Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by amyloid-β (Aβ) deposition in senile plaques colocalized with activated microglia and astrocytes. Recent studies suggest that CXCL8 is involved in the AD pathogenesis. The objective of this study was to determine the cellular sources of CXCL8 in the central nervous system during AD pathogenesis, and investigate the effects of CXCL8 on neuronal survival and/or functions. Our results showed significantly higher CXCL8 levels in AD brain tissue lysates as compared to those of age-matched controls. Upon Aβ and/or pro-inflammatory cytokine stimulation, microglia, astrocytes and neurons were all capable of CXCL8 production in vitro. Although CXCL8-alone did not alter neuronal survival, it did inhibit Aβ-induced neuronal apoptosis and increased neuronal brain-derived neurotrophic factor (BDNF) production. We conclude that microglia, astrocytes and neurons, all contribute to the enhanced CXCL8 levels in the CNS upon Aβ and/or pro-inflammatory cytokine stimulation. Further, CXCL8 protects neurons possibly by paracrine or autocrine loop and regulates neuronal functions, therefore, may play a protective role in the AD pathogenesis.
Keywords: Alzheimer’s disease (AD), amyloid-β (Aβ), human neuron, CXCL8, tumor necrosis factor-α (TNF-α), neuroprotection
Introduction
Alzheimer’s disease1 is a neurodegenerative disorder characterized by progressive memory loss, cognitive decline and widespread loss of neurons and their synapses in the cerebral cortex, entorhinal area, hippocampus, ventral striatum and basal forebrain [1]. An important pathological feature of AD is deposition of Aβ; a 40–42 amino acid peptide, proteolytically derived from amyloid precursor protein in senile plaques [1]. These depositions are reported to be surrounded by activated microglia [2] and astrocytes [3]. Although the cause of neuronal loss is still unclear, there are suggestions that Aβ can be directly toxic to neurons [4]. Inflammatory cytokines produced by activated microglia and astrocytes are also considered as significant factor in AD pathogenesis [1].
Growing evidences suggest that CXCL8, an inflammatory chemokine, and its receptors may play a role in AD pathogenesis. Recently, increased expression of CXCL8 in CSF [5] and its receptor, CXCR2, in senile plaques of AD brains has been reported [6]. In the periphery, neutrophils, monocytes and endothelial cells secrete CXCL8, which recruits neutrophils and monocytes to sites of inflammation [7]. In CNS, monocytes and microglia both have been shown to upregulate CXCL8 after exposure to Aβ [8,9]. Though astrocytes, microglia and neurons are all capable of producing CXCL8 [8,9], little is known about neuronal production of CXCL8 upon Aβ-injury and its consequent biological effects on neuronal survival/functions. CXCL8 may be neuroprotective rather than neurotoxic as no neurological deficits observed in cerebral malaria patients who had prolonged elevated CXCL8 levels in CSF [10].
We hypothesized that neurons also contribute to elevated CXCL8 levels during AD and may affect neuronal survival and function. Our results demonstrate that CXCL8 levels are significantly increased in AD brain tissues. Both Aβ and pro-inflammatory cytokines such as IL-1β or TNF-α can lead to neuronal CXCL8 production in vitro. CXCL8 inhibits Aβ-induced neuronal apoptosis and upregulates neuronal BDNF production. These findings suggest that CXCL8 plays a neuroprotective role in the AD pathogenesis probably by modulating neuronal functions.
Materials and Methods
Policy, ethics and conflict of interest
The work described in this article was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and the Uniform Requirements for manuscripts submitted to biomedical journals. The authors have no conflicts of interest to disclose that could have inappropriately influenced, or be perceived to have influenced, their work.
Isolation and cultivation of primary human astrocytes, neurons and microglia
Human astrocytes and neurons were isolated from first and early second trimester human fetal brain tissue from elective abortus specimens. These were obtained from the Laboratory of Developmental Biology, University of Washington, Seattle, WA in full compliance with the ethical guidelines of the NIH and the Universities of Nebraska and North Texas Health Science Center. Microglia, astrocytes and neurons were isolated and cultured separately as described previously [11,12].
Experimental treatments and evaluation of neuronal viability
Aβ1-42 and Aβscr (rPeptide, Bogart, GA) were solubilized (1mg/ml) in sterile 5mM HCL and sonicated for 1–5 min in water bath. The solution was then incubated overnight at 37°C to aggregate the proteins and stored at −80°C in aliquots until use. Cells were incubated with different concentrations of Aβ1-42 in triplicates for 24h either with TNF-α, CCL2 or CXCL8 with or without anti-CXCL8 monoclonal neutralizing antibodies (all from R&D systems, Minneapolis, MN). The MTT assay for neuronal viability, dsDNA ELISA and TUNEL for apoptosis were performed as previously described [11].
