Abstract
Rationale
Low aerobic exercise capacity is a powerful predictor of premature morbidity and mortality for healthy adults as well as those with cardiovascular disease For aged populations, poor performance on treadmill or extended walking tests indicates closer proximity to future health declines. Together, these findings suggest a fundamental connection between aerobic capacity and longevity.
Objectives
Through artificial selective breeding, we developed an animal model system to prospectively test the association between aerobic exercise capacity and survivability (aerobic hypothesis).
Methods and Results
Laboratory rats of widely diverse genetic backgrounds (N:NIH stock) were selectively bred for low or high intrinsic (inborn) treadmill running capacity. Cohorts of male and female rats from generations 14, 15 and 17 of selection were followed for survivability and assessed for age-related declines in cardiovascular fitness including maximal oxygen uptake (VO2max), myocardial function, endurance performance, and change in body mass. Median lifespan for low exercise capacity rats was 28-45% shorter than high capacity rats (hazard ratio, 0.06; P<.001). VO2max, measured across adulthood was a reliable predictor of lifespan (P<.001). During progression from adult to old age, left ventricular myocardial and cardiomyocyte morphology, contractility, and intracellular Ca2+ handling in both systole and diastole, as well as mean blood pressure, were more compromised in rats bred for low aerobic capacity. Physical activity levels, energy expenditure (VO2), and lean body mass were all better sustained with age in rats bred for high aerobic capacity.
Conclusions
These data obtained from a contrasting heterogeneous model system provide strong evidence that genetic segregation for aerobic exercise capacity can be linked with longevity and useful for deeper mechanistic exploration.
Keywords: Exercise capacity, longevity, cardiomyocyte function, rat models, aging
Introduction
Exercise capacity is a strong indicator of early morbidity and mortality1-8. For both healthy adults and those with cardiovascular disorders, low exercise capacity is a stronger predictor of decreased survival relative to other established risk factors, such as smoking, diabetes or hypertension7. In elders (adults 60 years or older), a higher capacity to perform a maximal treadmill exercise test to volitional exhaustion, a direct correlate of maximal oxygen consumption, is associated with reduction in all-cause mortality, independent of adiposity level9. For aged humans (>70 years old), a poor performance on an extended walking test positively correlates with closer proximity to future health declines and mortality10. The proposed genetic advantage associated with the exceptionally long-lived and their offspring, point to a lower prevalence and age of onset for cardiovascular disease11. These and several other studies provide support of a mechanistic theory that the diminished capacity for energy transfer of oxygen at all levels of biological organization underlies complex disease, accelerated aging, and diminished longevity (aerobic hypothesis)12. The broad premise between aerobic energy capacity and longevity is attractive because it has the power to explain the functional alterations that accumulate systematically across lifespan and influence essentially every intermediate phenotype within complex disease13.
To test this hypothesis beyond associational evidence we sought to develop a meaningful animal model. Aerobic capacity, as estimated from maximal oxygen consumption (VO2max) or an endurance run to exhaustion, is the summation of intrinsic (i.e., inborn) factors and the effects accrued in response to physical activity or training. As an initial strategy, we set out to explore the perhaps more direct factor of inheriting a low or high intrinsic aerobic phenotype. By selective breeding, we developed heterogeneous populations of laboratory rats that widely segregate for maximal treadmill running capacity as an animal model system (referred to as low capacity runners (LCR) and high capacity runners (HCR)14. As applied here, divergent selection from an outbred population is a powerful tool because it prospectively tests the hypothesis that an inherited capacity for oxygen metabolism sets a divide for longevity. If supported, the resulting rat lines provide unique contrasting heterogeneous (non-inbred) models for closer translational exploration of a mechanistic causation for aging.
As young adults (<6 months), LCR rats develop cardiovascular risk factors consistent with the metabolic syndrome including large gain in visceral adiposity, increased blood pressure, dyslipidemia, endothelial dysfunction occurring within carotid arteries, and insulin resistance15. Evaluation of the genotype-phenotype in LCR and HCR rats confirms that differences in gene expression networks in skeletal muscle related to oxidative phosphorylation and fatty acid metabolism correlate significantly with several exercise capacity and disease risk phenotypes such as physical activity levels, serum high density lipoproteins, and mitochondrial structure16. Here we provide data from the first sets of survivability studies and old-age comparisons between LCR and HCR rats to test the hypothesis that an intrinsic aerobic capacity sets an initial divide for longevity and age-related morbidity.
Methods
For detailed description, see Online Methods in Supplement Material available at http://circres.ahajournals.org
Rat Models of Aerobic Exercise Capacity
Rat founder population was from the genetically heterogeneous N:NIH out crossed stock17. We performed two-way artificial selective breeding14 on the trait of running capacity estimated from maximal distance run to exhaustion using a speed-ramped treadmill test. In contrast to inbred rodent models generated to curtail genetic complexity through strict brother-sister mating, we employed a rotational breeding scheme between 13 families which extends the possibility of various allelic combinations in each selected line. Retrospective estimates of the coefficient of inbreeding (F) 18 were close to the exact expected inbreeding estimate of less than 1% per generation (F = 0.179) we calculated prospectively19. More key features regarding the development of the rat model system is presented in the Online Supplemental Material, Online Table I & Table II, and Online Figure I.
For survivability studies, we followed a total of 139 aging male and female rats derived from generations 14 (studied in Trondheim, Norway), 15 and 17 (studied in Ann Arbor, MI, USA) that together had an over 7-fold difference in average aerobic running capacity between LCR and HCR populations (244 meters vs. 1960 meters, respectively). Based on veterinary inspections (all blinded for the rat line), aging rats were euthanized when natural death was deemed imminent or within the next few days, due to severe signs of morbidity or a moribund state that would constitute end-stage illness as defined by the University Committee on Care and Use of Animals and the Unit of Laboratory Medicine policy. Post-euthanasia, we used standard procedures for necropsy and extracted cells and tissues for further study.
