Abstract
Spontaneous aortic dissection in pregnancy is rare and life threatening for both the mother and the fetus. Most commonly, it is associated with connective tissue disorders, cardiac valve variants, or trauma. We present the case of a 23-year-old previously healthy woman, 36 weeks pregnant with a syncopal episode after dyspnea and vomiting. She subsequently developed cardiac arrest and underwent aggressive resuscitation, emergent thoracotomy, and cesarean delivery without recovery. On autopsy, she was found to have an aortic dissection of the ascending aorta. This case is presented to raise awareness and review the literature and the clinical approach to critical care for pregnant patients.
INTRODUCTION
Syncope and cardiopulmonary arrest in pregnancy narrow the differential diagnosis to the most severe, life-threatening clinical etiologies. The most common causes are venous thromboembolism and cardiac arrhythmias, followed by a broad differential diagnosis, including severe pregnancy-induced hypertension (pre-eclampsia/eclampsia), severe sepsis, amniotic fluid embolism, hemorrhage, trauma, and pre-existing heart disease.1,2
Aortic dissection in pregnancy is a rare, life-threatening condition most often associated with genetic or anatomic predisposition, such as Marfan syndrome or bicuspid aorta. A review in 2003 by Immer et al3 found that more than 50% of pregnant patients with aortic dissection had Marfan syndrome. However, even in the absence of risk factors, aortic dissection needs to be considered when evaluating syncope in the pregnant patient. We present a case of nontraumatic spontaneous aortic dissection in a previously healthy pregnant patient. We also review the clinical literature regarding the evaluation and management of a pregnant patient presenting in cardiac arrest with suspicion of aortic dissection.
CASE REPORT
A 23-year-old previously healthy Caucasian woman, G2P0A1, presented at 36 weeks gestation to the emergency department (ED) after sudden onset of dyspnea, nausea, and vomiting for 5 minutes, then collapse and unresponsiveness. Her pregnancy had been complicated by pregnancy-induced hypertension treated with labetalol 400 mg 3 times daily. She had no other medical, surgical, or drug-use history. On arrival, she was agitated, altered, uncooperative, and moving all extremities. Her heart rate was 151 beats per minute, blood pressure 118/78 mmHg, respiratory rate 32 breaths per minute, with SpO2 86% on a nonrebreather mask. The patient did not appear Marfanoid or dysmorphic, with a height of 5′7″ and weight 147 lbs. On physical exam, the patient had perioral cyanosis, tachycardia with distant heart sounds, no edema, and gravid abdomen.
The patient immediately underwent rapid sequence orotracheal intubation and was mechanically ventilated. Her initial hemoglobin (HemoCue) was 12.5 g/dL. The patient quickly became pulseless with pulseless electrical activity (PEA). Chest compressions were initiated. A bedside ultrasound examination showed a large clot inside the pericardium, pericardial fluid, and empty, poorly contracting ventricles. A pericardiocentesis was performed with 5 mL of blood aspirated. A thoracotomy was immediately performed by the ED physicians. The obstetrical team concurrently performed an emergent perimortem cesarean delivery. A baby boy was delivered, intubated, and transferred to the Neonatal Intensive Care Unit.
The thoracotomy had findings of blood in the pericardial sac and a boggy, fibrillating heart with no evidence of active bleeding or cardiac injury. Cardiac massage was initiated resulting in palpable carotid pulses. This was followed by intracardiac epinephrine injection, internal defibrillation, and intravenous bicarbonate infusion.
At this time, the cardiothoracic surgical team arrived. On further exploration of the chest cavity, the source of bleeding could not be isolated. The cardiothoracic surgeons then inserted a thoracostomy tube into the right chest, which drained 100 mL of serous fluid. The patient continued to decompensate and was pronounced dead 1 hour after her initial presentation to the ED.
On autopsy, the patient was found to have a 0.5-cm tear in the aortic adventitial tissue and a 3-cm ragged tear in the posterolateral aorta superior to the aortic valve and extending 3 cm vertically. The tear was not consistent with chest compressions performed during cardiopulmonary resuscitation. The heart had severe left ventricle hypertrophy and weighed 450 gm, which was much larger than the expected 250 gm.
The baby boy was discharged a month later to the care of his father.