Preparation of human brain tissue extracts
Human brain specimens from the frontal cortex and temporal cortex were provided by the NNTC and CNND Brain Bank [12]. All donors gave informed consent, which permits research use of their tissues and informed them of possible conflicts of interest. Brain lysates were prepared from specimens as previously described [13].
CXCL8 and BDNF measurements
CXCL8 and BDNF were measured in triplicates using commercially available ELISA kits (R&D Systems) according to the manufacturer’s specifications.
Immunocytochemistry
Adherent monolayer of human neurons in a 48-well plate (1×105 cells/well) was fixed and incubated with primary antibodies to human MAP-2 (1:100; Chemicon), CXCL8 (1:100; Pierce), and DAPI (1:300; Invitrogen, Carlsbad, CA). Alexa Fluor® 594 (goat anti-mouse; 1:100) was used for MAP-2 detection and Alexa Fluor® 488 (donkey anti-rabbit; 1:100) for CXCL8 (Invitrogen). Neuron culture purity was routinely >85% as assessed by GFAP (1:1000; Covance Research Products, Berkeley, CA) staining for astroctyes. Immunofluorescence micrographs were obtained on a Nikon Eclipse TE-300 microscope (Tokyo, Japan), 200X.
Statistics
All experiments are expressed as mean±SEM of triplicates and are representative of three different donors. All data was analyzed using GraphPad Prism 4.0 with ANOVA followed by Newman-Keuls post-hoc test or two-way ANOVA followed by Bonferroni post-hoc test. Mann-Whitney test and Pearson correlation coefficient were used for correlation analyses. Differences were considered significant at p<0.05.
Results
Increased CXCL8 levels in brain tissue of AD patients
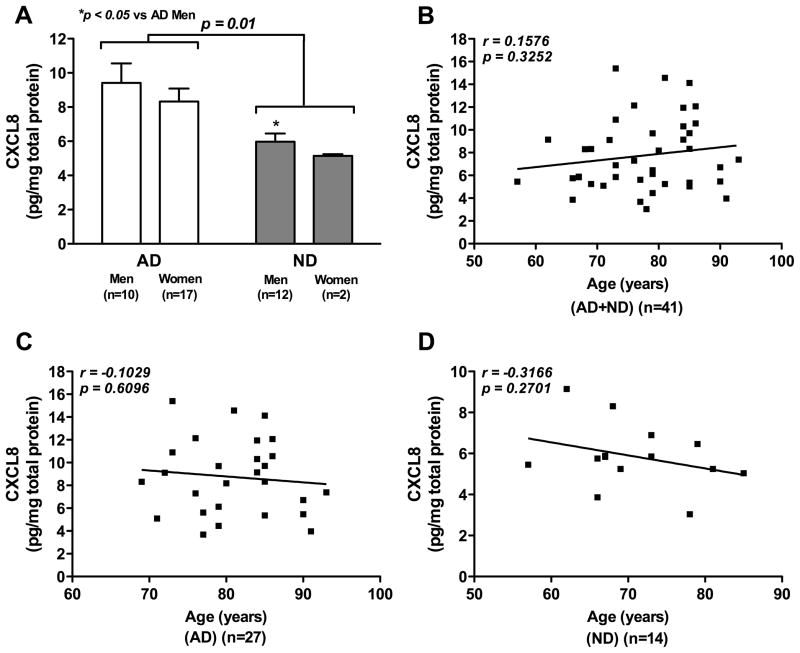
We first evaluated CXCL8 levels in brain tissue lysates from AD patients and age-matched controls. Brain tissue specimens (22 men and 19 women) from 27 AD patients (65.8%) and 14 ND controls (34.2%) with a mean age of 77.6 (±1.3) years were obtained based on clinical history, neuropathological diagnosis, gross and microscopic observations. AD and ND groups did not differ significantly with respect to PMI (mean±SEM, 7.1±1.1 h for AD, 10.0±2.3 h for ND). A statistical significant effect of disease on CXCL8 levels was observed in two-way ANOVA analysis, as the CXCL8 levels in AD patients were significantly higher than in ND controls (8.73±0.63 versus 5.86±0.42, F1,37 =7.20, p=0.01, Fig. 1A). However, no significant effect of gender was observed on disease (F1,37 =0.61, p=0.44) and brain CXCL8 levels (F1,37 =0.01, p=0.9205). Post hoc Bonferroni tests showed that the brain CXCL8 levels in men with AD were significantly higher than those in ND men (p<0.05, Fig. 1A). There was no difference in brain CXCL8 levels between the AD women and ND women (p>0.05). No significant correlation between age and brain CXCL8 levels emerged upon Pearson correlation coefficient analysis of the entire sample set (r=0.1576, p=0.3252, Fig. 1B). Similarly, no significant correlation between age and brain CXCL8 levels was found either in AD patients (r=−0.1029, p=0.6096, Fig. 1C) or in ND controls (r=−0.3166, p=0.2701, Fig. 1D).
