Dear Sirs,
Haemostasis requires fibrinogen conversion to fibrin and formation of a stable fibrin network. Fibrin network properties, including fibre thickness, branchpoint density, fibre density, mechanical stability, porosity, and resistance to lysis can differentiate plasma clots of healthy individuals from those with haemostatic or thrombotic disorders. Plasma from patients with a bleeding history produces thick, minimally-branched fibres in coarse, deformable networks that are highly susceptible to lysis, whereas plasma from patients with a personal or family history of thrombosis produces thin, highly-branched fibres in impermeable, rigid networks that are relatively resistant to fibrinolysis (1).
Most previous studies have examined fibrin networks produced in the absence of flow or cells. Networks formed under these conditions show an isotropic distribution of fibres with relatively uniform diameters. However, although reduced flow promotes fibrin deposition (2), it is unlikely that blood flow ever fully stops in vivo, even under “stasis”. Fibrin formation studied in systems modelling venous (10–100 s−1) or arterial (500–1,500 s−1) shear shows orientation of fibres along flow vectors (3). Few studies, however, have examined fibrin formation under very low flow rates expected during extravasation of blood into extravascular tissue factor (TF)-rich tissues. The impact of flow on fibrin morphology under these conditions is not known.
To mimic extravascular TF-bearing cells, immortalised human dermal fibroblasts (NHF1-hTert) were cultured on sterile Thermanox cover slips (Miles Laboratories, Elkhart, IN, USA) to ~90% confluence and washed in 10 mM sodium phosphate pH 7.4, 150 mM NaCl. “Stasis clots” were formed on these cells by incubating 150 µl re-calcified (10 mM, final) contact-inhibited, normal-pooled, platelet-free plasma (PFP) (4) with fibroblasts for 10 minutes (min). “Flow clots” were formed by pumping (0.1 ml/min) re-calcified PFP over fibroblasts for 10 min using a Genie Syringe Pump (Kent Scientific Corporation, Torrington, CT, USA) with the cover slip at a ~30° decline to allow plasma to slowly flow over fibroblasts and model haemostatic injury. For both conditions, clots were left still for 10 min, prepared for scanning electron microscopy (5), and viewed on a Zeiss Supra 25 Field Emission Scanning Electron Microscope (Carl Zeiss, Thorwood, NY, USA). Fibrin angular distribution was analysed using Image J (v1.41o, National Institutes of Health) by placing a random grid of two-pixel crosses on images (~48–54 crosses/image) and using the line tool to measure fibres intersecting cross midpoints. Raw orientation data were adjusted to reflect fibre angle magnitude relative to flow direction, collapsing angles to 0°–90°. A Chi Square Goodness of Fit test was used to test for normality of residuals in fibre angular distribution measurements. Fibre diameters were measured as described (6) and compared using a Mann-Whitney U Test.
