Abstract
During a microbubble infusion, guided high mechanical index impulses from a diagnostic two dimensional transducer improve microvascular recanalization in acute ST segment elevation myocardial infarction (STEMI). The purpose of this study was to further elucidate the mechanism of improved microvascular flow in normal and hyperlipidemic, atherosclerotic pigs. In 14 otherwise normal pigs, an acute left anterior descending thrombotic coronary occlusion was created. Pigs subsequently received aspirin, heparin and ½ dose fibrinolytic agent (Tenecteplase or tissue plasminogen activator), followed by randomization to either no additional treatment (Group I), or a continuous infusion of non-targeted microbubbles and guided high mechanical index impulses from a three dimensional transducer (3D/mechanical index; Group II). Epicardial recanalization rates, ST segment resolution, microsphere-derived myocardial blood flow (MBF), and ultimate infarct size using myocardial contrast echocardiography were compared. The same coronary thrombosis was created in a set of 12 hypercholesterolemic pigs who were then treated with the same pharmacologic and ultrasound regimen (Group III; n=6) or the pharmacologic regimen alone (Group IV; n=6). Epicardial recanalization rates in Group I and II pigs were the same (29%), however, peri-infarct MBF and ultimate infarct size improved following treatment in Group II pigs (p <0.01 versus Group I). In Group III pigs, epicardial recanalization was 100% (compared to 50% in Group IV), and there were significant reductions in ultimate infarct size (p=0.02 compared to Group IV). We conclude that guided high mechanical index impulses from a diagnostic transducer and non-targeted microbubbles improve peri-infarct microvascular flow in acute STEMI, even when epicardial recanalization does not occur.
Introduction
Microvascular thrombi play a major role in the no-reflow phenomena (1–3), and restoration of both microvascular and epicardial flow are critical to prevent post-infarction complications and left ventricular remodeling following acute myocardial infarction (4–8). During a continuous infusion of microbubbles, “guided” high mechanical index ultrasound impulses from a diagnostic transducer have improved microvascular flow in pigs with acute left anterior descending thrombotic occlusions (9). The term “guided” refers to the application of the high mechanical index impulses only when low mechanical index (MI) imaging indicates there are microbubbles present within the region of interest. Although platelet-targeted bubbles were shown to further improve the results, non-targeted microbubbles were also effective in improving microvascular flow, and are already commercially available. However, several pertinent questions and variables need to be examined prior to proceeding to clinical studies. First, what are the mechanisms of ultrasound and microbubble-induced improved myocardial blood flow? Secondly, what are the effects of underlying atherosclerosis? Previous animal models examining the effectiveness of ultrasound and microbubbles in this setting have been with histologically normal coronary arteries. Thirdly, could guided high mechanical index impulses from a three dimensional transducer achieve a similar result as the two dimensional impulses? This would avoid the requirement that one manually scan the risk area during the application of guided high mechanical index impulses. The purpose of this study was to examine the effects of guided high mechanical index impulses from a 3D transducer on microvascular and epicardial reflow in an established porcine model of acute coronary thrombosis, using non-targeted microbubbles that are similar to commercially available microbubbles, in a setting where the coronary arteries are either normal or atherosclerotic.
Methods
Animal Preparation/Protocol
This study was compliant with the guidelines of the Institutional Animal Care and Use Committee and the standards in the Guide for the Care and Use of Laboratory Animals. Animals were pre-anesthetized with an intramuscular mixture of Telazol (4.4mg/kg), ketamine (2.2 mg/kg), and xylazine (2.2 mg/kg), and then intubated. Following this, isoflurane inhaled anesthesia (induction at 4%, maintained at 1.0% to 1.8%) was administered. The percent oxygen mixture was kept at 24% during treatment periods in all pigs. Two femoral artery and venous catheters were placed for hemodynamic monitoring and infusions of microbubbles. An 8F guide catheter was introduced into the left main artery for digital angiograms and for balloon catheter insertion. Heart rate and oxygen saturation were also monitored throughout the entire experiment. Low-dose intravenous dobutamine (1 to 3 ug/kg per minute) was used to maintain systolic arterial pressure >80 mm Hg during the study protocol. Lidocaine boluses (40 mg IV followed by 20 mg IV boluses × 3) and a continuous lidocaine infusion (2 to 4 mg/min IV) were used in all animals to control arrhythmias.
Acute left anterior descending (LAD) thrombotic occlusions were created in 28 pigs. Two of these pigs died of refractory ventricular fibrillation before treatment could be initiated, leaving a total sample size of 26 pigs. In 12 of the pigs, pre-existing atherosclerotic lesions were created by a balloon injury in the left anterior descending on day zero, following by a high fat diet (15% Lard, 2% Cholesterol; Harlan Laboratories, Madison, WI) lasting 52 ± 21 days.
Coronary artery thrombotic occlusions were created in the 14 normal pigs by simulating the triad of Virchow (10). This consists of creating endothelial injury, stasis, and a hypercoagulable state. Endothelial injury was created by advancing a balloon catheter into into the left anterior descending, after the second diaganol branch, and inflating the balloon for 30 seconds three times at maximum diameters that were 110% of the measured coronary artery diameter. This balloon catheter was then withdrawn proximal to the injury site and partially inflated to reduce flow at the injury site. Then, small 0.1–0.2 milliliters of clotting venous blood from the pig were injected through the balloon catheter into the site of injury to create a hypercoagulable state.
