Abstract
Major depression (MD) is often associated with disturbances of the hypothalamic/pituitary/thyroid (HPT) axis. Unfortunately, whether this association is secondary to common underlying genetic variation or whether the MD-associated disturbances in HPT function are chronic or state-dependent is unknown. To examine these questions, we genotyped 12 single nucleotide polymorphisms identified in previous genome wide association analyses of thyroid function in DNA contributed by 1555 subjects from three longitudinal ethnically diverse studies that are well-characterized for lifetime major depression and thyroid function. We then examined associations between genetic variants and key outcomes of thyroid stimulating hormone (TSH), free thyroxine (FT4) and depression. We confirmed prior findings that two variants in deiodinase 1 (DIO1), including a variant in the 3’ UTR of DIO1 (rs11206244), were associated with altered free thyroxine (FT4) levels in both White and African American subjects. We also found that rs11206244 genotype was associated with lifetime MD in White female subjects, in particular those from high-risk cohorts. However, we found no association of current FT4 levels with lifetime MD in either ethnic group. We conclude that genetic variation influencing thyroid function is a risk factor for MD. Given the evidence from prior studies, further investigations of role of HPT variation in etiology and treatment of MD are indicated.
INTRODUCTION
Major depression (MD) affects nearly 15% of the United States population and is one of the largest socioeconomic burdens to industrialized economies (Greenberg and others 2003). MD is a heterogenous disorder that results from a complex interplay of genetic and environmental factors (Kendler and others 2003). In attempts to identify some of these genetic factors, a large number of molecular studies of MD have been conducted (Lewis and others 2010; Muglia and others 2010; Rietschel and others). Despite some promising findings, no common genetic variant has been unambiguously associated with MD.
Spurred by the well-established finding that altered thyroid function is often associated with symptoms of MD (Jackson 1998) and using the rationale that the identification of endophenotypes of MD may increase the likelihood of successfully identifying cryptic vulnerability factors, a number of investigative teams have interrogated the relationship between free thyroxine (FT4) or thyroid stimulating hormone (TSH) and MD in those subjects without autoimmune thyroid disease. Although not uniform, some studies, in particularly the larger studies, have produced strong evidence that increased FT4 levels are associated with either current or lifetime MD (Das and others 2007; Forman-Hoffman and Philibert 2006a; Philibert and others 2006; Williams and others 2009). Unfortunately, these findings, though promising, have flaws which limit their generalizability and were not genetically informed, thus missing a chance to explore whether genetic variation in the HPT pathway moderated these associations with MD. This missed opportunity is particularly significant because thyroid hormone augmentation is a commonly-used treatment for refractory MD. Accordingly, by understanding the relationship of HPT variation to MD, we could potentially better identify those individuals more or less likely to respond to thyroid hormone supplementation.
This lack of information on the relationship between HPT axis function and MD may be changing. Recently, at least three independent groups have conducted genome-wide studies of either TSH or FT4 in non-hypothyroid function and identified twelve single nucleotide polymorphisms (SNP) altering the serum levels of these critical hormones (Arnaud-Lopez and others 2008; Hwang and others 2007; Panicker and others 2008). In this study, we explore the relationships between MD, thyroid function, and these twelve SNPs using clinical and biological material from three independent, ethically diverse populations that are informative for both behavior and thyroid function. Specifically, we first test whether these SNPs are associated with altered TSH or FT4 activity in our population. Next, we test whether genotype at any of these SNPs whose biological effects on thyroid function are validated in our populations also alter vulnerability to MD. Finally, we test whether FT4 levels are associated with lifetime depression.
METHODS
The data for this study was ascertained from three populations, the Iowa Adoption Studies (IAS), the Family and Community Health Study (FACHS), and the Iowa Community Corrections Studies (ICCS). All procedures conducted in this study were approved by the University of Iowa, Iowa State University or the University of Georgia Institutional Review Boards.
