Abstract
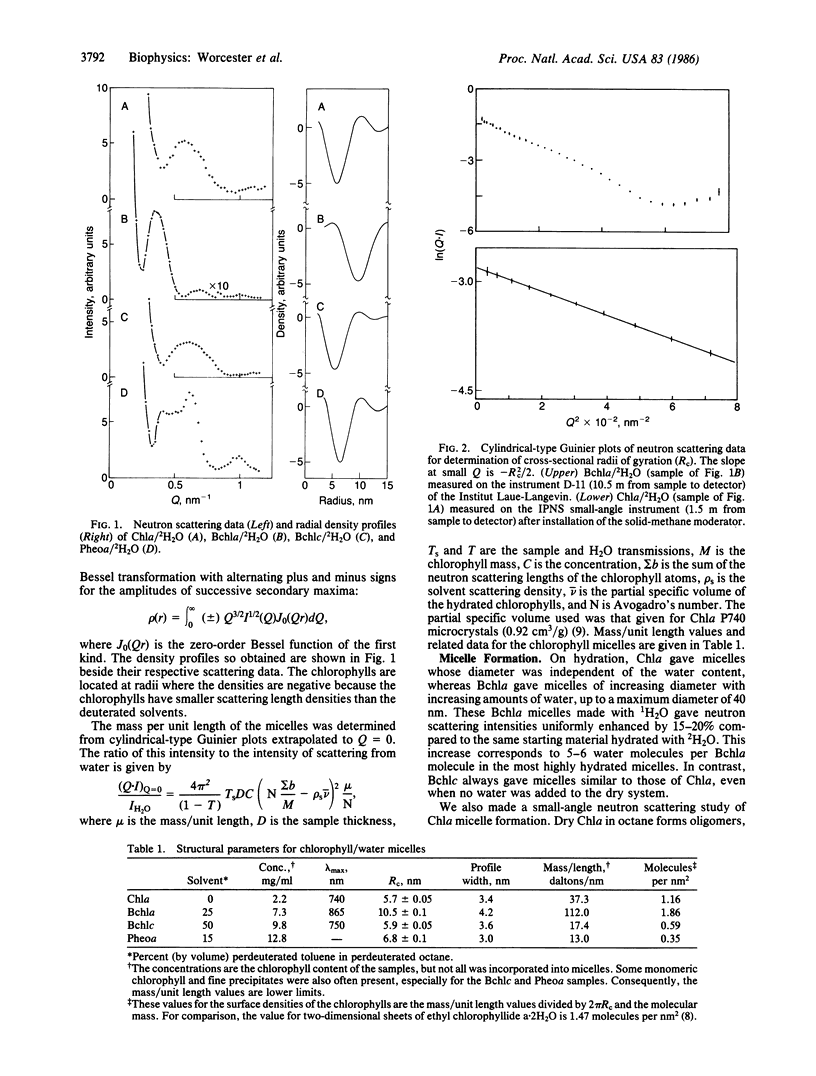
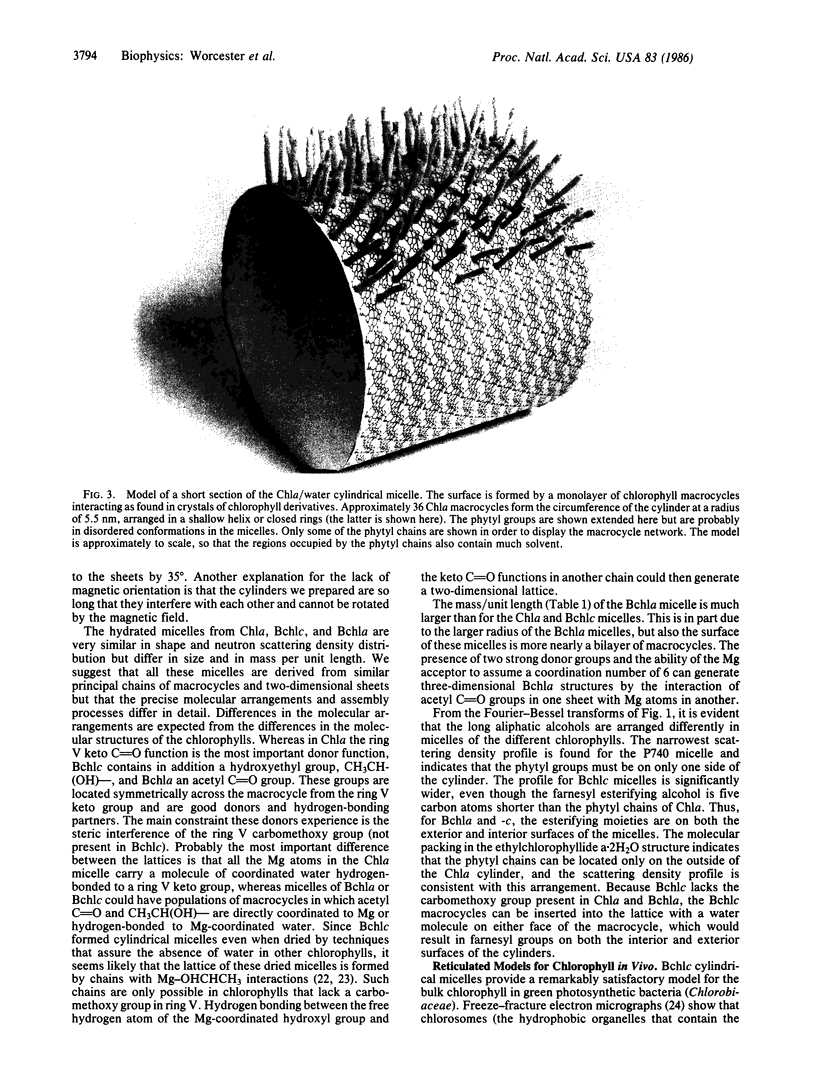
Micelles of hydrated chlorophyll a (P740), bacteriochlorophyll a (P865), bacteriochlorophyll c (P750), and pheophytin a prepared in organic media have been studied by small-angle neutron scattering to determine their shape, size, and mass per unit length. All of the micelles are hollow cylinders of well-defined size. The P740 and P750 cylinders are essentially monolayers of macrocycles crosslinked by water, probably in an arrangement similar to that of crystals of chlorophyll derivatives. The P865 micelle is more nearly a bilayer of macrocycles. We show that the curvature necessary to form cylinders probably results from intrinsic curvature of the five-coordinated chlorophyll macrocycle. Studies of P740 micelle formation and the disaggregating effects of another nucleophile (pyridine) are described. As the P750 micelles are nearly identical in size and optical spectra to the rod-shaped structures observed in chlorosomes, and the P865 micelles have optical properties very similar to the in vivo properties of the long-wavelength antenna of purple photosynthetic bacteria, we propose that features of the hydrated cylindrical micelles of these chlorophylls provide good models for antenna chlorophyll in photosynthetic bacteria.
Keywords: photosynthesis, energy transfer, chlorosomes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cruden D. L., Stanier R. Y. The characterization of chlorobium vesicles and membranes isolated from green bacteria. Arch Mikrobiol. 1970;72(2):115–134. doi: 10.1007/BF00409518. [DOI] [PubMed] [Google Scholar]