Once angiographic LAD occlusion was documented, it had to persist for at least 20 minutes. If spontaneous recanalization occurred, small thrombus injections were again administered through the balloon catheter until persistent occlusion was observed. Following this, pigs had baseline measurements of heart rate, oxygen saturation, arterial blood pressure, wall thickening, myocardial blood flow (MBF) with radiolabeled neutron-activated microspheres, perfusion defect size at plateau intensity (ultimate infarct size) with contrast low mechanical index imaging, and ST segment elevation on 12 lead EKG. Subsequently, 650 milligrams of aspirin was administered per nasogastric tube, followed by an intravenous heparin bolus (80 mg/kg), and a bolus injection of ½ dose fibrinolytic agents (0.25 mg/kg Tenecteplase or 1mg/kg tissue plasminogen activator (Genetech)). The normal pigs were then randomized to receive either no additional treatment (n=7 that composed a control group; subsequently referred to as Group I) or a continuous intravenous infusion of MRX-801 with intermittent high mechanical index impulses applied whenever microbubbles were visualized within the risk area (subsequently referred to as Group II).
In the 12 atherosclerotic pigs, coronary artery thrombi were created at 52 ± 21 days following the day zero balloon injury using the same protocol described for Group I and II pigs. The second balloon injury occurred at the same location as the day zero balloon injury. Specific angiographic markers were used (e.g. location in distance from a diagonal branch) to ensure that the thrombus initiation site on day 50 was at the site of balloon injury at day zero. Following a 20 minute documented angiographic occlusion, pigs then received the same aspirin, heparin, ½ dose lytic agent (Tenecteplase or tissue plasminogen activator). Subsequently, the pigs received either no additional treatment (Group III), or the same MRX-801 infusion with intermittent high mechanical index impulses applied to the risk area (Group IV).
Ultrasound and Microbubble Protocol
The microbubbles used for all studies were a lipid encapsulated formulation (MRX-801; NuvOx Pharma; Tucson, AZ). These microbubbles have a diameter of 1.0±0.1 μm, with concentration of 1.5 to 3.0 × 1010/ml. The microbubble infusion for both therapeutic and diagnostic studies were prepared by diluting 2 ml of the MRX-801 in 100 ml of 0.9% saline and infusing at a rate of 2.5–3.0 ml/min.
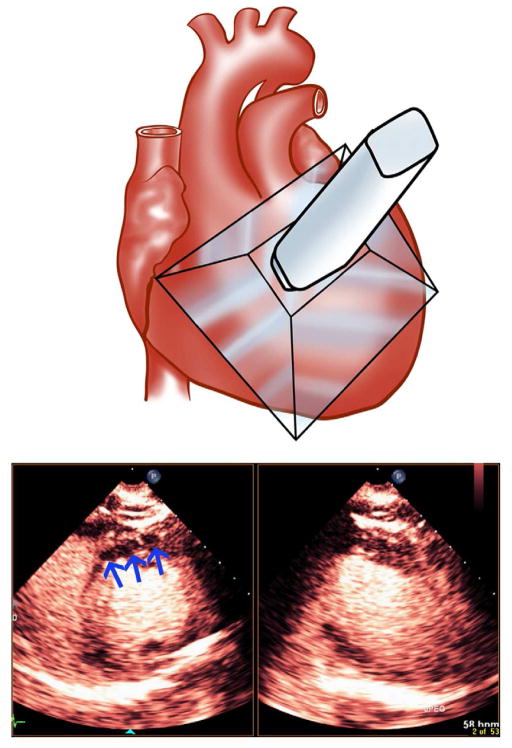
In pigs randomized to receive ultrasound, a real-time low mechanical index biplane image was obtained with a Matrix 1.6 Megahertz three dimensional transducer (Power Modulation at 0.2 mechanical index; Philips Healthcare), which permitted visualization of the perfusion defect size (Figure 1). A three dimensional array of high mechanical index impulses (1.2 mechanical index) was delivered from the same transducer at a frame rate of five Hertz for five seconds (total of 25 frames) after visualization of microbubbles within any portion of the peripheral or central portions of the risk area (defined by the extent of the wall thickening abnormality). The guided high MI impulses were applied for a time period that was considered sufficient to clear the myocardium (both normal and abnormal) of microbubbles, permitting the visual analysis of replenishment within the risk area and normal myocardial segments. Although the 25 frames of high MI impulses did occasionally result in some evidence of microbubble destruction within the left ventricular cavity, there was rapid reappearance of contrast within the cavity immediately following the return to low MI imaging. Risk area was divided into central and peripheral portions by arbitrarily dividing it into thirds, with the outer thirds being described as the peripheral portions. We observed no instances where, within 15 seconds, we did not observe at least some replenishment within at least the peripheral portion of the risk area. Treatments continued for 30 minutes. Venous samples for activated clotting times (ACTs) were obtained before initiation of treatment and at approximately 30 minutes into randomized treatments.
Figure 1.
Depiction of the transthoracic coverage of the 3D transducer used for the application of guided high mechanical index impulses following acute left anterior descending thrombotic occlusion. The blue arrows depict the hypoperfused zone identified with biplane low MI imaging following left anterior descending thrombotic occlusion.