The clinical characteristics of the IAS, FACHS and ICCS have been described in detail elsewhere (Cutrona and others 2005; Gunter and others 2009; Philibert 2006). But briefly, the IAS is a case and control adoption study of the genetic (G), environmental (E) and gene-environment interactions (GxE) in the etiology of common behavioral illness. Each of the IAS subjects who participated in this study has been characterized in 5 waves since their inception into the study. The clinical data for the current study is derived from interviews with the Semi-Structured Assessment of the Genetics of Alcoholism, version II (SSAGA-II) during the last two waves of the study (1999-2004 and 2004-2009) (Bucholz and others 1994). In addition, during the last wave phlebotomy was performed in order to provide DNA and sera for the current study. The FACHS is a longitudinal study of the effect of environmental stressors on physical and mental health outcomes in rural Georgia and Iowa African American families. The clinical data for this study were derived from either the CIDI (Anthony and others 1985) or the SSAGA-II administered during this most recent wave of the study (2007-2010). During the current wave of this study, phlebotomy was performed to provide biomaterial for DNA and sera. In addition, a small number of subjects declined phlebotomy but agreed to give a sample of saliva for DNA preparation. The ICCS is a longitudinal study of factors leading to recidivism in probationers parolees, and DUI-sentenced offenders from the 6th Judicial District in Iowa. The clinical and biological data for this study were derived from SSAGA-II and phlebotomy performed during the last wave of the interview.
DNA was prepared from whole blood or saliva as previously described. Determination of TSH and FT4 levels for 1447 subjects was performed by the University of Iowa Clinical Pathology Laboratories. However, secondary to the presence of autoimmune thyroid disease or medications known to interfere with thyroid function (e.g. lithium or levothyroxine), the values for 65 subjects were excluded from further analysis. Lifetime and current major depression for all of the subjects were determined as previously described using DSM-IV criteria (American Psychiatric Association 1994). Because of scheduling difficulties, the CIDI interview for a large portion of the FACHS population was not coincident with their phlebotomy. Therefore, these subjects were omitted from the current state analyses.
Genotyping of all DNA specimens was performed using Taqman® MGB assays (Applied Biosystems, Foster City, USA) and a Fluidigm Biomark Genetic Analysis System (Fluidigm, South San Francisco, CA). Approximately 10% of the samples were genotyped in replicate to insure accuracy.
Haploblock analysis and linkage disequilibrium analyses were conducted using Haploview (Barrett and others 2005). Data were analyzed using standard general linear model equations using JMP Version 7 (SAS Institute, Cary, NC) and an additive model with respect to the effects of genotype on thyroid function indices. Because TSH and FT4 values are not normally distributed, consistent with our prior practices (Forman-Hoffman and Philibert 2006b), these values were log transformed prior to analysis. As thyroid function and behavioral phenomenology differ between men and women, in keeping with our prior practices all analyses were conducted by gender. In analyses that included more than one cohort, results were controlled with respect to ethnicity and cohort using logistic regression. All reported p-values are two tailed.
RESULTS
The demographic properties of the cohorts, and the rates or values of clinical variables for each of the populations are described in Table I. As the Table describes, each of the populations is unique. The IAS subjects (n=561) are mostly white, 55% female and early middle aged. In contrast, the FACHS population (n=648) is largely African American, mostly female but is also in their late 40's. Finally, ICCS (n=338) is mostly male, 75% White but significantly younger than either the IAS or the FACHS participants (p<0.0001 for both the IAS and FACHS). Of significant note is the high rate of cigarette smoking in the past year (89%) in the ICCS population.
Table I.
Clinical and Demographic Characteristics of the Study Populations
| IAS | FACHS | ICCS | |
|---|---|---|---|
| Male | 259 | 165 | 220 |
| Female | 312 | 483 | 118 |
| Age | |||
| Male | 46 ± 8 | 49 ± 9 | 34 ± 10 |
| Female | 45 ± 7 | 47 ± 8 | 31 ± 9 |
| African American | 14 | 620 | 71 |
| White | 535 | 27 | 253 |
| Hispanic | 14 | 1 | 5 |
| American Indian | 2 | 0 | 4 |
| Asian | 0 | 0 | 1 |
| Unknown/other | 6 | 0 | 4 |
| Lifetime MD | |||
| Male | 71 (27%) | 13 (8%) | 74 (34%) |
| Female | 155 (50%) | 139 (29%) | 65 (55%) |
| Current MD | |||
| Male | 9 (3%) | NA | 15 (7%) |
| Female | 17 (5%) | NA | 15 (13%) |
| Smoking in the Past Year | 33% | 33% | 89% |
| TSH* | |||
| Male | 2.24 ± 1.19 (n=221) | 1.67 ± 0.87 (n=157) | 1.70 ± 1.07 (n=202) |
| Female | 2.10 ± 1.30 (n=247)1 | 1.57 ± 1.14 (n=458) | 1.59 ± 1.10 (n=104) |
| FT4* | |||
| Male | 1.19 ± 0.17 (n=219)2 | 1.18 ± 0.19 (n=157) | 1.18 ± 0.19 (n=203)2 |
| Female | 1.15 ± 0.17 (n=246) | 1.16 ± 0.18 (n=459) | 1.10 ± 0.15 (n=105)3 |
Does not include subjects excluded from the final analyses for medical reasons
Differs from that of ICCS and FACHS subjects at p<0.01.
