Abstract
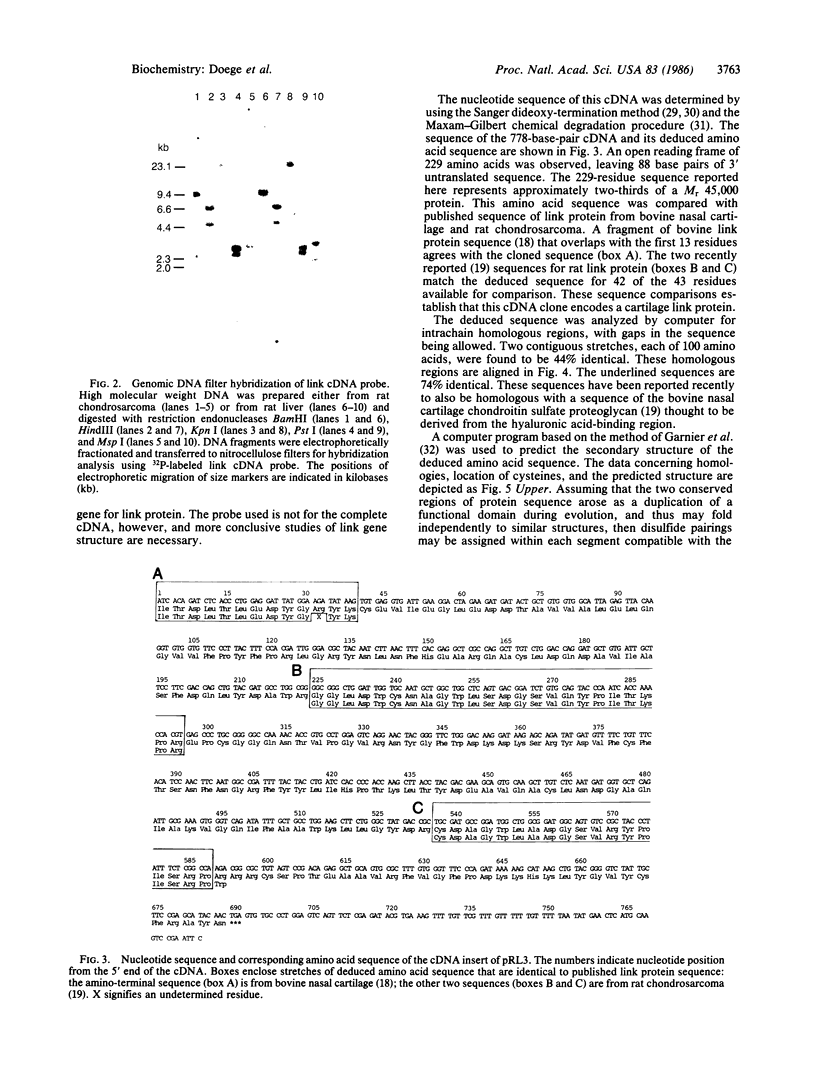
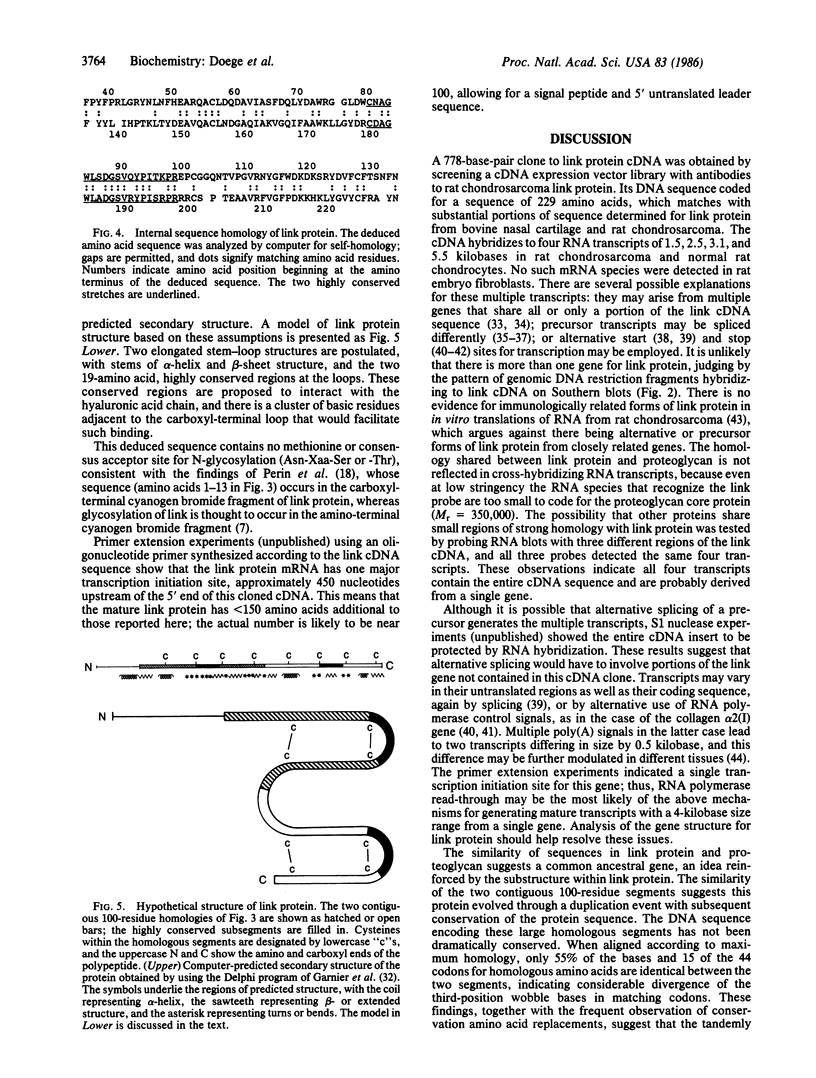
Link protein stabilizes the cartilage proteoglycan/hyaluronic acid aggregate by binding to both components. We screened a cDNA library prepared from rat chondrosarcoma mRNA in the lambda gt11 expression vector with monoclonal antibodies and polyclonal antisera to link protein. We obtained a clone for two-thirds of the link protein cDNA and identified it based on its deduced amino acid sequence. There are four RNA transcripts for link protein, ranging from 1.5 to 5.5 kilobases in size. The deduced amino acid sequence for link protein shows two domains of 100 residues each, which share 44% homology; within each domain is a 19-residue stretch 74% homologous with its counterpart. This structure indicates that link protein may have been formed by gene duplication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho S., Tate V., Boedtker H. Multiple 3' ends of the chicken pro alpha 2(I) collagen gene. Nucleic Acids Res. 1983 Aug 25;11(16):5443–5450. doi: 10.1093/nar/11.16.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. R., Caterson B. The isolation and characterization of the link proteins from proteoglycan aggregates of bovine nasal cartilage. J Biol Chem. 1979 Apr 10;254(7):2387–2393. [PubMed] [Google Scholar]
- Caterson B., Baker J. R., Christner J. E., Lee Y., Lentz M. Monoclonal antibodies as probes for determining the microheterogeneity of the link proteins of cartilage proteoglycan. J Biol Chem. 1985 Sep 15;260(20):11348–11356. [PubMed] [Google Scholar]
- Caterson B., Baker J. The interaction of link proteins with proteoglycan monomers in the absence of hyaluronic acid. Biochem Biophys Res Commun. 1978 Feb 14;80(3):496–503. doi: 10.1016/0006-291x(78)91596-6. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S., Kleinman H. K., Hassell J. R. Interaction of link protein with collagen. J Biol Chem. 1983 May 25;258(10):6226–6231. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Faltz L. L., Caputo C. B., Kimura J. H., Schrode J., Hascall V. C. Structure of the complex between hyaluronic acid, the hyaluronic acid-binding region, and the link protein of proteoglycan aggregates from the swarm rat chondrosarcoma. J Biol Chem. 1979 Feb 25;254(4):1381–1387. [PubMed] [Google Scholar]