Myocardial Blood Flow Measurements
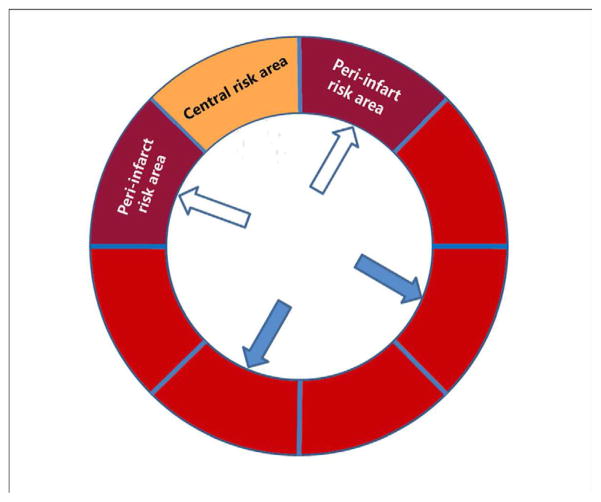
In order to determine how guided high mechanical index impulses and microbubbles affect peri-infarct MBF even in the absence of epicardial recanalization, MBF measurements were measured in Group I and II pigs using left atrial injections of neutron activated microspheres (15 micron diameter; Biopal; Worcester, MA) just prior to initiation of treatment, at 25 minutes into treatment, and at 60 minutes following completion of treatment. Following sacrifice, the excised heart was divided into eight subendocardial and sub-epicardial segments (Figure 2). MBF analysis from these eight segments demonstrate that there was a consistent segment with very low flow (corresponding to the central portion of the risk area), and two segments bordering on this central segment which had reduced flow, and hence we defined these as the peri-infarct regions. Peri-infarct MBF was defined as the ratio of averaged myocardial blood flow within the two bordering segments surrounding the segment with lowest blood flow divided by reference MBF within two normal segments in the lateral and inferolateral walls.
Figure 2.
The segmentation of the short axis of the left ventricle for myocardial blood flow measurements with radiolabelled microspheres. Peri-infarct flow was determined by the ratio of flow in the two peri-infarct zones (open arrows) divided by the normal zones (closed arrows).
Post Mortem Measurements
After the 90 minute angiograms and final MBF measurements, the pigs were sacrificed. In those pigs treated with high fat diets, the coronary arteries were dissected and analyzed with Hematoxylin/Eosin staining for presence of intra-coronary thrombus, dissection, and percent intimal hyperplasia.
Outcome Measurements
In all pigs, epicardial recanalization was assessed by angiography using left main coronary artery injections of five milliliters of iodinated contrast at 30, 60, and 90 minutes following initiation of treatment protocols. Assessments of flow in the left anterior descending were visually estimated by a blinded experienced reviewer using the TIMI criteria (11). Twelve lead EKG’s were obtained at baseline prior to treatment, and at 30, 60, and 90 minutes as well. Maximal ST segment elevation was compared at each time point. One pig in Group II was excluded from the ST segment recovery analysis because it did not exhibit ST deviation in any lead following angiographic occlusion of the LAD. Wall thickening within the central portion of the risk area on a two dimensional short axis view (mid- papillary muscle level) was computed off line by a blinded reviewer, who measured end-diastolic and end-systolic wall thickness prior to treatment, and at sixty minutes into treatment. Percent wall thickening was defined as the difference in thickness measurements divided by end-diastolic wall thickness × 100% (9). All measurements were made by an independent reviewer blinded to treatment protocol.
The risk area was approximated by defining the circumferential extent of the wall thickening abnormality (11). The effects of the guided high mechanical index impulses on ultimate infarct size were determined during continuous low mechanical index imaging and a continuous infusion of MRX-801 prior to initiation of treatment and at 90 minutes following treatment. To determine ultimate infarct size, planimetered measurements of end-systolic defect size (hypoperfused area) were determined at plateau intensity during the continuous infusion of microbubbles. This has been correlated with ultimate infarct size (12). All measurements were obtained by an experienced reviewer (SG) blinded to treatment assignment. MBF ratios in the peri-infarct zones (normalized to unaffected myocardium) were compared to baseline values at the end of the randomized treatment (30 minutes) and sixty minutes following completion of treatment.
Statistical Comparisons
Group I and II pig results were compared with each other, as were Group III versus Group IV. Comparisons of angiographic patency rates between the two treatment groups were made with a Chi Square test. Changes in percent wall thickening and ST segment resolution within groups were determined using paired t testing. Changes in peri-infarct radiolabelled MBF ratios, contrast echo-derived defect size at plateau intensity (ultimate infarct size) were also compared within groups using paired t testing. Inter- and intra-observer variabilities in perfusion defect size at plateau intensity were computed using a Bland Altman test.
Results
Hemodynamic and Histological Findings
Table 1 demonstrates arterial pressure, oxygen saturation, heart rate, left atrial pressures, and activated clotting time measurements at baseline and 90 minutes into treatment. No differences were observed in any of these parameters between Groups I and II, as well as between Groups III and IV. Median duration of thrombotic occlusion prior to treatment was 24 minutes (mean 26 ± 7 minutes), and was not different in the four groups (Table 1). In the atherosclerotic pigs, mean fasting cholesterol levels increased from 62 ± 28 mg/dl to 487 ± 216 mg/dl at 52 ± 21 days following initiation of the high cholesterol diet.