Differs from that of the female subjects in the cohort at p<0.01
Differs from that of IAS and FACHS male subjects at p<0.05
The rates of lifetime and current MD also varied among the cohorts. Consistent with their status as subjects from high risk populations, both males and females in the IAS and the ICCS had significantly higher rates of lifetime and current MD than the national average (~12% and 20%, respectively) (Kessler and others 2003). On the other hand, the FACHS sample, which is not intentionally enriched for behavioral disorders, had lifetime rates of depression more consistent with national norms.
The FT4 and TSH levels in those 1388 subjects who were not on medications known to interfere with thyroid function or having autoimmune thyroid disease are given in Table I. In contrast to the prior findings with respect to total T4 values (Forman-Hoffman and Philibert 2006b; Hollowell and others 2002), in two of the three cohorts examined, males had significantly higher FT4 levels than their female in the cohorts. Additionally, consistent with the prior literature (Hollowell and others 2002), the TSH level of the largely African American FACHS population was lower than that of subjects from the largely White, yet age-equivalent IAS population. In contrast to results with prior larger epidemiological studies (Hollowell and others 2002; Jorde and Sundsfjord 2006), neither smoking nor age was significantly associated with FT4 or TSH levels although there was a trend for age to be associated with TSH levels in the female subjects (p<0.10; results controlled for by ethnicity and cohort).
Because prior studies, including two by our group, have shown that elevated FT4 levels are associated with current MD, we next examined the relationship of current FT4 levels to lifetime categorical MD in all three cohorts to ascertain whether any MD- associated changes in FT4 levels are state or trait-dependent. Using data from all three cohorts, current FT4 levels were not associated with a lifetime history of MD in women or men (p<0.99 (n=313) and p<0.49 (n=141), respectively. Current TSH levels were also not associated with a lifetime history of MD in women or men (p<0.24, and p<0.23). These data and results support the supposition that if there are FT4 or TSH changes associated with MD, these changes are state-dependent. Unfortunately, current MD data are not available on FACHS subjects and the number of subjects without significant, potentially-interfering medical co-morbidities from the IAS and ICCS was insufficient to allow meaningful analysis of current MD status with FT4 levels.
The nominal p-values for the analyses of the relationship of SNP genotype to FT4 levels by cohort, gender and ethnicity is given in Table II. In general, the two SNPs chosen for their prior association with FT4 levels, rs11206244 and rs2235544, both of which are found in Deiodinase I, were significantly associated with FT4 levels overall, and separately, in African American and White subjects, in this study. However, in the “White only” sub-group, rs11206244 genotype was not significantly associated with FT4 levels (p<0.4). With respect to the rs11206244 CT polymorphism, the T allele was associated with increased FT4 levels. With respect to the rs2235544 AC polymorphism, the A allele was associated with increased FT4 levels. In contrast, the p-values of the SNPs chosen for their effect on TSH values, did not have significant relationships to FT4 levels with the exception of rs2523189 which had a nominal p-value of p<0.02 for all female subjects combined.
Table II.