- Fife R. S., Caterson B., Myers S. L. Identification of link proteins in canine synovial cell cultures and canine articular cartilage. J Cell Biol. 1985 Apr;100(4):1050–1055. doi: 10.1083/jcb.100.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht R. J., Adams S. L. Tissue specificity of type I collagen gene expression is determined at both transcriptional and post-transcriptional levels. Mol Cell Biol. 1984 Sep;4(9):1843–1852. doi: 10.1128/mcb.4.9.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell S., Baker J., Caterson B., Heinegård D., Rodén L. Link protein and a hyaluronic acid-binding region as components of aorta proteoglycan. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1823–1831. doi: 10.1016/s0006-291x(80)80111-2. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Jonas V., Lin C. R., Kawashima E., Semon D., Swanson L. W., Mermod J. J., Evans R. M., Rosenfeld M. G. Alternative RNA processing events in human calcitonin/calcitonin gene-related peptide gene expression. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1994–1998. doi: 10.1073/pnas.82.7.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. H., Hardingham T. E., Hascall V. C., Solursh M. Biosynthesis of proteoglycans and their assembly into aggregates in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1979 Apr 25;254(8):2600–2609. [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Kohno K., Sullivan M., Yamada Y. Structure of the promoter of the rat type II procollagen gene. J Biol Chem. 1985 Apr 10;260(7):4441–4447. [PubMed] [Google Scholar]
- Le Glédic S., Périn J. P., Bonnet F., Jollès P. Identity of the protein cores of the two link proteins from bovine nasal cartilage proteoglycan complex. Localization of their sugar moieties. J Biol Chem. 1983 Dec 25;258(24):14759–14761. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Dickson L. A., de Wet W. J., Bernard M. P., Chu M. L., Di Liberto M., Pepe G., Sangiorgi F. O., Ramirez F. Analysis of the 3' end of the human pro-alpha 2(I) collagen gene. Utilization of multiple polyadenylation sites in cultured fibroblasts. J Biol Chem. 1983 Aug 25;258(16):10128–10135. [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Neame P. J., Périn J. P., Bonnet F., Christner J. E., Jollès P., Baker J. R. An amino acid sequence common to both cartilage proteoglycan and link protein. J Biol Chem. 1985 Oct 15;260(23):12402–12404. [PubMed] [Google Scholar]