Fig. 1. CXCL8 levels in brain tissues of patients with AD and ND controls.
Human brain tissue lysates from patients with AD (n=27) and ND controls (n=14) were analyzed for both CXCL8 and total protein levels. Data were expressed as CXCL8 (ng/ml)/total protein (mg/ml). Data demonstrate CXCL8 levels in brain tissue of patients with AD and ND controls (A); correlation between brain CXCL8 levels and age for all donors (AD+ND; B); for patients with AD (C) and for ND controls (D).
CXCL8 production in response to Aβ or pro-inflammatory stimuli
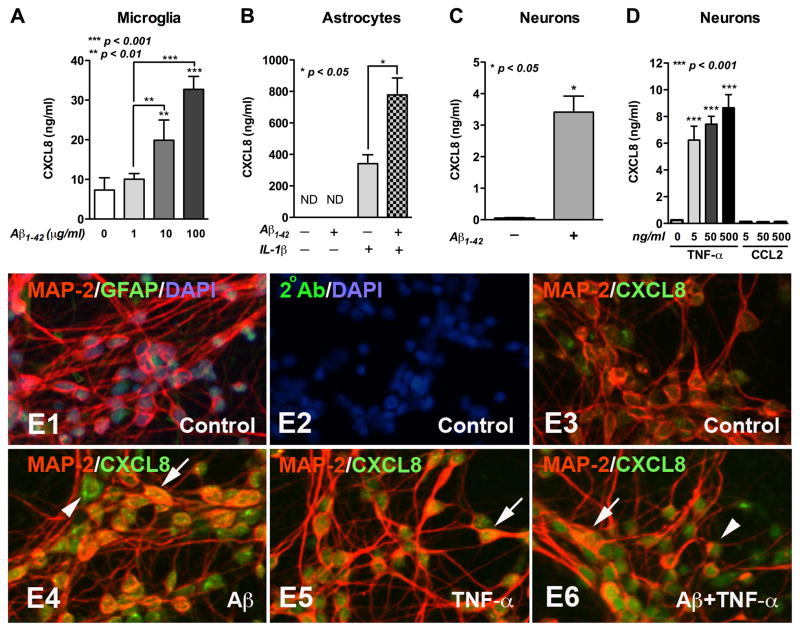
Human microglia, astrocytes and neurons were exposed to Aβ1-42 and/or pro-inflammatory cytokines for 24h as described [8] and CXCL8 levels were measured. Aβ1-42 induced a dose-dependent increase in microglial CXCL8 production (Fig. 2A). While 1 μg/ml Aβ1-42 showed no effect, 10 μg/ml Aβ1-42 significantly upregulated CXCL8 levels by 3.42-fold (p<0.01), and 100 μg/ml Aβ1-42 by 4.48-fold (p<0.001). In astrocytes IL-1β-alone predominantly upregulated CXCL8 production; Aβ1-42-alone (10 μg/ml) showed no effect (Fig. 2B). However, a significant synergistic effect of Aβ1-42 and IL-1β cotreatment was found (p<0.05). Interestingly, neuronal CXCL8 production was significantly incr eased by 42.1-fold as compared to the controls after exposure to Aβ1-42 (10μg/ml; p<0.05, Fig. 2C). However, a linear dose response to Aβ1-42 was not observed in neuronal CXCL8 production (data not shown). In addition, TNF-α induced a significant increase (26.3–36.6-fold) in neuronal CXCL8 levels (p<0.001, Fig. 2D), while CCL2 did not (p>0.05, Fig. 2D). Abundance of CXCL8 expression in neurons treated with Aβ1-42 (Fig. 2E4), TNF-α (Fig. 2E5), or Aβ1-42 with TNF-α (Fig. 2E6) as compared to control (Fig. 2E3) was also observed by immunostaining. CXCL8 colocalization with MAP-2 was visualized by bright yellow double staining (Arrows, Fig. 2E4–E6). In addition to MAP-2-bright cells, neuronal cell bodies that were MAP-2-dull were also positive for CXCL8 (Arrows head, Fig. 2E4, E6). Neuronal cultures were highly enriched in MAP-2-positive neurons and very few GFAP-positive astrocytes were observed (Fig. 2E1).