For age comparisons, young female rats from generation 14 were compared to old generation 14 females for indices of cardiovascular function including VO2max, Blood pressure, left ventricular cardiomyocyte morphology and contractility, Ca2+ handling, sarcoplasmic reticulum Ca2+ leak, and transverse (t)-tubule density. Older females (>16 months) from generation 22 were assessed for age-related changes in general fitness such as body composition, physical activity, and metabolic energy expenditure.
Measurements
Measurements of whole body composition and physiological cardiovascular and metabolic parameters during rest and maximal aerobic exercise; measurements of isolated cardiomyocyte contractile and intracellular Ca2+ handling; left ventricular levels of PGC-1α, UCP2, and TAS (total antioxidant status); and blood chemistries, serum levels, and cytokines are described in full details in Online Methods in Supplemental Material.
Statistical Analysis
Survival curves were generated by Kaplan-Meier method and survival rates determined by log-rank test (GraphPad Prism v.5.01, GraphPad Software, San Diego, CA). We tested for differences between experimental groups using two-tailed t-tests for unrelated observations. If the comparison between more than two groups (e.g. cardiovascular function data) indicated significance, we applied a Tukey all-pairwise multiple comparison procedure. Mean, standard deviations, and confidence intervals [%95CI] were calculated to describe populations. We used linear regression models to estimate the degree to which exercise capacity and body weight predict longevity. Difference were considered significant if the P value was <0.05.
Results
Aerobic Capacity and Survivability
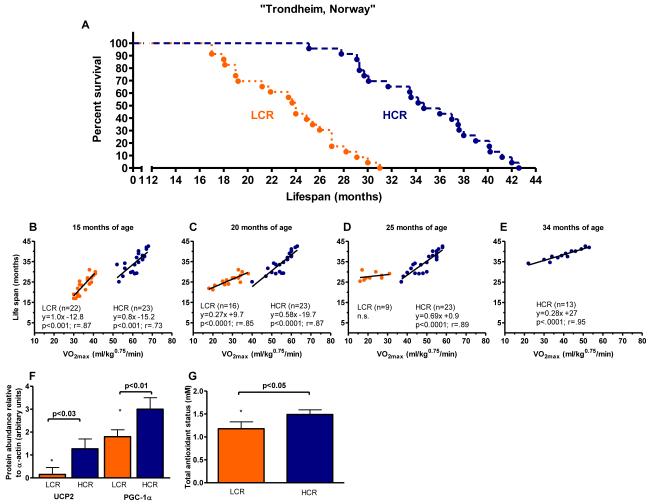
The first test of survivability in these rat models conducted at the Norwegian University of Science and Technology revealed that longevity segregated strongly with aerobic capacity between the populations. The median age of death for rats born as LCR was 24.0 months and 34.7 months for HCR, representing a 45% difference in life expectancy (p<0.001) and hazard ratio of 0.06 [95%CI 0.03 to 0.15] for survival of HCR over LCR (Figure 1A). Serial assessments of VO2max measured across lifespan (Figure 1B-E) were significant predictors of age at death for HCR at 15, 20, 25, and 34 months of age and for LCR at 15 and 20 months of age. At 15 months of age, each 10 ml/kg0.75/min increase in VO2max associated with 8 and 10 month extensions in lifespan for rats in the HCR (y = 0.82x -15.19) and LCR (y = 1.04x - 12.85) strains, respectively.
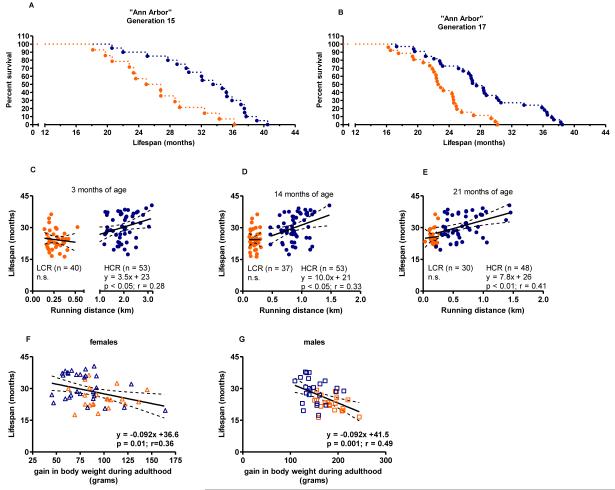
Figure 1. A prospective test of the “aerobic hypothesis” in rats selectively bred to contrast for intrinsic endurance exercise capacity.
Data collected in Trondheim, Norway. (A) Survival curves are significantly different between Low Capacity Runners (LCR; n = 23) and High Capacity Runners (HCR: n = 23). Logrank test; (P<0.0001). (B-E) Maximal oxygen consumption (VO2max) assessed longitudinally at 15, 20, 25, and 34 months of age predicted lifespan both between and within selected lines. (F) PGC-1α and UCP2 in left ventricular tissue. (G) Total antioxidant status in plasma (TAS).
Aging and decreased exercise capacity may be linked to skeletal- and cardiac muscle abnormalities related to mitochondrial biogenesis20 including an impaired defense against reactive oxygen species21. We examined: 1) the levels of peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α), a critical coordinator for activation of metabolic genes controlling substrate utilization and mitochondrial biogenesis, 2) uncoupling protein 2 (UCP2) which is suggested to play a key role for regulation of senescence22 in left ventricular tissue, and 3) total antioxidant status (TAS) in blood plasma. The levels of UCP2 (p<0.001), PGC-1α (p<0.001) and TAS (p<0.001) were lower in LCR (n = 8) relative to HCR (n = 7) (Figure 1F&G), suggesting greater mitochondrial capacity and defense against reactive oxygen species in HCR.