Results of Genotype Analyses with Respect to FT4 Levels
| IAS | FACHS | ICCS | All** | AA Only | White only | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | ||||
| SNP | Gene | Type* | N | 208 | 233 | 158 | 458 | 179 | 103 | 546 | 792 | 201 | 454 | 339 | 317 |
| rs6977660 | TMEM196 | TSH | 0.66 | 0.91 | 0.13 | 0.46 | 0.47 | 0.88 | 0.29 | 0.97 | 0.12 | 0.47 | 0.85 | 0.98 | |
| rs11206244 | DIO1 | FT4 | 0.8 | 0.05 | 0.04 | 0.11 | 0.24 | 0.11 | 0.06 | 0.003 | 0.03 | 0.02 | 0.4 | 0.02 | |
| rs10493147 | HSPA4 | TSH | 0.88 | 0.43 | 0.84 | 0.16 | 0.7 | 0.46 | 0.91 | 0.25 | 0.78 | 0.39 | 0.58 | 0.85 | |
| rs2235544 | DIO1 | FT4 | 0.13 | 0.03 | 0.1 | 0.16 | 0.06 | 0.03 | 0.03 | 0.001 | 0.05 | 0.03 | 0.02 | 0.005 | |
| rs2550298 | GNA01 | TSH | 0.91 | 0.65 | 0.86 | 0.46 | 0.72 | 0.45 | 0.81 | 0.85 | 0.82 | 0.52 | 0.93 | 0.43 | |
| rs9376165 | PDE7B | TSH | 0.08 | 0.12 | 0.12 | 0.69 | 0.7 | 0.01 | 0.2 | 0.16 | 0.77 | 0.93 | 0.12 | 0.11 | |
| rs1976324 | TSH | 0.82 | 0.4 | 0.08 | 0.58 | 0.93 | 0.36 | 0.58 | 0.37 | 0.13 | 0.1 | 0.95 | 0.23 | ||
| rs2294512 | DIO1 | TSH | 0.73 | 0.98 | 0.18 | 0.34 | 0.59 | 0.32 | 0.38 | 0.58 | 0.17 | 0.3 | 0.84 | 0.4 | |
| rs10512065 | GNAQ | TSH | 0.47 | 0.17 | 0.47 | 0.63 | 0.73 | 0.66 | 0.74 | 0.61 | 0.07 | 0.92 | 0.27 | 0.15 | |
| rs6026565 | GNAS | TSH | 0.87 | 0.32 | 0.57 | 0.16 | 0.86 | 0.9 | 0.45 | 0.53 | 0.55 | 0.11 | 0.75 | 0.69 | |
| rs2252696 | SLG TG | TSH | 0.2 | 0.31 | 0.62 | 0.57 | 0.3 | 0.56 | 0.3 | 0.18 | 0.8 | 0.28 | 0.35 | 0.3 | |
| rs2523189 | GNAI1 | TSH | 0.51 | 0.39 | 0.47 | 0.03 | 0.06 | 0.94 | 0.84 | 0.02 | 0.4 | 0.18 | 0.29 | 0.23 | |
Denotes whether the prior genome wide association was with TSH or FT4.
Controlled for with respect to ethnicity, cohort and smoking status.
The nominal p-values for the analyses of the relationship of SNP genotype to TSH levels by cohort, gender and ethnicity is given in Table III. The frequency of the SNP, the type of polymorphism and allele frequencies for each SNP are given in Supplemental Table I. In contrast to the findings with respect to FT4, none of the SNPs chosen for their prior associations with TSH levels, including those which had significant effects on FT4 levels, were consistently associated with TSH levels in the current study. Therefore, these SNPs were eliminated from further consideration in subsequent analyses.
Table III.
