Abstract
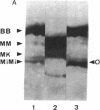
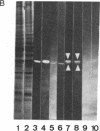
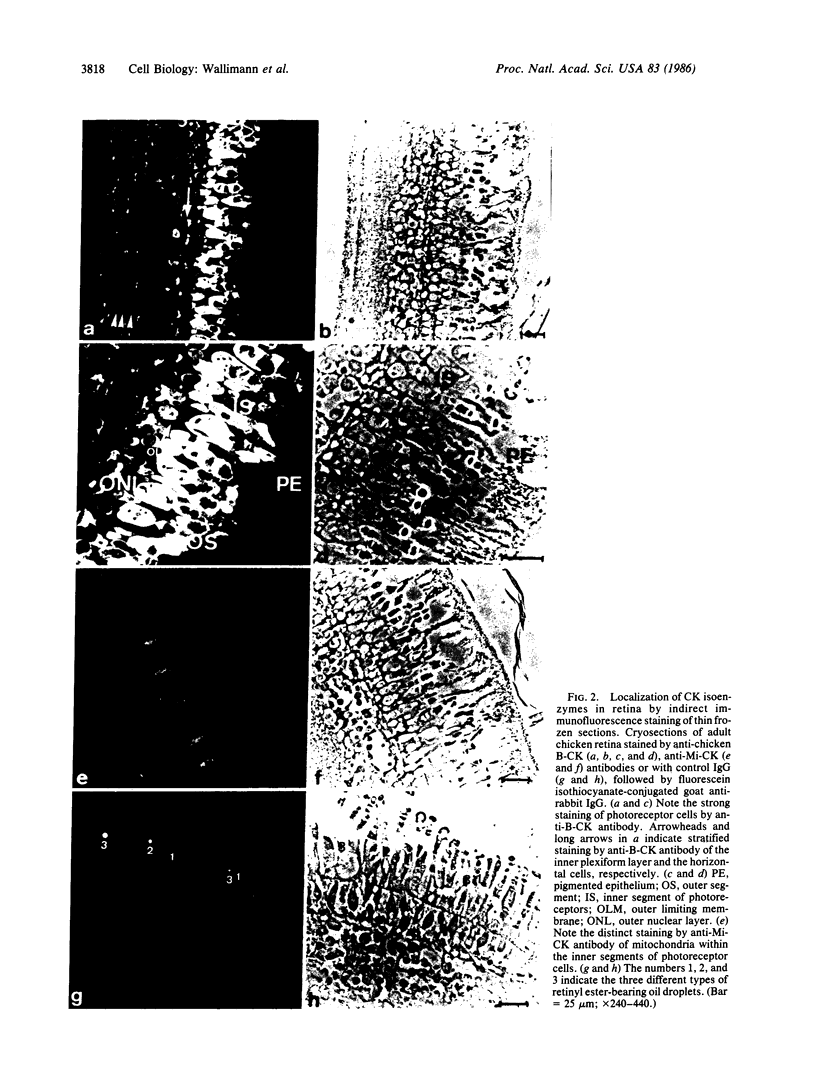
Two isoforms of creatine kinase (CK; ATP:creatine N-phosphotransferase, E.C. 2.7.3.2), brain type (BB-CK) and mitochondrial type (MiMi-CK), but not the muscle types (MM- or hybrid MB-CK), were identified by cellulose polyacetate electrophoresis and immunoblots in retina from adult chickens. Indirect immunofluorescence labeling of cryosections of retinas revealed high concentrations of BB-CK in both rod and cone photoreceptor cells. Most of the fluorescence staining with anti-B-CK antibodies was found within the myoid and the ellipsoid portions of inner segments and the peripheral region of the outer segments. Significant staining with anti-B-CK antibodies was also found in horizontal cells and in the optical nerve fibers, with additional stratified staining in the inner plexiform layer. MiMi-CK was solely demonstrated in the ellipsoid portion of the photoreceptor cells. The presence of high concentrations of compartmentalized CK isoenzymes within photoreceptor cells (approximately equal to 30 enzyme units/mg) as well as the relatively high concentration of total creatine in these cells (approximately equal to 10-15 mM) indicates an important physiological function for CK and phosphocreatine in the energy transduction of vision.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour R. L., Sotak C. H., Levy G. C., Chan S. H. Use of gated perfusion to study early effects of anoxia on cardiac energy metabolism: a new 31P NMR method. Biochemistry. 1984 Dec 4;23(25):6053–6062. doi: 10.1021/bi00320a024. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J., Mieskes G., Wallimann T. Creatine kinase activity in the Torpedo electrocyte and in the nonreceptor, peripheral v proteins from acetylcholine receptor-rich membranes. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5440–5444. doi: 10.1073/pnas.80.17.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981 Jan 30;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Borroni E. Role of creatine phosphate in the discharge of the electric organ of Torpedo marmorata. J Neurochem. 1984 Sep;43(3):795–798. doi: 10.1111/j.1471-4159.1984.tb12801.x. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Rubin L. J. A direct demonstration that inositol-trisphosphate induces an increase in intracellular calcium in Limulus photoreceptors. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1137–1142. doi: 10.1016/0006-291x(84)91402-5. [DOI] [PubMed] [Google Scholar]
- Cohen A., Buckingham M., Gros F. A modified assay procedure for revealing the M form of creatine kinase in cultured muscle cells. Exp Cell Res. 1978 Aug;115(1):201–206. doi: 10.1016/0014-4827(78)90417-2. [DOI] [PubMed] [Google Scholar]
- Dontsov A. E., Zak P. P., Ostrovskii M. A. Regeneratsiia ATP v naruzhnykh segmentakh fotoretseptorov liagushki. Biokhimiia. 1978;43(4):592–596. [PubMed] [Google Scholar]
- Eggleton P., Elsden S. R., Gough N. The estimation of creatine and of diacetyl. Biochem J. 1943;37(5):526–529. doi: 10.1042/bj0370526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Lehninger A. L. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J Biol Chem. 1973 Jul 10;248(13):4803–4810. [PubMed] [Google Scholar]
