Abstract
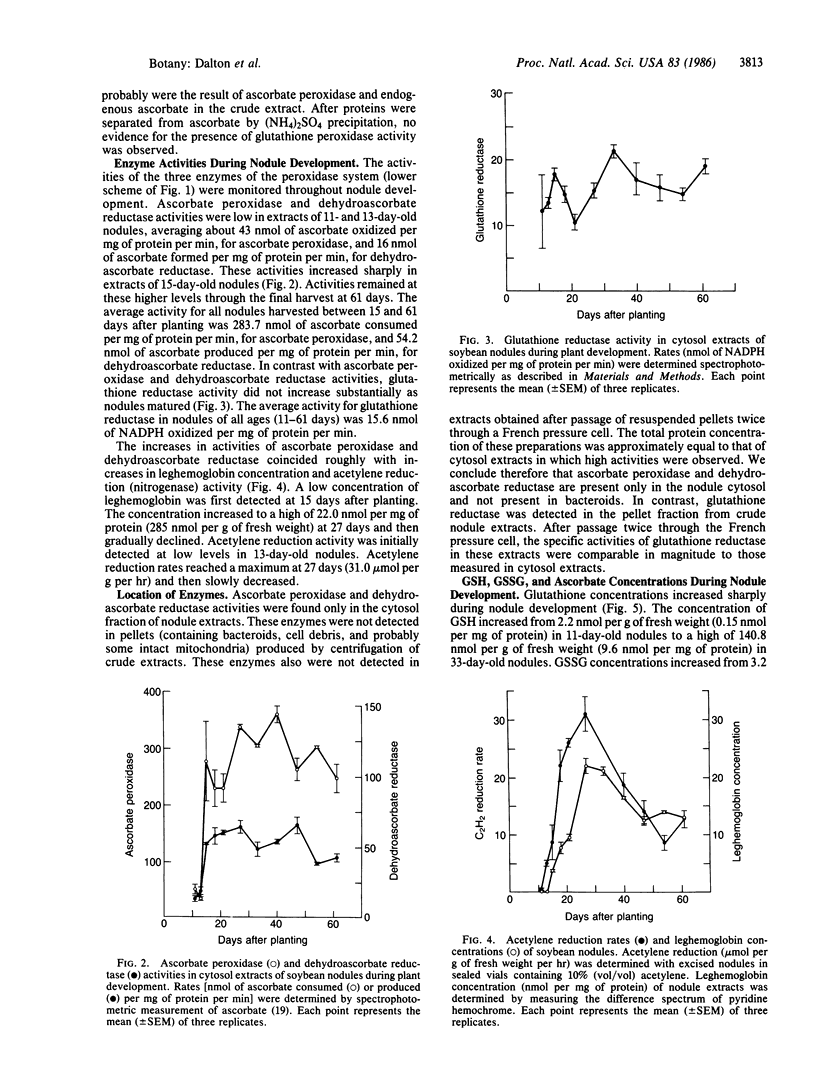
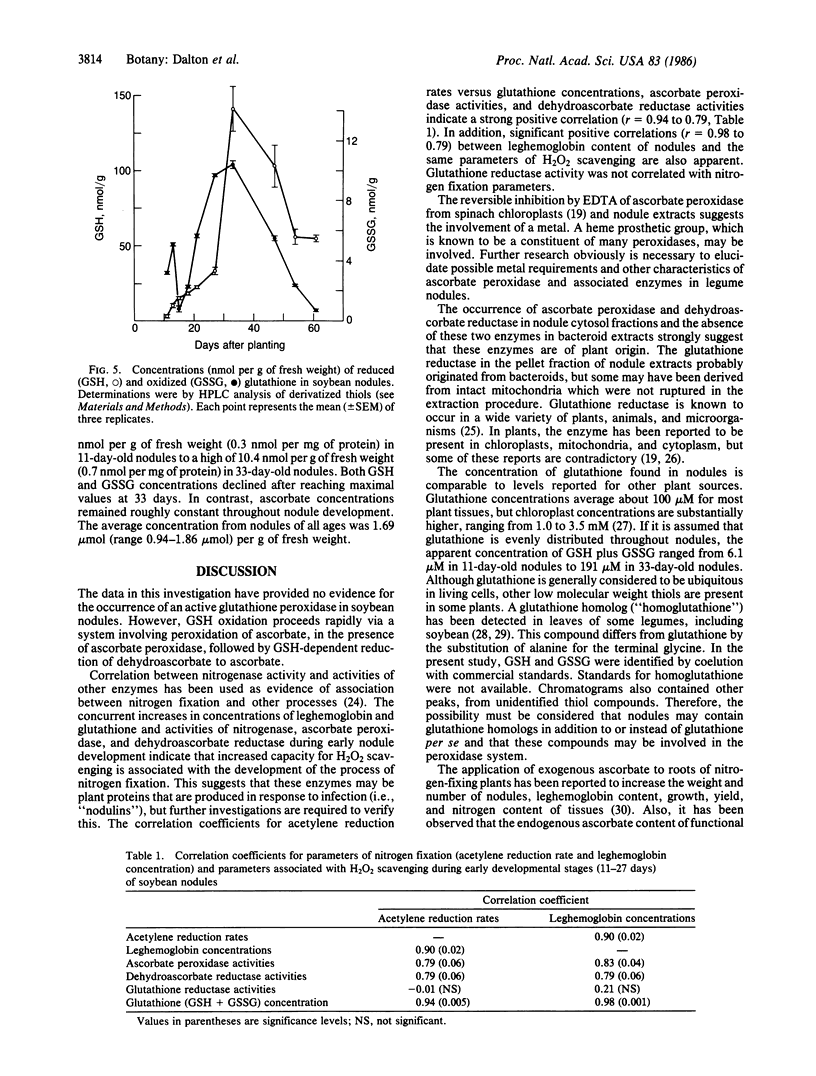
The critical problem of oxygen toxicity for nitrogen-fixing organisms may be related to damage caused by oxygen radicals and peroxides. An enzymatic mechanism is described for removal of peroxides in root nodules of soybean (Glycine max). The system utilizes ascorbate as an antioxidant and glutathione as a reductant to regenerate ascorbate. The enzymes involved are ascorbate peroxidase (ascorbate:hydrogen-peroxide oxidoreductase, EC 1.11.1.7), dehydroascorbate reductase (glutathione:dehydroascorbate oxidoreductase, EC 1.8.5.1), and glutathione reductase (NADPH:oxidized-glutathione oxidoreductase, EC 1.6.4.2). The reactions are essentially the same as those involving scavenging of H2O2 in chloroplasts. Glutathione peroxidase (glutathione:hydrogenperoxide oxidoreductase, EC 1.11.1.9) was not detected. During the course of early nodule development, ascorbate peroxidase and dehydroascorbate reductase activities and total glutathione contents of nodule extracts increased strikingly and were positively correlated with acetylene reduction rates and nodule hemoglobin contents. The evidence indicates an important role of glutathione, ascorbate, ascorbate peroxidase, dehydroascorbate reductase, and glutathione reductase as components of a peroxide-scavenging mechanism in soybean root nodules.
Keywords: nitrogen fixation, oxygen toxicity, antioxidant
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CARNEGIE P. R. ISOLATION OF A HOMOLOGUE OF GLUTATHIONE AND OTHER ACIDIC PEPTIDES FROM SEEDLINGS OF PHASEOLUS AUREUS. Biochem J. 1963 Dec;89:459–471. doi: 10.1042/bj0890459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevon J. J., Frazier L., Russell S. A., Evans H. J. Respiratory and Nitrogenase Activities of Soybean Nodules Formed by Hydrogen Uptake Negative (Hup) Mutant and Revertant Strains of Rhizobium japonicum Characterized by Protein Patterns. Plant Physiol. 