Table 1.
Hemodynamic Characteristics
| Group I | Group II | Group III | Group IV | |||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Heart rate (beat/minute) | 109 ± 17 | 111 ± 12 | 109 ± 23 | 98 ± 18 | 98 ± 16 | 103 ± 14 | 106 ± 23 | 100 ± 17 |
| Systolic arterial pressure (mmHg) | 107 ± 5 | 104 ± 13 | 113 ± 17 | 112 ± 9 | 104 ± 13 | 100 ± 8 | 107 ± 11 | 103 ± 10 |
| Diastolic arterial pressure (mmHg) | 73 ± 7 | 69 ± 12 | 76 ± 13 | 76 ± 10 | 68 ± 12 | 63 ± 12 | 74 ± 7 | 69 ± 10 |
| SpO2 (%) | 100 ± 0 | 99 ± 2 | 100 ± 1 | 99 ± 1 | 100 ± 0 | 100 ± 1 | 100 ± 1 | 99 ± 2 |
| Activated Clotting Time (second) | 104 ± 15 | *134 ± 16 | 100 ± 8 | *147 ± 28 | 107 ± 11 | *191 ± 19 | 108 ± 8 | *174 ± 28 |
p<0.01 compared to before treatment
Four of the pigs in the study required 360 Joules direct current cardioversion to convert ventricular fibrillation to sinus rhythm. Three pigs developed ventricular tachycardia which terminated spontaneously. The 40 mg IV bolus was administered to all pigs immediately after the onset of isolated ventricular depolarizations. Twenty milligram boluses (up to five) were given subsequent to this at 10–15 minute intervals, along with a 3–4 milligram per minute lidocaine drip.
Post-mortem histological examinations of the coronary arteries in Group IV pigs revealed eccentric plaques at the left anterior descending balloon injury site in five of the six pigs placed on a high fat diet, with stenosis severity ranging from 0–25% in two pigs, 26–50% in two pigs, and 50–75% in one pig. Focal areas of healed dissection in the LAD (from the day 1 balloon injury) were seen in three Group III pigs. Coronary artery histology were available in five of the six control Group III pigs. Two of the five exhibited either focal eccentric atherosclerotic lesions or intimal polymorphic leukocytes, and all five had thrombus at the site of the lesion. No Group III pigs had evidence of healed dissection.
Angiographic, Electrocardiographic, and Wall Thickening Measurements
Table 2 displays the angiographic. EKG, and WT recovery within the risk area following treatment in Groups I and II. Baseline TIMI scores were zero in all pigs prior to treatment. Angiographic recanalization rates and TIMI 3 flow were the same in the Group I and Group II pigs (2/7, or 29%, in each group) at 60 and 90 minutes into treatment. There were no differences in measured ST segment elevation or WT within the risk area prior to initiation of treatment in Group I and Group II (Table 2). However, Group II pigs had significant reductions in ST segment elevation already at 30 minutes following initiation of treatment. Wall thickening within the risk area improved only in the Group II pigs.
Table 2.
Wall thickening (WT), ST segment changes, and angiographic recanalization rates before and following treatment
| Group I | Group II | |
|---|---|---|
| WT | ||
| Baseline WT (%) | 3 ± 6 | 6 ± 5 |
| 60 minutes WT (%) | 5 ± 4 | 12 ± 6* |
| EKG | ||
| Baseline ST Elevation (mm) | 5.8 ± 2.0 | 7.4 ± 2.3 |
| 30 minutes ST Elevation (mm) | 5.1 ± 3.1 | 4.4 ± 2.5* |
| 60 minutes ST Elevation (mm) | 4.2 ± 2.1 | 3.9 ± 2.5* |
| 90 minutes ST Elevation (mm) | 3.7 ± 2.1* | 3.5 ± 2.0* |
| Angiographic recanalization | ||
| 30 min recanalization | 2 (29%) | 4 (57%) |
| 60 min recanalization | 2 (29%) | 2 (29%) |
| 90 min recanalization | 2 (29%) | 2 (29%) |
p <0.05 compared to Baseline
Table 3 displays the same data for the atherosclerotic pigs. Epicardial recanalization and TIMI 3 flow was present in all six Group IV pigs, compared to 3/6 for control Group III atherosclerotic pigs (p=0.09). Similar to Group II pigs, only pigs treated with guided high mechanical index impulses and microbubbles had recovery of ST segments, and improvements in wall thickening.
Table 3.
Comparisons of ST segment resolution, WT recovery, and angiographic recanalization rates in atherosclerotic pigs.
| Group III | Group IV | |
|---|---|---|
| WT | ||
| Baseline WT (%) | 7 ± 2 | 6 ± 7 |
| 60 minutes WT (%) | 11 ± 8 | 18 ± 13* |
| EKG | ||
| Baseline ST Elevation (mm) | 3.9 ± 1.6 | 4.6 ± 2.6 |
| 30 minutes ST Elevation (mm) | 3.0 ± 1.2 | 1.0 ± 1.2* |
| 60 minutes ST Elevation (mm) | 2.3 ± 0.9 | 0.6 ± 0.9* |
| 90 minutes ST Elevation (mm) | 2.6 ± 1.4 | 0.6 ± 0.9* |
| Angiographic recanalization | ||
| 30 minutes recanalization | 3 (50%) | 6 (100%) |
| 60 minutes recanalization | 3 (50%) | 6 (100%) |
| 90 minutes recanalization | 3 (50%) | 6 (100%) |
p <0.05 compared to Baseline
The total duration of high mechanical index exposure did not differ between the normal pigs and atherosclerotic pigs treated with ultrasound (14.8 ± 3.5 minutes for Group I and 13.7 ± 1.5 minutes Group III).