Fig. 2. CXCL8 production from neural cells.
Human microglia (A), astrocytes (B), or neurons (C, D) were treated with different concentrations of Aβ1-42 (A), Aβ1-42 (10 μg/ml) and/or IL-1β (20ng/ml; B), Aβ1-42 (10 μg/ml; C), or different concentrations of TNF-α or CCL2 (D) for 24 h. Supernatants were collected and CXCL8 levels were measured by ELISA. Data are expressed as CXCL8 ng/ml/million cells, presented as mean±SEM of triplicates and are representative of three different donors. Human neurons treated with Aβ1-42 (10 μg/ml; E4), TNF-α (50ng/ml; E5), or Aβ1-42 and TNF-α simultaneously (E6) for 24 h, immunostained with antibodies for MAP-2 (red: neuronal marker; E1, E3–E6), GFAP (green: astrocyte marker; E1), and CXCL8 (green; E3–E6). DAPI was used to stain nuclei (blue; E1, E2). Original magnification ×200.
CXCL8 inhibits Aβ-induced neuronal apoptosis
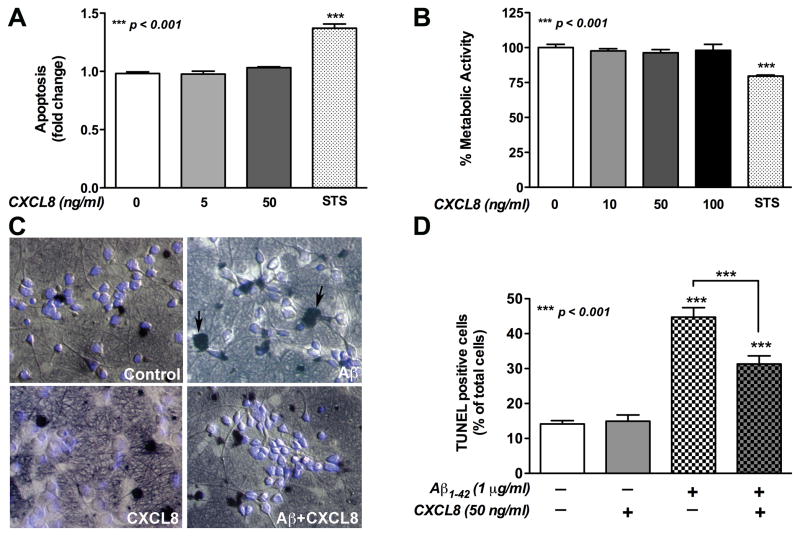
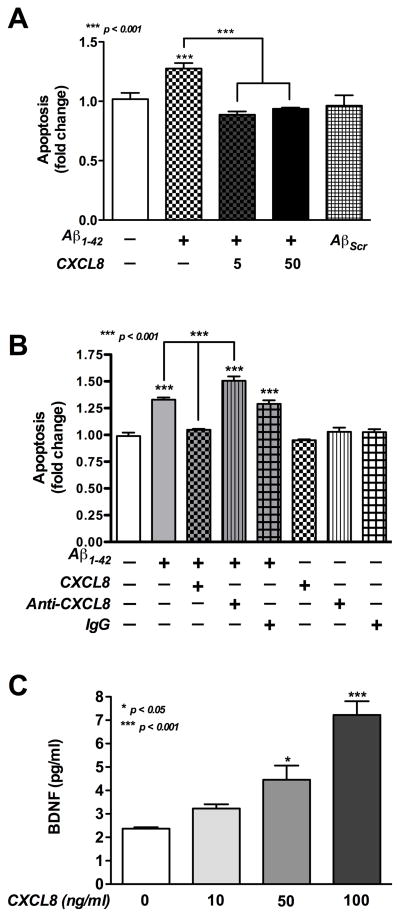
MTT and dsDNA ELISA showed that human neurons treated with CXCL8-alone have no significant effect on viability or apoptosis, while the positive control, STS has (p<0.001, Fig. 3A, B). Two separate assays, TUNEL staining and dsDNA ELISA were used to quantify Aβ-induced neuronal cell death. It was observed by TUNEL staining that Aβ1-42-alone significantly increased neuronal apoptosis (p<0.001, Fig. 3C, D) as compared to control. Co-incubation of Aβ1-42 with CXCL8 significantly decreased the apoptosis levels (p<0.001, Fig. 3C, D). Similar to above, CXCL8-alone did not show cytotoxicity as detected by TUNEL staining (p>0.05, Fig. 3C, D). A ten-fold lower amount of CXCL8 was co-incubated with Aβ1-42 to investigate its physiological importance in Aβ-induced neurotoxicity. It was also observed by dsDNA ELISA that Aβ1-42 treatment led to a significant increase in neuronal apoptosis (p<0.001, Fig. 4A) compared to control. Further, as low as 5ng/ml of CXCL8 significantly decreased Aβ1-42-induced apoptosis (p<0.001). As expected neuronal apoptosis in AβScr-treated neurons was not significantly different from untreated controls (p>0.05).
