribute to the loss during subsequent conditioning depolarizations.
Figure 3 plots the membrane potential measured during this protocol (Fig. 3A), the clamp current elicited by eight test depolarizations to −7 mV in the control solution (Fig. 3B), and the clamp current elicited in the same cell by the same test depolarizations 15–19 min after beginning the application of 13 µM SKF-38393 (Fig. 3C). For clarity, only the current activated by the conditioning depolarizations; the rising phase, peak, and most of the decline of current activated by each test depolarization; and the corresponding traces of membrane potential are shown. The INa amplitude declined as the conditioning depolarization duration increased (Fig. 3B). Because this decline continued when the conditioning depolarization was increased from 2 to 100 msec, the loss of current was more gradual than “fast” inactivation at −52 mV (Hodgkin and Huxley 1952), as gradual as “slow inactivation” in some preparations (Martina and Jonas 1997; Mickus et al. 1999), and less gradual than “ultra-slow inactivation” (Fox 1976). Consistent with the measurements of slow inactivation in various cells (Rudy 1975; Howe and Ritchie 1992), the current kinetics during test depolarizations did not change in any conspicuous way despite this fall in amplitude.
SKF-38393 accelerated this fall in INa amplitude (Fig. 3C, D), consistent with the possibility that D1-type dopamine receptor activation increased the rate at which Na+ channels became unavailable for activation. To show the time-dependence of this effect, the lines connecting each control INa peak in Figure 3B were superimposed over the SKF-38393 data in Fig. 3C after normalizing the amplitudes of the currents activated without conditioning depolarizations. This shows that INa inactivated in the presence of SKF-38393 as quickly as the control current did for the first few msec at −52 mV. Then, as the conditioning depolarization exceeded 10 msec, the current in SKF-38393 declined faster than the control. The plot of normalized current amplitudes from all cells tested this way (n=4) shows this as a statistically significant difference between the control and modulated current, when the duration of the conditioning depolarization reached 30 and 100 msec (Fig. 3D; P < 0.05, Student’s t-test, unpaired).
After cells dwelled for more than 30 msec at −52 mV, the acceleration of inactivation accounted for a substantial fraction of the total reduction in current. For example, for the cells represented in Fig. 3D, the mean raw current amplitudes (control vs. SKF-38393) differed by 34% when currents were activated by depolarizations after a 100-msec conditioning depolarization to −52 mV (e.g., Figs. 3B and C). As noted above, these currents differed by 24% when activated by depolarizations from −72 mV. The acceleration of inactivation at −52 mV thus accounted for roughly one-third [i.e., (34% − 24%)/(34%)] of the reduction in current by SKF at this timepoint. The lines drawn through the data in Fig. 3D suggest that this fraction might be larger after longer times at −52 mV.
Because –72 mV is more negative than the INa activation threshold in retinal ganglion cells of the species used here (Hidaka and Ishida 1998; Hayashida et al. 2004), the reduction of INa at this potential in Figs. 1 and 3 are consistent with the possibility that D1-type dopamine receptor and PKA activation facilitate “closed-state inactivation”, i.e. a kinetic transition from a closed state to an inactivated state (Armstrong and Bezanilla 1977). The current amplitude in SFK-38393 was not restored to the control amplitude by 100-msec conditioning hyperpolarizations to –87 mV (data not shown). Our results are therefore consistent with the possibility that SKF-38393 facilitates entry of retinal ganglion cell Na+ channels into at least one “slow” inactivated state that requires longer times for recovery. For two reasons, we did not attempt to measure the rate of entry into or recovery from this process, or the voltage sensitivity of this process. First, recovery from “slow” inactivation can continue for as long as several minutes at negative voltages (Fox 1976), whereas ganglion cells do not hyperpolarize for such prolonged times in response to light flashes or fluctuating stimuli (e.g., Saito 1983; Sakuranaga et al. 1987). Secondly, complete recovery generally requires that the channels be hyperpolarized below −100 mV (e.g., Chandler and Meves 1970; Brismar 1977; Rudy 1981), whereas ganglion cells do not traverse such negative voltages during their responses to light (e.g., Zaghloul et al. 2003).