Results of Genotype Analyses with Respect to TSH Levels
| IAS | FACHS | ICCS | All** | AA Only | White only | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | ||||
| SNP | Gene | Type* | N | 208 | 233 | 158 | 458 | 179 | 103 | 546 | 792 | 201 | 454 | 339 | 317 |
| rs6977660 | TMEM196 | TSH | 0.53 | 0.54 | 0.66 | 0.14 | 0.66 | 0.14 | 0.34 | 0.08 | 0.85 | 0.07 | 0.22 | 0.86 | |
| rs11206244 | DIO1 | FT4 | 0.69 | 0.89 | 0.69 | 0.52 | 0.69 | 0.52 | 0.59 | 0.10 | 0.59 | 0.16 | 0.87 | 0.52 | |
| rs10493147 | HSPA4 | TSH | 0.94 | 0.42 | 0.37 | 0.61 | 0.37 | 0.61 | 0.74 | 0.90 | 0.77 | 0.4 | 0.72 | 0.57 | |
| rs2235544 | DIO1 | FT4 | 0.95 | 0.93 | 0.91 | 0.11 | 0.91 | 0.11 | 0.78 | 0.26 | 0.3 | 0.13 | 0.81 | 0.24 | |
| rs2550298 | GNA01 | TSH | 0.74 | 0.99 | 0.97 | 0.61 | 0.97 | 0.61 | 0.76 | 0.66 | 0.98 | 0.17 | 0.88 | 0.82 | |
| rs9376165 | PDE7B | TSH | 0.49 | 0.38 | 0.04 | 0.34 | 0.04 | 0.34 | 0.46 | 0.03 | 0.72 | 0.37 | 0.33 | 0.11 | |
| rs1976324 | TSH | 0.22 | 0.67 | 0.25 | 0.29 | 0.25 | 0.29 | 0.22 | 0.40 | 0.07 | 0.28 | 0.62 | 0.91 | ||
| rs2294512 | DIO1 | TSH | 0.18 | 0.55 | 0.05 | 0.03 | 0.05 | 0.03 | 0.74 | 0.10 | 0.72 | 0.33 | 0.07 | 0.91 | |
| rs10512065 | GNAQ | TSH | 0.54 | 0.87 | 0.56 | 0.28 | 0.56 | 0.28 | 0.38 | 0.68 | 0.66 | 0.3 | 0.4 | 0.56 | |
| rs6026565 | GNAS | TSH | 0.67 | 0.07 | 0.58 | 0.65 | 0.59 | 0.65 | 0.79 | 0.05 | 0.99 | 0.49 | 0.82 | 0.38 | |
| rs2252696 | SLG TG | TSH | 0.77 | 0.11 | 0.52 | 0.81 | 0.52 | 0.81 | 0.86 | 0.97 | 0.58 | 0.31 | 0.67 | 0.23 | |
| rs2523189 | GNAI1 | TSH | 0.2 | 0.98 | 0.16 | 0.36 | 0.16 | 0.36 | 0.86 | 0.97 | 0.22 | 0.37 | 0.51 | 0.52 | |
Denotes whether the prior genome wide association was with TSH or FT4.
Controlled for by ethnicity, cohort and smoking status.
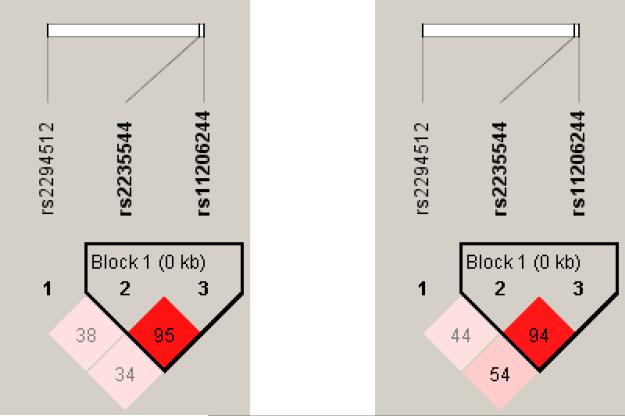
Since the two of the three SNPs from DIO1 were significantly associated with FT4 function, we next conducted linkage disequilibrium analysis of the locus using Haploview in order to discern whether the reliable imputation of haplotypes would be possible. Figure 1 depicts the linkage disequilibrium of the three SNPs in the African American and White subjects while Supplemental Table II gives the haplotype frequencies in these two ethnic groups as per Haploview. The two SNPs strongly associated with FT4 levels, rs11206244 and rs2235544, were in tight linkage disequilibrium with each other in both African American and White subjects and with the T allele of rs11206244 being exclusively associated with the A allele of rs2235544. In contrast, rs2294512, which was not associated with FT4 levels, demonstrated less linkage disequilibrium with the other two DIO1 SNPs, thus not allowing accurate imputation of haplotypes in the absence of other genetic information.
Figure 1.
Linkage disequilibrium and haplotype structure of DIO1 in African-American (right) and White (left) subjects. Disequilibrium is expressed as D’.