Fig. 3. The effects of CXCL8 on Aβ-induced human neuronal apoptosis.
Human neurons were treated with Aβ1-42 (1 μg/ml) and/or CXCL8 (5, 10, 50, 100ng/ml) for 24 h. Effect of CXCL8-alone on neuronal apoptosis/viability was determined by dsDNA ELISA (A) and MTT assay (B). The base line metabolic activity in untreated, control neurons was considered as 100%. Aβ-induced cell apoptosis and CXCL8-mediated neuroprotection was determined by TUNEL and DAPI staining (C). Images are representative of 30 random pictures (original magnification, 200X) taken from triplicate wells (10 each) from each treatment condition. Dark black nuclei (apoptotic, arrows C) and bright blue nuclei (healthy) were counted to determine the total number of cells in each image. Percent TUNEL positive cells relative to total cells was calculated for each image and graphed in panel D. STS (0.3μM) was used as a positive control. Data are presented as mean±SEM of triplicates and are representative of three different donors.
Fig. 4. The self-protective effect of CXCL8 on Aβ-induced human neuronal apoptosis and BDNF production.
Human neurons were treated with Aβ1-42 (1 μg/ml) and/or CXCL8 (5, 10, 50, 100ng/ml) with or without anti-CXCL8 antibodies (0.2 μg/ml) and scrambled Aβ (AβScr) as a control. Neurons apoptosis was determined by dsDNA ELISA after 24 h (A, B). Pre-immune IgG antibodies were used as a control. Culture supernatants were analyzed for BDNF levels (C). Data are presented as mean±SEM of triplicates and are representative of three different donors.
CXCL8 protects neurons by self-protective loop and BDNF production
To further investigate CXCL8 self-protective mechanism, we neutralized exogenous CXCL8 with anti-CXCL8 monoclonal neutralizing antibodies. Co-culture of neurons with Aβ1-42 and anti-CXCL8 antibodies (0.2 μg/ml) significantly increased neuronal apoptosis (p<0.001) compared to Aβ1-42, Aβ1-42 with pre-immune IgG antibodies and untreated control. Anti-CXCL8 antibodies-alone do not have any significant effect on neuronal apoptosis (Fig. 4B).
We then studied whether CXCL8 was able to influence neuronal BDNF production. As shown in Fig. 4C, treatment with CXCL8 induces a dose-dependent increase in neuronal BDNF production. Although the increase was not significant at 10ng/ml, treatment with both 50 and 100ng/ml CXCL8 significantly increased BDNF production as compared to control (p<0.05 and p<0.001, respectively). As the primary neuronal cultures have minimal astrocyte contamination (Fig. 2E1), we tested the astrocytes response to CXCL8 stimulation at similar concentrations (0.1–100ng/ml) for 24 h. We observed that astrocytes do not respond to CXCL8 (data not shown). These data suggest that increase in BDNF production is primarily due to CXCL8 effects on neurons.
Discussion
The results from the current study demonstrated that CXCL8 levels were increased in brain tissue lysates from AD patients as compared to age-matched controls. Aβ- or pro-inflammatory cytokines such as IL-1β- or TNF-α induced CXCL8 production by microglia, astrocytes and neurons in vitro. Although CXCL8 did not significantly influence neuron survival, it inhibited Aβ-induced neurotoxicity and increased neuronal BDNF production, indicating its protective role in AD pathogenesis.