Lastly, we tested the recovery from open-state inactivation, because this is likely to contribute to losses of excitability during repetitive spiking (Ruff 1996). In the two-pulse protocol used for this, cells were depolarized for 2-msec from the holding potential to −7 mV to activate and inactivate INa; returned to the holding potential for various amounts of time; and depolarized again to −7 mV to test the amount of INa available for activation (cf. Rudy 1981; Fleidervisch et al. 1996; Mickus et al. 1999). Cells were kept at the holding potential for 6 sec after each test depolarization before starting the next episode, so that the amplitude of INa activated by each conditioning depolarization was constant, and the loss of current during one conditioning depolarization was unlikely to contribute to the rate of recovery following subsequent conditioning depolarizations. The data in Figure 3E–I are formatted as in Fig. 3A–D. Panels E–G plot the recordings of membrane potential, control INa, and INa in SKF-38393 (20 µM, 13–17 min after beginning the application) from a single cell; the lines connecting the current peaks in panel F are superimposed over the data in panel G; and panels H and I show the values collected from all cells (n=5) studied this way.
Two results emerge from these plots. First, we find that INa recovers almost completely from inactivation within 1 sec. This is neither as fast as recovery from fast inactivation, nor as slow as the slow recovery that occurs over seconds in other cells at voltages similar to the ones we have made our measurements at (e.g., Fig. 9 of Howe and Ritchie 1992; see also Figs. 6 and 8 of Mickus et al. 1999). Although we did not test the effect of longer conditioning depolarizations to −7 mV, a study of cat retinal ganglion cells reported recovery rates that are no slower than the values we find, after depolarizations to −5 mV for as long as 1 sec (Kaneda and Kaneko 1991). Slower recovery has been found in salamander retinal ganglion cells after 500-msec depolarizations to −20 mV (Kim and Rieke 2003). However, the external Na+ concentration was substantially lower than in our recordings and the cat study, leaving the extent of the difference among these results, if any, unclear (Townsend and Horn 1997; Kuo and Liao 2000). Second, we find that SKF-38393 slowed the rate of recovery from inactivation induced by brief depolarizations, and that there is less current available for activation in the SKF-38393-containing solution compared to the control values, for 2–1000 msec after the conditioning depolarization. The slower recovery in SKF-38393 was especially apparent within the first 30 msec after the conditioning pulse in the raw data (Fig. 3G). The difference in recovery rates was estimated by fitting the normalized amplitudes by the sum of two exponential time functions (Fig. 3H). The fast and slow time constants obtained from these fits are 3.6 and 360 msec for recovery in SKF-38393 (red curve), versus 2.7 and 240 msec for the control data (black curve). [Thus, the fast and slow rate constants in SKF-38393 were 24% and 35% smaller than the corresponding control values.] The log-log plot in Fig. 3I allows the recovery process over a wide range of time, as well as the difference between the currents during the first 30 msec after the conditioning depolarization, to be seen more clearly. Although the current amplitudes at 0 msec are not plotted (because the scale is logarithmic), they overlap (Fig. 3G,H), and therefore the difference in amplitudes observed at the subsequent timepoints shows that SKF-38393 slows the recovery rate. For example, the current recovers 80% of its amplitude within ~10 msec after the conditioning depolarization in the control solution, whereas this amount of recovery does not occur until 20–30 msec after the conditioning depolarization in the presence of SKF (see the blue arrows and the times marked “𝖺” and “𝖻” in Fig. 3I).
CONCLUSIONS
The major result of this study is that D1-type dopamine receptor-related ligands (SKF-38393, 8-bromo-cAMP, and Sp-5,6-DCl-cBIMPS) reduce the amplitude of voltage-gated Na+ current by at least three mechanisms: accelerating the entry into inactivation, slowing recovery from inactivation, and increasing closed-state inactivation. As discussed below, this is the first report to our knowledge that a dopamine receptor agonist and PKA activators modulate the rates of entry into, and recovery from, inactivation.