Finally, we examined the relationship of SNP genotype to lifetime MD. Because of the tight linkage disequilibrium of the two SNPs, we focused on rs11206244 because it is found in the 3’UTR of DIO1 and prior evidence suggests that this cSNP may be functional (Gereben and others 2008; Panicker and others 2008). Briefly, using a logistic regression model controlling for cohort, in White subjects, rs11206244 genotype is significantly associated with lifetime MD in women (p<0.004), but not men (p<0.74). However, in African American subjects, rs11206244 was not associated with MD in women (p<0.28) or men (p<0.18).
CONCLUSION
In summary, we confirm prior reports that T allele of the DIO1 SNP rs11206244 is associated with increased FT4 levels and show that this genotype is also associated with lifetime MD in White female subjects from high risk cohorts. However, we did not find that current FT4 levels are associated with lifetime history of MD. Limitations of the current study include the diverse nature of the cohorts, the limited scope of the findings with respect to MD, and we note that all p values reported are nominal.
The overarching aim of this study was to test whether genetic variation that affects thyroid function also influences vulnerability to depression. As an initial step to establish a firm biological basis for our study, we genotyped 12 SNPs, including two selected for their association with FT4 (Panicker and others 2008) and 10 selected for their association with TSH (Arnaud-Lopez and others 2008; Hwang and others 2007) in genome-wide analyses, and examined their association with thyroid indices in our populations. The two SNPs associated with FT4 levels, including the cSNP rs11206244, in prior studies were also associated with FT4 levels in our population. These affirmative findings are gratifying because DIO1, which is a critical gene in thyroid hormone production and is responsible for the conversion of thyroxine to triiodothyronine (Gereben and others 2008), has strong face validity as a candidate gene for MD given the broad literature supporting altered thyroid hormone action in the etiology of MD, which includes large epidemiologic studies and meta-analyses (Forman-Hoffman and Philibert 2006b; Williams and others 2009)
In this study, we focused on one of those two DIO1 variants, the rs11206244 polymorphism for three reasons. First, because it is a cSNP found in the 3’UTR of DIO1, that has been hypothesized to be functional in molecular analyses (Gereben and others 2008) presumably by modifying the stability of the mRNA (Merritt and others 2008). Second, genotype at this SNP has been associated with response to response to thyroid hormone augmentation (Cooper-Kazaz and others 2009). Third, our genetic information was not sufficient to reliably impute more exacting haplotypes. Nevertheless, the current findings confirm the prior findings by two prior groups that the T allele of this polymorphism is associated with increased FT4 but not TSH levels (Panicker and others 2008; Peeters and others 2003). However, it should be noted that other polymorphisms, including the intronic rs2235544 polymorphism, are also in linkage disequilibrium with rs11206244 and that further studies to determine the nature of the functional variant in this haploblock are indicated.
In contrast, none of the 10 SNPs associated with TSH levels in prior studies were consistently associated with TSH levels in our populations. Because our clinical pathology laboratory uses the same third-generation TSH assay that the prior studies used and our studies employed a sufficient number of subjects to detect the effect sizes reported in the prior studies, it is unlikely that the failure to replicate the prior TSH findings results from TSH measurement errors or lack of power. Instead, we note that the two prior genome-wide studies of TSH used distinctly different population sets and did not replicate one another. We suggest that the effect sizes associated with these 10 SNPs may be smaller than previously anticipated and that more exacting analyses of our data set may reveal more subtle relationships. However, the simple main effects are not significant in our subjects.
A critical question is why the association with depression is manifest only in the White female subjects. The observation that review of the GAIN study of major depression data available on the dbGaP website does not show a significant association for this SNP tends to suggest that the current findings may be a false positive (Boomsma and others 2008). However, the GAIN analyses are still largely underpowered and there are many other possible explanations including that differing genotype frequencies or haplotype structure between the two ethnic groups may obscure real effects. We believe that the reason for the discrepancies between the various sets of results may be related to the high rate of stress in the high-risk ICCS and IAS cohorts which allows differential biology to become manifest. Both the stress diathesis (Moffitt and others 2005) and plasticity models (Belsky and Pluess 2009) postulate that the effects of genetic variants are only manifest in the presence of stressors. It very well may be that the excessive amounts of stress and the lack of psychosocial support present in these high- risk groups may allow otherwise silent genetic vulnerability factors to become manifest as evidenced by their extremely high rates of MD. Further gene-environment studies will be necessary to verify this point and to uncover possible protective mechanisms are indicated.