DISCUSSION
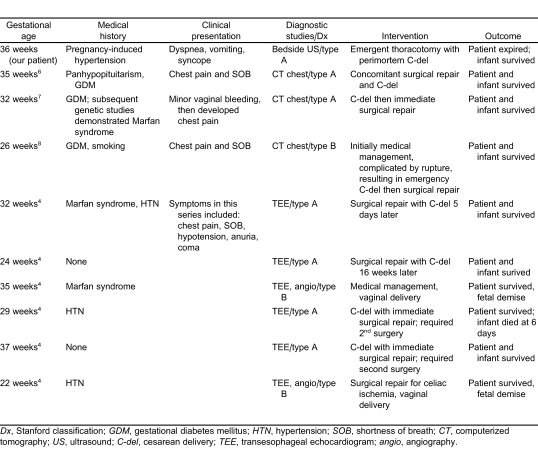
Dissection of the aorta is characteristically a disease associated with multiple risk factors and is very rare in young women under the age of 40 years.4 A review of the International Registry of Acute Aortic Dissection showed only 2 of 346 women were pregnant. The most common risk factors for women were older age and chronic hypertension.5 This patient's case is unique in that she had no known risk factors predisposing her to aortic dissection compared to other published cases (Table).
Aortic dissection in pregnancy occurs most commonly in the third trimester due to the hyperdynamic state and hormonal effect on vasculature.9 It often presents with sudden severe, tearing chest pain, vomiting, and syncope, most often from acute pericardial tamponade.10 Bronchospasm may also occur due to irritation of the vagal nerve from the intimal tearing. A diastolic murmur would signify aortic insufficiency.
Emergency physicians are required to have skills to resuscitate the pregnant patient in cardiopulmonary arrest and to diagnose the underlying cause. Transesophageal echocardiography (TEE) is the gold standard for diagnosing aortic aneurysm or dissection.1 However, due to the critical condition of the patient and the lack of immediate access, TEE is less likely to be available in the ED setting. Bedside ultrasound is readily available, and visualization of pericardial effusion by ultrasound has a high specificity.11 An electrocardiogram may be normal or show signs of left ventricular hypertrophy or acute myocardial infarction if the coronary arteries are involved. Abnormalities on chest radiography are only apparent in 85% of cases.1 Thus, for a critically ill pregnant patient with suspected aortic dissection presenting in the acute setting, ultrasound visualization of pericardial effusion is the most efficient, effective method of diagnosis and facilitates early therapeutic intervention.
Pericardiocentesis is the initial treatment indicated to relieve external pressure on the heart and regain cardiac output.12 This is performed either using the subxiphoid blind approach or with transthoracic ultrasound-guided entry through the chest wall, using a large bore needle. In this case, the pericardiocentesis produced only 5 mL of blood on aspiration with no resolution of the PEA rhythm. Due to the hypercoagulable state of pregnancy, the blood surrounding the heart clots quickly, thus causing cardiac tamponade.
An open thoracotomy is indicated to evacuate clots and resolve pericardial tamponade that cannot be drained via pericardiocentesis.13 Thoracotomy has a poor prognosis for successful resuscitation and is a last-resort therapy for a patient with cardiac arrest and pericardial tamponade.
With the onset of cardiopulmonary arrest, emergent cesarean delivery in the pregnant patient beyond 24 weeks gestation is recommended. After 4 minutes of resuscitation without improvement, the cesarean delivery should be performed.2,14,15 In a study by Katz et al2 in 2005, no patient undergoing perimortem cesarean delivery was found to have worsening hemodynamic status postdelivery. Prior to 24 weeks gestation, the risk of fetal demise is high, and delivery does not demonstrate improvement in maternal circulation.2 In our case, the patient did have initial improvement in hemodynamic stability after successful emergent cesarean delivery. However, continued resuscitative efforts were unsuccessful.
Our patient was remarkably different from other pregnant patients reported in the literature. She had no evidence of Marfan syndrome or other connective tissue disorders, no bicuspid valve on autopsy, and no prior history of chronic hypertension. Most cases in the literature focus on patients that are at high risk for aortic dissection and discuss appropriate surgical intervention prior to acute complications. In the ED setting, it is important to maintain a high suspicion for this diagnosis as this represents one of the rapidly fatal causes of death in pregnancy. Early intervention may lead to successful treatment. In the case described, all the procedures and interventions were performed within 1 hour of presentation, and although the patient was unable to be resuscitated, it resulted in the survival of her infant.