The effects we show here are consistent with previous demonstrations that PKA activation reduces INa amplitude without marked changes in kinetics or activation range, and that the voltage-sensitivity of steady-state inactivation measured with brief conditioning voltages shifts left-ward slightly (Li et al. 1992; Gershon et al. 1992; Schiffmann et al. 1995; Smith and Goldin 1997; Cantrell et al. 1999; Maurice et al. 2001). Because changes in whole-cell current amplitude generally reflect changes in channel gating voltage-sensitivity (rather than single-channel conductance), one possible interpretation of our data is that activating either D1-type dopamine receptors or PKA in retinal ganglion cells (i) reduces the number of Na+ channels available for activation at the resting potential, (ii) does not markedly change the probability that the remaining Na+ channels open or close during depolarizations from the resting potential, and (iii) produces a time-dependent reduction in the number of Na+ channels available for activation during subthreshold depolarizations.
SKF-38393, 8-bromo-cAMP, and Sp-5,6-DCl-cBIMPS reduced INa by 10–50% of the control amplitude. Previous studies have reported similar reductions of INa by PKA activation in other preparations (e.g., Li et al. 1992; Gershon et al. 1992; Cantrell et al. 1999), and to our knowledge, PKA activation has never been reported to abolish INa totally. This would be expected to alter individual spikes and volleys of spikes in several ways. The simplest would resemble effects of losing inward current by “slow” inactivation, namely a decrease in spike rate of rise (Narahashi 1964) and a decrease in spike amplitude (e.g., Colbert et al. 1997). How these effects contribute to retinal ganglion cell function remains to be seen. On the other hand, SKF-38393 reduced the INa elicited by small as well as large depolarizations (Fig. 2A, B), implying that dopamine could raise spike threshold (by increasing the amount of inward current necessary to counterbalance outward currents). This is consistent with the inhibition of spikes in ganglion cells of the species studied here (Vaquero et al. 2001; Hayashida and Ishida 2003) and in other species (e.g., Straschill and Perwein 1969). A corollary of selectively reducing INa is that, in cells where INa does not contribute significantly to the resting conductance, dopamine could inhibit spikes without changing resting potential (e.g., Stanzione et al. 1984; Schiffmann et al. 1995; Vaquero et al. 2001).
In addition to raising spike threshold by reducing INa without changing the activation range, our results are consistent with the possibility that dopamine fosters accumulation of inactivation, i.e. that it increased the rate of inactivation at subthreshold potentials, and slowed recovery from inactivation after brief depolarizations. Previous studies reported qualitatively and quantitatively different effects of dopamine receptor and PKA activation on INa. Although we find that SKF-38393 slowed the recovery from inactivation during the first 2–1000 msec after a brief conditioning depolarization, Schiffmann et al. (1995) found that dopamine reduced INa without altering the rate of recovery from inactivation, Carr et al. (2003) found no significant effect of PKA activation on the rate of recovery from inactivation, and Wicher (2001) showed that PKA activation slowed the recovery of INa from fast, rather than slow, inactivation. Furthermore, while our results show that SKF-38393 increased the amount by which INa inactivates if cells dwell at −52 mV for as little as 30 msec, and for 2 msec at −7 mV, Cantrell et al (1999) reported that PKA activation did not affect the amount of current inactivated by conditioning depolarizations to either −40 or 0 mV, and Carr et al. (2003) found no effect of PKA activation until conditioning depolarizations to −20 mV exceeded 1 sec. Is it possible that PKA modulates different rates (fast, slow, and very slow) of INa inactivation because these differentially influence the spiking behavior of different cells? This is not yet known for ganglion cells, at least in part because the extent that other currents are modulated by dopamine, and the possibility that dopamine modulates much slower INa inactivation, remain to be examined. However, the duration of spike bursts in these cells (e.g., 5 spikes at 100–200 Hz; Berry et al. 1997) is similar to the time at which dopamine receptor activation begins to enhance inactivation, viz. after cells spend more than 10–30 msec at −52 mV (Fig. 3A–D). Cells spiking at 100–200 Hz may also be primed to lose INa because dopamine receptor activation markedly slows recovery from inactivation during the first 5–10 msec after single brief depolarizations (see Fig. 3E–I). Slower losses of INa might, by contrast, delay the truncation of spiking (e.g., compare Figs. 5 and 9 of Carr et al., 2003).