CXCL8 is an inflammatory chemokine, which recently reported to be increased in CSF of patients with mild cognitive impairment and AD [5]. However, few studies have examined the CXCL8 levels in brain tissue lysates from AD patients. The in vitro neurotoxicity and induction of CXCL8 production in human microglia or astrocytes by Aβ previously has been reported [14,15]. However, little is known about whether neurons can produce chemokines in response to Aβ stimulation. Our data demonstrate that CXCL8 levels are elevated in AD brains. We confirm that microglial and astrocytes produce CXCL8 upon Aβ or inflammatory cytokines stimulation. Importantly, for the first time we demonstrate that Aβ upregulates neuronal production of CXCL8. Further, we did not observe significant changes in CXCL8 production by astrocytes in response to Aβ-alone until cotreated with IL-1β. Therefore, neuronal response to Aβ by producing CXCL8 could be an important event in disease pathogenesis.
In addition, TNF-α, a prototypical inflammatory cytokine associated with AD pathogenesis, also led to significant enhancement in neuronal CXCL8. TNF-α was produced by microglia upon Aβ-exposure, which led to neuronal cell death [16]. Our data demonstrate that TNF-α, but not CCL2, is capable of inducing neuronal CXCL8 production in vitro. This is in parallel with a previous report where TNF-α induced chemokine production in neural precursor cells [17]. Taken together, our data indicate that during AD pathogenesis, human neurons can produce inflammatory mediators such as CXCL8 upon stimulation induced either by neurotoxins such as Aβ or pro-inflammatory cytokines such as TNF-α.
The direct effect of CXCL8 on neurons was unclear. CXCL8 was shown to increase apoptosis in primary rat neurons [18]. However, a protective effect of CXCL8 was reported in murine neonatal hippocampal neurons [19]. Further, no effects of CXCL8 on cell viability were found in the human neuronal cell line NT2-N [20]. Because of differences in sensitivities towards Aβ between human and mouse neurons (approximately 500-fold), there is possibility of secondary necrosis while studying Aβ-induced neurotoxicity in mouse neurons [21]. Therefore, we investigated these findings directly on human neurons. Our results show that CXCL8-alone has neither cytotoxic nor proliferative effects on human neurons in vitro. Interestingly, when human neurons were treated with CXCL8-neutralizing antibodies there was no significant increase in apoptosis. Thus, during normal conditions, CXCL8 has no significant role in neuron viability, which also may explain their very low level of CXCL8 production during normal conditions.
In this study, we also observe that CXCL8 partially inhibits Aβ-induced neuronal cell death supporting Watson and Fan, 2005 [19]. However, in contrast to murine neonatal neurons, human neurons were very sensitive to Aβ. In order to focus on the apoptotic effects of Aβ without any significant contribution of necrosis, low concentrations of Aβ were used in all experiments. Further, CXCL8 may be neuroprotective to Aβ-induced toxicity through a self-protective loop since CXCL8-neutralization significantly increased apoptosis in neurons cotreated with Aβ and anti-CXCL8 compared Aβ-alone. In vitro, it seems that the neuronal CXCL8 response is quantitatively insufficient to overcome Aβ-mediated toxicity. While in vivo this may depend on the tissue microenvironment and other factors. We also did not observe a Aβ-dose dependent increase in neuronal CXCL8 production, which may be due to need of chronic exposure of Aβ or a threshold necessary for Aβ activation along with other mechanisms in vivo. Interestingly, it has been reported that only untreated, not Aβ-treated, astrocytes are protective to Aβ-induced neuronal toxicity in rat hippocampal culture [22]; therefore, self-protection of neurons by CXCL8 production could be a very important phenomenon.
We postulated that CXCL8 protects neurons through an autocrine mechanism and that it induces the synthesis and/or release of neurotrophic factors. It has been reported that CXCL8 protects murine neonatal hippocampal neurons through CXCR2 receptor [19], possibility by induction of the synthesis or release of neurotrophic factors. However, it has not been investigated in detail thus far. Therefore, we assayed for release of a neurotrophic factor. BDNF, a member of the neurotrophin family of growth factors, has been shown to improve the survival and function of neurons in the CNS, particularly in brain regions susceptible to degeneration in AD [23,24]. Recently, the release of mature BDNF by primary neurons has been demonstrated [25]. Therefore, the effect of CXCL8 on neuronal BDNF production may contribute to its neuroprotective effects against Aβ-induced neurotoxicity. Interestingly, CXCL8-alone did not alter neuronal survival, indicating that other intracellular mechanisms are probably involved in the neuroprotective effects observed. Indeed, expression of both CXCR1 and CXCR2 on neurons and CXCL8-induced intracellular Ca2+ signaling in neurons were demonstrated [26]. Thus, CXCL8-induced intracellular signaling may be one potential mechanism for its neuroprotective effects. Taken together, our data suggest that CXCL8 is able to mediate neuroprotective effects via influencing neuronal functions. In vivo, upon injury/stimulation mediated by Aβ or TNF-α, neuronal CXCL8 production may serve as protective paracrine and autocrine loops against Aβ- or TNF-α-induced neurotoxicity.