Differences in Cardiomyocyte and Myocardial Structure and Function
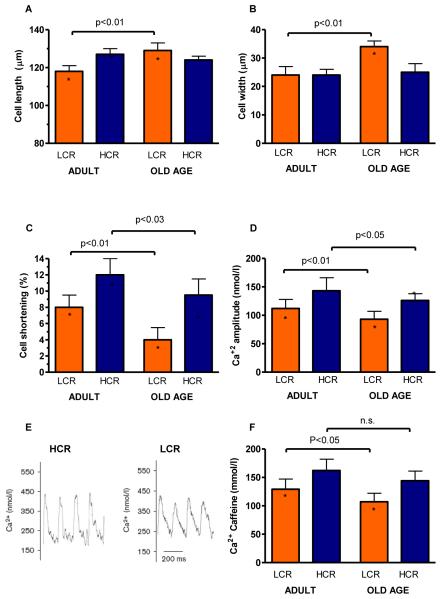
Effective cardiac function is a primary determinant of VO2max and a prerequisite for healthy aging23. We therefore evaluated left ventricular cardiomyocyte morphology, contractility, and intracellular Ca2+ handling between LCR and HCR in adult rats (15-20 months) versus rats at old age (>25 months) as markers of evidence for pathological changes26. During progression from adult to old age, cardiac cell width and length were unchanged in HCR whereas both width and length, including the width/length ratio, increased in LCR with aging (Figure 2A&B); indicative of pathologic remodelling. Aging produced a 50% and 26% decrease in left ventricular cardiomyocyte fractional shortening in HCR and LCR, respectively (p<0.001; Figure 2C). Accordingly, intracellular Ca2+ transient (CaT) amplitude was also more compromised in LCR than HCR (p=0.002; Figure 2D, CaT example recordings are shown in Figure 2E). The substrate for this effect is the sarcoplasmic reticulum Ca2+ load, which we measured by applying caffeine to cardiomyocytes. We found that sarcoplasmic reticulum Ca2+ load was more compromised in LCR rats than HCR rats already at adult age (20% lower in LCR; Figure 2F), and that aging further reduced sarcoplasmic reticulum Ca2+ load in LCR by 17%, whereas no aging-mediated reduction was observed in HCR.
Figure 2. Properties of cardiac ventricular cardiomyocytes were more compromised as a function of age in LCR compared with HCR rats.
(A&B) Cell length and width increased in old LCR but not in old HCR. (C) Isolated cell shortening greater in HCR than LCR in both adult and old age (D) Amplitude of Ca2+ transients decreased with aging in LCR but not HCR. (E) Example signals of Ca2+ transients. (F) Sarcoplasmic reticulum Ca2+ load measured after caffeine application was reduced in LCR vs. HCR cells, and deteriorated with aging in LCR, but not HCR cells. Adult: 15-20 months; Old Age: >25 months. *: p<0.05 age-matched LCR vs. HCR.
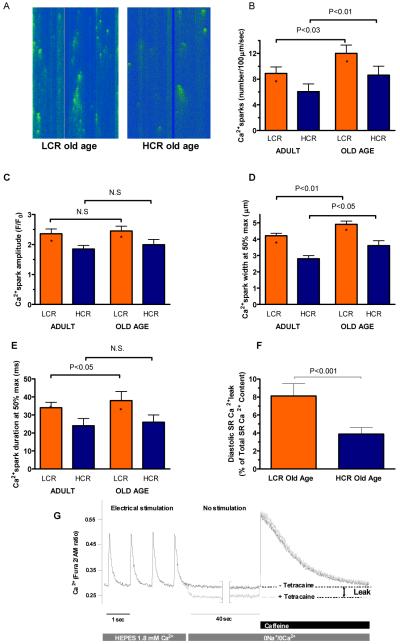
Next, we measured rate of diastolic re-lengthening, and found that time to 50% re-lengthening was impaired in cardiomyocytes from adult LCR compared to HCR rats, but that no age-dependent decline occurred; thus, the difference between LCR and HCR rats was sustained throughout life (Figure 3A). The similar difference between LCR and HCR cells including sustained difference throughout life was also found for the rate of CaT decay, measured as the time to 50% decay (Figure 3B). The reduced rate of sarcoplasmic reticulum Ca2+ uptake in diastole may also at least partly explain the reduced CaT amplitude27. Consistent with reduced CaT decay, we measured a higher diastolic Ca2+ concentration (p<0.001) in cells from LCR rats relative to HCR, which in LCR, but not HCR, also increased with aging (Figure 3C). However, adjacent to impaired Ca2+ uptake, increased diastolic [Ca2+] may also be explained by increased ryanodine receptor-mediated Ca2+ leak events from the sarcoplasmic reticulum. Thus, we measured the frequency and characteristics of spontaneous Ca2+ sparks in quiescent cardiomyocytes (Figure 4A). Ca2+ spark frequency was higher in cells from LCR relative to HCR in both adult and aged rats (p<0.001), whereas aging increased the Ca2+ spark frequency in both strains (Figure 4B). Diastolic Ca2+ leak by sparks was not off-set by changed characteristics of individual sparks, as Ca2+ spark amplitude, and width- and duration at half-maximum amplitude indicated that Ca2+ sparks were smaller and thus released less Ca2+/spark in HCR relative to LCR, and that age-dependent changes tended to be comparable between LCR and HCR (Figure 4C-E). As a last test of this phenomenon, we measured [Ca2+] in quiescent cells over a prolonged period of time (40 seconds) with and without tetracaine so as to quantify the total sarcoplasmic reticulum ryanodine receptor-mediated Ca2+ leak in old LCR and HCR rats. This showed that at old age, LCR cells lost ~8% of sarcoplasmic reticulum Ca2+, whereas HCR cells lost ~4% during the protocol, after normalizing for differences in sarcoplasmic reticulum Ca2+ content (Figure 4F, Figure 4G provides example traces to illustrate the protocol). Thus, impaired sarcoplasmic reticulum Ca2+ control in diastole in LCR, including some accentuation during aging, appears to be caused by several mechanisms such as reduced uptake and increased leak, of which at least some of it occurs in the form of Ca2+ sparks, i.e. localized ryanodine receptor-mediated Ca2+ leak events. Diastolic Ca2+ leak has also been suggested to initiate ventricular arrhythmias and thus underlie a proportion of sudden cardiac deaths24. This is of interest, because previous studies showed LCR are more prone to ischemia-mediated lethal cardiac arrhythmias than HCR28.