Lastly, our results show that this inhibition is slow both in onset and the time to reach steady state. The INa amplitude did not fall within the first several seconds of applying SKF-38393, 8-bromo-cAMP, and Sp-5,6-DCl-cBIMPS, and the steady-state response to these agents was reached around 2–10 minutes thereafter. This is not a peculiarity of retinal ganglion cell Na+ channels, as the response of Na+ channels in at least some preparations to SKF-38393, PKA activation (Li et al. 1993; Schiffmann et al. 1995; Smith and Goldin 1997; Zhang et al. 1998), and protein kinase C activation (Sigel and Baur 1988; Numann et al. 1991), as well as the effect of dopamine on other channels in other retinal neurons (e.g., Piccolino et al., 1984), have all been found to follow similarly slow timecourses. This does not necessarily pose a functional disadvantage: while the response reaches steady-state too slowly to signal moment-to-moment changes in distributions of light falling on the retina in situ, this would be compatible with the gradual changes in light sensitivity mediated by dopamine released endogenously under natural conditions (e.g., Manglapus et al. 1999).
ACKNOWLDEGEMENTS
This work was supported by NIH grant EY 08120 (to A.T.I.) and National Eye Institute Core grant P30 EY12576. We thank Dr. Philippe Ascher for discussion and comments on our manuscript.
REFERENCES
- Armstrong CM, Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian V, Berry MJ., 2nd A test of metabolically efficient coding in the retina. Network. 2002;13:531–552. [PubMed] [Google Scholar]
- Barlow HB, Levick WR, Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res (Suppl) 1971;3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Warland DK, Meister M. The structure and precision of retinal spike trains. Proc Natl Acad Sci U SA. 1997;94:5411–5416. doi: 10.1073/pnas.94.10.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Slow mechanism for sodium permeability inactivation in myelinated nerve fibre of Xenopus laevis. J Physiol. 1977;270:283–297. doi: 10.1113/jphysiol.1977.sp011952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Scheuer T, Catterall WA. Voltage-dependent neuromodulation of Na+ channels by D1-like dopamine receptors in rat hippocampal neurons. J Neurosci. 1999;19:5301–5310. doi: 10.1523/JNEUROSCI.19-13-05301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Chandler WK, Meves H. Slow changes in membrane permeability and long-lasting action potentials in axons perfused with fluoride solutions. J Physiol. 1970;211:707–728. doi: 10.1113/jphysiol.1970.sp009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Magee JC, Hoffman DA, Johnston D. Slow recovery from inactivation of Na+ channels underlies the activity-dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 1997;17:6512–6521. doi: 10.1523/JNEUROSCI.17-17-06512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science. 1975;188:270–273. doi: 10.1126/science.804181. [DOI] [PubMed] [Google Scholar]
- Ellerkmann RK, Riazanski V, Elger CE, Urban BW, Beck H. Slow recovery from inactivation regulates the availability of voltage-dependent Na(+) channels in hippocampal granule cells, hilar neurons and basket cells. J Physiol. 2001;532:385–397. doi: 10.1111/j.1469-7793.2001.0385f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish IA, Friedman A, Gutnick MJ. Slow inactivation of Na+ current and slow cumulative spike adaptation in mouse and guinea-pig neocortical neurones in slices. J Physiol (Lond) 1996;493:83–97. doi: 10.1113/jphysiol.1996.sp021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JM. Ultra-slow inactivation of the ionic currents through the membrane of myelinated nerve. Biochim Biophys Acta. 1976;426:232–244. doi: 10.1016/0005-2736(76)90334-5. [DOI] [PubMed] [Google Scholar]
- Gershon E, Weigl L, Lotan I, Schreibmayer W, Dascal N. Protein kinase A reduces voltage-dependent Na+ current in Xenopus oocytes. J Neurosci. 1992;12:3743–3752. doi: 10.1523/JNEUROSCI.12-10-03743.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, Ishida AT. Dopaminergic modulation of spike-generation mechanism in retinal ganglion cells. Program # 264.17. Society for Neuroscience Abstract Viewer/Itinerary Planner. Washington, DC: 2003. [Google Scholar]