Traditionally, CXCL8 is viewed as an inflammatory chemokine. Our data provide new evidence on the potential neuroprotective role of neuroinflammatory responses. Furthermore, the evidence that neurons produce CXCL8 upon injury/stimulation indicates that neurons are active players in neuroinflammatory responses, and may indeed work in a self-protective loop. Data regarding the effects of CXCL8 on neurons was not clear thus far. Observed discrepancies may be a result of differences in experimental design (in vivo versus in vitro), neuronal cells selected (primary neurons versus neuronal cell lines), the presence or absence of glial cells, treatment duration (acute versus chronic), dose of CXCL8 used (from ng/ml to μg/ml), and biological indicators (morphological changes, cell viability, or electrophysiological measurements). Therefore, the neuroprotective or detrimental effects of cytokines and chemokines, including CXCL8 in the CNS are dependent upon dose, treatment duration, conditioning, and presence of other cells or molecules. All of these factors need to be taken into account in further studies investigating the biological effects of cytokines and chemokines on neuronal function and/or survival.
In conclusion, our data demonstrate that neurotoxins such as Aβ, as well as pro-inflammatory cytokines such as TNF-α, induce CXCL8 production in neurons and Aβ and/or IL-1β in glial cells or in vitro. Enhanced CXCL8 levels in brain tissue lysates from AD patients were also found. Higher levels of CXCR2, the CXCL8 receptor, have been reported in the senile plaques of AD brains [6]. We thus, propose that during the process of AD; neurons, astrocytes, and microglia all contribute to the CXCL8 increase in the CNS. Glial and neuronal CXCL8 production serve as paracrine or autocrine neuroprotective mechanisms probably by regulating neuronal functions in the pathological process of AD. Investigating the regulation of CXCL8-CXCR1/2 and intracellular signaling mediated through these ligand-receptor interactions may provide better understanding of the pathological process of AD and a potential target for disease therapy.
Research highlights.
AD brain tissue has enhanced CXCL8 levels.
Neurons also produce CXCL8 upon Aβ-injury or stimulation by TNF-α.
CXCL8 protects neurons from Aβ-induced toxicity and enhances BDNF levels.
Capacity of self-protection in neurons against Aβ has implications in AD.
Acknowledgments
This study was supported by grants from the NINDS, RO1 NS48837, NIMH, MH087345 to A. Ghorpade and additional support from J.E.S. Edwards Foundation, Fort Worth, TX. The project entitled “Laboratory of Developmental Biology” was supported by NIH 5R24HD0008836 from NICHD. Also supported by NIMH and NINDS funding to: Manhattan HIV Brain Bank, U01MH083501, R24MH59724; Texas NeuroAIDS Research Center U01MH083507, R24 NS45491; National Neurological AIDS Bank 5U01MH083500, NS38841; California NeuroAIDS Tissue Network U01MH083506, R24MH59745; Statistics and Data Coordinating Center U01MH083545, N01MH32002. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NICHD, NNTC or NIH. The authors thank the families of donors to the CNND Brain Bank, the Laboratory of Developmental Biology and the NNTC for their contribution to this research.