- Govindjee, Cederstrand C., Rabinowitch E. Existence of Absorption Bands at 730-740 and 750-760 Millimicrons in Algae of Different Divisions. Science. 1961 Aug 11;134(3476):391–392. doi: 10.1126/science.134.3476.391. [DOI] [PubMed] [Google Scholar]
- JACOBS E. E., VATTER A. E., HOLT A. S. Crystalline chlorophyll and bacteriochlorophyll. Arch Biochem Biophys. 1954 Nov;53(1):228–238. doi: 10.1016/0003-9861(54)90248-9. [DOI] [PubMed] [Google Scholar]
- Katz J. J., Norris J. R., Shipman L. L., Thurnauer M. C., Wasielewski M. R. Chlorophyll function in the photosynthetic reaction center. Annu Rev Biophys Bioeng. 1978;7:393–434. doi: 10.1146/annurev.bb.07.060178.002141. [DOI] [PubMed] [Google Scholar]
- Kratky C., Dunitz J. D. Ordered aggregation states of chlorophyll a and some derivatives. J Mol Biol. 1977 Jun 25;113(2):431–442. doi: 10.1016/0022-2836(77)90151-6. [DOI] [PubMed] [Google Scholar]
- LIPPINCOTT J. A., AGHION J., PORCILE E., BERTSCH W. F. The preparation and characterization of chloroplast fragments having an absorbancy maximum at 7400 A. Arch Biochem Biophys. 1962 Jul;98:17–27. doi: 10.1016/0003-9861(62)90140-6. [DOI] [PubMed] [Google Scholar]
- Olson J. M. Chlorophyll organization in green photosynthetic bacteria. Biochim Biophys Acta. 1980 Dec 22;594(1):33–51. doi: 10.1016/0304-4173(80)90012-9. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Golecki J. R., Drews G. Supramolecular organization of chlorosomes (chlorobium vesicles) and of their membrane attachment sites in Chlorobium limicola. Biochim Biophys Acta. 1980 Jan 4;589(1):30–45. doi: 10.1016/0005-2728(80)90130-9. [DOI] [PubMed] [Google Scholar]
- Worcester D. L. Structural origins of diamagnetic anisotropy in proteins. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5475–5477. doi: 10.1073/pnas.75.11.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]