- Hayashida Y, Partida GJ, Ishida AT. Dissociation of retinal ganglion cells without enzymes. J Neurosci Meth. 2004;137:25–35. doi: 10.1016/j.jneumeth.2004.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden WL, Jr, Dowling JE. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond B Biol Sci. 1978;201:27–55. doi: 10.1098/rspb.1978.0031. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Ishida AT. Voltage-gated Na+ current availability after step- and spike-shaped conditioning depolarizations of retinal ganglion cells. Pflügers Arch. 1998;436:497–508. doi: 10.1007/s004240050664. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952;116:497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston AH, Madden JA. Environmental temperature and plasma electrolyte regulation in the carp, Cyprinus carpio. Nature. 1968;217:969–970. [Google Scholar]
- Howe JR, Ritchie JM. Multiple kinetic components of sodium channel inactivation in rabbit Schwann cells. J Physiol (Lond) 1992;455:529–566. doi: 10.1113/jphysiol.1992.sp019315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida AT, Cohen BN. GABA-activated whole-cell currents in isolated retinal ganglion cells. J Neurophysiol. 1988;60:381–396. doi: 10.1152/jn.1988.60.2.381. [DOI] [PubMed] [Google Scholar]
- Kara P, Reid RC. Efficacy of retinal spikes in driving cortical responses. J Neurosci. 2003;23:8547–8557. doi: 10.1523/JNEUROSCI.23-24-08547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratyuk T, Rossie S. Depolarization of rat brain synaptosomes increases phosphorylation of voltage-sensitive sodium channels. J Biol Chem. 1997;272:16978–16983. doi: 10.1074/jbc.272.27.16978. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Liao SY. Facilitation of recovery from inactivation by external Na+ and location of the activation gate in neuronal Na+ channels. J Neurosci. 2000;20:5639–5646. doi: 10.1523/JNEUROSCI.20-15-05639.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, West JW, Lai Y, Scheuer T, Catterall WA. Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron. 1992;8:1151–1159. doi: 10.1016/0896-6273(92)90135-z. [DOI] [PubMed] [Google Scholar]
- Li M, West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993;261:1439–1442. doi: 10.1126/science.8396273. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lasater EM. Calcium currents in turtle retinal ganglion cells. II. Dopamine modulation via a cyclic AMP-dependent mechanism. J Neurophysiol. 1994;71:743–752. doi: 10.1152/jn.1994.71.2.743. [DOI] [PubMed] [Google Scholar]
- Manglapus MK, Iuvone PM, Underwood H, Pierce ME, Barlow RB. Dopamine mediates circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1999;19:4132–4141. doi: 10.1523/JNEUROSCI.19-10-04132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Jonas P. Functional differences in Na+ channel gating between fast-spiking interneurones and principal neurones of rat hippocampus. J Physiol. 1997;505:593–603. doi: 10.1111/j.1469-7793.1997.593ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickus T, Jung H-Y, Spruston N. Properties of slow, cumulative sodium channel inactivation in rat hippocampal CA1 pyramidal neurons. Biophys J. 1999;76:846–860. doi: 10.1016/S0006-3495(99)77248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Ferrer C, Yazulla S, Studholme KM, Haak-Frendscho M. Dopamine D1-receptor immunolocalization in goldfish retina. J Comp Neurol. 1999;411:705–714. doi: 10.1002/(sici)1096-9861(19990906)411:4<705::aid-cne14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Threshold potential in anodally restored lobster giant axons. J Cell Physiol. 1964;64:239–248. doi: 10.1002/jcp.1030640208. [DOI] [PubMed] [Google Scholar]
- Numann R, Catterall WA, Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991;254:115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- Piccolino M, Neyton J, Gerschenfeld HM. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984;4:2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinagel P, Godwin D, Sherman SM, Koch C. Encoding of visual information by LGN bursts. J Neurophysiol. 1999;81:2558–2569. doi: 10.1152/jn.1999.81.5.2558. [DOI] [PubMed] [Google Scholar]
- Rudy B. Slow recovery of the inactivation of sodium conductance in Myxicola giant axons. J Physiol. 1975;249:22P–24P. [PubMed] [Google Scholar]