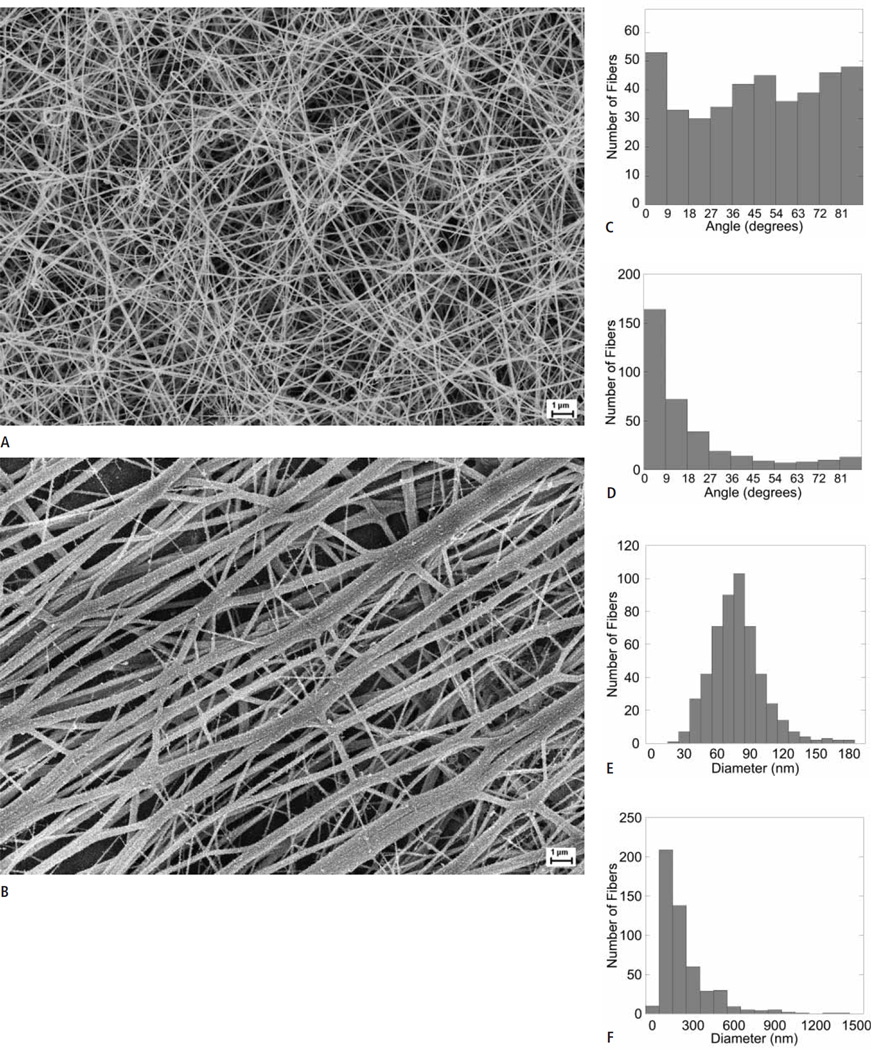
Networks formed under stasis exhibited the characteristic isotropic fibre distribution seen in previous studies (►Fig. 1A, C). In contrast, networks formed under flow showed anisotropy, with fibres significantly (p<10−6) aligned parallel to flow vectors. Fibres in “flow clots” exhibited peak angles <10° relative to the line of flow (0°) (Fig. 1B, D).
Figure 1. Flow significantly influences fibrin structure.
A, B)Representative scanning electron micrographs of networks formed under stasis (A) and flow (B) (n = 3). Clots were imaged at 49,680X, with a 10 mm working distance and a 5 kV accelerating voltage. Scale bar = 1 µm. C, D)Angular distribution measurements indicating the isotropic network produced under stasis (C) and anisotropic network produced under flow (D). Histograms (E and F) indicating significantly skewed distribution patterns and thicker diameter measurements of fibres present in clots produced under flow (F) compared to those produced during stasis (E). Note the different x-axis in E and F.
Consistent with prior studies, the diameter distribution of fibres formed under stasis was Gaussian, with a mean of 79 ± 5.7 nm (Fig. 1E). In contrast, “flow clots” showed a prominent network of thick fibres, as well as fibre “aggregates” in which multiple individual fibres were coalesced into bundles, several of which had total diameters as large as 1 µm. These large bundles were treated as single “fibres” in diameter measurements. Clots formed under flow also contained a minor network of thin fibres that connected individual thick fibres (Fig. 1B). The diameter distribution of fibres formed in flow was asymmetrical with a strong right skew (Fig. 1F) and mean and median diameters of 226.1 ± 180.5 and 168.8 nm, respectively. “Flow clots” demonstrated significantly (p<0.001) thicker fibres than “stasis clots,” with the thickest fibres parallel to flow vectors and finer fibres generally perpendicular to flow lines (Fig. 1B).