- Odermatt E., Tamkun J. W., Hynes R. O. Repeating modular structure of the fibronectin gene: relationship to protein structure and subunit variation. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6571–6575. doi: 10.1073/pnas.82.19.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Brown M., Dziewiatkowski D. D. The link protein in proteoglycan aggregates from the Swarm rat chondrosarcoma. J Biol Chem. 1977 Sep 25;252(18):6470–6477. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L. Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules. Cell. 1985 Jul;41(3):657–663. doi: 10.1016/s0092-8674(85)80046-5. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Cöster L., Hassell J. R. Mammalian eyes and associated tissues contain molecules that are immunologically related to cartilage proteoglycan and link protein. J Cell Biol. 1982 Jun;93(3):910–920. doi: 10.1083/jcb.93.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Reiner A., Mort J. S., Tang L. H., Choi H. U., Rosenberg L. C., Caputo C. B., Kimura J. H., Hascall V. C. Cartilage link proteins. Biochemical and immunochemical studies of isolation and heterogeneity. J Biol Chem. 1984 Dec 10;259(23):14849–14856. [PubMed] [Google Scholar]
- Périn J. P., Bonnet F., Pizon V., Jollès J., Jollès P. Structural data concerning the link proteins from bovine nasal cartilage proteolycan complex. FEBS Lett. 1980 Oct 6;119(2):333–336. doi: 10.1016/0014-5793(80)80283-3. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Poole A. R., Mort J. S. The heterogeneity of link proteins isolated from human articular cartilage proteoglycan aggregates. J Biol Chem. 1982 Oct 25;257(20):11908–11914. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. D., Martin G. R., Miller E. J., Dorfman A., Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975 Jan;166(1):181–186. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayagopal P., Radhakrishnamurthy B., Srinivasan S. R., Berenson G. S. Isolation and characterization of a link protein from bovine aorta proteoglycan aggregate. Biochim Biophys Acta. 1985 Mar 29;839(1):110–118. doi: 10.1016/0304-4165(85)90188-6. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]