Figure 3. Diastolic properties of cardiac ventricular cardiomyocytes were more compromised in adult LCR compared with HCR rats, and this difference was sustained or accentuated with aging.
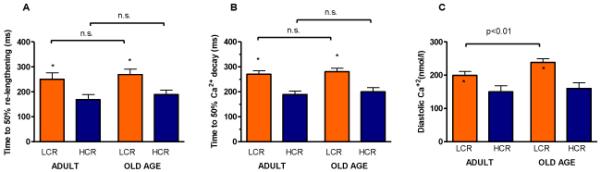
(A&B) Cell relaxation and rate of Ca2+ transient decay were impaired in LCR rats compared to HCR rats, but did not change with aging. (C) Diastolic Ca2+ concentration was higher in adult LCR rats compared to adult HCR, and increased with aging in LCR, but not HCR rats. Adult: 15-20 months; Old Age: >25 months. *: p<0.05 age-matched LCR vs. HCR.
Figure 4. Sarcoplasmic reticulum Ca2+ leak was more compromised in LCR rats compared with HCR rats, and this difference was sustained with aging.
(A) Example confocal images of Ca2+ sparks from quiescent cells. (B-E) Ca2+ spark frequency, amplitude, and width and duration at half-maximum amplitude were higher in LCR than HCR rats, indicating increased loss of sarcoplasmic reticulum Ca2+. (F&G) Quantitative assessments of sarcoplasmic reticulum Ca2+ leak confirmed that cells of old LCR rats had greater Ca2+ leak than HCR equivalents. Adult: 15-20 months; Old Age: >25 months. *: p<0.05 age-matched LCR vs. HCR.
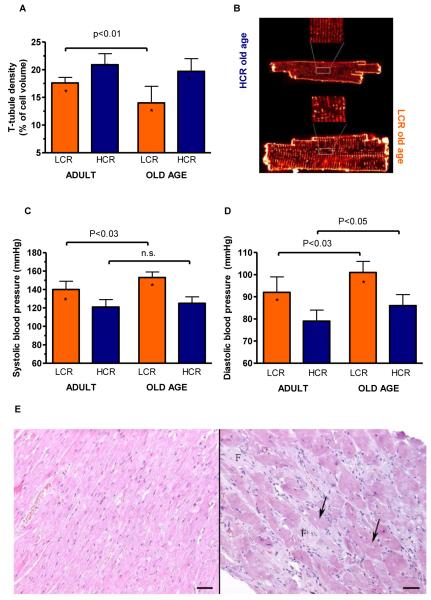
Although the abundance of Ca2+ cycling proteins within the T-tubular system suggests a coordinating role, the fate of the T-tubular density with aging is unknown. We found T-tubule density for HCR was higher than LCR and did not decline with age (p=0.1860), whereas in LCR, it decreased by 26% (p<0.001; Figure 5A&B).
Figure 5. Evidence to suggest mild pathological remodelling in LCR over HCR rats.
(A) Transverse (T) tubule density relative to cardiomyocyte cell size decreased in old LCR but not old HCR rats. (B) Representative examples of cardiomyocytes stained with Di-8-ANEPPS for imaging of T-tubules in old LCR and HCR rats. (C) Higher systolic and (D) diastolic blood pressures in the LCR vs. HCR might be responsible for part of the cardiac cellular changes. *: p<0.05 age-matched LCR vs. HCR. (E) Light micrographs (hematoxylin and eosin stained) of myocardium from representative HCR (left) and LCR (right, both old age). Vacuolization marked with arrows and interstitial fibroplasia marked by letter F. Bar = 50 μm.
The remodelling of the LCR hearts was likely influenced by higher systolic and diastolic pressures (Figure 5C&D) along with a greater rise in systolic blood pressures with aging (p=0.047). In agreement with these physiological findings, more cardiomyocyte degeneration and necrosis were observed in myocardial specimens from LCR (16 of 19) compared to HCR (7 of 16) (p<0.03; Figure 5E). Loss of cells and enlargement of the remaining cardiomyocytes may represent the structural basis for the reduced energy capacity of aged tissues25. As a final test, we performed echocardiography in a subset of anesthetized LCR and HCR rats of old age. No major systolic dysfunction was indicated; however, during resting conditions, LCR presented with higher systolic work than HCR, indicating a greater chronic cardiac stress (Online Table III). Also, LCR rats presented with signs of diastolic dysfunction, as evaluated by the E/A ratio30 (Mean (SD): 0.9 (0.1) and 1.5 (0.5); p<0.05, in LCR and HCR, respectively).
Fitness and Survivability
Because gene-environment interactions can produce traits that are idiosyncratic to a particular laboratory setting, we repeated the test of survivability in a larger population comprised of rats derived from generations 15 and 17 birthed and housed in the vivarium at the University of Michigan. We found the LCR cohort from generation 15 (n=14) died at a median age of 25.6 months and the generation 15 HCR cohort (n=20) at 34.1 months (33% difference; p<0.001) (Figure 6A). The median survival of LCR and HCR from generation 17 was 22.7 and 27.5, respectively, p<0.001 (21% difference) (Figure 6B). Combined, the survival data generated a hazard ratio of 0.26 [%95CI 0.16 to 0.44; p<0.01) for the HCR outliving the LCR rats. Thus, survival curves (Figures 1 & 6) from three populations encountering different environments indicate that HCR rats outlive LCR counterparts by 5-10 months. Standard necropsy profiles (Online TableIV) demonstrates that age-related lesions were not different in incidence or severity between the LCR and HCR rats, suggesting that an overt disease condition was not over-represented in rats bred for low intrinsic capacity. Differences in patterns of survival noted between generations 15 and 17 may be partly a reflection of the wider differential for aerobic exercise capacity between the LCR and HCR cohorts. The average running capacity for Generation 15 LCR cohort was significantly less than from the Generation 17 cohort (198.6 (30 SD) vs. 313 (80SD); p< 0.001). Likewise, for HCR rats, Generation 15 average running distance was significantly higher than generation 17 (2378 (405SD) vs. 1661 (362); p< 0.001).