Footnotes
Abbreviations: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 4′,6-diamidino-2-phenylindole (DAPI), Alzheimer’s disease (AD), amyloid-β (Aβ), brain-derived neurotrophic factor (BDNF), Center for Neurovirology and Neurodegenerative Disorders (CNND), central nervous system (CNS), cerebrospinal fluid (CSF), double-stranded DNA fragmentation (dsDNA), enzyme-linked immunosorbent assay (ELISA), glial fibrillary acidic protein (GFAP), microtubule-associated protein 2 (MAP-2), National Institutes of Health (NIH), National NeuroAIDS Tissue Consortium (NNTC), non-demented (ND), one-way analysis of variance (ANOVA), scrambled Aβ (Aβscr), standard error of mean (SEM), staurosporine (STS), terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL), tumor necrosis factor (TNF).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 3.Pike CJ, Cummings BJ, Cotman CW. Early association of reactive astrocytes with senile plaques in Alzheimer’s disease. Exp Neurol. 1995;132:172–179. doi: 10.1016/0014-4886(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 4.Yankner BA. Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 5.Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, Guidi I, Blankenstein MA, Bresolin N, Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 6.Xia M, Qin S, McNamara M, Mackay C, Human BT. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimer’s disease. Am J Pathol. 1997;150:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 7.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 8.Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GM, Jr, Brachova L, Yan SD, Walker DG, Shen Y, Rogers J. Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia. 2001;35:72–79. doi: 10.1002/glia.1072. [DOI] [PubMed] [Google Scholar]
- 9.Tixier E, Galmiche JP, Neunlist M. Intestinal neuro-epithelial interactions modulate neuronal chemokines production. Biochem Biophys Res Commun. 2006;344:554–561. doi: 10.1016/j.bbrc.2006.03.159. [DOI] [PubMed] [Google Scholar]
- 10.John CC, Panoskaltsis-Mortari A, Opoka RO, Park GS, Orchard PJ, Jurek AM, Idro R, Byarugaba J, Boivin MJ. Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. Am J Trop Med Hyg. 2008;78:198–205. [PMC free article] [PubMed] [Google Scholar]
- 11.Deshpande M, Zheng J, Borgmann K, Persidsky R, Wu L, Schellpeper C, Ghorpade A. Role of activated astrocytes in neuronal damage: potential links to HIV-1-associated dementia. Neurotox Res. 2005;7:183–192. doi: 10.1007/BF03036448. [DOI] [PubMed] [Google Scholar]
- 12.Ghorpade A, Bruch L, Persidsky Y, Chin B, Brown WH, Borgmann K, Persidsky R, Wu L, Holter S, Cotter R, Faraci J, Heilman D, Meyer V, Potter JF, Swindells S, Gendelman HE. Development of a rapid autopsy program for studies of brain immunity. J Neuroimmunol. 2005;163:135–144. doi: 10.1016/j.jneuroim.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Suryadevara R, Holter S, Borgmann K, Persidsky R, Labenz-Zink C, Persidsky Y, Gendelman HE, Wu L, Ghorpade A. Regulation of tissue inhibitor of metalloproteinase-1 by astrocytes: Links to HIV-1 dementia. Glia. 2003;44:47–56. doi: 10.1002/glia.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- 15.Ehrlich LC, Hu S, Sheng WS, Sutton RL, Rockswold GL, Peterson PK, Chao CC. Cytokine regulation of human microglial cell IL-8 production. J Immunol. 1998;160:1944–1948. [PubMed] [Google Scholar]
- 16.Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheng WS, Hu S, Ni HT, Rowen TN, Lokensgard JR, Peterson PK. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J Leukoc Biol. 2005;78:1233–1241. doi: 10.1189/jlb.0405221. [DOI] [PubMed] [Google Scholar]
- 18.Thirumangalakudi L, Yin L, Rao HV, Grammas P. IL-8 induces expression of matrix metalloproteinases, cell cycle and pro-apoptotic proteins, and cell death in cultured neurons. J Alzheimers Dis. 2007;11:305–311. doi: 10.3233/jad-2007-11307. [DOI] [PubMed] [Google Scholar]
- 19.Watson K, Fan GH. Macrophage inflammatory protein 2 inhibits beta-amyloid peptide (1–42)-mediated hippocampal neuronal apoptosis through activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Mol Pharmacol. 2005;67:757–765. doi: 10.1124/mol.104.004812. [DOI] [PubMed] [Google Scholar]
- 20.Froyland E, Pedersen ED, Kvissel AK, Almaas R, Pharo A, Skalhegg BS, Mollnes TE, Rootwelt T. Effect of acidosis on IL-8 and MCP-1 during hypoxia and reoxygenation in human NT2-N neurons. Brain Res. 2006;1113:64–73. doi: 10.1016/j.brainres.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 21.Wei L, Ying DJ, Cui L, Langsdorf J, Yu SP. Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res. 2004;1022:54–61. doi: 10.1016/j.brainres.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 22.Paradisi S, Sacchetti B, Balduzzi M, Gaudi S, Malchiodi-Albedi F. Astrocyte modulation of in vitro beta-amyloid neurotoxicity. Glia. 2004;46:252–260. doi: 10.1002/glia.20005. [DOI] [PubMed] [Google Scholar]
- 23.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 24.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008 doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- 26.Puma C, Danik M, Quirion R, Ramon F, Williams S. The chemokine interleukin-8 acutely reduces Ca(2+) currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J Neurochem. 2001;78:960–971. doi: 10.1046/j.1471-4159.2001.00469.x. [DOI] [PubMed] [Google Scholar]