CONCLUSION
Pregnant patients with syncope warrant immediate evaluation for possible life-threatening events. This case report of a pregnant patient with no risk factors for aortic dissection emphasizes the importance of maintaining a broad differential diagnosis and utilizing the necessary clinical tools to further direct patient care and necessary interventions.
Footnotes
Supervising Section Editor: Rick A. McPheeters, DO
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
Table.
Comparison of case reports of pregnant patients with thoracic aortic aneurysm/dissection.
REFERENCES
- 1.Chen K, Varon J, Wenker OC, et al. Acute thoracic aortic dissection: the basics. J Emerg Med. 1997;;15:859–867. doi: 10.1016/s0736-4679(97)00196-0. [DOI] [PubMed] [Google Scholar]
- 2.Katz V, Balderston K, DeFreest M. Perimortem cesarean delivery: were our assumptions correct? Am J Obstet Gynecol. 2005;;192:1916–1920. doi: 10.1016/j.ajog.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003;;76:309–314. doi: 10.1016/s0003-4975(03)00169-3. [DOI] [PubMed] [Google Scholar]
- 4.Zeebregts CJ, Schepens MA, Hameeteman TM, et al. Acute aortic dissection complicating pregnancy. Ann Thorac Surg. 1997;;64:1345–1348. doi: 10.1016/S0003-4975(97)00916-8. [DOI] [PubMed] [Google Scholar]
- 5.Nienaber CA, Fattori R, Mehta RH, et al. Gender-related differences in acute aortic dissection. Circulation. 2004;;109:3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C. [DOI] [PubMed] [Google Scholar]
- 6.Shihata M, Pretorius V, MacArthur R. Repair of an acute type A aortic dissection combined with an emergency cesarean section in a pregnant woman. Interact Cardiovasc Thorac Surg. 2008;;7:938–940. doi: 10.1510/icvts.2008.182766. [DOI] [PubMed] [Google Scholar]
- 7.Papatsonis DN, Heetkamp A, van den Hombergh C, et al. Acute type A aortic dissection complicating pregnancy at 32 weeks: surgical repair after cesarean section. Am J Perinatol. 2009;;26:153–157. doi: 10.1055/s-0028-1095184. [DOI] [PubMed] [Google Scholar]
- 8.Stout CL, Scott EC, Stokes GK, et al. Successful repair of a ruptured Stanford type B aortic dissection during pregnancy. J Vasc Surg. 2010;;51:990–992. doi: 10.1016/j.jvs.2009.10.121. [DOI] [PubMed] [Google Scholar]
- 9.Collins D. Aetiology and management of acute cardiac tamponade. Crit Care Resusc. 2004;;6:54–58. [PubMed] [Google Scholar]
- 10.Kim TE, Smith DD. Thoracic aortic dissection in an 18-year-old woman with no risk factors. J Emerg Med. 2010;;38:e41–e44. doi: 10.1016/j.jemermed.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez C, Shuler K, Hannan H, et al. C.A.U.S.E.: cardiac arrest ultra-sound exam—a better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation. 2008;;76:198–206. doi: 10.1016/j.resuscitation.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Seferovic PM, Ristic AD, Imazio M, et al. Management strategies in pericardial emergencies. Herz. 2006;;31:891–900. doi: 10.1007/s00059-006-2937-0. [DOI] [PubMed] [Google Scholar]
- 13.Harper RJ. Roberts JR, Hedges JR, editors. Pericardiocentesis. Clinical Procedures in Emergency Medicine. 4th ed. Philadelphia, PA: Saunders; 2004. pp. 305–322.
- 14.Warraich Q, Esen U. Perimortem caesarean section. J Obstet Gynaecol. 2009;;29:690–693. doi: 10.3109/01443610903165511. [DOI] [PubMed] [Google Scholar]
- 15.Atta E, Gardner M. Cardiopulmonary resuscitation in pregnancy. Obstet Gynecol Clin North Am. 2007;;34:585–97. doi: 10.1016/j.ogc.2007.06.008. xiii. [DOI] [PubMed] [Google Scholar]