Myocardial Blood Flow Measurements
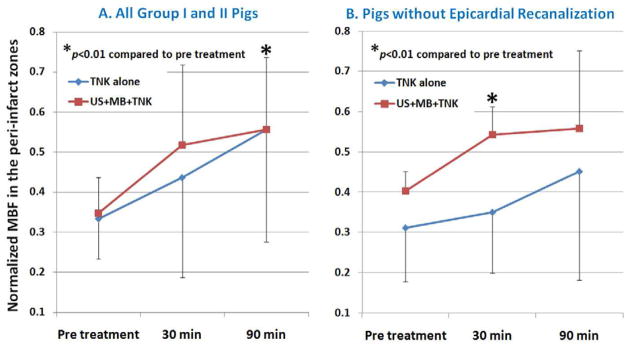
Microsphere-derived MBF measurements were obtained in six normal Group I pigs and five Group II pigs. Figure 3a demonstrates the changes in peri-infarct MBF ratios at rest, 30, and 90 minutes into treatment in all Group I and II pigs, while Figure 3b demonstrates the changes in pigs where epicardial recanalization did not occur. There were no differences in the peri-infarct MBF ratios at rest. However, at the end of the ultrasound treatment (30 minutes), there was a consistent increase in MBF in the peri-infarct zones in Group II pigs, including those who did not have epicardial recanalization (Figure 3b).
Figure 3.
Changes in peri-infarct MBF in Group I and Group II pigs before and after randomized treatment to either tenecteplase (TNK) alone versus TNK with diagnostic ultrasound and intravenous non-targeted microbubbles (Group II). The improvement in peri-infarct MBF occurred was seen at 30 minutes into treatment, even in those without epicardial recanalization (Figure 3b).
Ultimate Infarct Size by Myocardial Contrast Echocardiography
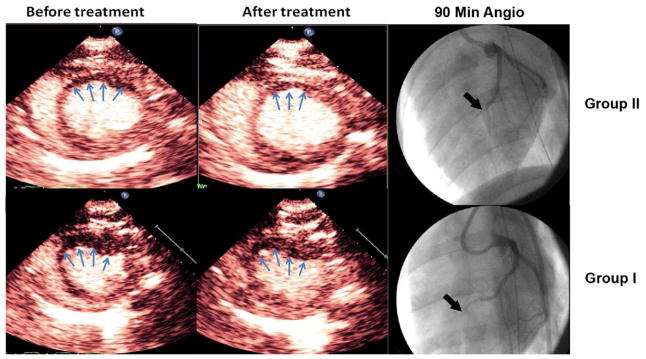
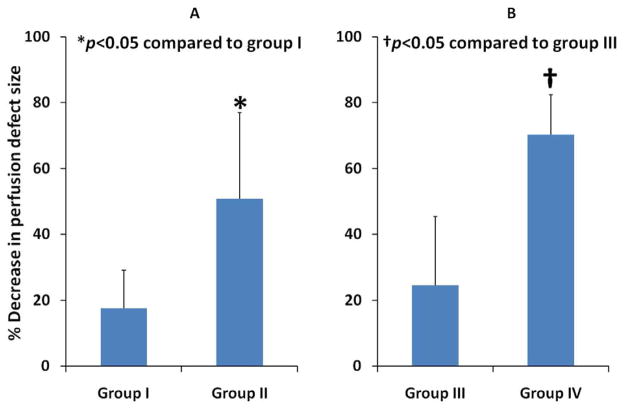
Mean planimetered defect size at the plateau intensity, prior to treatment, was smaller in the Group III and IV pigs (2.7 ± 0.3 cm2 for Group I, 2.7 ± 0.3 cm2 for Group II, 1.55 ± 0.36 cm2 for Group III, and 1.63 ± 0.52 cm2 for Group IV; p<0.05; Group III and IV compared to other groups). Both Group II and Group IV pigs had significant decreases in plateau defect size after treatment (p<0.001 for both groups compared to their respective control groups). Defect size, after therapy, was significantly smaller in Group II pigs (2.2 ± 1.3 cm2 for Group I, 1.6 ± 0.6 cm2 for Group II; p=0.005). Although perfusion defect sizes prior to treatment were similar in the atherosclerotic pigs, perfusion defect size following treatment was significantly smaller in Group IV pigs (0.53 ± 0.43 cm2 Group IV versus 1.12 ± 0.37 cm2 Group IIII; p=0.02). Figure 4 displays an example in the reduction in plateau myocardial contrast defect size at 90 minutes into treatment in a Group I versus a Group II pig. Figure 5 depicts examples of the reduction in defect size in hypercholesterolemic atherosclerotic Group IV pigs. Figures 6a and 6b display the mean reductions in planimetered plateau defect size in the four groups. Of note, significant reductions in defect size were also observed in the five Group II pigs that did not have epicardial recanalization (p =0.03 compared to Group I pigs). These reductions occurred along the peripheral margins of the risk area, corresponding to the peri-infarct zones where radiolabeled microspheres detected improved MBF.