1982 Nov;70(5):1341–1346. doi: 10.1104/pp.70.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Schubert K., Tolbert N. E. Isolation and characterization of infected and uninfected cells from soybean nodules : role of uninfected cells in ureide synthesis. Plant Physiol. 1983 Apr;71(4):869–873. doi: 10.1104/pp.71.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRICE C. A. A new thiol in legumes. Nature. 1957 Jul 20;180(4577):148–149. doi: 10.1038/180148a0. [DOI] [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Production of superoxide radicals by soluble hydrogenase from Alcaligenes eutrophus H16. Biochem J. 1981 Jan 1;193(1):99–107. doi: 10.1042/bj1930099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. E., Wilson C. W., 3rd Ascorbic acid content of some tropical fruit products determined by high-performance liquid chromatography. J Agric Food Chem. 1982 Mar-Apr;30(2):394–396. doi: 10.1021/jf00110a046. [DOI] [PubMed] [Google Scholar]
- Tappel A. L. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52:506–513. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- Tappel A. L. Selenium--glutathione peroxidase: properties and synthesis. Curr Top Cell Regul. 1984;24:87–97. [PubMed] [Google Scholar]
- Tel-Or E., Huflejt M., Packer L. The role of glutathione and ascorbate in hydroperoxide removal in cyanobacteria. Biochem Biophys Res Commun. 1985 Oct 30;132(2):533–539. doi: 10.1016/0006-291x(85)91166-0. [DOI] [PubMed] [Google Scholar]