Figure 6. Survival features in LCR and HCR rats support intrinsic aerobic capacity as a biomarker of aging.
(A-B) Survival curves for rats aged at the University of Michigan, Ann Arbor confirmed that HCR (n= 53) live longer than LCR (n=40). Logrank test; (P<0.0001). (C-E) Treadmill running distances at 3, 14, and 21 months of age were statistical predictors of age at death in HCR but not LCR rats. (F&G) Gains in body weight between 3 and 14 months of age associated with a decrease in survival in both females and males. Linear regressions are shown with 95% confidence intervals.
To test for a connection between age-related declines in general fitness and longevity, we measured maximal exercise capacity and body weight across adulthood. Figures 6C-E each displays the relationship between maximal running distance (km) at 3, 14 and 21 months of age and rat longevity. At middle age (14 months), a 0.5 km further running distance associated with a 5.0 month extension in life expectancy in HCR rats. The range in running capacity was much narrower for the LCR rats and did not predict longevity. It should also be noted that in comparison to the relationship between longevity and VO2max (Figures 1B-E), the longevity-running distance (Figures 6C-E) presents with greater variability. Whereas VO2max provides a direct measure of aerobic capacity, maximal running distance to exhaustion may be influenced by factors other than VO2max, such as anaerobic capacity, efficiency and oxygen cost of running, resistance to fatigue, as well as psychological and motivational factors.
Consistent with previous reports that energy consumption is not different between LCR and HCR rats25,26, we found no difference in weight gain from 3 to 14 months of age (data not shown). Yet, when evaluated as a combined population (Figure 6 F&G), we discovered that for every 10-gram gain in weight during adulthood, there was nearly a one-month decline in lifespan for both females (y = −0.092x + 36.61) and males (y = −0.09x + 41.55). These results suggest that exercise capacity and gain in body weight on an ad libitum diet can predict longevity in rats.
To investigate whether LCR rats manifest metabolic or inflammatory phenotypes as a likely determinant for multi-system declines, blood samples were taken from this cohort when approaching two years of age (Mean (SD): 21. 9 (1.0) months). Blood chemistries (Online Table V) indicate that LCR rats measured higher for triglycerides and glucose levels (p<0.001 and p=0.05, respectively) and that HCR were significantly higher for alanine aminotransferase (ALT) (p=0.005). The profile of pro- and anti-inflammatory cytokines by bead-based multiplex assay (Online Table VI) showed that LCR rats had higher levels of pro-inflammatory cytokines than HCR rats, with TNF-α showing the largest magnitude of difference (Mean (SD): 223.5 (104.3) vs. 33.96 (31.74) pg/ml, p<0.001). Compared to the HCR, LCR rats had increased IL-4 (Mean (SD): 544 (53) vs. 411 (117) pg/ml, p=0.007) and IL-10 (1881 (742) vs. 1192 (580) pg/ml, p=0.04), likely representing compensatory anti-inflammatory mechanisms.
Aerobic Capacity and Aging
The interactions between cardiovascular fitness, metabolic changes, and mortality is most difficult to interpret in elderly populations27. Therefore, identifying suitable animal models in which non-invasive quantitative measures of co-occurring disease processes28, functional impairment29, and differential for mortality coalesce is necessary in order to better understand and develop geriatric biology and medicine. The fact that selection for intrinsic exercise capacity in rats produced models that contrast for energy capacity, body mass, increased risk for complex disease and life span, proposes that the LCR/HCR model system may be useful for tracking phenotypic alterations during late-life. A group of age-matched female LCR and HCR rats from generation 22 were evaluated across 8 weeks beginning at 16 months of age. Summary of our results is shown in Table 1. Between 16 to 18 months of age, both the LCR and HCR strains were weight stable (p=0.42). Using NMR-based analysis, we confirmed that the wide differential for body mass between the models was due to a lower % fat body mass and higher % lean body mass in HCR compared to LCR (Mean (SD): 8.80 (2.83) % vs. 23.33 (9.38) % fat body mass; p<0.001 in HCR and LCR, respectively, and 78.79 (2.12) % vs. 66.04 (7.32) % lean body mass in HCR and LCR, respectively; p<0.001). Physical activity levels monitored while pair-housed in home cage were not different between LCR and HCR (counts/kg/min/cage over 24 hours; p=0.13). Activity levels were also recorded while rats were housed singly for over 48 hour period in an integrated open-circuit calorimeter unit equipped with an optical beam activity monitoring device (Table 1). HCR rats exhibited a total activity count of 622.84 (66.92), which was 38% more than LCR (451.40 (78.79); p<0.001). For measures of energy expenditure, VO2, adjusted for body weight or lean body mass differences, and VCO2 were significantly higher in HCR rats. However, RQ between LCR and HCR rats was not different (0.97 (0.04) vs. 0.96 (0.03); p=0.28), indicating no certain differential metabolic differences between the LCR and HCR before 18 months of age.