These findings have significant implications for polymerisation kinetics, mechanical stability, and fibrinolytic susceptibility of clots formed in flowing blood in both extravascular and intravascular settings. In the current model of fibrin formation, thrombin converts fibrinogen to fibrin via protofibril formation, lateral aggregation of protofibrils to fibres, and formation of a branched fibrin network (7, 8). Flow/shear modulates the kinetics of both fibrin monomer formation and polymerisation (9). Increasing shear from 10–100 s−1 decreases fibre formation by depleting monomer below a necessary threshold. However, increasing thrombin generation or decreasing shear increases local fibrin monomer concentrations, allowing formation of protofibrils and subsequently, fibrin fibres (9). During stasis, lateral association of protofibrils would presumably occur via interactions arising from the proximity of growing protofibrils and fibres and/or motion within the network (8). Our data show low flow promotes formation of thick fibre bundles, suggesting flow-induced alignment of growing fibres enhances lateral associations.
Previous studies have correlated fibrin structure with network mechanical properties (6, 10–12). Most studies have examined isotropic clots made under stasis; however, the highly anisotropic clots observed in this report would have mechanical properties that differ dramatically from isotropic clots (13). The hydrodynamic drag forces on individual fibres and shear stresses affecting the entire clot would be optimally resisted by fibres oriented along flow lines. Moreover, these fibres would undergo tensile stretching, rather than bending, under flow-induced stresses. Thus, unlike isotropic networks in which softer bending modes of fibres determine the low strain shear stiffness of the clot, stiffness in clots formed under flow would be determined by tensile stiffness of thick fibres aligned with flow. Since fibrin segments exhibit higher stiffness under tensile stretching than in bending (14), networks in which thick fibres are predominantly aligned along flow vectors would be more resistant to deformation than isotropic networks formed under stasis.
Fibre diameter and network density play significant roles in clot dissolution (15). Compared to thin fibres, thick fibres support faster plasmin generation rates. Plasmin lyses fibrin via laterally transecting individual fibres. Thin fibres lyse faster than thick fibres; however, coarse networks of thick fibres lyse faster than tight networks of thin fibres (16). Our data suggest networks formed under static conditions – which exhibit a relatively homogeneous distribution of fibre diameters – would support uniform plasmin generation and lysis throughout the clot. In contrast, in heterogeneous networks produced under flow, thick fibres aligned with flow would support rapid plasmin generation but lyse more slowly, whereas thin fibres running perpendicular to streamlines would support lower plasmin generation but lyse more rapidly, causing uneven lysis within the clot. Additionally, visual inspection indicated larger pores in clots formed under flow, suggesting cells would more easily penetrate clots produced under flow and further influence lysis patterns.
Together, these effects of flow on fibrin formation and mechanical and fibrinolytic stability would be expected to have significant consequences for clot quality in vivo. Structural features that destabilise the fibrin network may increase bleeding risk but decrease thrombosis. Microenvironments of laminar flow, turbulent flow, or stasis during clotting may promote structural heterogeneity that influences cell migration into the wound during healing of haemostatic clots or cause embolisation of intravascular thrombi. Although fibre diameter and other parameters differ in clots from patient plasmas versus controls, these parameters may be more or less discernable in clots formed under flow. Finally, both platelets and factor XIII may uniquely modulate fibrin characteristics under flow (17, 18). Because techniques typically used to evaluate clots (i.e. turbidity, thrombelastography) are not performed on anisotropic networks, these technologies have not identified influences of flow on fibrin quality. Development of novel technologies may be warranted for these assessments. In sum, our findings demonstrate significant effects of flow on both fibrin fibre and network characteristics, establish flow as an important component of not only thrombosis, but also haemostatic clot formation, and identify important avenues of investigation for future research.