If the current findings at rs11206244 with respect to lifetime MD are validated, it will be important to understand the environmental conditions under which the effects are most pronounced. For example, from a public health perspective, it will be important to know whether dietary, psychosocial or chronological influences alter the effects of DIO1 genetic variation. Likewise, from the medical point of view, because thyroid hormone augmentation of antidepressant treatment is an important strategy in the treatment of refractory MD and rs11206244 genotype is associated with response to this augmentation(Cooper-Kazaz and others 2009), it will be important to know whether genotyping HPT axis variation may help personalize treatment of MD.
In summary, we report that genotype at the DIO1 variant rs11206244 is associated with lifetime MD in White demales in high risk cohorts. We suggest that further studies of genetic variation in HPT axis genes in the etiology of MD are indicated.
Supplementary Material
ACKNOWLEDGEMENTS
The work in this study was supported by DA015789 and MH080898 to Dr. Philibert and 2 R01 MH62666 to Dr. Cutrona. Additional support for these studies was derived from the Center for Contextual Genetics and Prevention Science (Grant Number P30 DA027827, Dr. Brody) funded by the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Philibert had full access to all data and takes complete responsibility for the integrity of the findings.
Supported by DA015789, MH080898, 2 R01 MH62666 and P30 DA027827
Footnotes
A portion of this work will be presented at the American Society for Human Genetics meeting in Washington DC in November 2010.
REFERENCES
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorder. Fourth Edition American Psychiatric Association; Washington D.C.: 1994. [Google Scholar]
- Anthony J, Folstein M, Romanoski A. Comparison of lay Diagnostic Interview Schedule and a standardized psychiatric diagnosis. Archives of General Psychiatry. 1985;42:667–675. doi: 10.1001/archpsyc.1985.01790300029004. [DOI] [PubMed] [Google Scholar]
- Arnaud-Lopez L, Usala G, Ceresini G, Mitchell BD, Pilia MG, Piras MG, Sestu N, Maschio A, Busonero F, Albai G, et al. Phosphodiesterase 8B Gene Variants Are Associated with Serum TSH Levels and Thyroid Function. The American Journal of Human Genetics. 2008;82(6):1270–1280. doi: 10.1016/j.ajhg.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Willemsen G, Sullivan PF, Heutink P, Meijer P, Sondervan D, Kluft C, Smit G, Nolen WA, Zitman FG, et al. Genome-wide association of major depression: description of samples for the GAIN Major Depressive Disorder Study: NTR and NESDA biobank projects. Eur J Hum Genet. 2008;16(3):335–42. doi: 10.1038/sj.ejhg.5201979. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr., Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cooper-Kazaz R, van der Deure WM, Medici M, Visser TJ, Alkelai A, Glaser B, Peeters RP, Lerer B. Preliminary evidence that a functional polymorphism in type 1 deiodinase is associated with enhanced potentiation of the antidepressant effect of sertraline by triiodothyronine. J Affect Disord. 2009;116(1-2):113–6. doi: 10.1016/j.jad.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Cutrona CE, Russell DW, Brown PA, Clark LA, Hessling RM, Gardner KA. Neighborhood context, personality, and stressful life events as predictors of depression among African American women. Journal of Abnormal Psychology. 2005;114:3–15. doi: 10.1037/0021-843X.114.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das BK, Baral N, Shyangwa PM, Toora BD, Lamsal M. Altered serum levels of thyroxine, triiodothyroinine and thyroid stimulating hormone in patients with depression. Kathmandu Univ Med J (KUMJ) 2007;5(3):330–4. [PubMed] [Google Scholar]