Table 1. Comparison of Cardiovascular Fitness in Aged LCR vs. HCR Rats.
| Characteristic | LCR | HCR | p value | |
|---|---|---|---|---|
| Age, days | Days | 488.4 (0.7) | 487.9 (1.1) | 0.13 |
| Body weight | N | 15 | 16 | |
| Week 1, g | 347.4 (57.7) | 236.5 (23.1) | <0.001 | |
| Week 8, g | 350.4 (54.25) | 242.4 (24.46) | <0.001 | |
| Gain, g | 2.95 (11.13) | 5.89 (8.73) | 0.42 | |
| NMR Body Composition | N | 15 | 16 | |
| Body weight, g | 349.1 (58.72) | 239.5 (21.77) | <0.001 | |
| Fat,% | 23.3 (9.38) | 8.8 (2.83) | <0.001 | |
| Lean, % | 66.0 (7.32) | 78.7 (2.12) | <0.001 | |
| Fluid, % | 7.4 (0.62) | 7.5 (0.21) | 0.45 | |
| Home-Cage Activitya | N | 14 | 16 | |
| Dark cycle | Total Movement, ct/kg/min | 19.9 (13.4) | 33.0 (15.9) | 0.11 |
| Light cycle | Total Movement, ct/kg/min | 7.3 (3.1) | 8.5 (3.7) | 0.51 |
| 24-hour | Total Movement, ct/kg/min | 13.8 (7.7) | 21.1 (9.6) | 0.13 |
| CLAMS Activity | N | 14 | 14 | |
| Dark cycle | X-Horizontal Movement,ct/hr | 222.5 (68.7) | 316.0 (73.4) | 0.002 |
| Z-Vertical Movement, ct/hr | 413.9 (128.0) | 574.1 (130.5) | <0.003 | |
| Total Movement, ct/hr | 631.2 (121.4) | 858.7 (109.9) | <0.001 | |
| Light cycle | X-Horizontal Movement,ct/hr | 77.4 (15.9) | 117.5 (24.9) | <0.001 |
| Z-Vertical Movement, ct/hr | 127.2 (43.7) | 180.9 (57.8) | 0.010 | |
| Total Movement, ct/hr | 257.9 (46.2) | 368.7 (73.8) | <0.001 | |
| 24-hour | X-Horizontal Movement,ct/hr | 152.2 (41.8) | 220.2 (41.6) | <0.001 |
| Z-Vertical Movement,ct/hr | 275.1 (84.16) | 384.9 (21.5) | 0.001 | |
| Total Movement,ct/hr | 451.4 (78.8) | 622.8 (66.9) | <0.001 | |
| CLAMS Metabolic | N | 14 | 14 | |
| Dark cycle | VO2,,ml/kg/hr | 1310.4 (222.9) | 1634.5 (485.4) | 0.03 |
| VO2 0.75BW.,ml/kg0.75BW | 5608.0 (773.6) | 6872.8 (731.7) | <0.001 | |
| VO2 lean ,ml/kglean | 1966.2 (221.1) | 2199.4 (206.1) | 0.008 | |
| VCO2,ml/kg/hr | 1287.7 (247.6) | 1688.9 (184.4) | <0.001 | |
| RQ | 0.98 (0.04) | 0.97 (0.02) | 0.15 | |
| Light cycle | VO2,,ml/kg/hr | 1080.8 (181.6) | 1365.1 (403.9) | 0.024 |
| VO2 0.75BW,,ml/kg0.75BW | 4614.2 (644.1) | 5749.4 (561.9) | <0.001 | |
| VO2 lean ,ml/kglean | 1618.6 (204.7) | 1840.6 (170.1) | 0.004 | |
| VCO2,ml/kg/hr | 1033.3 (204.3) | 1385.4 (155.2) | <0.001 | |
| RQ | 0.96 (0.05) | 0.95 (0.03) | 0.53 | |
| 24-hour | VO2,,ml/kg/hr | 1199.9 (200.4) | 1505.4 (445.5) | 0.027 |
| VO2 0.75BW,,ml/kg0.75BW | 5130.5 (696.4) | 6335.7 (561.9) | <0.001 | |
| VO2 lean ,ml/kglean | 1799.0 (207.1) | 2027.4 (172.7) | 0.005 | |
| VCO2,ml/kg/hr | 1166.2 (224.5) | 1544.0 (166.5) | <0.001 | |
| RQ | 0.97 (0.04) | 0.96 (0.03) | 0.28 | |
| Blood Sugar | N | 15 | 16 | |
| Random Glucose,mg/dL | 106.9 (8.4) | 91.5 (6.5) | <0.001 |
Rats housed 2 per cage. Abbreviations: g, grams; ct/kg/min, counts per kilogram per minute; ct/hr, counts per hour.
Discussion
Our results, using a heterogeneous animal model system that divides widely for intrinsic (i.e. untrained) aerobic exercise capacity, provide first demonstration that an inherent aerobic capacity phenotype and co-segregated sub-phenotypes can be linked with health and survivability. This so named aerobic hypothesis of longevity might be of clinical value because as a minimum, it fulfills the fundamental criteria for service as a biomarker of aging as suggested by The American Federation for Aging Research. That is, one’s current endurance exercise capacity predicts the rate of aging, represents a basic underlying process, can be tested repeatedly without harm, and can be evaluated in other animal models30.
In accord with the aerobic hypothesis and current knowledge in cardiovascular health, our data reveal that rats with low intrinsic aerobic capacity and reduced longevity display a reduced ability for mitochondrial regeneration, decreased metabolic control in the heart, and a reduced antioxidant status. Consistent with these results, we previously demonstrated that pathways preferential for fatty acid β-oxidation are down-regulated and carbohydrate metabolic pathways are up-regulated in hearts of LCR relative to HCR31. This switch of the LCR to glucose metabolism may represent a compensatory shift to improved energy efficiency, reminiscing early stages of heart failure32. Additionally, evaluations of gene expression differences within skeletal muscle have demonstrated that oxidative phosphorylation and lipid metabolism segregated with aerobic running performance and disease risk phenotypes. Expression-phenotype correlations, together with diminished skeletal muscle capillarity and mitochondrial area in LCR rats support the general hypothesis that an inherited intrinsic aerobic capacity can underlie disease risks. In humans, higher life expectancy and reduced mortality risk for both elite endurance athletes33 and twin pairs34 with greater aerobic fitness, is also in accord with a central role for an intrinsic endurance capacity.