- Rudy B. Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J Physiol. 1978;283:1–21. doi: 10.1113/jphysiol.1978.sp012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Inactivation in Myxicola giant axons responsible for slow and accumulative adaptation phenomena. J Physiol. 1981;312:531–549. doi: 10.1113/jphysiol.1981.sp013642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL. Sodium channel slow inactivation and the distribution of sodium channels on skeletal muscle fibres enable the performance properties of different skeletal muscle fibre types. Acta Physiol Scand. 1996;156:159–168. doi: 10.1046/j.1365-201X.1996.189000.x. [DOI] [PubMed] [Google Scholar]
- Saito H-A. Morphology of physiologically identified X-, Y-, and W-type retinal ganglion cells of the cat. J Comp Neurol. 1983;221:279–288. doi: 10.1002/cne.902210304. [DOI] [PubMed] [Google Scholar]
- Sakuranaga M, Ando Y, Naka K-I. Dynamics of the ganglion cell response in the catfish and frog retinas. J Gen Physiol. 1987;90:229–259. doi: 10.1085/jgp.90.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf CL, Bullock JO. Internal cesium alters sodium inactivation in Myxicola. Biophys J. 1978;23:473–477. doi: 10.1016/S0006-3495(78)85463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Lledo PM, Vincent JD. Dopamine D1 receptor modulates the voltage-gated sodium current in rat striatal neurones through a protein kinase A. J Physiol. 1995;483:95–107. doi: 10.1113/jphysiol.1995.sp020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Desdouits F, Menu R, Greengard P, Vincent JD, Vanderhaeghen JJ, Girault JA. Modulation of the voltage-gated sodium current in rat striatal neurons by DARPP-32, an inhibitor of protein phosphatase. Eur J Neurosci. 1998;10:1312–1320. doi: 10.1046/j.1460-9568.1998.00142.x. [DOI] [PubMed] [Google Scholar]
- Schubert B, VanDongen AM, Kirsch GE, Brown AM. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989;245:516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R. Activation of protein kinase C differentially modulates neuronal Na+, Ca2+, and gamma-aminobutyrate type A channels. Proc Natl Acad Sci USA. 1988;85:6192–6196. doi: 10.1073/pnas.85.16.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Goldin AL. Phosphorylation at a single site in the rat brain sodium channel is necessary and sufficient for current reduction by protein kinase A. J Neurosci. 1997;17:6086–6093. doi: 10.1523/JNEUROSCI.17-16-06086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzione P, Calabresi P, Mercuri N, Bernardi G. Dopamine modulates CA1 hippocampal neurons by elevating the threshold for spike generation: an in vitro study. Neuroscience. 1984;13:1105–1116. doi: 10.1016/0306-4522(84)90291-4. [DOI] [PubMed] [Google Scholar]
- Straschill M, Perwein J. The inhibition of retinal ganglion cells by catecholeamines and gamma-aminobutyric acid. Pflügers Arch. 1969;312:45–54. doi: 10.1007/BF00588530. [DOI] [PubMed] [Google Scholar]
- Stys PK, Lopachin RM., Jr Elemental composition and water content of rat optic nerve myelinated axons during in vitro post-anoxia reoxygenation. Neuroscience. 1996;73:1081–1090. doi: 10.1016/0306-4522(96)00114-5. [DOI] [PubMed] [Google Scholar]
- Toib A, Lyakhov V, Marom S. Interaction between duration of activity and time course of recovery from slow inactivation in mammalian brain Na+ channels. J Neurosci. 1998;18:1893–1903. doi: 10.1523/JNEUROSCI.18-05-01893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend C, Horn R. Effect of alkali metal cations on slow inactivation of cardiac Na+ channels. J Gen Physiol. 1997;110:23–33. doi: 10.1085/jgp.110.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature. 1998;395:384–387. doi: 10.1038/26487. [DOI] [PubMed] [Google Scholar]
- Vaquero CF, Pignatelli A, Partida GJ, Ishida AT. A dopamine- and protein kinase A-dependent mechanism for network adaptation in retinal ganglion cells. J Neurosci. 2001;21:8624–8635. doi: 10.1523/JNEUROSCI.21-21-08624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher D. Peptidergic modulation of insect voltage-gated Ca(2+) currents: role of resting Ca(2+) current and protein kinases A and C. J Neurophysiol. 2001;86:2353–2362. doi: 10.1152/jn.2001.86.5.2353. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Zucker CL. Synaptic organization of dopaminergic interplexiform cells in the goldfish retina. Vis Neurosci. 1998;1:13–29. doi: 10.1017/s0952523800000997. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J Neurosci. 1998;18:488–498. doi: 10.1523/JNEUROSCI.18-01-00488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]