Acknowledgements
We thank Dr. Susan Lord for her thoughtful contributions. This study was supported by research funding from the NIH (R01HL094740 to ASW, T32ES007017 to RAC, P41EB002025 to Dr. Richard Superfine/MRF), the Gustavus and Louise Pfeiffer Research Foundation (ASW), and the National Hemophilia Foundation (ASW).
References
- 1.Wolberg AS, Aleman MM. Influence of cellular and plasma procoagulant activity on the fibrin network. Thromb Res. 2010;125:S35–S37. doi: 10.1016/j.thromres.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartner HR. The role of blood flow in platelet adhesion, fibrin deposition, and formation of mural thrombi. Microvasc Res. 1973;5:167–179. doi: 10.1016/0026-2862(73)90069-1. [DOI] [PubMed] [Google Scholar]
- 3.Wielders SJ, Broers J, ten Cate H, et al. Absence of platelet-dependent fibrin formation in a patient with Scott syndrome. Thromb Haemost. 2009;102:76–82. doi: 10.1160/TH08-11-0719. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RA, Overmyer KA, Bagnell CR, et al. Cellular procoagulant activities dictate clot structure and stability as a function of distance from the cell surface. Arterio Thromb Vasc Biol. 2008;28:2247–2254. doi: 10.1161/ATVBAHA.108.176008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolberg AS, Allen GA, Monroe DM, et al. High dose factor VIIa enhances clot stability in a model of hemophilia B. Brit J Haematol. 2005;131:645–655. doi: 10.1111/j.1365-2141.2005.05820.x. [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Mockros LF, Weisel JW, et al. Structural origins of fibrin clot rheology. Biophys J. 1999;77:2813–2826. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisel JW, Phillips GN, Jr, Cohen C. A model from electron microscopy for the molecular structure of fibrinogen and fibrin. Nature. 1981;289:263–267. doi: 10.1038/289263a0. [DOI] [PubMed] [Google Scholar]
- 8.Chernysh IN, Weisel JW. Dynamic imaging of fibrin network formation correlated with other measures of polymerization. Blood. 2008;111:4854–4861. doi: 10.1182/blood-2007-08-105247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neeves KB, Illing DA, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98:1344–1352. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janmey PA, Amis EJ, Ferry JD. Viscoelastic properties of fibrin clots in large shearing deformations. J Rheology. 1982;26:599–600. [Google Scholar]
- 11.Gerth C, Roberts WW, Ferry JD. Rheology of fibrin clots. II. Linear viscoelastic behavior in shear creep. Biophys Chem. 1974;2:208–217. doi: 10.1016/0301-4622(74)80046-3. [DOI] [PubMed] [Google Scholar]
- 12.Collet JP, Allali Y, Lesty C, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26:2567–2573. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- 13.Namani R, Wood MD, Sakiyama-Elbert SE, et al. Anisotropic mechanical properties of magnetically aligned fibrin gels measured by magnetic resonance elastography. J Biomech. 2009;42:2047–2053. doi: 10.1016/j.jbiomech.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collet JP, Shuman H, Ledger RE, et al. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci USA. 2005;102:9133–9137. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisel JW, Litvinov RI. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6:161–180. doi: 10.2174/187152508784871963. [DOI] [PubMed] [Google Scholar]
- 16.Collet JP, Park D, Lesty C, et al. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterio Thromb Vasc Biol. 2000;20:1354–1361. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- 17.Collet JP, Montalescot G, Lesty C, et al. A structural and dynamic investigation of the facilitating effect of glycoprotein IIb/IIIa inhibitors in dissolving platelet-rich clots. Circ Res. 2002;90:428–434. doi: 10.1161/hh0402.105095. [DOI] [PubMed] [Google Scholar]
- 18.Mutch NJ, Koikkalainen JS, Fraser SR, et al. Model thrombi formed under flow reveal the role of factor XIII-mediated cross-linking in resistance to fibrinolysis. J Thromb Haemost. 2010;8:2017–2024. doi: 10.1111/j.1538-7836.2010.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]