Despite the fact the experimental regime that resulted in the observed divide for longevity was strictly confined to aerobic capacity, the possibility remains that subsequent phenotypic developments within each strain of rats constitute the immediate reason for the divide for longevity; for example, the chronically elevated blood pressure in LCR vs. HCR rats may be a likely candidate, as myocardial remodeling in response to hypertension is well-documented40. It should though be noted that the increase in both systolic and diastolic blood pressures in LCR vs. HCR rats, including the aging-associated accentuation, is relatively small. In fact, the observed blood pressures compare to values normally obtained in normotensive control rats, whereas hypertensive rats present with ~30-50 mmHg higher values than those observed in the current LCR rats41. Similarly, the observed cardiomyocyte remodeling is less than normally observed in heart failure rats42, whereas the necropsy and pathology investigations could neither find any over-representation of disease in LCR vs. HCR rats. Thus, it is unlikely that parameters such as blood pressure or myocardial remodeling alone lead to the observed difference in longevity. In contrast, we propose that the earlier death in LCR rats may have occurred due to a variety of aging-related mechanisms that lead to expected failure of one or more organs. Finally, it remains to be determined whether the aerobic hypothesis for longevity is mechanistically pre-determined by the inborn genome, or whether lifespan may be extended by regular exercise training. This has not yet been studied, but it is noteworthy that exercise training in several different models of overt cardiovascular disease, in which myocardial dysfunction surpasses that of the current LCR rats, leads to reversal of contractile42-44 and diastolic43,45 Ca2+ handling dysfunctions, as well as improved survival46. These studies effectively suggest that inhibition or reversal of phenotypes related to morbidity and mortality may be achievable with exercise training.
The selective breeding paradigm in rats is a powerful approach because it provides an unbiased prospective test of the association between exercise capacity and longevity. As a model system, concurrent breeding of LCR and HCR rats at every generation allows the lines to serve as reciprocal controls for environmental changes across time. Nevertheless, the outcome that selection for intrinsic aerobic capacity produced rat lines that contrast for longevity provides strong evidence, but not unqualified proof, that an inherited capacity for energy metabolism mechanistically contributes to longevity. A major presumed value for developing genetically-derived animal models is their ultimate use to identify combinations of allelic variants causative of differences for complex traits. This goal has not been attained previously, partly because of shortcomings in the choice of animal models for genetic evaluation. The utility of comparing rodent inbred strains, while bringing the benefit of experimental and analytical simplicity, is limited because only two genotypes are represented. In practice, two-way artificial selective breeding produces divergent heterogeneous populations that have contrasting arrangements of naturally occurring alleles that are co-selected at multiple interacting loci and enriched at each successive generation. During their development, the LCR and HCR models have maintained over 80% of the genetic variation of the original founder population. Maintenance of genetic sophistication in a contrasting animal model system opens the possibility of discovering novel epistatically linked regulatory networks, modifier genes, synergistic actions, and compensatory variants35 for unraveling biological complexity of aging.
Supplementary Material
Novelty and Significance.
“What is known?”
Aging and longevity represent the most complex and least understood of human phenotypes.
Several large-scale clinical studies over the past two decades show that low exercise capacity is a stronger predictor of morbidity and mortality than other commonly reported risk factors such as hypertension, type II diabetes, obesity and smoking.
Dysfunctional aerobic energy metabolism has been reported in essentially all age-related disease conditions including type 2 diabetes, heart disease, cardiac arrhythmias, inflammation, and neurodegenerative diseases.
“What new information does this article contribute?”
We developed a contrasting animal model system via genetic selection and conducted the first set of survivability studies and old-age comparisons to test the hypothesis that an intrinsic (inborn) capacity for aerobic metabolism sets an initial divide for longevity and a compression of age-related morbidity.
This study provides the first demonstration of the “aerobic hypothesis of longevity” and as such, is a significant step forward in the study of longevity mechanisms and aging biology, especially the role of the myocardium and the circulatory system.
The complexity and difficulty of studying aging and longevity in humans make animal models an attractive alternative for in-depth exploration and hypothesis testing. The clinical observation of a strong statistical link between low exercise capacity and increased mortality lead us to propose that low capacity for oxygen metabolism mechanistically underlies accelerated aging and diminished longevity (aerobic hypothesis). As a test of this hypothesis we selectively bred rats across several generations to produce strains that differ in their intrinsic (inherent) capacity for treadmill endurance running. In agreement with the aerobic hypothesis, selective breeding experiments revealed a robust association between low intrinsic aerobic capacity and decreased longevity. Other traits associating with low aerobic capacity included reduced maximal oxygen uptake, decreased indices of mitochondrial function, low cardiac myocyte function and calcium signaling, diminished physical activity and increased body mass segregated with selection for low aerobic running capacity. The aerobic hypothesis is consistent with the fundamental criteria for service as a biomarker of aging as suggested by The American Federation for Aging Research. That is, aerobic endurance capacity predicts the rate of aging, which represents a basic underlying process, and could be tested repeatedly without harm, and evaluated in animals.
Acknowledgements
We thank Dr Ingolf Hansen in Trondheim, Norway for excellent veterinary services, and Nathan Kanner and Ashley Duval in Ann Arbor, Michigan for care and maintenance of the LCR/HCR rat colony.