- Korenbrot J. I. Signal mechanisms of phototransduction in retinal rod. CRC Crit Rev Biochem. 1985;17(3):223–256. doi: 10.3109/10409238509113605. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Brien D. F. The chemistry of vision. Science. 1982 Dec 3;218(4576):961–966. doi: 10.1126/science.6291153. [DOI] [PubMed] [Google Scholar]
- Porrello K., Cande W. Z., Burnside B. N-ethylmaleimide-modified subfragment-1 and heavy meromyosin inhibit reactivated contraction in motile models of retinal cones. J Cell Biol. 1983 Feb;96(2):449–454. doi: 10.1083/jcb.96.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks V. A., Rosenshtraukh L. V., Smirnov V. N., Chazov E. I. Role of creatine phosphokinase in cellular function and metabolism. Can J Physiol Pharmacol. 1978 Oct;56(5):691–706. doi: 10.1139/y78-113. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Metabolite channeling: a phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell. 1985 May;41(1):325–334. doi: 10.1016/0092-8674(85)90085-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. C., Wallimann T., Eppenberger H. M. A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):702–705. doi: 10.1073/pnas.70.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A., Montal M. Light-regulated biochemical events in invertebrate photoreceptors. 2. Light-regulated phosphorylation of rhodopsin and phosphoinositides in squid photoreceptor membranes. Biochemistry. 1984 May 22;23(11):2347–2352. doi: 10.1021/bi00306a004. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Eppenberger H. M. Localization and function of M-line-bound creatine kinase. M-band model and creatine phosphate shuttle. Cell Muscle Motil. 1985;6:239–285. doi: 10.1007/978-1-4757-4723-2_8. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Moser H., Zurbriggen B., Wegmann G., Eppenberger H. M. Creatine kinase isoenzymes in spermatozoa. J Muscle Res Cell Motil. 1986 Feb;7(1):25–34. doi: 10.1007/BF01756199. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Schlösser T., Eppenberger H. M. Function of M-line-bound creatine kinase as intramyofibrillar ATP regenerator at the receiving end of the phosphorylcreatine shuttle in muscle. J Biol Chem. 1984 Apr 25;259(8):5238–5246. [PubMed] [Google Scholar]
- Wallimann T., Turner D. C., Eppenberger H. M. Localization of creatine kinase isoenzymes in myofibrils. I. Chicken skeletal muscle. J Cell Biol. 1977 Nov;75(2 Pt 1):297–317. doi: 10.1083/jcb.75.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Walzthöny D., Wegmann G., Moser H., Eppenberger H. M., Barrantes F. J. Subcellular localization of creatine kinase in Torpedo electrocytes: association with acetylcholine receptor-rich membranes. J Cell Biol. 1985 Apr;100(4):1063–1072. doi: 10.1083/jcb.100.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. Passage of newly formed protein through the connecting cilium of retina rods in the frog. J Ultrastruct Res. 1968 Jun;23(5):462–473. doi: 10.1016/s0022-5320(68)80111-x. [DOI] [PubMed] [Google Scholar]