Figure 4.
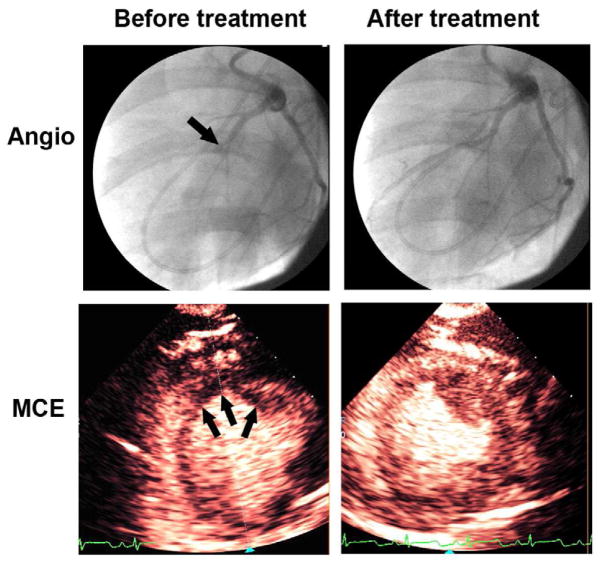
Changes in plateau intensity defect size (ultimate infarct size) in a normal pig treated with 3D/MB with ½ dose TNK (Group II) versus TNK alone (Group I). Note the reduction in contrast defect size (arrows) in the 3D/MB treated pig (top panels), compared to the pig treated with TNK alone (arrows; bottom panels).
Figure 5.
An example of the reduction in plateau contrast defect size in a Group IV pig following ½ dose TNK and 3D/MB. Note the near complete resolution of the perfusion defect that was evident prior to treatment (arrows), signifying a marked reduction in ultimate infarct size. The top panels demonstrate the coronary angiogram before and after treatment, in which epicardial recanalization occurs (arrows).
Figure 6.
The improvement in planimetered defect size at plateau contrast intensity (ultimate infarct size) in the Group II (Figure 6a) and Group IV pigs treated with 3D/MB (Figure 6b). The improvement in defect size in Group II pigs was observed even without epicardial recanalization in five of the seven pigs treated.
Discussion
This study demonstrates that guided high mechanical index impulses from a 3D diagnostic ultrasound probe, combined with intravenous non-targeted microbubbles, can improve microvascular flow and function within the risk area following acute LAD thrombotic occlusions in both normal and hypercholesterolemic pigs with underlying coronary atherosclerosis. The beneficial effects of the guided high mechanical index impulses and microbubbles appeared to be the greatest in pigs with underlying atherosclerosis, resulting in higher epicardial recanalization rates and greater reductions in ultimate infarct size. The improvements in peri-infarct MBF and reductions in ultimate infarct size occurred independent of epicardial recanalization, suggesting that ultrasound-induced improvements in microvascular flow during STEMI are not dependent on restoration of anterograde flow in the thrombosed coronary artery.
Previously it has been shown that guided high mechanical index impulses from a two-dimensional transducer and either non-targeted or glycoprotein 2b/3a targeted microbubbles improve microvascular reperfusion following LAD thrombotic occlusion induced in otherwise normal pigs (9). However, the mechanism for improved microvascular flow was not clear. One would have to use high mechanical index impulses to examine myocardial blood flow changes with contrast echo, since this requires analyzing contrast replenishment kinetics following the high mechanical index impulses. However, these high mechanical index impulses are potentially therapeutic, and thus we cannot use them in a control group. This is why in the present study we had to have an independent measure of myocardial blood flow in the normal pigs (where epicardial recanalization rates were low), to understand how myocardial blood flow within the risk area was affected by the high mechanical index impulses. The peri-infarct segments, which are the collateral-dependent zones of the risk area (13,14), exhibited sustained increases in MBF when guided high mechanical index impulses were applied during a continuous infusion of microbubbles even in pigs who did not have epicardial recanalization, indicating collateral flow was induced by ultrasound-mediated destruction of the microbubbles.
Ultrasound induced improvements in myocardial perfusion downstream from an occlusion have been demonstrated by others. Siegel, et al. found that low frequency ultrasound can increase capillary cross sectional area within acutely ischemic myocardium downstream from a coronary ligation (15), resulting in improvements in tissue pH that persisted even after removal of the ultrasound. This beneficial effect of ultrasound was reversed with nitric oxide inhibitors, similar to what has also been observed with ultrasound in ischemic skeletal muscle (16). Other potential explanations for improved microvascular flow in the absence of epicardial recanalization may have been ultrasound-induced coronary artery vasodilatation. Low frequency ultrasound has induced transient increases in coronary vessel diameters (17). In the setting of arterial thrombotic occlusions, low mechanical index ultrasound imaging studies have shown that acute arterial thrombi still have small micro-channels that cannot be seen with angiography, but which permit microbubbles to penetrate the interior of a thrombus (18). Thus, an increase in coronary diameter induced by ultrasound could have improved local delivery of both microbubbles and lytic agent through these micro-channels, resulting in improved flow in the downstream microvasculature, even without angiographic evidence of coronary artery reperfusion.