- Forman-Hoffman V, Philibert RA. Lower TSH and higher T4 levels are associated with current depressive syndrome in young adults. Acta Psychiatr Scand. 2006a;114(2):132–9. doi: 10.1111/j.1600-0447.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- Forman-Hoffman V, Philibert RA. Lower TSH and higher T4 levels are associated with current depressive syndrome in young adults. Acta Psychiatrica Scandinavica. 2006b;0(0) doi: 10.1111/j.1600-0447.2005.00703.x. Epub ahead of publication. [DOI] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. Cellular and Molecular Basis of Deiodinase-Regulated Thyroid Hormone Signaling. Endocr Rev. 2008;29(7):898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465–75. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Gunter TD, Philibert R, Hollenbeck N. Medical and psychiatric problems among men and women in a community corrections residential setting. Behav Sci Law. 2009;27(5):695–711. doi: 10.1002/bsl.887. [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T4, and Thyroid Antibodies in the United States Population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- Hwang S-J, Yang Q, Meigs J, Pearce E, Fox C. A genome-wide association for kidney function and endocrine-related traits in the NHLBI's Framingham Heart Study. BMC Medical Genetics. 2007;8(Suppl 1):S10. doi: 10.1186/1471-2350-8-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson IM. The thyroid axis and depression. Thyroid. 1998;8(10):951–6. doi: 10.1089/thy.1998.8.951. [DOI] [PubMed] [Google Scholar]
- Jorde R, Sundsfjord J. Serum TSH levels in smokers and non-smokers. The 5th Tromso study. Exp Clin Endocrinol Diabetes. 2006;114(7):343–7. doi: 10.1055/s-2006-924264. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The Structure of Genetic and Environmental Risk Factors for Common Psychiatric and Substance Use Disorders in Men and Women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). Jama. 2003;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Ng MY, Butler AW, Cohen-Woods S, Uher R, Pirlo K, Weale ME, Schosser A, Paredes UM, Rivera M, et al. Genome-Wide Association Study of Major Recurrent Depression in the U.K. Population. Am J Psychiatry. 2010;167(8):949–957. doi: 10.1176/appi.ajp.2010.09091380. [DOI] [PubMed] [Google Scholar]
- Merritt C, Rasoloson D, Ko D, Seydoux G. 3' UTRs are the primary regulators of gene expression in the C. elegans germline. Current biology : CB. 2008;18(19):1476–82. doi: 10.1016/j.cub.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62(5):473–81. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Muglia P, Tozzi F, Galwey NW, Francks C, Upmanyu R, Kong XQ, Antoniades A, Domenici E, Perry J, Rothen S, et al. Genome-wide association study of recurrent major depressive disorder in two European case-control cohorts. Mol Psychiatry. 2010;15(6):589–601. doi: 10.1038/mp.2008.131. [DOI] [PubMed] [Google Scholar]
- Panicker V, Cluett C, Shields B, Murray A, Parnell KS, Perry JRB, Weedon MN, Singleton A, Hernandez D, Evans J, et al. A Common Variation in Deiodinase 1 Gene DIO1 Is Associated with the Relative Levels of Free Thyroxine and Triiodothyronine. J Clin Endocrinol Metab. 2008;93(8):3075–3081. doi: 10.1210/jc.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, Visser TJ. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88(6):2880–8. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- Philibert R. Merging Genetic and Environmental Effects in the Iowa Adoption Studies: Focus on Depression. Ann Clin Psychiatry. 2006;18(4):219–22. doi: 10.1080/10401230600948399. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Gunter T, Black DW, Hollenbeck N, Secrest D, Barkhurst A, Adams W. Association of elevated free T4 levels with depressive symptoms in patients with psychotic disorders. Schizophr Res. 2006;87(1-3):334–5. doi: 10.1016/j.schres.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Rietschel M, Mattheisen M, Frank J, Treutlein J, Degenhardt F, Breuer R, Steffens M, Mier D, Esslinger C, Walter H, et al. Genome-Wide Association-, Replication-, and Neuroimaging Study Implicates HOMER1 in the Etiology of Major Depression. Biological Psychiatry. doi: 10.1016/j.biopsych.2010.05.038. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Williams MD, Harris R, Dayan CM, Evans J, Gallacher J, Ben-Shlomo Y. Thyroid function and the natural history of depression: findings from the Caerphilly Prospective Study (CaPS) and a meta-analysis. Clinical Endocrinology. 2009;70(3):484–492. doi: 10.1111/j.1365-2265.2008.03352.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.