Sources of Funding
This work utilized the Animal Phenotyping Core of the Michigan Diabetes Research and Training Center funded by NIH5P60 DK20572 from the National Institute of Diabetes and Digestive and Kidney Diseases. Studies supported by National Center for Research Resources of the National Institutes of Health (R24 RR017718 to L.G.K. and S.L.B.), University of Michigan Institute of Gerontology, Nathan Shock Center Pilot Grant Program (P30 AG013283 to L.G.K.) and Geriatric Center, The Claude D. Pepper Older Americans Independence Center Pilot Grant (P30 AG024824 to L.G.K. and S.X.L), Norwegian Council on Cardiovascular Disease and Norwegian Research Council Funding for Outstanding Young Investigators (U.W.), K.G. Jebsen Foundation, Simon Fougner Hartmanns Family Foundation, Foundation for Cardiovascular Research at St. Olav’s Hospital (O.J.K., Ø.E. and U.W.), and British Heart Foundation (O.J.K. and G.L.S.).
Non-Standard Abbreviations and Acronyms
- VO2max
maximal oxygen uptake
- LCR
Low Capacity Runners
- HCR
High Capacity Runners
- T-tubule
transverse tubule
- CaT
Ca2+ transient
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- UCP2
uncoupling protein 2
- TAS
total antioxidant status
- IL
Interleukin
- IFN-γ
Interferon gamma
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- TNF-α
Tumor necrosis factor alpha
- ALT
alanine aminotransferase
- E/A ratio
ratio between early (E) and late (atrial - A) ventricular filling velocity
- RQ
respiratory quotient, calculated as VCO2/VO2
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr., Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 2.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr., Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 3.Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27:83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR. Exercise capacity and the risk of death in women: The st james women take heart project. Circulation. 2003;108:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 5.Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V, Singh S. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- 6.Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994;330:1549–1554. doi: 10.1056/NEJM199406023302201. [DOI] [PubMed] [Google Scholar]
- 7.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 8.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged norwegian men. N Engl J Med. 1993;328:533–537. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 9.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 11.Sebastiani P, Solovieff N, Puca A, Hartley SW, Melista E, Andersen S, Dworkis DA, Wilk JB, Myers RH, Steinberg MH, Montano M, Baldwin CT, Perls TT. Genetic signatures of exceptional longevity in humans. Science. 2010 doi: 10.1126/science.1190532. [DOI] [PubMed] [Google Scholar]
- 12.Koch LG, Britton SL. Aerobic metabolism underlies complexity and capacity. J Physiol. 2008;586:83–95. doi: 10.1113/jphysiol.2007.144709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 16.Kivela R, Silvennoinen M, Lehti M, Rinnankoski-Tuikka R, Purhonen T, Ketola T, Pullinen K, Vuento M, Mutanen N, Sartor MA, Reunanen H, Koch LG, Britton SL, Kainulainen H. Gene expression centroids that link with low intrinsic aerobic exercise capacity and complex disease risk. FASEB J. doi: 10.1096/fj.10-157313. fj.10-157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen C, Spuhler K. Development of the national institutes of health genetically heterogeneous rat stock. Alcohol Clin Exp Res. 1984;8:477–479. doi: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- 18.Plum M. Computation of inbreeding and relationship coefficients - in populations with a relatively small number of different male ancestors. J Hered. 1954;45:92–94. [Google Scholar]
- 19.Falconer DS, Mackay TFC. Introduction to quantitative genetics. Addison Wesley Longman, Ltd.; Essex: 1996. [Google Scholar]
- 20.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: The central role of pgc-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 21.Tosato M, Zamboni V, Ferrini A, Cesari M. The aging process and potential interventions to extend life expectancy. Clin Interv Aging. 2007;2:401–412. [PMC free article] [PubMed] [Google Scholar]
- 22.Dietrich MO, Horvath TL. The role of mitochondrial uncoupling proteins in lifespan. Pflugers Arch. 2010;459:269–275. doi: 10.1007/s00424-009-0729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 24.Maier LS, Bers DM, Brown JH. Calmodulin and ca2+/calmodulin kinases in the heart - physiology and pathophysiology. Cardiovasc Res. 2007;73:629–630. doi: 10.1016/j.cardiores.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587:1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;293:E31–41. doi: 10.1152/ajpendo.00500.2006. [DOI] [PubMed] [Google Scholar]
- 27.McAuley PA, Kokkinos PF, Oliveira RB, Emerson BT, Myers JN. Obesity paradox and cardiorespiratory fitness in 12,417 male veterans aged 40 to 70 years. Mayo Clinic Proceedings. 85:115–121. doi: 10.4065/mcp.2009.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM, Cardiovascular Health Study Collaborative Research Group Risk factors for 5-year mortality in older adults: The cardiovascular health study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 29.Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, Tylavsky F, Bauer DC, Goodpaster BH, Harris TB. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- 30.Johnson TE. Recent results: Biomarkers of aging. Exp Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Bye A, Langaas M, Hoydal MA, Kemi OJ, Heinrich G, Koch LG, Britton SL, Najjar SM, Ellingsen O, Wisloff U. Aerobic capacity-dependent differences in cardiac gene expression. Physiol Genomics. 2008;33:100–109. doi: 10.1152/physiolgenomics.00269.2007. [DOI] [PubMed] [Google Scholar]
- 32.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 33.Kujala UM, Tikkanen HO, Sarna S, Pukkala E, Kaprio J, Koskenvuo M. Disease-specific mortality among elite athletes. JAMA. 2001;285:44–45. doi: 10.1001/jama.285.1.44. [DOI] [PubMed] [Google Scholar]
- 34.Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: The roles of genetics and childhood environment. Am. J. Epidemiol. 2002;156:985–993. doi: 10.1093/aje/kwf151. [DOI] [PubMed] [Google Scholar]
- 35.Pickrell JK, Coop G, Novembre J, Kudaravalli S, Li JZ, Absher D, Srinivasan BS, Barsh GS, Myers RM, Feldman MW, Pritchard JK. Signals of recent positive selection in a worldwide sample of human populations. Genome Res. 2009;19:826–837. doi: 10.1101/gr.087577.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.