The effectiveness of guided high mechanical index impulses and non-targeted microbubbles in restoring epicardial coronary flow in otherwise normal pigs was not different than what has been seen previously (9). However, in pigs with hypercholesterolemia and underlying atherosclerotic plaque, ultrasound and non-targeted microbubbles were also effective at improving epicardial recanalization rates. There are several potential explanations for this. First, increased lipid levels have been associated with higher endogenous levels of tissue plasminogen activator (19). In the presence of these higher endogenous lytics, the shearing of the thrombus by cavitation induced by the guided high mechanical index impulses could have increased the exposure of the plasminogen activator to fibrin embedded within the thrombus. A second potential explanation is that the increased plaque volume within the vessel may have reduced the size of thrombus required to occlude the coronary artery. Thus, less thrombus dissolution may have been required to restore epicardial flow. Thirdly, since the pigs on an atherosclerotic diet were larger than the normal Group I or II pigs after their high cholesterol diet, their weight-adjusted doses of both lytic agents and heparin were higher, which may have improved their efficacy in dissolving the thrombus. This is why a control group that underwent the same atherosclerotic diet and balloon injury protocol was added (Group III). This group, although receiving higher weight adjusted doses of fibrinolytic agents and heparin, still had only a 50% epicardial recanalization rates and less improvement in microvascular volume (Table 3).
Atherosclerotic pigs had a smaller baseline perfusion defect size prior to treatment when compared to normal pigs, indicating collateral flow was more developed in the atherosclerotic pigs. Despite this, improvements in ultimate infarct size and reductions in ST segments were only seen in pigs receiving ultrasound and microbubbles. Clinically this may have significance, since both ST segment resolution and improved blood flow to the risk area are associated with reduced left ventricular remodeling (20).
Limitations of The Study
We only used a thirty minute ultrasound treatment period in the current study, to match what had been attempted in previous animal studies. To our knowledge, no one has used extended microbubble infusions beyond a 30 minute treatment period in this acute myocardial infarction model. It is possible that longer treatment periods may further improve microvascular outcome. In the clinical setting, this may be helpful since microvascular emboli would still be expected to occur after primary percutaneous interventions (21). Further study is needed to explore what effects longer treatment times have on microvascular flow, and how this affects outcome in the setting of primary percutaneous interventions.
Although the three dimensional guided high MI impulses, in the presence of microbubbles, were successful in restoring both microvascular and epicardial blood flow in this study, we did not directly compare our results with what could have been achieved with a two dimensional transducer. Nonetheless, the three dimensional transducer has practical advantages, in that the entire volume of the risk area can be covered during the high MI impulses, without the need to manually move the probe during the high MI applications.
Conclusions
The utilization of guided high mechanical index impulses from a 3D transducer improves microvascular flow rates following STEMI in pigs. The improvement in microvascular flow was in the peri-infarct segments, and occurred independent of epicardial recanalization. Greater reductions in perfusion defect size and epicardial recanalization rates are observed in the setting of hypercholesterolemia and underlying plaque.
This study utilized intravenous non-targeted microbubbles that are very similar to what is already commercially available, and guided 3D high mechanical index impulses from a clinical diagnostic ultrasound system. The three dimensional probe used in this study had a practical advantage in that high mechanical index impulses could be delivered to the entire risk area without probe manipulation.
It has been shown that 65% of patients treated with state-of-the-art percutaneous coronary interventions in acute STEMI have persistent microvascular obstruction (21). Our study shows this microvascular obstruction could be reduced significantly with the addition of guided high mechanical index impulses and intravenous microbubbles, thus preventing the adverse remodeling that leads to reductions in left ventricular ejection fraction and development of congestive heart failure. This safe and effective therapy, therefore, could not only prevent significant complications in patients with STEMI, but also reduce the enormous costs associated with these complications.
Acknowledgments
This work was supported by the Nation Heart, Lung, and Blood Institute at the National Institutes of Health [2R44 HL071433-02], in part by the National Institutes of Health (NIH/NIBIB R01 EB009050-03); and by Theodore Hubbard Foundation, Omaha, NE; with instrumentation support from Philips Medical Solutions.
Footnotes
Conflict of Interest
Thomas R Porter, MD has the following conflict of interests to disclose:
1) Grant Support – Lantheus Medical Imaging
2) Grant Support – NuvOx Pharma Inc.
3) Equipment Support – Philips Medical Solution
4) Consultant – Lantheus Medical Imaging
Evan Unger, MD has the following conflict of interest to disclose:
1) Shareholder – NuvOx Pharma Inc.
Terry Matsunaga, Pharm D has the following conflict of interest to disclose:
1) Shareholder – NuvOx Pharma Inc.
All other authors have no conflict of interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakuma T, Sari I, Goodman CN, Lindner JR, Klibanov AL, Kaul S. Simultaneous integrin αVβ3 and glycoprotein IIb/IIIa inhibition causes reduction in infarct size in a model of acute coronary thrombosis and primary angioplasty. Cardiovasc Res. 2005;66:552–561. doi: 10.1016/j.cardiores.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Sakuma T, Leong-Poi H, Fisher NG, Goodman NC, Kaul S. Further insights into the no-reflow phenomenon after primary angioplasty in acute myocardial infarction: the role of microthromboemboli. J Am Soc Echocardiogr. 2003;16:15–21. doi: 10.1067/mje.2003.44. [DOI] [PubMed] [Google Scholar]
- 3.Tillmanns H, Leinberger H, Neumann FJ, Steinhausen M, Parekh N, Zimmerman R, et al. Myocardial microcirculation in the beating heart – In vivo microscopic studies. In: Spaan JAE, Bruschke AVG, Gittenberger-de Groot AC, editors. Coronary Circulation. Dordrecht, The Netherlands: Martin Nijhoff Publishers; 1987. pp. 89–94. [Google Scholar]
- 4.Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, et al. Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 5.Khumri TM, Nayyar S, Idupulapati M, Magalski A, Stoner CN, Kusnetzky LL, et al. Usefulness of myocardial contrast echocardiography in predicting late mortality in patients with anterior wall acute myocardial infarction. Am J Cardiol. 2006;98:1150–1155. doi: 10.1016/j.amjcard.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 6.Kaul S. Evaluating the “no reflow” phenomenon with myocardial contrast echocardiography. Basic Res Cardiol. 2006;101:391–399. doi: 10.1007/s00395-006-0618-z. [DOI] [PubMed] [Google Scholar]
- 7.Hayat SA, Senior R. Myocardial contrast echocardiography in ST elevation myocardial infarction: ready for prime time. Eur Heart J. 2008;29:299–314. doi: 10.1093/eurheartj/ehm621. [DOI] [PubMed] [Google Scholar]
- 8.Galiuto L, Garramone B, Scara A, Rebuzzi AG, Crea F, La Torre G, et al. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the multicenter AMICI study. J Am Coll Cardiol. 2008;51:552–559. doi: 10.1016/j.jacc.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 9.Xie F, Lof J, Matsunaga T, Zutshi R, Porter TR. Diagnostic ultrasound combined with glycoprotein Iib/IIIa targeted microbubbles improve microvascular recovery following acute coronary thrombotic occlusions. Circulation. 2009;119:1358–1360. doi: 10.1161/CIRCULATIONAHA.108.825067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol. 2008;143:180–90. doi: 10.1111/j.1365-2141.2008.07323.x. [DOI] [PubMed] [Google Scholar]
- 11.The TIMI Study Group. The thrombolysis in myocardial infarction (TIMI) trial. Phase 1 findings. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 12.Coggins MP, Sklenar J, Le DE, Wei K, Lindner JR, Kaul S. Non-invasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateral-derived myocardial blood flow. Circulation. 2001;104:2471–2477. doi: 10.1161/hc4501.098954. [DOI] [PubMed] [Google Scholar]
- 13.Le DE, Jayaweera AR, Wei K, Coggins MP, Lindner JR, Kaul S. Changes in myocardial blood volume over a wide range of coronary driving pressures: role of capillaries beyond the autoregulatory range. Heart. 2004;90:1199–1205. doi: 10.1136/hrt.2003.020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter TR, Li S, Kilzer K, Deligonul U. Effect of significant two-vessel versus one-vessel coronary artery stenosis on myocardial contrast defects observed with intermittent harmonic imaging after intravenous contrast injection during dobutamine stress echocardiography. J Am Coll Cardiol. 1997;30:1399–406. doi: 10.1016/s0735-1097(97)00290-8. [DOI] [PubMed] [Google Scholar]
- 15.Siegel RJ, Suchkova VN, Miyamoto T, Luo H, Baggs RB, Neuman Y, et al. Ultrasound energy improves myocardial perfusion in the presence of coronary occlusion. J Am Coll Cardiol. 2004;44:1454–1458. doi: 10.1016/j.jacc.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 16.Sukova VN, Baggs RB, Francis CW. Effect of 40 kHz ultrasound on acute thrombotic ischemia in a rabbit femoral artery thrombosis model: enhancement of thrombolysis and improvement in capillary muscle perfusion. Circulation. 2000;101:2296–2301. doi: 10.1161/01.cir.101.19.2296. [DOI] [PubMed] [Google Scholar]
- 17.Iida K, Luo H, Kohsuke H, Akima T, Shah PK, Naqvi TZ, Siegel RJ. Noninvasive low-frequency ultrasound energy causes vasodilation in humans. J Am Coll Cardiol. 2006;48:532–7. doi: 10.1016/j.jacc.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Xie F, Lof J, Everbach C, He A, Bennett RM, Matsunaga T, et al. Treatment of acute intravascular thrombi with diagnostic ultrasound and intravenous microbubbles. J Am Coll Cardiol Img. 2009;2:511–518. doi: 10.1016/j.jcmg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Geppert A, Graf S, Beckmann R, Hornykewycz S, Schuster E, Binder BR, et al. Concentration of endogenous tPA antigen in coronary artery disease. Relation to thrombotic events, aspirin treatment, hyperlipidemia, and multivessel disease. Arterioscler Thomb Vasc Biol. 1998;18:1634–42. doi: 10.1161/01.atv.18.10.1634. [DOI] [PubMed] [Google Scholar]
- 20.Funaro S, La Torre G, Madonna M, Galiuto L, Scara A, Labbadia A, et al. Incidence, determinants, and prognostic value of reverse left ventricular remodeling after primary percutaneous intervention: results of acute myocardial infarction contrast imaging (AMICI) multicenter registry. European Heart J. 2009;30:566–75. doi: 10.1093/eurheartj/ehn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial non-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]